Abstract
Human studies suggest that a variety of prenatal stressors are related to high risk for cognitive and behavioral abnormalities associated with psychiatric illness (Markham and Koenig, 2011). Recently, a down-regulation in the expression of GABAergic genes (i.e., glutamic acid decarboxylase 67 and reelin) associated with DNA methyltransferase (DNMT) overexpression in GABAergic neurons has been regarded as a characteristic phenotypic component of the neuropathology of psychotic disorders (Guidotti et al., 2011).
Here, we characterized mice exposed to prenatal restraint stress (PRS) in order to study neurochemical and behavioral abnormalities related to development of schizophrenia in the adult. Offspring born from non-stressed mothers (control mice) showed high levels of DNMT1 and 3a mRNA expression in the frontal cortex at birth, but these levels progressively decreased at post-natal days (PND) 7, 14, and 60. Offspring born from stressed mothers (PRS mice) showed increased levels of DNMTs compared to controls at all time-points studied including at birth and at PND 60. Using GAD67-GFP transgenic mice, we established that, in both control and PRS mice, high levels of DNMT1 and 3a were preferentially expressed in GABAergic neurons of frontal cortex and hippocampus. Importantly, the overexpression of DNMT in GABAergic neurons was associated with a decrease in reelin and GAD67 expression in PRS mice in early and adult life. PRS mice also showed an increased binding of DNMT1 and MeCP2, and an increase in 5-methylcytosine and 5-hydroxymethylcytosine in specific CpG-rich regions of the reelin and GAD67 promoters. Thus, the epigenetic changes in PRS mice are similar to changes observed in the post-mortem brains of psychiatric patients. Behaviorally, adult PRS mice showed hyperactivity and deficits in social interaction, prepulse inhibition, and fear conditioning that were corrected by administration of valproic acid (a histone deacetylase inhibitor) or clozapine (an atypical antipsychotic with DNA-demethylation activity). Taken together, these data show that prenatal stress in mice induces abnormalities in the DNA methylation network and in behaviors indicative of a schizophrenia-like phenotype. Thus, PRS mice may be a valid model for the investigation of new drugs for schizophrenia treatment targeting DNA methylation.
Keywords: schizophrenia, DNA methyltransferase, prenatal stress, epigenetic, antipsychotic, valproic acid
1. Introduction
Brain development comprises a time- and region-specific complex sequence of neuronal events, involving neurogenesis and neuronal differentiation that result in the generation of the normal structure and function of the adult brain. It is now accepted that stress and environmental factors can lead in adulthood to neuropsychiatric disorders including schizophrenia (SZ) and bipolar (BP) disorders by disrupting the sequence of these neurodevelopmental events (Howes et al., 2004; Weinberg and Lipska 1995; Lewis and Levitt 2002; Barker, 2003; Rapaport et al., 2005). For example, the exposure of pregnant women to psychological stress, malnutrition, or viral infection during pregnancy is associated with an increased incidence of SZ later in life (Brown et al., 1996, 2011; Koenig et al., 2002; Mednick et al., 1994; Izumoto et al., 1999; Susser et al., 1996). Animal studies confirm the sensitivity of the developing brain to environmental insults. For example, exposure of rats or mice to stresses, immune challenges, infections and malnutrition during pregnancy leads to disruption of behavioral and neurochemical parameters in adult offspring that mimic aspects of major neuropsychiatric disorders (Borrell et al., 2002; Moreno et al., 2011; Fatemi et al., 2008; Fortier et al., 2007; Kinnumen et al., 2003; Koenig et al., 2005; Winter et al., 2008; Zuckerman and Weiner, 2005; Shi et al., 2003). The above studies and the lack of definitive genetic abnormalities causally related to psychotic disorders suggest that the etiopathogenesis of SZ and BP disorders may be at least in part epigenetic (Petronis et al., 1999).
The epigenetic role of environmental factors in the pathogenesis of SZ was suggested a decade ago in epidemiological studies by Gottesman et al. (1994). At the molecular level epigenetic studies in SZ and BP disorder patients have focused on promoter cytosine methylation, a covalent modification of DNA, in which a methyl group is transferred from S-adenosyl-methionine (SAM) to the C-5 position of cytosine, by a family of DNA-methyltransferases (i.e., DNMT1, 3a, 3b, 3L). We and others have shown that DNMT1 and DNMT3a are highly expressed in brain and these enzymes are overexpressed in GABAergic neurons of SZ and BP disorder patients (Veldic et al. 2004, 2005, 2007; Ruzicka et al., 2007; Guidotti et al., 2011). Likely, as a consequence of the increased expression of DNA-methyltransferases, reelin and other GABAergic or glutamatergic gene promoters are hypermethylated and their expression is downregulated in SZ (Veldic et al., 2005; Mill et al. 2008; Ruzicka et al., 2007; Kundakovic et al., 2009; Grayson et al., 2005, 2006; Zhang et al., 2010; Weaver, 2007; Sweatt, 2009).
Considering that high levels of DNMT are expressed in neuroprogenitor cells and DNMT expression as well as DNA methylation can be increased in post-mitotic neurons by early-life stressors (Murgatroyd et al., 2009; Meaney and Ferguson-Smith, 2010; Weaver et al., 2007), we propose that a putative mechanism by which adverse prenatal experiences could provoke neuropsychiatric disturbances is by modifying DNA methylation modulating the expression of DNMT in GABAergic neurons. However, to our knowledge, there is no evidence in mice regarding: (i) the expression and function of DNMT in the developing brain, (ii) DNMT changes in response to prenatal stress, or (iii) the effect of epigenetic changes on behaviors related to SZ.
Here, we characterize a model of prenatal stress (PRS) in mice seeking to establish whether restraint stress during pregnancy results in an epigenetic GABAergic dysfunction that persists in the brain of adult offspring and contributes to behaviors related to SZ and/or other related neuropsychiatric disorders.
2. Methods
2.1. Prenatal Stress Paradigms
Pregnant Swiss-albino-ND4 mice (Harlan, Indianapolis) were individually housed with a 12-h light-dark cycle and food and water ad libitum. Pregnant mice were divided into two groups: one left undisturbed throughout gestation and one subjected to a restraint stress using a plastic tube (10 cm × 3 cm) for 30 min twice daily from embryonic day 7 to 21 as described previously (Matrisciano et al., 2011). Offspring not used for neurochemical measurements were weaned from their natural mothers after 21 days; male PRS and control mice were housed separately (5 per cage), and left undisturbed for an additional 40-50 days until behavioral testing.
2.2. Quantitative competitive RT-PCR measurement of DNMT1 and DNMT3a mRNA levels
Total RNA was extracted from mouse frontal cortex (FC) (2 mm anterior to bregma) and hippocampus with Trizol reagent (Invitrogen, Carlsbad, CA). The absolute amount of DNMT1 and 3a mRNA was measured by RT-PCR using co-linear internal standards generated by internal deletions. The internal standards (IS) were designed by deleting 100- to 150-bp fragments from the middle of the target cDNA sequence and were generated by an overlap extension PCR with internal deletion primers (Auta et al., 2007). Amplification of cDNA was carried out employing the following primers: DNMT1 – forward: 5′ TGACAGTGGTGCTGAAGAAGCCAT 3′, reverse: 5′ AGAATGGAGCCTCGAATTCTGAGA 3′; DNMT3a – forward: 5′ AACAACAACTGCTGCAGGTGCTTT 3′, reverse: 5′ ACTCCTGGATATGCTTCTGTGTGA 3′. Concentrations of DNMT1 and DNMT3a mRNA were calculated from a known amount of IS and corrected for nuclear specific enolase (NSE) using the following primers – forward: 5′ AATCCAAGTTTGGGGCCAATGCCA 3′, reverse: 5′ TCTTTTCCGTGTAGCCAGCCTTGT 3′. The amplification products of IS and of the target gene were separated on agarose gels. The ratio of target IS/target cDNA equal to one establishes the concentration of target gene present in the sample.
