Abstract
RNA-binding proteins (RBPs) play a major role in control of mRNA turnover and translation rates. We examined the role of the RBP human antigen R (HuR) during cholestatic liver injury and hepatic stellate cells (HSC) activation. HuR silencing attenuated fibrosis development in vivo after BDL, reducing liver damage, oxidative stress, inflammation, and collagen and α-SMA (α-smooth muscle actin) expression. HuR expression increased in activated HSC from BDL mice and during HSC activation in vitro, and HuR silencing markedly reduced HSC activation. HuR regulated platelet-derived growth factor (PDGF)-induced proliferation and migration, and controlled expression of several mRNAs involved in these processes (Actin, MMP9, Cyclin D1 and B1). These functions of HuR were linked to its abundance and cytoplasmic localisation, controlled by PDGF, via ERK and PI3K activation, and ERK-LKB1 activation respectively. More importantly, we identified the tumor suppressor LKB1 as a novel downstream target of PDGF-induced ERK activation in HSC. HuR also controlled transforming growth factor beta (TGF-β-induced profibrogenic actions by regulating expression of TGF-β, α-SMA, and p21. This was likely due to an increased cytoplasmic localisation of HuR, controlled by TGF-β-induced p38 MAPK activation. Finally, we found that HuR and LKB1 (Ser428) levels were highly expressed in activated HSC in human cirrhotic samples. Conclusion: Our results show that HuR is important for pathogenesis of liver fibrosis development in the cholestatic injury model, for HSC activation, and for the response of activated HSC to PDGF and TGF-β.
Keywords: TGF-β, PDGF, HSC, LKB1, cirrhosis
INTRODUCTION
Hepatic fibrosis is the common consequence of chronic liver diseases such as viral and autoimmune hepatitis, alcohol consumption, biliary obstruction, and non-alcoholic fatty liver disease (1). Hepatic stellate cells (HSC) are the major producers of collagen in the damaged liver (2). In healthy liver, HSC have a quiescent phenotype, accumulating retinoids (vitamin A) and expressing markers characteristic of adipocytes (3). After continued liver damage, these quiescent HSC are exposed to apoptotic hepatocytes, ROS, and inflammatory and profibrogenic factors, and undergo a process of activation to a myofibroblastic phenotype. These activated HSC increase proliferation and migration, acquire contractility and pro-inflammatory properties, and express myogenic markers like α-SMA to become the major col1a1-producing cells (4).
In the liver, levels of many mRNAs are regulated in response to fibrosis-inducing injuries (5). RBPs can promote rapid spatiotemporal expression of proteins by binding to U-and AU-rich elements (AREs) in mRNAs (6). HuR, a member of the Hu/Elav family, is a ubiquitously expressed RBP that is predominantly (>90%) localized in the nucleus of most unstimulated cells. In response to proliferative, stress, apoptotic, differentiation, senescence, inflammatory and immune stimuli, HuR is exported to the cytoplasm, increasing the half-life and/or the rate of translation of target mRNAs (6). Several studies have shown that HuR has important functions in hepatocytes, including HGF-induced hepatocyte proliferation (7), differentiation (8) and apoptosis (9), and during hepatocyte malignant transformation (8,10). Also, HuR expression is upregulated in HCC tissue compared to normal tissues (10), suggesting that it could represent a novel target for liver damage research.
The aims of the current work were to study the role of HuR in liver fibrosis and in HSC activation, and examine its role in controlling the functions of two principal mediators of HSC activation, PDGF and TGF-β.
MATERIAL AND METHODS
Reagents
TGF-β and PDGF were from Peprotech. SB203580 and BAY 11-7082 were from Calbiochem, U0126 from Promega and LY 294002 from SIGMA.
Human Samples
Surgically resected liver tumor specimens from 16 cirrhotic patients (hepatitis C, n=7; alcoholic, n= 9) were examined. Informed consent to all clinical investigations, in accordance with the principles in the Declaration of Helsinki, was provided. The institutional review board of the Hospital Clínic de Barcelona approved the protocol.
HSC Isolation
Animals were maintained in the CIC bioGUNE animal facility with appropriate approvals from the institutional review committee on animal use. HSC were isolated from liver of male Sprague-Dawley rats, bile duct ligated (BDL) and sham operated mice as described (11).
Bile duct ligation
BDL was performed in 12-week-old mice by tying the common bile duct using a non-absorbable filament. Mice (n=8) were injected via the tail vein with 200 μl of a 0.75 μg/μl solution of HuR specific Sh RNA (sense 5′-gatgcagagagagcaatca-3′) or control Sh RNA (pSM2c Open Biosystems).
Carbon Tetrachloride (CCl4) treatment
Rats (n=5) were treated with CCl4 diluted 1:1 in corn oil (0.5 μl of CCl4/g b.wt) by intraperitoneal injection twice a week for 6 weeks. Control animals received vehicle alone (n=5).
Viral Infection
Cells were treated with short-hairpin lentiviral particles against HuR [CCGGCCCACAAATGTTAGACCAATTCTCGAGAATTGGTCTAACATTTGTGGGTTTTTG], or against LKB1 [CCGGCATCTACACTCAGGACTTCACCTCGAGGTGAAGTCCTGAGTGT-AGATGTTTTT] in the presence of hexadimethrine bromide (8 μg/ml). For control cells, HSC were infected with pLKO.1 lentiviral vector (SIGMA). After 24h transduction, the cells were selected using puromycin (1.25 μg/ml).
Migration assay
Migration using the “scratch-assay” was performed in LKB1- and HuR-silenced cells seeded onto PDL-coated dishes, as described (13).
RNA isolation and real-time PCR (qPCR)
PCR was performed with primers described in Supplementary Table I.
RNA immunoprecipitation-qPCR (RIP-qPCR)
Immunoprecipitation (IP) of endogenous RNA–protein complexes were performed as described (8,10).
Western blot analysis
Total proteins were extracted in RIPA buffer. Cytoplasmic and nuclear lysates were prepared with the subcellular proteome extraction kit (Calbiochem). Immunoblotting analysis was performed with specific antibodies (Supplementary Table II).
Immunohistochemistry
Detailed immunohistochemistry protocol of paraffin-embedded sections is provided in Supplemental Material and Methods.
