Abstract
The extracellular enzyme matrix metalloproteinase-9 (MMP-9) is overexpressed in atherosclerotic plaques and in metastatic cancers. The enzyme is responsible for rupture of the plaques and for the invasion and metastasis of a large number of cancers. The ability of ultrasonic excitation to induce thermal and mechanical effects has been used to release drugs from different carriers. However, majority of these studies were performed with low frequency ultrasound (LFUS) at kHz frequencies. Clinical usage of LFUS excitations will be limited due to harmful biological effects. Herein, we report our results on the release of encapsulated contents from substrate lipopeptide incorporated echogenic liposomes triggered by recombinant human MMP-9. The contents release was further enhanced by the application of diagnostic frequency (3 MHz) ultrasound. The echogenic liposomes were successfully imaged employing a medical ultrasound transducer (4 – 15 MHz). The conditioned cell culture media from cancer cells (secreting MMP-9) released the encapsulated dye from the liposomes (30 – 50%) and this release is also increased (50 – 80%) by applying diagnostic frequency ultrasound (3 MHz) for 3 minutes. With further developments, these liposomes have the potential to serve as multimodal carriers for triggered release and simultaneous ultrasound imaging.
Keywords: Echogenic liposomes, MMP-9 triggered release, Ultrasound triggered release
1. INTRODUCTION
Liposomes are nano- to micrometer-size vesicles with a hydrated lipid bilayer encapsulating an aqueous phase. Due to their structural similarity with biological cells, liposomes show attractive features as drug carriers (e.g., lesser toxicity, increased uptake and longer circulation time). Hence, liposomes have been extensively investigated for targeted drug-delivery applications1. Currently, about 10 liposomal formulations are approved by the US Food and Drug Administration for human use. Conjugation with targeting ligands leads to active targeting of the liposomes to the intended sites for drug delivery and imaging2. However, after reaching the intended sites, most of the liposomes release the contents passively and this process is often slow3. The rate of release from the liposomes can be increased by the application of external triggers, e.g., temperature4, pH5, light6, ultrasound7, metal ions8 and enzymes9. Recently, we have demonstrated enzymatic release of liposomal contents in the presence of recombinant as well as cancer cell secreted matrix metalloproteinase-9 (MMP-9)10.
MMP-2 and -9 are members of Zn2+ and Ca2+ dependent family of enzymes responsible for degradation of gelatin and collagen (IV and V) in the extracellular matrix11. MMPs play an important role in a variety of normal physiological processes, e.g., embryonic development, tissue metamorphosis, angiogenesis, wound healing, ovulation etc. 12 Increased expression levels of MMP-9 and MMP-2 correlate with arthritis, atherosclerosis, cancer and other diseases13. These two enzymes hydrolyze and weaken the fibrous caps of the plaques, leading to plaque rupture14. MMP-9 is also involved in progression and metastasis of many cancers and are being considered as biomarkers for various types of cancers15. Inhibitors of these enzymes are currently in clinical trials for adjuvant therapy of various cardiovascular diseases and cancers16.
The ability of ultrasonic excitation to induce thermal and/or mechanical effects has been used to release drugs from different carriers such as polymeric assemblies, micelles, emulsions, microcapsules, microspheres and liposomes. However, majority of these studies were performed with low frequency ultrasound (LFUS) at kHz frequencies17. Although, inertial/transient cavitation thresholds are much lower at such frequencies18 favoring ultrasound mediated destruction and subsequent release from liposomes19, clinical usage of similar excitations will be limited due to harmful biological effects. The release is considerably diminished for MHz range excitation20. Diagnostic frequency ultrasound has been used for drug delivery and imaging employing micron-sized bubbles21 and microbubbles conjugated to liposomes22.
Recently, a modified protocol for preparing acoustically reflective liposomes (echogenic liposomes or ELIPs) has been reported23. The preparation protocol ensures that the liposomes entrap air pockets, although exact location of the entrapped air has not yet been exactly ascertained.21c, 23c, 24. It strengthens the mechanical coupling with ultrasound resulting in strong ultrasound echoes. Ultrasound excitation can also be used to destabilize the bilayer membrane and release of contents from liposomes10b. Since ELIPs retain all the desired properties of normal liposomes, they have been extensively investigated for simultaneous imaging and drug delivery applications employing diagnostic frequency ultrasound (1 – 10 MHz)24-25. However, often the amount of contents released by ultrasound excitation of the ELIPs is not optimal, ranging from 20 – 50%26. Herein, we demonstrate that the combination of enzymatic triggering (by MMP-9) and ultrasound excitation leads to considerably higher amounts of contents release from echogenic liposomes. The echogenic liposomes were successfully imaged employing a medical ultrasound transducer (4 – 15 MHz). We also demonstrate that conditioned cell culture media from cancer cells (secreting MMP-9) released the encapsulated dye from the liposomes (30 – 50%) and this release is also increased (50 – 80%) by applying diagnostic frequency ultrasound (3 MHz) for 3 minutes.