2.3. Western blot analysis
Western blot analysis was performed as described previously (Matrisciano et al., 2002). Briefly, mouse FC was homogenized at 4°C in RIPA lysis buffer containing 1 mM of a protease inhibitor cocktail (Sigma), pH 7.4. Twenty micrograms of protein were resuspended in a SDS-bromophenol blue reducing buffer. Western blot analysis was carried out using 4-12% tris-glycine gels (Invitrogen). After blotting onto a nitrocellulose filter (0.2 μm pore size; Invitrogen), blots were incubated for one hour at room temperature with primary antibodies directed against DNMT1 (mouse monoclonal; 0.5 μg/ml; Imagenex), reelin (1:5000, a generous gift of A.M. Goffinet, University of Namur, Brussels), and GAD67 (mouse monoclonal, 1 μg/ml; Millipore) in Tris-saline/Tween 20 buffer as described by Matrisciano at al. (2011). The same blots were incubated for 1 hr at room temperature with mouse monoclonal antibodies directed against β-actin (mouse monoclonal; 0.5 μg/ml; Sigma) in TTBS buffer (100 mM Tris-HCl; 0.9% NaCl; 0.1% Tween 20; pH 7.4). After three washes with TTBS buffer, blots were incubated for one hour with peroxidase-conjugated secondary antibodies (Sigma). Densitometric analysis was performed using a Storm 860 (Molecular Dynamics, Sunnyvale, California) with IMAGEQUANT analysis software and the values were expressed as an optical density (OD) ratio with respect to β-actin
2.4. MeDIP and HMeDIP (methylated and hydroxymethylated DNA immunoprecipitation) analysis of reelin and GAD67 promoters
We analyzed the ratio of 5′ methylated cytosines (5mC) or 5′ hydroxymethylated cytosines (5HMC) to the unmethylated cytosines of mouse reelin (−432 to −252) and GAD67 (−840 to −768) CpG-enriched promoter fragments. Mouse monoclonal 5MC (Diagenode) or 5HMC (Active Motif) antibodies were used for the immunoprecipitation. According to Gavin et al. (2012), we performed the MeDIP procedure because bisulfite conversion and most enzyme-dependent methods are incapable of distinguishing 5MC from the approximately 20% of methylcytosines in the brain that are 5HMC (Globish et al., 2010). These two cytosine modifications may have very different functions and genomic locations (Valinluck et al., 2004; Jin et al., 2011; Guo et al., 2011). Genomic DNA was extracted from the mouse FC and sonicated to produce a fragment size of 200-600 bp. After ethanol precipitation, 3 μg of sonicated DNA were diluted to 300 μl in TE buffer and heat-denatured at 95 °C for ten min. Then, 30 μl of sonicated solution were removed and stored at −20 °C to be used to quantify the total amount of promoter before immunoprecipitation (input). The remaining solution was incubated overnight at 4°C with mouse anti-5MC or anti-5HMC antibodies. The immunoprecipitated DNA was released from the antibody complex by proteinase-K digestion. After phenol-chloroform extraction and ethanol precipitation, the DNA pellet was resuspended in 20 μl of DEPC water. CpG-rich GAD67 and reelin gene promoters were measured by Q-PCR. An immunoprecipitation negative control (no antibody added) was included in each assay and did not produce any detectable signal.
2.5. ChIP Assay: measurements of DNMT1 and Methyl CpG binding protein 2 (MeCP2) binding to reelin and GAD67 promoters
We performed these experiments as previously described by Matrisciano et al. (2011). Briefly, about 10 mg of tissue was used for this procedure. Tissue slices were incubated with 500 μl of PBS containing 1% formaldehyde at 37 °C for 10 min, supplemented with a protease inhibitors cocktail (Sigma), and after three washes with cold PBS, tissue was homogenized in 300 μl of SDS lysis buffer (supplied by ChIP kit, Upstate). To obtain consistent chromatin fragmentation, the lysates were sonicated for 15 min on ice (Sonic Dismembrator, Model 500, Fisher Scientific). The ChIP procedure was carried out by using the ChIP assay kit and protocol provided by the manufacturer (Upstate, no. 17-295). The concentrations of DNMT1 (mouse monoclonal; Imagenex) and MeCP2 (rabbit polyclonal; Upstate) antibodies were 1 μg/ml. An aliquot (2%) of the sonicated lysate without antibody (Input) was used to quantify the total amount of DNA in sample extracts before immunoprecipitation. At the end of ChIP procedure, the protein/DNA cross-linked nucleosomal chromatin complex immunoprecipitated by specific antibody was reverse cross-linked with NaCl at a final concentration of 100 mM at 65 °C overnight. Samples were then treated with proteinase-K. Protein-free DNA was extracted in phenol/chloroform and precipitated and washed in ethanol. The extract was used for detection and quantification of reelin and GAD67 gene promoters.
CpG-rich reelin [(−432 to −252bp) (forward: GGGCGGCGGGCCCCGAGG, reverse: AGAGACCGACGGGCTGCC)] and GAD67 [(from −840 to −768 bp) (forward: GAGGAGAGCGGGCCAAGA, reverse: GTGCCGCTCCACACGCC)] promoter fragments (see Fig. 4 for detailed sequences of the promoter regions) were measured by Q-PCR as previously described (Matrisciano et al., 2011). The percent methylated vs. unmethylated promoter was calculated by the following equation: % (meDNA-IP/total input) = 2^[(Ct(10%input) – 3.32) – Ct (meDNA – IP)] × 100% (MagMeDIP kit instruction manual, Diagenode).
Fig. 4.
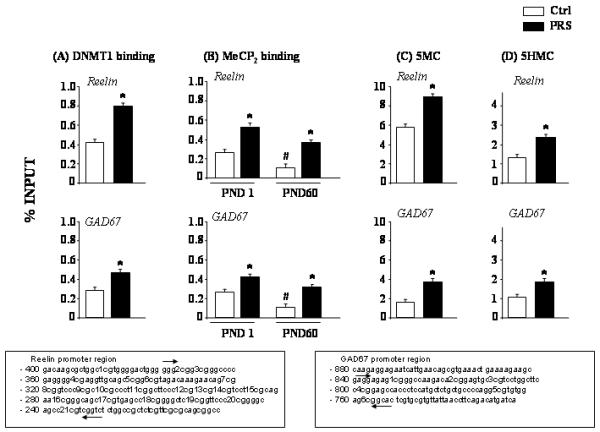
PRS causes an increase of: A) DNMT1 binding to specific reelin (−432 to −252) and GAD67 (−154 to +21) promoter regions in the FC of PND 60 mice; B) MeCP2 binding to the same reelin and GAD67 promoter regions in the FC of PND1 and PND60 mice. C) 5-methylcytosine binding at reelin and GAD67 promoters in the FC of PND 60 mice, and D) 5-hydroxymethylcytosine binding at reelin and GAD67 promoters in the FC of PND 60 mice. Values are means ± S.E.M. of 6 mice. p < 0.05. (Student’s t test) vs. the corresponding control (Ctrl) values (*) or vs. saline (#).