RESULTS
HuR expression in HSC from human chronic liver diseases
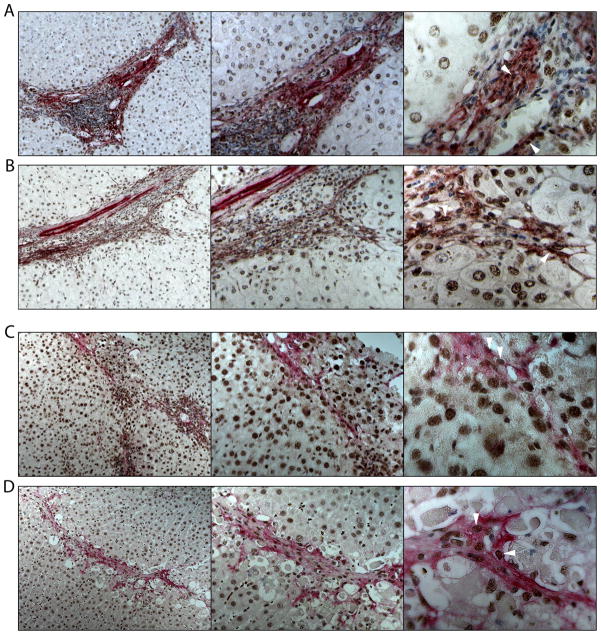
We found that activated HSC (α-SMA+ cells) strongly expressed HuR in surgically resected liver samples from patients with alcoholic (Figure 1A) and hepatitis C (VHC) cirrhosis (Figure 1B). Similarly, activated HSC expressed HuR in the nucleus of liver sections from two animal models of induced fibrosis, bile duct ligated (BDL) mice (Figure 1C) and rats treated with CCl4 (5) (Figure 1D), suggesting that HuR could play a role during HSC activation.
Figure 1. HuR in human cirrhosis, BDL-mice and CCl4-treated rats.
IHC showing expression of HuR (DAB+ cells, brown, arrowheads) in activated HSC (α-SMA+ cells, alkaline phosphatase, red) in liver sections from patients with (A) alcoholic and (B) VHC cirrhosis. Representative pictures of 9 alcoholic and 7 VHC samples are shown. (C), BDL-mice, 7 days after surgery (n=5) (D) and CCl4-treated rats (n=5).
HuR silencing attenuated hepatic fibrosis in BDL-mice
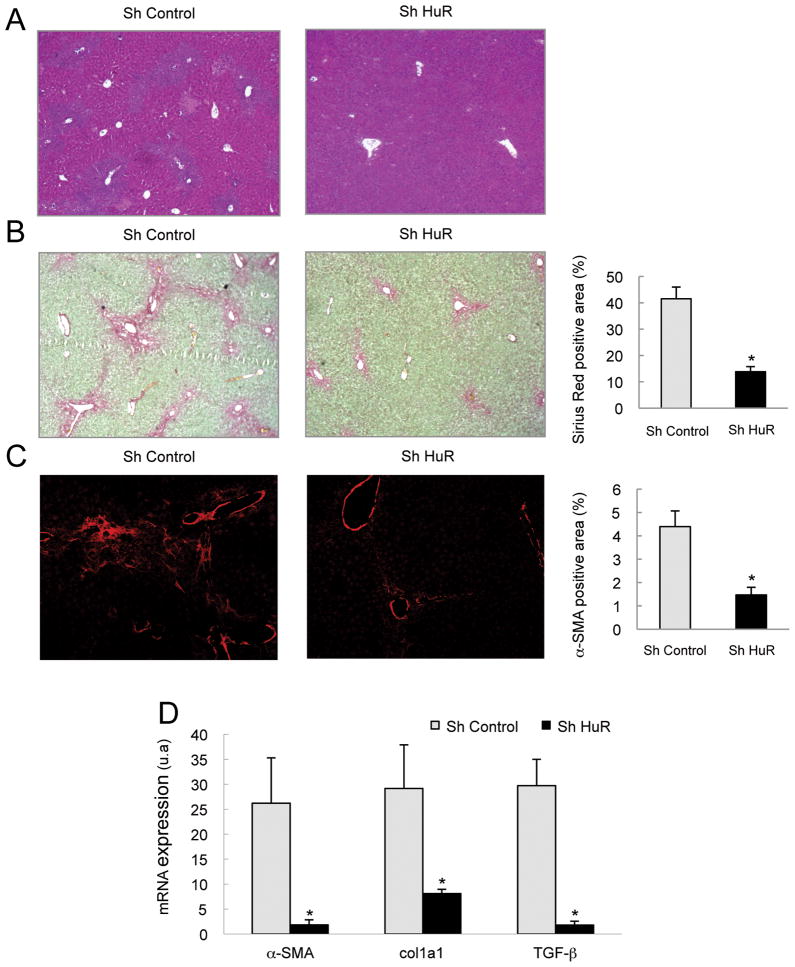
To confirm the role of HuR in liver fibrosis, we silenced HuR in vivo in BDL mice. Thus, mice were injected in the tail vein with a HuR-specific or control Sh RNA at time 0h, days 3 and 6 after BDL, and then sacrificed 9 days after BDL. HuR silencing was confirmed by RT-PCR and Western Blot in whole liver extracts (Supplementary Figures 1A and 1B) and specifically in HSC by immunohistochemistry (Supplementary Figures 1C). HuR silencing resulted in reduced histological liver damage, as seen by hematoxilin/eosin staining (Figure 2A) and decreased ALT and bilirubin serum levels (Supplementary Figure 1D and 1E). Notably, fibrosis development in these mice was significantly attenuated as shown by reduced collagen deposition (Figure 2B), α-SMA expression (Figure 2C), and col1a1, α-SMA and TGF-β mRNA levels (Figure 2D).
Figure 2. HuR silencing attenuated liver damage and hepatic fibrosis in BDL-mice.
Livers from BDL mice, injected with Sh control or Sh HuR plasmids, were extracted and the following analyses performed: (A) Hematoxilin/eosin staining, (B) Sirius red staining and quantification of positive areas (C) α-SMA immunostaining and quantification of positive areas and (D) qPCR of fibrogenic genes. (*p<0.05).