2. MATERIALS AND METHODS
2.1 Synthesis of lipopeptide (LP4)
The lipopeptide LP4 was synthesized using a microwave assisted peptide synthesizer (Liberty, CEM Corporation, Matthews, SC) following a reported protocol27. Commercially available Fmoc-protected amino acids (0.1 mM) (Peptides International, KY, USA) were used in the synthesis employing Fmoc-Gly-CLEAR acid resin (Peptides International, KY, USA) as the solid support. A mixture of 1-hydroxybenzotriazole (HOBT, AK Scientific, CA) and O-(benzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium hexafluorophosphate (HBTU, ChemPep, FL) was used as coupling agent in 5 fold excess. Coupling steps were performed with 20 W microwave power for 5 minutes at 50 °C except for arginine (25 °C for 25 minutes). Diisopropylethylamine was used as the activator base during the peptide coupling reactions. The Fmoc deprotection was carried out by 5% piperazine (TCI America) using N,N-dimethylformamide as the solvent (Macron Chemicals, NJ). The peptide was cleaved from resin using a mixture of trifluoroacetic acid (TCI America), triisopropyl silane and water (95:2.5:2.5) for three hours at room temperature with constant stirring. Subsequently, the reaction mixture was filtered and the filtrate was treated with cold ether to precipitate the crude peptide, dried and stored at −20 °C until use.
2.2 Purification of crude lipopeptide
Purification of the crude LP4 was conducted by reverse phase high performance liquid chromatography (Shimadzu Scientific Instruments) using a diphenyl semipreparatory column (Grace Vydac, 300 Å pore diameter silica, 5 Qm particle size, 10 × 250 mm) as the stationary phase. A linear gradient (0-70%) of acetonitrile in water was used at a flow rate of 8 mL/min over 45 minutes. Trifluoroacetic acid (25 mM) was added to both solvents and was monitored at 235 nm using a UV detector. After evaporating solvents from the purified product, the purity was determined using MALDI TOF mass spectrometry with an AB 4800 MALDI TOF/TOF Mass Analyzer using α-cyano-4-hydroxy-cinnamic acid as the ionizing matrix. The dried powder was stored in freezer (−20 °C) until use.
2.3 Circular Dichroism (CD) Spectroscopy
CD spectra were recorded (to ascertain the triple helical structure of LP4) using a Jasco J-815 CD spectrometer with 1 mm path length quartz cuvette. The lipopeptide LP4 (0.5 mg/mL) was dissolved in 4 mM phosphate buffer (pH = 4.0) and stored at 4°C overnight before recording the spectra. For each spectrum, 12 accumulations were carried out at scanning speed of 50 nm/min in order to obtain a good signal to noise ratio. For liposome incorporated LP4, 1 mg/mL concentration was used and 36 accumulations were performed to reduce noise. The CD spectrum of MMP-9 was subtracted from CD spectrum of ELIPs treated with MMP-9. Melting temperature of LP4 was calculated by plotting the intensity at 225 nm against temperature.
2.4 Preparation of dye encapsulated Echogenic Liposomes (ELIPs)
Stock solutions of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC, Avanti Polar Lipids) was prepared (1 mg/mL) by dissolving in chloroform and stored in freezer. Solutions of POPC (2 mg) and LP4 (2.6 mg) were mixed in the molar ratio of 70:30 respectively in a 10 mL round bottom flask. A thin film at the bottom of the flask was formed by evaporating the solvent at 40 °C using a rotary evaporator. In order to remove any residual solvents, the flask was placed under high vacuum overnight. Subsequently, the dried film was hydrated with 100 mM carboxyfluorescein (>90% fluorescence is quenched) in HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid) buffer (25 mM, pH = 8) with added ions (Ca2+ and Zn2+) and 2 mL of 0.64 M mannitol (final concentration 0.32 M) at 50 °C. Mannitol is a weak cryoprotectant; we have recently observed that finite amount of mannitol during preparation is critical for ensuring the echogenicity of ELIPs23c. The lipids were hydrated for 3 hours and the resultant multilamellar vesicles were bath sonicated for 10 minutes. The liposomal solution was exposed to three freeze (−70 °C) and thaw cycles. Subsequently, the liposomes were extruded first through 800 nm and then 200 nm polycarbonate filters (Nuclepore, Whatman) 15 times at 60 °C using a mini-extruder (Avanti Polar Lipids). To remove the unencapsulated dye, the liposomes were gel filtered using Sephadex G-100 column conditioned with HEPES buffer (25 mM, pH = 8). The osmolarity of the eluent buffer was adjusted (540 mOsm/kg with 0.17 M NaCl solution in HEPES buffer, pH = 8,) to that of the liposomal solution to ensure the minimum leakage of dye from liposomes due to osmotic shock. A similar procedure was followed to prepare the liposomes without any encapsulated dye using HEPES buffer without carboxyfluorescein. ELIPs for CD spectroscopic studies were prepared by the same method using a 4 mM phosphate buffer (pH = 4.0).