2.6 Confocal Fluorescence microscopy in GAD67-GFP knock-in mice
To identify the location of DNMT with respect to neurons of defined neurotransmitter phenotype, we used heterozygous knock-in enhanced green fluorescent protein-glutamic acid decarboxylase 67 C57BL/6 mice (referred to as GAD67-GFP mice), in which the cDNA encoding enhanced GFP is inserted into the GAD67 ATG start codon by homologous recombination (Tamamaki et al., 2003). These mice were obtained with permission of Dr. Yuchio Yanagawa, Department of Genetic and Behavioral Neuroscience, 11 Gunma University Graduate School of Medicine, Maebashi 371-8511, Japan. Heterozygous GAD67-GFP breeder males were mated with wild type C57BL/6 females and offspring were genotyped at birth. Two primer pairs were used for genotyping so as to identify both alleles (GAD67-GFP and GAD67/GAD67) of all offspring (Tamamaki et al., 2003). For the experiments described, 7 and 60 day old animals were used. Coronal slices (nominally 20 to 25 μm thick) of fixed mouse brains were cut using a cryostat (Richard Allen Scientific, USA). Immunofluorescence labeling was performed as recently described (Kadriu et al. 2011). The following antibodies were used: (i) polyclonal anti-GFP (diluted 1:250; Abcam; Cambridge, MA), (ii) monoclonal anti-DNMT1 (diluted 1:500; Imgenex, San Diego, CA), and (iii) monoclonal anti-DNMT3a (diluted 1:500; Imgenex, San Diego, CA).
To test the specificity of the immunological detection, the primary antibody was omitted yielding no detectable fluorescent staining. Antibody specificity was evaluated by Western analyses of cortical extracts. Following separation on 4-20% SDS polyacrylamide gels and blotting to nitrocellulose, major immunoreactive bands of the expected molecular size were detected with all antibodies used. For more details on the method see Kadriu et al. (2011).
2.7. Behavioral tests
Non-stressed (Control) and prenatally stressed (PRS) male mice at PND 60 were used to examine the behavioral characteristics under basal conditions (drug- or vehicle-free). For this purpose, we measured in order the 1) locomotor activity, 2) social interaction with an intruder (unfamiliar, non-aggressive male mouse 60 days old, Swiss albino ND4 strain) in a novel environment, 3) prepulse inhibition at startle, and 4) contextual fear conditioning. All behavioral tests were performed on consecutive days. In the drug administration experiments, mice were injected with valproic acid (70 mg/kg, i.p.; twice a day for 5 days), or clozapine (5 mg/kg, s.c.; twice a day, for 5 days), or vehicle and then, 24 hours after the last drug injection, animals were tested for locomotor activity, social interaction, PPI, and fear conditioning over the next four days with drugs being administered after completion of each daily test. In another set of experiments, control and PRS mice were injected with clozapine (5 mg/kg, s.c., twice a day) or vehicle for five days and then with a single injection of MK-801 (0.1 mg/Kg s.c.) or vehicle 30 min before the test. All animals were housed in the experimental room an hour prior to the test session for adaptation.
2.7.1 Locomotor activity
A computerized Animal Activity Monitoring System with VersaMax software (AccuScan Instruments, Columbus, Ohio) was used for the quantification and tracking of locomotor activity in mice as described previously (Carboni et al., 2004). Each activity cage consisted of a Perspex box (20 × 20 × 20 cm) surrounded by horizontal and vertical infrared sensor beams. The total number of interruptions of the horizontal sensors was taken as a measure of horizontal activity whereas that of vertical sensors was used as a measure of vertical activity. The percentage of distance traveled in the center of the field with respect to the margins was also calculated. Activity was recorded for 20 min.
2.7.2 Stereotypic behavior
Stereotypy was measured using AccuScan software. Repeated beam breaks on the same beam (or set of beams) were recorded as an index of stereotypic activity. Activity was recorded for 20 min.
2.7.3 Social interaction in a novel environment
We used the experimental paradigm described by Tremolizzo et al. (2005). In brief, individual mice were placed in a novel cage together with an unfamiliar, non-aggressive 60-day old male mouse of the same strain used as an intruder and the interactions between the two mice (initiated by the experimental mouse) were recorded for 10 min with a digital webcam (Logitech, Inc, California, USA). The time spent in social interaction (sec/10 min) was scored by two well-trained blind operators. Social interaction was defined by body contact including inspection and ano-genital sniffing. Reliability of measurements was assessed by correlating the scores of two raters.
2.7.4 Prepulse inhibition of startle (PPI)
Startle was recorded using the SR-Lab Startle Response System (San Diego, California). The startle box was programmed to record five 120 db startle pulses (broadband noise 30 msec in duration), at the beginning and at the end of the 20 min session. The PPI trial sequence consisted of the presentation of 50 pseudo-random trials, 10 for each of the following trial types: (i) no stimulus; (ii) a startle-only pulse at 120 db, and prepulse-startle trials with a (iii) 74 db-, (iv) 78 db-, or (v) 82 db-prepulse. The time between the offset of the prepulse and the onset of the startle pulse was 100 msec. Startle amplitude was defined as the maximum amplitude within a 100 msec window following the presentation of the startle pulse. Intertrial intervals were random (mean ITI = 15 sec). The amount of inhibition was calculated as the following ratio: mean startle for startle-only trials minus mean startle for prepulse-trials divided by mean startle for startle-only trials multiplied by 100.
2.7.5 Contextual Fear Conditioning
We used the experimental paradigm described by Pibiri et al. (2008). The fear-conditioning apparatus consisted of a transparent acrylic chamber measuring 25 cm wide, 18 cm high, and 21 cm deep (San Diego Instrument, Inc., San Diego, CA). The cage floor consisted of stainless steel rods connected to an electric shock generator. A small fan was located on the top wall of the enclosure. A speaker placed on a side wall of the conditioning chamber delivered the auditory tone. The chamber was surrounded by a frame with 16 infrared photo beams. Computer software controlled the delivery of electric foot shocks and auditory stimuli and recorded beam interruptions and latencies to beam interruptions (freezing time).
Training session: mice were placed into the chamber and allowed to explore it for 2 min. After this time, they received an acoustic tone (conditioned stimulus, CS) (30s, 85 DB) co-terminated with an unconditioned stimulus (US) (electric footshock, 2 s, 0.5 mA). The tone plus the foot shock were repeated for three times every 2 min. After the last tone + shock delivery, mice were allowed to explore the context for an additional minute prior to removal from the training chamber.
Test session: 24 hr after training, mice were placed in the same chamber and freezing behavior was measured for 5 min without tone or footshock presentation. Freezing was defined by the absence of any movement except for those related to respiration while the animal was in a stereotyped crouching posture. To measure extinction, mice were re-exposed over the next nine days to the conditional context without receiving footshock or acoustic tone.
2.8. Statistical analysis
Results are expressed as mean ± SEM. Experimental differences were assessed by Student’s t-test, one way ANOVA followed by multiple comparisons with the Bonferroni test, or two way repeated measures ANOVA followed by multiple comparisons (Bonferroni or Student-Newman-Keuls comparison) as indicated in figure legends. The criterion for significance was p < 0.05, two tailed. SigmaStat 2.03 statistical software (SPSS inc.) was used for the analysis.