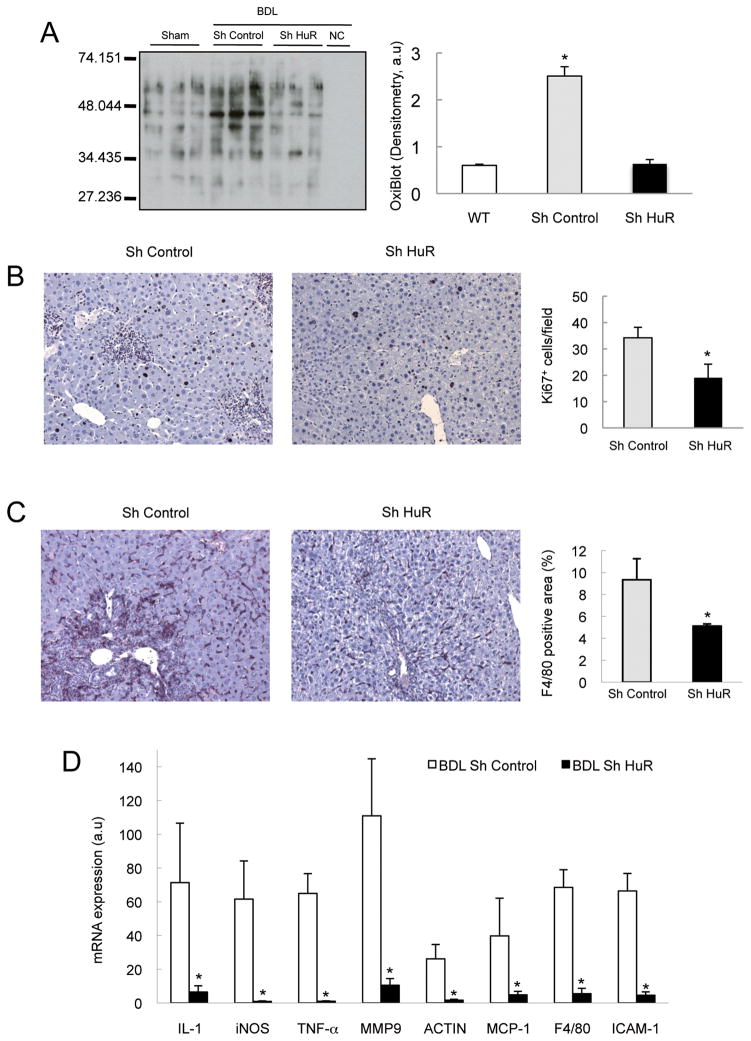
HuR silencing also led to reduced protein oxidation (Figure 3A and Supplementary Figure 1F and 1G), proliferation (Figure 3B), macrophage infiltration (Figure 3C), and lower expression of genes involved in inflammation (iNOS, IL-1α, TNF-α) and infiltration (MCP-1, F4/80, ICAM-1, MMP9 and Actin) (Figure 3D). Altogether, our results suggest that HuR plays a crucial role in the pathogenesis of cholestatic liver injury.
Figure 3. HuR silencing attenuated oxidative stress and hepatic inflammation in BDL- mice.
(A) Quantification of protein oxidation by WB, (B) analysis of proliferation by Ki67 IHC and quantification of positive cells, (C) analysis of macrophage infiltration determined by F4/80 IHC and quantification of positive areas (D) qPCR of inflammatory genes. (*p<0.05).
HuR expression during HSC activation
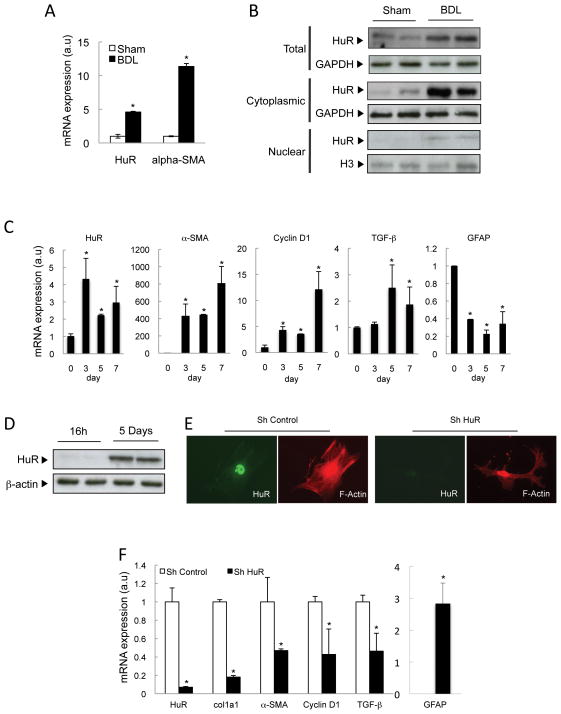
The data above suggest that HuR could be regulating HSC activation and fibrosis development either directly, and/or indirectly by a decrease in liver damage and inflammation. To characterize the effect of HuR in HSC activation only, we examined its expression in primary HSC isolated from sham and BDL mice, nine days after surgery. HuR mRNA levels increased in HSC isolated from BDL mice, correlating with HSC activation, as seen by induction of α-SMA mRNA expression (Figure 4A). Total, cytoplasmic and nuclear HuR protein levels were also upregulated (Figure 4B).
Figure 4. HuR is expressed in activated HSC.
(A) qPCR and (B) WB analysis in HSC from sham and BDL mice 9 days after surgery. (C) qPCR and (D) WB analysis in culture-activated HSC. (E) HuR and F-actin staining and (F) qPCR analysis of selected mRNAs after HuR silencing in culture-activated HSC (7 days). *p<0.05
Similarly, during in vitro activation of primary HSCs on plastic surface (14), HuR mRNA levels also increased after 3, 5 and 7 days in culture compared to quiescent HSC (day 1), correlating with HSC activation, as seen by induction of α-SMA, cyclin D1 and TGF-β, and down-regulation of GFAP (Glial fibrillary acidic protein) expression (Figure 4C). This up-regulation of HuR was confirmed by Western blotting in 5-days cultured HSC compared to quiescent HSC (Figure 4D). HuR silencing in primary HSC, as confirmed by immunocytochemsitry (Figure 4E), induced morphological changes (F-actin immunostaining) (Figure 4E), significantly reduced levels of activation (α-SMA, col1a1 and TGF-β) and proliferation markers (cyclin D1), and markedly increased expression of the quiescent marker GFAP (15) (Figure 4F). Taken together, our data show that HuR could play a role during HSC activation.