3. Results
3.1. Increased expression of DNMT1 and 3a in frontal cortex (FC) and hippocampus of PRS mice
We first measured DNMT1 and DNMT3a mRNA levels in the FC of offspring born from non stressed mothers (control mice) at post natal days (PND) 1, 7, 14 and 60. Although, DNMT1 mRNA levels were considerably higher than DNMT3a mRNA levels, the expression of both was markedly elevated at PND1 but then decreased dramatically and progressively up to PND 60 (Fig 1A). In the FC of offspring born from stressed mothers (PRS mice), we found that DNMT1 (Fig 1A top) and DNMT3a (Fig 1A bottom) mRNA levels were significantly higher at PND 1, 7, 14, and 60, compared to controls.
Fig. 1.
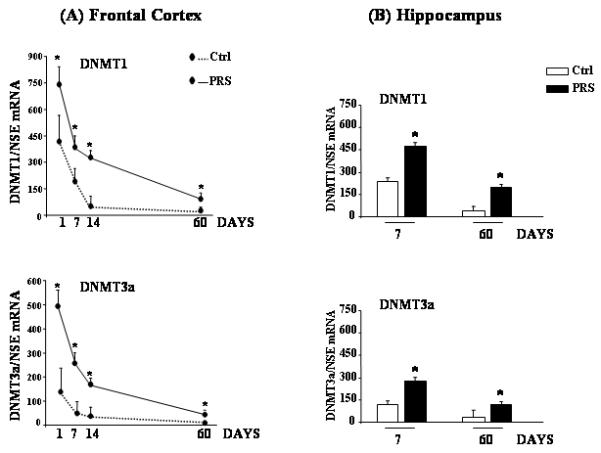
Prenatal stress (PRS) causes an early and long lasting increase in the expression of DNMT1 and 3a in the mouse frontal cortex and hippocampus. DNMT1 and 3a mRNA levels in the frontal cortex (FC) of control (Ctrl) and PRS mice at PND 1, 7, 14, and 60 are shown in (A); levels in the hippocampus of Ctrl and PRS mice at PND 7 and 60 are shown in (B). NSE and GPDH were utilized as internal controls. All values (A, B) are means ± S.E.M. of six mice. *p < 0.05. (Student’s t test) vs. the corresponding Ctrl value.
We also measured the DNMT mRNA levels in the hippocampus at PND 7 and 60. As shown in Fig. 1B, PRS mice showed a marked increase in DNMT1 (Top) and DNMT3a (bottom) mRNA levels compared to control mice. As in the FC, DNMT1 mRNA levels were higher than DNMT3a mRNA levels in the hippocampus (Fig. 1B).
3.2. DNMT is primarily expressed in GABAergic interneurons
We have previously reported that DNMT1 and 3a are highly localized in GABAergic neurons in the cortex and hippocampus of adult human and mouse brains (Veldic et al., 2005; Ruzicka et al., 2007; Satta et al., 2008, Kadriu et al., 2011). Here, using GAD67/GFP knock-in mice that are extremely sensitive to stress exposure (Uchida et al., 2011), we confirmed the almost complete co-localization of GAD67 and DNMT1 protein in cortical and hippocampal neurons of 7 day old control and PRS mice (Fig. 2). Similar results were obtained for DNMT3a (data not shown). Hence, the higher levels of DNMT in the cortex and hippocampus of 7 day old control and PRS mice as compared to the respective 60 day old mice, presumably reflect the presence in brain of GABAergic neurons overexpressing DNMT.
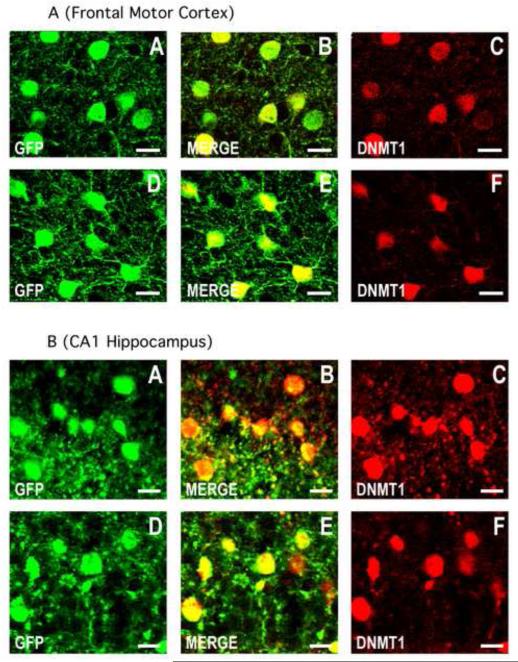
Fig. 2.
DNMT1 is co-expressed with GFP in cortico-limbic GABAergic neurons of the GAD67-GFP knock-in mouse. Confocal double immunofluorescence labeling of GFP (green), DNMT1 (red), and merged images in orange (center panels).
2A) A-C: motor cortex layer II of a 7 day old PRS mouse; D-F: motor cortex layer II of a 7 day old control mouse. Coronal sections correspond roughly to bregma −1.4 mm. 2B) A-C: CA1 field of the hippocampus of the PRS mouse; D-F: CA1 field of the hippocampus of the control mouse. Coronal sections correspond roughly to bregma −2 mm. Scale bars for all panels represent 20 μm.
3.3. Altered expression of schizophrenia-related genes in the frontal cortex of PRS mice
Earlier reports suggest that an increase in DNMT levels is associated with a down-regulation of the gene encoding the GABA-synthesizing enzyme GAD67, or the trophic protein reelin in post-mortem brain tissue from patients affected by SZ or BP disorders (Guidotti et al., 2011) and in brain of rats exposed to early-life postnatal stress (Zhang et al., 2010). Here, we report that, associated with an increased level of DNMT, 60 day old PRS mice show a marked decrease in GAD67 and reelin protein levels in the FC (Fig. 3), when compared to control mice.
Fig. 3.
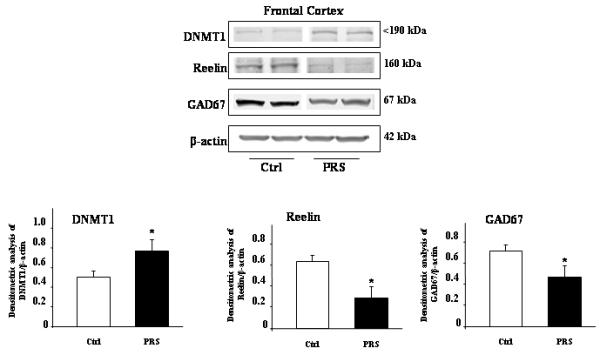
PRS induced changes in the DNMT1, reelin, and GAD67 protein levels in the mouse frontal cortex. Immunoblot analysis shows an increase in the protein levels of DNMT1, and a marked decrease in reelin and GAD67 protein levels in 60-day old PRS mice compared to controls. The representative immunoblots show a major band of 190 kDa for DNMT1, 160 kDa for reelin, and 67 kDa for GAD67. All values are means ± S.E.M. of five mice. *p < 0.05. (Student’s t test) vs. the corresponding Ctrl value. All data were normalized by β-actin protein levels.
3.4. DNMT binding to GABAergic gene promoters
To test whether the overexpression of DNMT1 in FC of PRS mice correlates with an increased binding of DNMT1 to specific reelin and GAD67 CpG-rich promoter sequences, we measured the binding of DNMT1 to these promoters by ChIP assay. We showed that in the FC of 60 day old PRS mice, the binding of DNMT1 to the same reelin (−432 to −252); and GAD67 (−154 to +21) promoter regions was increased (Fig 4A).
To study whether the binding of DNMT1 correlates with changes in the methylation of these promoters we assessed: a) binding of MeCP2 to specific CpG-rich reelin and GAD67 promoter regions, and b) MeDIP and HMeDIP measurements of reelin and GAD67 promoters.
As shown in Fig. 4B, at PND1, when the levels of DNMT were highest, and at PND60 when the levels of DNMT were lowest, the binding of MeCP2 to specific reelin and GAD67 promoter regions was highest at day 1 and markedly decreased at day 60. Moreover, the MeCP2 binding to GAD67 and reelin gene promoters was significantly higher in PRS mice (Fig. 4B). We recently observed that changes in MeCP2 binding in PRS mice occur in the absence of changes in MeCP2 levels (Matrisciano et al., 2011). In Figs. 4C and D we show that the 5MC and 5 HMC covalent modifications at the reelin and GAD67 promoters were increased in 60 day old PRS mice compared to controls.
3.5. PRS mice show a schizophrenia-like behavioral phenotype
We made the consistent observation that PRS mice were born 12 to 18 hours before control mice and had lower (10-15%) body weight at birth (control mice weight: 4.06 ± 0.4 gr) as previously reported by Torche and Kleinhaus, (2011). However, PRS mice had no obvious differences in body weight, auditory sensitivity, pain sensitivity, or motor coordination when compared with offspring born from unstressed mothers at PND 60. In contrast, offspring of stressed pregnant mothers at 2-3 month of age (late adolescence/early adulthood in mice) demonstrate alterations in behavioral tests.
3.5.1. Spontaneous locomotor activity
In Fig. 5A, we report that 60-70 day old PRS male mice showed a robust and persistent hyperactivity as measured by horizontal activity in an open field arena over the 20 minutes of the test. These mice were also characterized by an increase in the percentage of distance traveled in the center of the field with respect to the margins.
Fig 5.
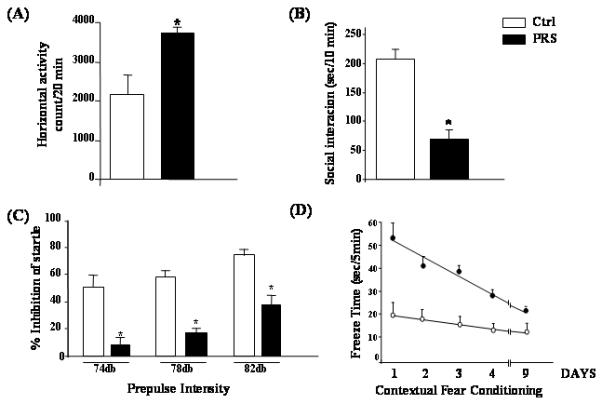
PRS is associated with schizophrenia-like behavioral abnormalities. Sixty-day old PRS mice compared to controls were characterized by: (A) an increase in locomotor activity t23 = 2.466, p = 0.02); (B) a decrease in social interaction (t16 = 3.455, p < 0.001); (C) a deficit in prepulse inhibition of startle (2 factor ANOVA: F1,16 for group = 14.826, p = 0.001, F2,16 for prepulse intensity = 12.503, p < 0.001, group by intensity interaction not significant; and (D) a deficit in contextual fear conditioning (2-factor ANOVA on freezing on re-exposure to the context over the first three days following conditioning F1,9 = 6.735, p = .029); Bonferroni comparison: t(10) 2.59, p = 0.029 Further, regression analysis revealed that contextual fear extinguished in controls to PRS level within 9 days following conditioning (F1,9 = 5.334, p = 0.002).
3.5.2. Social interaction
Social withdrawal is a frequent negative symptom of SZ. In mice, social withdrawal can be evaluated using the social interaction test (Tremolizzo et al., 2005). The same PRS mice subjected to the social interaction test showed a significant decrease in social interactions with an unfamiliar mouse during a 10-min test period compared to controls (Fig. 5B).
3.5.3. Deficits in attention and information processing
Deficits in information processing and attention have been considered central features of SZ which may lead to stimulus overload, cognitive fragmentation, and thought disorders. A well established method for evaluating the status of information processing across species including humans and mice is prepulse inhibition of startle (PPI). Our 60-70 day old PRS mice showed a significant PPI deficit (Fig. 5C; 2-way repeated measure ANOVA followed by Bonferroni comparison), a condition that is similar to the deficit in sensory-motor gating seen in SZ patients (reviewed by Braff et al., 2001).
Another paradigm useful for evaluating the integrity of learning and memory processes in cortico-limbic structures is fear conditioning. In our experiments normal mice exhibited freezing behavior if re-exposed after 24 hours to the context in which they had previously received an electric shock. This freezing response extinguished with repeated re-exposure to the context without footshock or acoustic tone (Fig. 5D). PRS mice demonstrated a significant decrease in fear conditioning response and no extinction (Fig. 5D; 2-way repeated measure ANOVA followed by Bonferroni comparison) which may be reminiscent of abnormal cognitive processing in SZ (Amann et al., 2010). There was no difference in freezing behavior measured during the habituation phase or during the training session suggesting that PRS mice fail to exhibit alterations in unconditioned fear-related behavior, pain or noise perception.
3.5.4. Stereotypic behavior
PRS mice show an increase in stereotypy when compared to control mice. PRS mice responded to low doses (0.1 mg/kg s.c.) of the NMDA receptor antagonist MK-801 with a further increase in stereotypy whereas control mice failed to respond to this low dose of MK-801 (Fig. 6).
Fig. 6.

Increased stereotypic behavior in PRS mice induced by NMDA receptor blockade with a low dose of MK-801. Stereotypy counts are shown in control and PRS mice pretreated with clozapine (5 mg/kg. s.c. twice a day for 5 days) and given a single s.c. injection of MK-801 (0.1 mg/kg) 24 hours after the last injection of clozapine and 30 min before the behavioral test. Values are means and ± S.E.M. Two way repeated measures ANOVA: Group effect (F3,18 = 5.736, p = 0.006; MK-801 effect (F1,18 = 5.939, p = 0.025); Group × MK-801 interaction (F3,18 = 3.198, p = 0.48). Newman-Keuls post hoc comparisons: vs. control mice treated with vehicle (*) or vs. PRS mice treated with MK-801 (**).
3.6. Valproate (VPA) and clozapine correct SZ-like behavior in PRS mice
To test whether the behavioral alterations of offspring of PRS mice were mediated by epigenetic mechanisms including an increase in DNMTs, an increase of GABAergic promoter methylation, and a downregulation of the GABAergic gene expression, we evaluated the behavior of these mice following repeated treatment with the histone deacetylase (HDAC) inhibitor VPA and the chromatin remodeling antipsychotic drug clozapine (Guidotti et al., 2011). We have previously reported that VPA, which is known to induce promoter demethylation by activating DNA-demethylation mechanisms (Szyf, 2011; Guidotti et al., 2009, 2011), corrects the reelin and GAD67 promoter hypermethylation, the decrease of GAD67 and reelin expression, and the PPI and social interaction deficits induced by protracted (one week) methionine treatment (Tremolizzo et al. 2005). Here, we show that VPA corrected the hyperactivity (Fig. 7A), the social interaction (Fig. 7B), and PPI (Fig. 7C) deficits in offspring of prenatally stressed mothers at doses that had no major effect in control mice.