Role of HuR in PDGF-induced migration and proliferation
We next examined if HuR activity controlled the functions of two principal mediators of HSC activation, PDGF and TGF-β. PDGF potently promotes HSC migration and proliferation during fibrosis (16). HuR silencing in primary HSC isolated from BDL mice (Supplementary Figure 2A) significantly reduced their migratory rate both basally (Supplementary Figure 2C) and after PDGF treatment (Supplementary Figure 2D), and decreased BrDU incorporation after PDGF stimulation (Supplementary Figure 2E).
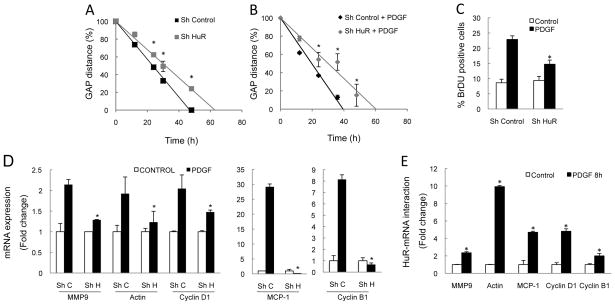
HuR silencing in a cell line of activated HSC, CFSC-8B cells (12) (Supplementary Figure 2B) also blocked PDGF-induced migration and proliferation (Figure 5A and 5B). In CFSC-8B cells, HuR silencing prevented PDGF-induced increase in mRNA levels of genes regulating proliferation (cyclin D1 and B1), migration [MMP9 and Actin (17)], and infiltration [MCP-1 (18)] (Figure 5D) as well as cyclin D1 protein (Supplementary Figure 2B). RIP-qPCR analyses revealed a significantly increased binding of HuR to these mRNAs after PDGF stimuli (Figure 5E).
Figure 5. PDGF-induced responses are mediated by HuR.
HuR silencing in CFSC-8B cells reduced PDGF-mediated (A,B) migration, as shown by slower rate of the gap closure at different time-points, (C) proliferation, as shown by percentage of BrDU+ cells, 24h after treatment, (D) up-regulation of specific mRNAs (Sh C = Sh Control, Sh H = Sh HuR). (E) RIP-qPCR showing binding of selected genes with HuR after PDGF stimulation. *p<0.05.
These data demonstrate the importance of HuR in PDGF-mediated HSC proliferation and migration.
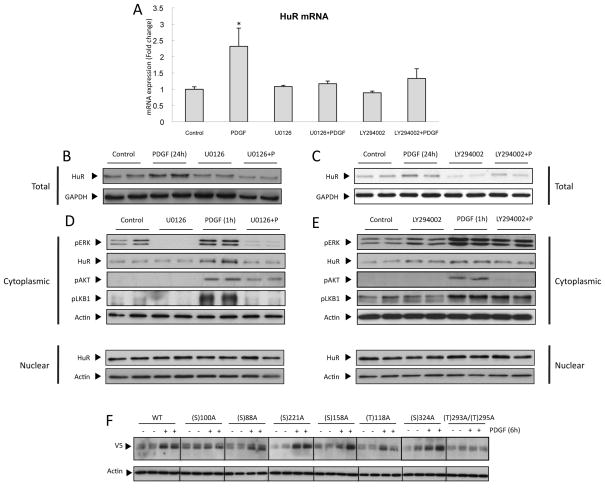
Role of PI3K and ERK in PDGF-induced HuR
The abundance and subcellular localisation of HuR are important determinants of its activity (19,20). PDGF treatment increased the expression of HuR mRNA (Figure 6A) and protein (Figure 6B and 6C) levels in CFSC-8B cells as well as its cytoplasmic localization (Figure 6D). Inhibition of both ERK and PI3K blocked PDGF-induced up-regulation of HuR mRNA and protein (Figure 6A, 6B and 6C), thus controlling HuR abundance. Recently, it was reported that HuR transcription is controlled by NFκB/p65 (21). We found that both ERK and PI3K induced nuclear translocation of the NFκB subunit p65 in response to PDGF (Supplementary Figure 3A and 3C), and inhibition of this translocation by BAY117802 treatment prevented PDGF-mediated up-regulation of HuR protein expression (Supplementary Figure 3B and 3D).
Figure 6. Role of PI3K and ERK in PDGF-induced HuR.
(A) qPCR and (B,C) WB analysis of total protein extracts showing that ERK inhibition using UO126 and PI3K inhibition using LY-294002, prevents PDGF-induced increase in HuR expression. (D,E) WB showing that ERK inhibition, but not PI3K inhibition, prevents PDGF-induced HuR cytoplasmic translocation. (F) WB showing V5 expression in cytoplasmic extracts of PDGF-treated CFSC-8B cells, transfected with plasmids containing wild-type or mutant HuR. *p<0.05.
Conversely, we found that cytoplasmic localisation of HuR was mediated by the ERK pathway only, and not by the PI3K pathway, unlike above (Figure 6D and 6E, Supplementary Figure 3E). Post-translational modifications of HuR such as phosphorylation play an important role in its subcellular localisation (19,20). We performed mutagenesis of six serine (S) and two threonines (T) residues to the non-phosphorylable residue alanine (A) of HuR protein. Mutation of serine residue 100 and threonine residues 293 or 295 prevented the translocation to the cytosol of the mutant protein after PDGF treatment (Figure 6F and Supplementary Figure 3F), without affecting the nuclear levels (data non shown) suggesting that these phosphorylation sites are important for PDGF-induced HuR nucleo-cytoplasmic shuttling.