Fig. 7.
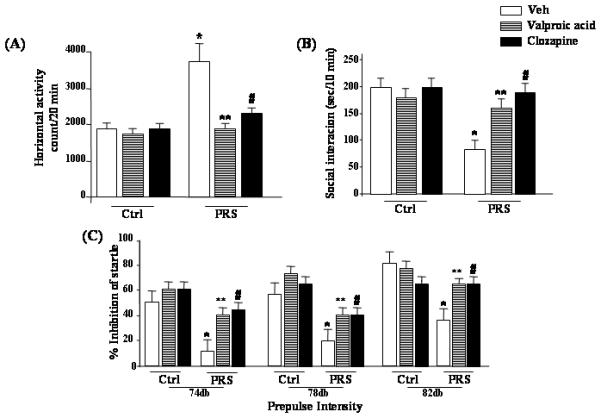
Schizophrenia-like behavioral abnormalities in PRS mice are reversed by treatment with valproic acid (70 mg/kg, i.p.; twice a day for 5 days), or clozapine (5 mg/kg, s.c.; twice a day, for 5 days). Data of locomotor activity (A), social interaction (B), and PPI (C), 24 hours after the last drug injection, are shown. One way ANOVA comparing controls (NS) veh, PRS veh, PRS VPA and PRS clozapine: (A) locomotor activity (F3,33 = 8.919, p = 0.001), (B) social interaction (F3,28 = 17.233, p < 0.001, and (C) PPI (74db F3,42 = 5.189, p = 0.004; 78db F3,42 = 9.625, p < 0.001; 82db F3,42 = 6.582, p < 0.001). Post-hoc Newman-Keuls comparisons indicated that PRS veh mice differed from controls veh mice (*), and from PRS mice treated with VPA (**) or clozapine (#). Analysis for PRS mice considered yielded a similar result while ANOVAs for controls were not significant.
Clozapine, which in clinically relevant doses also induces chromatin remodeling and facilitates DNA promoter demethylation, (Guidotti at al. 2009) also corrected the behavioral abnormalities observed in adult PRS mice (Fig. 7A-C). In addition, a low dose (0.1 mg/kg) of the NMDA receptor antagonist, MK-801, significantly increased stereotypic behavior in vehicle-treated PRS mice but not in clozapine pretreated PRS mice or in controls (Fig. 6).
4. Discussion
Several lines of evidence point to SZ as a neurodevelopmental disorder in which stress or environmental insults during pregnancy or in early-life contribute to the onset of the disease by altering epigenetic DNA marking preferentially at cortical and hippocampal GABAergic neurons (Zhang et al., 2010; Guidotti et al., 2011, Benes, 2011; Grayson, 2010; Brown, 2011; Fatemi et al., 2008; Markham and Koenig, 2011; Howes et al., 2004).
In rats, exposure to stress during gestation induces marked changes in the behavior of the offspring that are reminiscent of the positive, negative and cognitive symptoms present in SZ and BP disorder patients (Lemaire et al., 2000; Koenig et al., 2005). Our results provide further evidence that restraint stress during pregnancy in mice leads to a clozapine- and VPA-sensitive behavioral psychosis-like phenotype in offspring (PRS mice) strongly suggesting that PRS mice represent a valid behavioral animal model of SZ and BP disorders.
Here, we demonstrate for the first time that, in PRS mice, “psychotic-like” behavior (hyperactivity, enhanced response to MK-801, and a deficit in social interaction, PPI, and fear conditioning) measured in the adult is associated with a persistent upregulation of DNMT1 and 3a in cortical and hippocampal GABAergic neurons during neurodevelopment and by a sustained binding of DNMT to GABAergic gene promoters. These epigenetic events are associated with hypermethylation of reelin, GAD67, and likely other GABAergic promoters (as determined by the MeDIP, HMeDIP assays and by the increase in MeCP2 binding) and with a persistent downregulation of the expression of the respective genes. Importantly, the epigenetic phenotype present in cortex and hippocampus of PRS mice is reminiscent of the epigenetic phenotype shown in the brain of patients with SZ and BP disorders (Guidotti et al., 2011; Roth et al., 2009). When the postmortem brains of SZ and BP disorder patients are compared with that of non psychotic subjects, a GABAergic neuropathology is detected in the prefrontal cortex and hippocampus (Guidotti et al. 2005). This GABAergic neuropathology is not associated with neuronal loss but is characterized by decreased 1) GAD67, the rate limiting step enzyme in GABA synthesis (Akbarian et al. 1995, Volk et al., 2000; Benes et al., 2007; Huang and Akbarian, 2007; Guidotti et al., 2000); 2) reelin, the extracellular matrix protein that regulates dendritic spine maturation and glutamate receptor structure and function (Guidotti et al., 2000, Fatemi et al. 2000); and 3) other genes encoding markers of GABAergic interneurons, including the NMDA receptor subunit NR2A (Woo et al., 2008), the nAChR subunits α4, β2, and α7 (Breese et al., 2000), the high affinity GABA transporter, somatostatin, and cholecystokinin (Lewis et al., 2005, Benes et al., 2007).
We have recently shown that DNMT is highly expressed in telencephalic GABAergic neurons of the adult mammalian brain and is preferentially overexpressed in GABAergic neurons of SZ and BP disorder patients (Veldic et al., 2007; Zhubi et al., 2009; Ruzicka et al., 2007; Kadriu et al., 2011). Comparing the expression of DNMT, reelin, GAD67 and other GABAergic genes in laser microdissected GABAergic interneurons and pyramidal neurons of SZ patients, we were able to establish that the overexpression of DNMT and the down-regulation of GABAergic genes in GABAergic interneurons are closely related events (Ruzicka et al., 2007).
It is plausible that in the human and mouse brain, DNMT-induced GABAergic deficits could be the basis for the disturbance of the reciprocal interaction between GABAergic, glutamatergic and monoaminergic neurons that likely represents the mechanism underlying the exacerbation of psychotic episodes elicited by NMDA receptor antagonists to SZ and BP disorder patients. (Meltzer et al., 2011; Javitt, 2007; Breese et al., 2002, Lisman et al., 2008). GABAergic-glutamatergic-monoaminergic interaction deficits may also explain the stereotypic hypersensitivity to administration of low doses of the NMDA receptor antagonist MK-801 in PRS mice (see Fig. 6).
In view of the evidence that alterations in DNA methylation are involved in the etiopathogenesis of SZ (Mill et al., 2008, Veldic et al., 2005; Grayson, 2006; Tremolizzo et al., 2005) and that high levels of DNMT are expressed in the developing brain (Fig. 1) (Guidotti et al., 2011; Zhang et al., 2010; Weaver et al., 2007), a putative mechanism by which adverse prenatal experiences could perturb GABA-glutamate interactions and exacerbate the stereotypic behavior induced by the NMDA receptor antagonist MK-801 may involve alterations in DNA methylation and the expression of DNMT in cortico-limbic GABAergic neurons.