Role of p-LKB1 in PDGF induced HuR
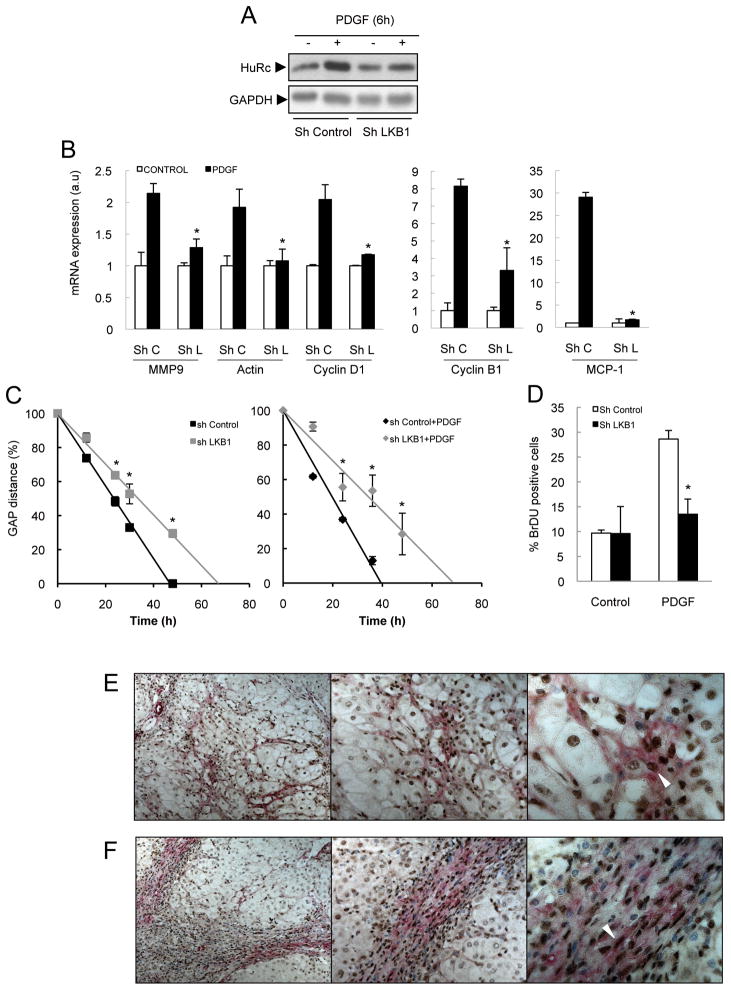
Recent studies have shown that PDGF induces LKB1 (Ser428) phosphorylation, via ERK-induced activation, in a cell type dependent manner (22). Here, using the CFSC-8B cell line, we found that PDGF-induced LKB1 phosphorylation was blocked by the MEK inhibitor U0126 (Figure 6D and Supplementary Figure 3E). No regulation by the PI3-Kinase inhibitor LY294002 was observed (Figure 6E and Supplementary Figure 3E). LKB1 silencing did not affect PDGF-induced ERK and AKT phosphorylation (Supplementary Figure 4A), showing that LKB1 is a downstream kinase of ERK. Importantly, LKB1 knockdown (Supplementary Figure 4A) prevented HuR cytoplasmic localization (Figure 7A and Supplementary Figure 4B), and blocked PDGF-induced cyclin D1 protein expression (Supplementary Figure 4C and 4D), and MMP9, actin, MCP-1, cyclin D1 and cyclin B1 mRNA expression (Figure 7B). Finally, basal and PDGF-induced HSC migration (Figure 7C) and PDGF-induced proliferation (Figure 7D) were both reduced after LKB1 silencing.
Figure 7. LKB1 is a downstream target of ERK in PDGF-treated cells.
LKB1 silencing in CFSC-8B cells reduces PDGF-induced (A) HuR translocation, (B) up-regulation of specific mRNAs (C) migration using the scratch assay, and (D) proliferation, as measured by BrDU incorporation, IHC showing expression of p-LKB1 (DAB+ cells, brown, arrowheads) in activated HSC (α-SMA+ cells, alkaline phosphatase, red) in liver sections from patients with alcoholic (E) and VHC cirrhosis (F). Representative pictures of 9 alcoholic and 7 VHC samples are shown. *p<0.05.
It is known that LKB1 phosphorylates and regulates AMPK, and recent studies have shown that activation of AMPK in HSC leads to reduction of induced proliferation and migration of HSC (23,24). Here, however, we show that in activated HSC (CFSC-8B), PDGF induced pLKB1 without affecting pAMPK levels (Supplementary Figure 5A), and that AMPK silencing did not affect PDGF-induced HuR cytosolic translocation (Supplementary Figure 5B). Altogether our results suggest that in activated HSC, AMPK does not mediate LKB1-induced HuR translocation in response to PDGF.
In primary HSC isolated from BDL mice, PDGF-induced HuR cytosolic localization was also accompanied by LKB1 phosphorylation (Supplementary Figure 3G) and LKB1 silencing (Supplementary Figure 6A) also reduced migration both basally and after PDGF treatment (Supplementary Figure 6B and 6C) and inhibited PDGF-induced proliferation (Supplementary Figure 6D). Finally, we found strong LKB1 phosphorylation in activated HSC (α-SMA+ cells) from BDL mice and CCl4-treated rats (Supplementary Figure 6E), and more importantly in human cirrhotic samples (Figure 7E). In summary, our data suggest that LKB1 activation by ERK could be mediating PDGF-induced proliferation and migration in HSC by regulating, at least partly, cytoplasmic localisation of HuR and expression of its critical target genes.
Role of HuR in TGF-β induced profibrogenic response
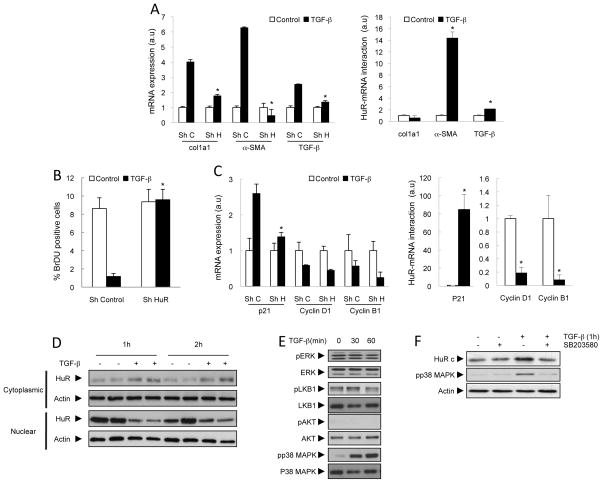
TGF-β is another major mediator of liver fibrogenesis (25). HuR silencing in the CFSC-8B cell line markedly reduced up-regulation of col1a1, α-SMA and TGF-β mRNA after TGF-β treatment (Figure 8A). RIP-qPCR analysis showed thatα-SMA and TGF-β, but not col1a1, were bound to HuR in TGF-β–stimulated cells (Figure 8A).
Figure 8. TGF-β-induced fibrogenesis is mediated by HuR.
(A) qPCR showing reduced expression of profibrogenic mRNAs after HuR silencing and RIP-qPCR showing binding of selected genes with HuR after TGF-β stimulation in CFSC-8B cells. (B) HuR silencing prevents the anti-proliferative effects of TGF-β, as measured by BrDU incorporation. (C) qPCR showing reduced p21 expression after HuR silencing, and RIP-qPCR showing its binding to HuR after TGF-β stimulation. WB showing TGF-β-induced (D) HuR translocation and (E) p38 MAPK activation. (F) WB showing that p38 MAPK inhibition prevents TGF-β-induced HuR translocation. *p<0.05.
In HSC, TGF-β also plays a major role in inhibiting proliferation in HSC (26). TGF-β treatment decreased levels of the cell cycle activators cyclin D1 and B1, whilst increasing levels of the cell cycle inhibitor p21 (Supplementary Figure 7A and 7B). HuR knockdown abrogated the anti-proliferative effects of TGF-β in primary HSC from BDL mice (Supplementary Figure 7C) and in the CFSC-8B cell line (Figure 8B). This anti-proliferative effect of TGF-β was likely due to reduced p21 levels (Figure 8C). RIP-qPCR showed that TGF-β treatment induced an increased binding of HuR to p21, whilst reducing the interaction of cyclin D1 and B1 mRNA with HuR (Figure 8C).
TGF-β treatment did not regulate HuR at mRNA and protein levels, unlike PDGF (Supplementary Figure 7D and 7E). However, TGF-β induced increased cytoplasmic localisation of HuR both in primary HSC (Supplementary Figure 3G) and in the CFSC-8B cell line (Figure 8D and Supplementary Figure 7F). This translocation is unlikely to be mediated by ERK, AKT or LKB1 since TGF-β did not activate any of these kinases (Figure 8E). However, TGF-β activated p38 MAPK (Figure 8E) and inhibition of this pathway prevented TGF-β-induced HuR translocation (Figure 8F). TGF-β did not affect phosphorylation at any of the 8 residues that we previously tested for PDGF-induced translocation (data not shown) suggesting that TGF-β and PDGF mediates HuR translocation by different post-translational modifications.
In summary, we found that the profibrogenic and anti-proliferative actions of TGF-β could be controlled by HuR-mediated regulation of critical genes.
DISCUSION
Liver fibrosis and cirrhosis result from the majority of chronic liver insults and represent a difficult clinical challenge. Recent studies have shown that HuR regulates Angiotensin II-induced kidney fibrosis (27) and ventricular remodeling after myocardial infarction (28). However, HuR functions during liver fibrosis development are unknown. Several studies have shown that HuR regulates expression of several mRNAs encoding pro-inflammatory cytokines (TNF-α, IL-6, TGF-β and INF-γ), proinflammatory mediators (iNOS) and chemoattractant factors (MCP-1) (29). Most of these factors are involved in the pathogenesis of liver fibrosis (4). Here, we show that HuR silencing in a cholestactic liver injury model (BDL) reduces expression of several of these genes leading to decreased liver damage, oxidative stress, inflammation, macrophage infiltration and liver fibrosis development. This suggests that HuR silencing would have a beneficial effect after cholestactic liver injury.
More importantly, our study also shows that HuR regulates HSC activation, which likely results in the reduced fibrosis observed in vivo after HuR silencing. HSC activation is highly regulated with hundreds of genes up- and down-regulated (5). Modulation of mRNA stability and translation rates plays an important role in regulation of gene expression during liver fibrosis development and hepatic stellate activation (1). Here, we show that HSC activation in vitro and in vivo after BDL is accompanied by an increase in HuR. HuR silencing significantly reduces expression of HSC activation markers. Importantly, we observed that HuR mediates the response of two of the principal mediators of HSC activation, PDGF and TGF-β (30,31). These data, together with the finding that HSC from human samples of hepatic cirrhosis expressed HuR, suggest that HuR has a significant role in fibrosis development after liver injury by controlling HSC activation itself in addition to liver damage and inflammation.
HuR regulates PDGF-induced proliferation and migration, controlling the expression of several genes involved in these processes. PDGF binding to its receptor leads to the sequential activation of Raf-1, MEK and ERK-1 and -2. ERK signalling is involved in PDGF-stimulated mitogenesis, migration and chemotaxis. PI3K also mediates PDGF-induced proliferation, migration and chemotaxis, at least in part via ERK-independent pathways (30). Here, we demonstrated that ERK1/2, but not PI3K, regulates cytoplasmic translocation of HuR. PDGF also induces LKB1 (Ser428) phosphorylation via ERK activation (22). LKB1 has been classically described as a tumor suppressor (32) but seems to have the opposite role in liver, controlling HuR nucleo-cytoplasmic shuttling and proliferation in HGF-stimulated hepatocytes and during apoptosis in hepatoma cell lines (8,9). Here, we also identified LKB1 as a downstream target of ERK1/2 in PDGF-stimulated HSC, and silencing LKB1 significantly reduced PDGF-induced migration and proliferation. These functions of LKB1 are possibly mediated by HuR activity, since LKB1 regulates nucleo-cytoplasmic shuttling of HuR and both regulate expression of a common set of mRNAs. It is known that LKB1 phosphorylates and regulates AMPK, however we observed that PDGF-induced HuR cytosolic localization was independent of AMPK activity. This observation is in agreement with previous work describing that AMPK exerts anti-proliferative properties in HSC (23,24), and with studies in melanoma cells, which show that LKB1 can be active without affecting AMPK activity (22). Previous studies have shown that PI3K and ERK are activated in HSC in vivo following liver injury (33,34). Here we found that similarly, LKB1 (Ser428) phosphorylation, is also expressed in vivo in activated HSC in two animal models of hepatic fibrosis (BDL and CCl4) and importantly in human cirrhotic patients.