To further investigate the hypothesis that prenatal stress may be responsible for the epigenetic alterations of GABA-glutamate neuron interactions in PRS mice, we administered VPA and clozapine in doses that are known to act on chromatin remodeling inducing reelin and GAD67 promoter demethylation (Guidotti et al., 2009). Under these conditions, VPA and clozapine abolished the hyperactivity, stereotypy, social interaction, and PPI shown in PRS mice whereas no effects were observed in control mice. Furthermore, clozapine blocked the increased stereotyped behavior in PRS mice induced by low doses of MK-801. It is noteworthy that the doses of VPA and clozapine active on behavior in PRS mice fail to have a significant effect on the behavior of control mice, suggesting a specificity of action on the epigenetic mechanisms that underlie the behavioral pathology in PRS mice. We are now testing this hypothesis experimentally. PRS mice and postmortem brain of SZ patients have in common increased levels of DNMT. From the data presented in Fig. 1, which show higher levels of DNMT in cortex and hippocampus from birth to adulthood, we can infer that in PRS mice the increase in DNMT is probably the result of changes occurring during embryonic life. The importance of stress during the embryonic period is indicated by our finding that DNMT was elevated in PRS mice even on Day 1 before differences in maternal care could be a significant factor. Further, it has been reported in Swiss mice that stressed mothers raising stressed pups exhibit maternal care comparable to that of non-stressed mothers raising non-stressed pups (Meek et al., 2001).
We cannot establish at the present time if a similar time course in changes in DNMT occur in the human brain but it is conceivable that similar neurodevelopmental changes can occur as a response to stressful situations either in utero or during early post-natal life, thus preventing the reduction in DNMT expression that occurs with development. This hypothesis is supported by reports that as in our mouse model, the exposure of pregnant women to psychological stress, malnutrition, or viral infection during pregnancy is associated with an increased incidence of psychosis later in the life of offspring (Mittal et al., 2008; Markham and Koenig, 2011; Howes et al., 2004). Hence, the neurochemical, behavioral, and pharmacological responses observed in adult offspring of mothers exposed to stress during pregnancy appear to parallel some of the responses obtained in the adult onset of SZ and strongly support the prenatal stress model in mice as a pertinent endophenotypic epigenetic animal model of psychosis.
4.1. Conclusions
Taken together, the effects of prenatal stress on behavior are indicative of neocortical inhibitory/excitatory circuit imbalance and changes in the expression of DNMT, GAD67, and reelin, suggesting that PRS mice can be a valid animal model to study the epigenetic mechanisms underlying SZ and BP disorders. PRS mice are suitable for validating new compounds with potential antipsychotic activity acting at an epigenetic level as shown for mGlu2/3 metabotropic glutamate receptor agonists (Matrisciano et al. 2011).
Highlights.
We characterized mice exposed to prenatal restraint stress (PRS) in order to study neurochemical and behavioral abnormalities related to development of schizophrenia in the adult life.
We measured the DNMT, reelin, and GAD67 levels as markers of schizophrenia.
Using GAD67-GFP transgenic mice, we established that, in both control and PRS mice, high levels of DNMT1 and 3a were preferentially expressed in GABAergic interneurons.
We examined the DNA methylation network in prenatally stressed mice and controls.
Acknowledgments
This work was supported in part by the National Institutes of Health, National Institute of Mental Health (Grant MH0708551 to AG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest
All Authors (FM, PT, ID, BK, DG, FN, GA) deny any conflict of interest for the preparation of this paper.
References
- Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin S,G, Bunney WE, Jr, Jones EG. GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb. Cortex. 1995;5:550–60. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- Auta J, Chen Y, Ruzicka WB, Grayson DR. Nucleic acid quantitation using the competitive polymerase chain reaction: Practical neurochemistry methods. In: Baker G, Dunn S, Holt A, Lajtha A, editors. The handbook of Neurochemistry and Molecular Neurobiology. Springer; New York: 2007. pp. 341–361. [Google Scholar]
- Barker DJ. The developmental origins of adult disease. Eur. J. Epidemiol. 2003;18:733–6. doi: 10.1023/a:1025388901248. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc. Natl. Acad. Sci. U S A. 2007;104:10164–9. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Regulation of cell cycle and DNA repair in post-mitotic GABA neurons in psychotic disorders. Neuropharmacology. 2011;60:1232–42. doi: 10.1016/j.neuropharm.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–15. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr Res. 2001;49:171–8. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–64. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Moy SS. Integrative role for serotonergic and glutamatergic receptor mechanisms in the action of NMDA antagonists: potential relationships to antipsychotic drug actions on NMDA antagonist responsiveness. Neurosci. Biobehav. Rev. 2002;26:441–55. doi: 10.1016/s0149-7634(02)00011-8. [DOI] [PubMed] [Google Scholar]
- Brown AS, Susser ES, Butler PD, Richardson, Andrews R, Kaufmann CA, Gorman JM. Neurobiological plausibility of prenatal nutritional deprivation as a risk factor for schizophrenia. The Journal of Nervous and Mental Disease. 1996;184:71–85. doi: 10.1097/00005053-199602000-00003. [DOI] [PubMed] [Google Scholar]
- Brown AS. Further evidence of infectious insults in the pathogenesis and pathophysiology of schizophrenia. Am. J. Psychiatry. 2011;168:764–6. doi: 10.1176/appi.ajp.2011.11050722. [DOI] [PubMed] [Google Scholar]
- Carboni G, Tueting P, Tremolizzo L, Sugaya I, Davis J, Costa E, Guidotti A. Enhanced dizocilpine efficacy in heterozygous reeler mice relates to GABA turnover downregulation. Neuropharmacology. 2004;46:1070–81. doi: 10.1016/j.neuropharm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol. Psychiatry. 2000;5:654–63. 571. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, Smee DF, Pearce DA, Winter C, Sohr R, Juckel G. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Scizophr. Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav. Brain Res. 2007;181:270–7. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Gavin DP, Sharma RP, Chase KA, Matrisciano F, Dong E, Guidotti A. Growth Arrest and DNA-Damage-Inducible, Beta (GADD45b)-Mediated DNA Demethylation in Major Psychosis. Neuropsychopharmacology. 2012;37:531–42. doi: 10.1038/npp.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globisch D, Münzel M, Müller M, Michalakis S, Wagner M, Koch S, Brückl T, Biel M, Carell T. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS One. 2010;5:e15367. doi: 10.1371/journal.pone.0015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II. Complications to the complex inheritance f schizophrenia. Clinical Genetics. 1994;46:116–123. doi: 10.1111/j.1399-0004.1994.tb04213.x. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proc. Natl. Acad. Sci. U S A. 2005;102:9341–6. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Chen Y, Costa E, Dong E, Guidotti A, Kundakovic M, Sharma RP. The human reelin gene: transcription factors (+), repressors (−) and the methylation switch (+/−) in schizophrenia. Pharmacol Ther. 2006;111:272–86. doi: 10.1016/j.pharmthera.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Grayson DR. Schizophrenia and the epigenetic hypothesis. Epigenomics. 2:3414. doi: 10.2217/epi.10.22. 1010. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch. Gen. Psychiatry. 2000;7:1061–9. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology. 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Kundakovic M, Satta R, Grayson DR, Costa E. Characterization of the action of antipsychotic subtypes on valproate-induced chromatin remodeling. Trends Pharmacol. Sci. 2009;30:55–60. doi: 10.1016/j.tips.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, Tueting P. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 2011;60:1007–16. doi: 10.1016/j.neuropharm.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Wu X, Ren L, Liu G, Li L. Emerging roles of TET proteins and 5-hydroxymethylcytosines in active DNA demethylation and beyond. Cell Cycle. 2011;10:2662–8. doi: 10.4161/cc.10.16.17093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: the impact of environmental factors. Int. J. Neuropsychopharmacol. 2004;7:S7–S13. doi: 10.1017/S1461145704004122. [DOI] [PubMed] [Google Scholar]