TGF-β1 is another major mediator of liver fibrogenesis (35). We found that TGF-β1 treatment increased binding of HuR to several target mRNAs such asα-SMA and TGF-β, and HuR silencing significantly reduced their expression. Increasing evidence supports a mechanism by which autocrine production of TGF-β is required to maintain the pathogenic myofibroblast phenotype in several cell types (36). We found that col1a1 was significantly reduced after HuR silencing likely due to reduced TGF-β autocrine secretion, rather than by regulation of its stability and translation since we did not find increased binding of col1a1 to HuR. TGF-β1 is also an important negative regulator of proliferation in activated HSC (25). Our results showed that TGF-β increased the stabilization or translation of p21 mRNA increasing its binding to HuR. Conversely, we observed a markedly reduced association between HuR and cyclin D1 and cyclin B1 mRNAs in response to TGF-β. The TGF-β-induced decrease in proliferation was abrogated by HuR silencing, suggesting that HuR is an important mediator of the anti-proliferative effects of TGF-β.
This role of HuR in TGF-β-treated cells is in sharp contrast to its effects in PDGF-treated cells, where we showed that HuR positively regulated HSC proliferation. Whilst PDGF activates the ERK-LKB1 signalling pathway to promote HuR translocation, TGF-β induced HuR translocation via p38 MAPK activation. In addition, TGF-β does not phosphorylate the same residues of HuR protein that control its cytoplasmic translocation, induced by PDGF. Thus, it is possible that the specific post-translational modification of HuR induced by the two signals could determine its binding to different mRNA targets. Similarly, PDGF and TGF-β have contrasting roles in regulating the levels of HuR. PDGF, via ERK- and PI3K-mediated activation of NFκB, is sufficient to increase HuR transcription. This is in agreement with other studies, which show that NFκB activity is regulated by cytokines in activated HSC (11), and that p65 binds to the HuR promoter in gastric tumour cells (21).
HuR has been implicated in several biological events such as carcinogenesis, cell proliferation, differentiation, and inflammation (29). However both low and high levels of HuR have been correlated with good prognosis in cancer, making careful designs of interventions to modulate HuR functions necessary. These generate the need to study the advantages or disadvantages of HuR silencing in different pathologies, as well as the identification of its specific mediators (29). Here, we have demonstrated that HuR silencing has pleiotropic and beneficial functions during cholestactic liver injury and HSC activation. Importantly, we find that HuR levels in human cirrhotic samples strongly correlate with the degree of HSC activation, suggesting that it could be a valuable therapeutic target for treatment of liver fibrosis, and possibly its progression to hepatocellular carcinoma in humans.
Supplementary Material
Acknowledgments
Financial support: This work is supported by NIH grants RO1AT-1576 and RO1AT-004896 (to S.C.L., M.L.M-C. and J.M.M.), SAF 2011-29851 (to J.M.M), ETORTEK-2011, Sanidad Gobierno Vasco 2008, Educación Gobierno Vasco 2011 (PI2011/29) and FIS (PI11/01588) (to M.L.M.-C), Sanidad Gobierno Vasco 2012 (to MVR), FIS PS09/00094, Fundación Científica de la Asociación Española Contra el Cáncer (Cancer Infantil) (to AW), and the Program Ramón y Cajal, Ministry of Science and Innovation, Spain (to AW and NB). FIS PS09/02010 (to NB) and FIS PS09/01164 (to J.C.). Ciberehd is funded by the Instituto de Salud Carlos III.
Abbreviations
- AREs
AU-rich elements
- CCl4
carbon tetrachloride
- col1a1
collagen type I α1
- ECM
extracellular matrix proteins
- ERK
Extracellular signal-regulated kinases
- GFAP
Glial fibrillary acidic protein
- HSC
hepatic stellate cells
- HuR
human antigen R
- MEK
MAPK/ERK kinases (MAP2Ks)
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NAFLD
non-alcoholic fatty liver disease
- p38 MAPK
p38 mitogen-activated protein kinases
- PI 3-kinases or PI3Ks
Phosphatidylinositol 3-kinases
- PDGF
platelet-derived growth factor
- Raf-1
RAF proto-oncogene serine/threonine-protein kinase
- qPCR
real-time PCR
- RIP-qPCR
RNA immunoprecipitation of ribonucleotide complexes coupled to qPCR
- RBPs
RNA-binding proteins
- STK11
Serine/threonine kinase 11
- LKB1
or liver kinase B1
- TGF-β
Transforming growth factor beta
- HCV
viral hepatitis
- α-SMA
α-smooth muscle actin
References
- 1.Friedman SL. Transcriptional regulation of stellate cell activation. J Gastroenterol Hepatol. 2006;21 (Suppl 3):S79–83. doi: 10.1111/j.1440-1746.2006.04585.x. [DOI] [PubMed] [Google Scholar]
- 2.Milani S, Herbst H, Schuppan D, Hahn EG, Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989;10:84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- 3.Hendriks HF, Verhoofstad WA, Brouwer A, de Leeuw AM, Knook DL. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985;160:138–49. doi: 10.1016/0014-4827(85)90243-5. [DOI] [PubMed] [Google Scholar]
- 4.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–46. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol Life Sci. 2008;65:3168–81. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Chantar ML, Vazquez-Chantada M, Garnacho M, Latasa MU, Varela-Rey M, Dotor J, et al. S-adenosylmethionine regulates cytoplasmic HuR via AMP-activated kinase. Gastroenterology. 2006;131:223–32. doi: 10.1053/j.gastro.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Vazquez-Chantada M, Fernandez-Ramos D, Embade N, Martinez-Lopez N, Varela-Rey M, Woodhoo A, et al. HuR/methyl-HuR and AUF1 regulate the MAT expressed during liver proliferation, differentiation, and carcinogenesis. Gastroenterology. 2010 May;138(5):1943–53. doi: 10.1053/j.gastro.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Lopez N, Varela-Rey M, Fernandez-Ramos D, Woodhoo A, Vazquez-Chantada M, Embade N, et al. Activation of LKB1-Akt pathway independent of phosphoinositide 3-kinase plays a critical role in the proliferation of hepatocellular carcinoma from nonalcoholic steatohepatitis. Hepatology. 2010;52:1621–31. doi: 10.1002/hep.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Embade N, Fernandez-Ramos D, Varela-Rey M, Beraza N, Sini M, de Juan VG, et al. Mdm2 regulates HuR stability in human liver and colon cancer through neddylation. Hepatology. 2011 doi: 10.1002/hep.24795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellerbrand C, Jobin C, Iimuro Y, Licato L, Sartor RB, Brenner DA. Inhibition of NFkappaB in activated rat hepatic stellate cells by proteasome inhibitors and an IkappaB super-repressor. Hepatology. 1998;27:1285–95. doi: 10.1002/hep.510270514. [DOI] [PubMed] [Google Scholar]
- 12.Greenwel P, Schwartz M, Rosas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991;65:644–53. [PubMed] [Google Scholar]
- 13.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann J, Gressner AM, Weiskirchen R. Immortal hepatic stellate cell lines: useful tools to study hepatic stellate cell biology and function? J Cell Mol Med. 2007;11:704–22. doi: 10.1111/j.1582-4934.2007.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carotti S, Morini S, Corradini SG, Burza MA, Molinaro A, Carpino G, et al. Glial fibrillary acidic protein as an early marker of hepatic stellate cell activation in chronic and posttransplant recurrent hepatitis C. Liver Transpl. 2008;14:806–14. doi: 10.1002/lt.21436. [DOI] [PubMed] [Google Scholar]
- 16.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84:1786–93. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenagy RD, Hart CE, Stetler-Stevenson WG, Clowes AW. Primate smooth muscle cell migration from aortic explants is mediated by endogenous platelet-derived growth factor and basic fibroblast growth factor acting through matrix metalloproteinases 2 and 9. Circulation. 1997;96:3555–60. doi: 10.1161/01.cir.96.10.3555. [DOI] [PubMed] [Google Scholar]
- 18.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007;22 (Suppl 1):S73–8. doi: 10.1111/j.1440-1746.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- 19.Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal. 2008;20:2165–73. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Doller A, Schlepckow K, Schwalbe H, Pfeilschifter J, Eberhardt W. Tandem phosphorylation of serines 221 and 318 by protein kinase Cdelta coordinates mRNA binding and nucleocytoplasmic shuttling of HuR. Mol Cell Biol. 2010;30:1397–410. doi: 10.1128/MCB.01373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang MJ, Ryu BK, Lee MG, Han J, Lee JH, Ha TK, et al. NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology. 2008;135:2030–42. 2042, e1–3. doi: 10.1053/j.gastro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Esteve-Puig R, Canals F, Colome N, Merlino G, Recio JA. Uncoupling of the LKB1- AMPKalpha energy sensor pathway by growth factors and oncogenic BRAF. PLoS One. 2009;4:e4771. doi: 10.1371/journal.pone.0004771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caligiuri A, Bertolani C, Tosti C, et al. Adenosine Monophosphate–Activated Protein Kinase Modulates the Activated Phenotype of Hepatic Stellate Cells. Hepatol. 2008;47:669–676. doi: 10.1002/hep.21995. [DOI] [PubMed] [Google Scholar]
- 24.Adachi M, Brenner DA. High Molecular Weight Adiponectin Inhibits Proliferation of Hepatic Stellate Cells via Activation of Adenosine Monophosphate–Activated Protein Kinasa. Hepatol. 2008;47:677–685. doi: 10.1002/hep.21991. [DOI] [PubMed] [Google Scholar]
- 25.Breitkopf K, Haas S, Wiercinska E, Singer MV, Dooley S. Anti-TGF-beta strategies for the treatment of chronic liver disease. Alcohol Clin Exp Res. 2005;29:121S–131S. doi: 10.1097/01.alc.0000189284.98684.22. [DOI] [PubMed] [Google Scholar]
- 26.Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793–807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 27.Doller A, Gauer S, Sobkowiak E, Geiger H, Pfeilschifter J, Eberhardt W. Angiotensin II induces renal plasminogen activator inhibitor-1 and cyclooxygenase-2 expression post-transcriptionally via activation of the mRNA-stabilizing factor human-antigen R. Am J Pathol. 2009 Apr;174(4):1252–63. doi: 10.2353/ajpath.2009.080652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnamurthy P, Lambers E, Verma S, Thorne T, Qin G, Losordo DW, Kishore R. Myocardial knockdown of mRNA-stabilizing protein HuR attenuates post-MI inflammatory response and left ventricular dysfunction in IL-10-null mice. FASEB J. 2010 Jul;24(7):2484–94. doi: 10.1096/fj.09-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srikantan S, Gorospe M. HuR function in disease. Front Biosci. 2012 Jan 1;17:189–205. doi: 10.2741/3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–73. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Borkham-Kamphorst E, Herrmann J, Stoll D, et al. Dominant-negative soluble PDGF-beta receptor inhibits hepatic stellate cell activation and attenuates liver fibrosis. Lab Invest. 2004;84:766–77. doi: 10.1038/labinvest.3700094. [DOI] [PubMed] [Google Scholar]
- 32.Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, Sharpless NE, et al. Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature. 2002;419:162–7. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 33.Marra F, Arrighi MC, Fazi M, et al. Extracellular signal-regulated kinase activation differentially regulates platelet-derived growth factor’s actions in hepatic stellate cells, and is induced by in vivo liver injury in the rat. Hepatology. 1999;30:951–8. doi: 10.1002/hep.510300406. [DOI] [PubMed] [Google Scholar]
- 34.Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, et al. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor’s actions on hepatic stellate cells. Gastroenterology. 1997;112:1297–306. doi: 10.1016/s0016-5085(97)70144-6. [DOI] [PubMed] [Google Scholar]
- 35.Yata Y, Gotwals P, Koteliansky V, Rockey DC. Dose-dependent inhibition of hepatic fibrosis in mice by a TGF-beta soluble receptor: implications for antifibrotic therapy. Hepatology. 2002;35:1022–30. doi: 10.1053/jhep.2002.32673. [DOI] [PubMed] [Google Scholar]
- 36.Dabiri G, Tumbarello DA, Turner CE, Van de Water L. Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the TGF-beta1 autocrine loop. J Invest Dermatol. 2008 Oct;128(10):2518–25. doi: 10.1038/jid.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.