- Huang HS, Akbarian S. GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS One. 2007;2:e809. doi: 10.1371/journal.pone.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumoto Y, Inoue S, Yasuda N. Scizophrenia and the influenza epidemics of 1957 in Japan. Biol. Psych. 1999;46:119–124. doi: 10.1016/s0006-3223(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int. Rev. Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- Jin SG, Wu X, Li AX, Pfeifer GP. Genomic mapping of 5-hydroxymethylcytosine in the human brain. Nucleic Acids Res. 2011;39:5015–24. doi: 10.1093/nar/gkr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, Guidotti A, Chen Y, Grayson DR. The DNA methyltransferases1 (DNMT1) and 3a (DNMT3a) co-localize with GAD67-positive neurons in the GAD67-GFP mouse brain. J. Comp. Neurol. 2011 Dec 1; doi: 10.1002/cne.23020. doi: 10.1002/cne.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen AK, Koenig JI, Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. J. Neurochem. 2003;86:736–48. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Kirkpatrick B, Lee P. Glucocorticoid hormones and early brain development in schizophrenia. Neuropsychopharmacology. 2002;27:309–318. doi: 10.1016/S0893-133X(01)00396-7. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behave. Brain Res. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol. Pharmacol. 2009;75:342–54. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl. Acad. Sci. U S A. 2000;97:11032–7. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Scizophrenia as a disorder of neurodevelopment. Annu. Rev. Neurosci. 2002;25:409–32. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–42. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SA, Hutten MO, Machon RA. Prenatal influenza infections and adult schizophrenia. Schizophr. Bull. 1994;20:450–452. doi: 10.1093/schbul/20.2.263. [DOI] [PubMed] [Google Scholar]
- Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology. 2011;214:89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Storto M, Ngomba RT, Cappuccio I, Caricasole A, Scaccianoce S, Riozzi B, Melchiorri D, Nicoletti F. Imipramine treatment up-regulates the expression and function of mGlu2/3 metabotropic glutamate receptors in the rat hippocampus. Neuropharmacology. 2002;42:1008–15. doi: 10.1016/s0028-3908(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Tueting P, Maccari S, Nicoletti F, Guidotti A. Pharmacological Activation of Group-II Metabotropic Glutamate Receptors Corrects a Schizophrenia-Like Phenotype Induced by Prenatal Stress in Mice. Neuropsychopharmacology. 2011 Nov 16; doi: 10.1038/npp.2011.274. doi: 10.1038/npp.2011.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Ferguson-Smith AC. Epigenetic regulation of the neural transcriptome: the meaning of the marks. Nat. Neurosci. 2010;13:1313–8. doi: 10.1038/nn1110-1313. [DOI] [PubMed] [Google Scholar]
- Meek LR, Dittel PL, Sheehan MC, Chan JY, Kjolhaug SR. Effects of stress during pregnancy on maternal behavior in mice. Physiol. Behav. 2001;72:473–9. doi: 10.1016/s0031-9384(00)00431-5. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Horiguchi M, Massey BW. The role of serotonin in the NMDA receptor antagonist models of psychosis and cognitive impairment. Psychopharmacology. 2011;213:289–305. doi: 10.1007/s00213-010-2137-8. [DOI] [PubMed] [Google Scholar]
- Mill J, Tang T, Kaminsky Z, Khare T, Yazdanpanah S, Bouchard L, Jia P, Assadzadeh A, Flanagan J, Schumacher A, Wang SC, Petronis A. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am. J. Hum. Genet. 2008;82:696–711. doi: 10.1016/j.ajhg.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr. Bull. 2008;34:1083–94. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Kurita M, Holloway T, López J, Cadagan R, Martínez-Sobrido L, García-Sastre A, González-Maeso J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HT2A and mGlu2 receptors in the adult offspring. J. Neurosci. 2011;31:1863–72. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat. Neurosci. 2009;12:1559–66. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Petronis A, Paterson AD, Kennedy JL. Schizophrenia: an epigenetic puzzle? Schizophr. Bull. 1999;25:639–55. doi: 10.1093/oxfordjournals.schbul.a033408. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc. Natl. Acad. Sci. U S A. 2008;105:5567–72. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update. Mol. Psychiatry. 2005;10:434–49. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Sodhi M, Kleinman JE. Epigenetic mechanisms in schizophrenia. Biochim. Biophys. Acta. 2009;1790:869–77. doi: 10.1016/j.bbagen.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol. Psychiatry. 2007;12:385–97. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- Satta R, Maloku E, Zhubi A, Pibiri F, Hajos M, Costa E, Guidotti A. Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc. Natl. Acad. Sci. U S A. 2008;105:16356–61. doi: 10.1073/pnas.0808699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, Gorman JM. Schizophrenia after prenatal famine. Further evidence. Arch. Ge. Psychiatry. 1996;169:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- Szyf M. DNA methylation, the early-life social environment and behavioral disorders. J. Neurodev. Disord. 2011;3:238–49. doi: 10.1007/s11689-011-9079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry. 2009;65:191–7. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Torche F, Kleinhaus K. Prenatal stress, gestational age and secondary sex ratio: the sex-specific effects of exposure to a natural disaster in early pregnancy. Hum. Reprod. 2011 Dec 7; doi: 10.1093/humrep/der390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremolizzo L, Doueiri MS, Dong E, Grayson DR, Davis J, Pinna G, Tueting P, Rodriguez-Menendez V, Costa E, Guidotti A. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol. Psychiatry. 2005;57:500–9. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Uchida T, Oki Y, Yanagawa Y, Fukuda A. A heterozygous deletion in the glutamate decarboxylase 67 gene enhances maternal and fetal stress vulnerability. Neurosci Res. 2011;69:276–82. doi: 10.1016/j.neures.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Valinluck V, Tsai HH, Rogstad DK, Burdzy A, Bird A, Sowers LC. Oxidative damage to methyl-CpG sequences inhibits the binding of the methyl-CpG binding domain (MBD) of methyl-CpG binding protein 2 (MeCP2) Nucleic Acids Res. 2004;32:4100–8. doi: 10.1093/nar/gkh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc. Natl. Acad. Sci. U S A. 2005;102:2152–7. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, Costa E. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr. Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch. Gen. Psychiatry. 2000;57:237–45. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Weaver IC. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: let’s call the whole thing off. Epigenetics. 2007;2:22–8. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- Weaver IC, D’Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J. Neurosci. 2007;27:1756–68. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs and schizophrenia: a search for common ground. Schizophrenia Research. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Woo TU, Kim AM, Viscidi E. Disease-specific alterations in glutamatergic neurotransmission on inhibitory interneurons in the prefrontal cortex in schizophrenia. Brain Res. 2008;1218:267–77. doi: 10.1016/j.brainres.2008.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C, Djodari-Irani A, Sohr R, Morgenstern R, Feldon J, Juckel G, Meyer U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int. J. Neuropsychopharmacol. 2008;12:513–24. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J. Neurosci. 2010;30:13130–7. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhubi A, Veldic M, Puri NV, Kadriu B, Caruncho H, Loza I, Sershen H, Lajtha A, Smith RC, Guidotti A, Davis JM, Costa E. An upregulation of DNA-methyltransferase 1 and 3a expressed in telencephalic GABAergic neurons of schizophrenia patients is also detected in peripheral blood lymphocytes. Schizophr. Res. 2009;111:115–22. doi: 10.1016/j.schres.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J. Psychiatr. Res. 2005;39:311–23. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]