2.5 Measurement of Size Distribution
Particle size distribution (PSD) of ELIPs was measured using a Dynamic Light Scattering (DLS) instrument (Malvern Zetasizer Nano-ZS90). DTS 0012 polystyrene latex disposable sizing cuvette (RI: 1.59) was used and the measurements were conducted at a scattering angle of 90°. Samples (0.1 mg/mL in HEPES buffer) were equilibrated for 120 seconds and 12 readings were then taken for a single sample at constant temperature (25 °C). Each batch of ELIPs was tested for polydispersity and each experiment repeated three times to ensure reproducibility of the results.
2.6 Transmission Electron Microscopy (TEM)
The ELIPs samples were dispersed to 1 mg/mL concentration and dropped onto 300 mesh Formvar coated copper grids previously coated with 0.01% poly-L-lysine and allowed to stand for 1 min before wicking off with filter paper. After air drying for 2 minutes, the samples were negatively stained with 1% phosphotungstic acid for 1.5 minutes and subsequently wicked off with filter paper and allowed to dry before viewing. The samples were observed using a JEOL JEM-2100-LaB6 transmission electron microscope operating at 200 kV.
2.7 Release studies with recombinant MMP-9
The release experiments were carried out using a microplate multidetection instrument (Spectramax M5, Molecular devices) employing the liposomes prior to freeze drying at 25 °C. Release was monitored by recording the fluorescence emission intensity at 518 nm with excitation wavelength 480 nm. All experiments were conducted in triplicate. Each well contained 20 μL of 0.1 mg/mL ELIPs in HEPES buffer (25 mM, pH = 8.0, osmolarity 540 mOsm/kg adjusted with 0.17 M NaCl in HEPES buffer, pH = 8) and 16 μL of 25 μM MMP-9 (final concentration: 2 QM). Release of dye was observed over 60 minutes. The emission intensity was recorded in one minute interval. After 1 h, 10 μL of Triton-X100 was added to disrupt all the liposomes and the emission intensity was measured (excitation: 480 nm). This fluorescence intensity was treated as total (100%) release. The percent release was calculated using the formula:
2.8 Measurement of Echogenicity of ELIPs
For echogenicity experiments, the freeze-dried ELIPs sample was suspended in a solution of phosphate buffered saline (PBS) with 0.5% by weight of bovine serum albumin (BSA). We observed that the liposome solution prior to freeze drying was not echogenic. Echogenicity of the freeze dried and then reconstituted ELIPs was investigated using an in vitro acoustic setup (Figure 1) which is capable of measuring non linear response from contrast agents. The setup employs two single element spherically focused immersion transducers (Panametrics NDT) confocally positioned at right angles. This type of transducer placement ensures the similarity of scattered signals to backscattered echoes28 and also provides high spatial resolution29. Each transducer has an individual diameter of 1.27 cm and focal length of 3 cm. The sample is held in a rectangular chamber. Holes were drilled on adjacent walls for insertion of transducers. Complete immersion of the transducers required 100 mL of solution. Appropriate amount of ELIPs was dissolved in 100 mL of PBS-BSA solution to yield a final lipid concentration of 10 Qg/mL which is low enough to ensure absence of multiple scattering effects. The transmitting transducer has a nominal center frequency of 3.87 MHz with a −6 dB bandwidth of 86.4%. The receiving transducer has a center frequency of 5.54 MHz with an 85% bandwidth. The transmitting transducer was excited at 3.5 MHz with a 32 cycle sinusoidal wave. A 0.4 mm needle hydrophone (PZT-Z44-0400, Onda Corporation, CA, USA) was used to calibrate transducers. A programmable function generator was utilized (Model 33250A; Agilent, Santa Clara, CA) to generate the wave which was amplified using a power amplifier (Model A-300; ENI, Rochester, NY) and fed to the transmitting transducer. The scattered signal was received using the other transducer and a pulser/receiver (Model 5800; Panametrics-NDT, Waltham, MA) with a 20 dB gain. A digital oscilloscope (Model TDS2012; Tektronix, Beaverton, OR) was used to observe the signal in real time. Scattered voltage-time responses were acquired from the oscilloscope using LabView (Version 6.0.3; National Instruments, Austin, TX) via a GPIB IEEE 488 cable and GPIB card and saved on a PC for post experimental analysis using MATLAB (MathWorks, Natick, MA). Fast Fourier Transforms of 50 oscilloscope acquisitions were obtained and averaged in frequency domain which was then converted to dB scale using unit reference. Responses at fundamental, second and sub harmonic frequencies were extracted from the resultant data set and plotted.
Figure 1.
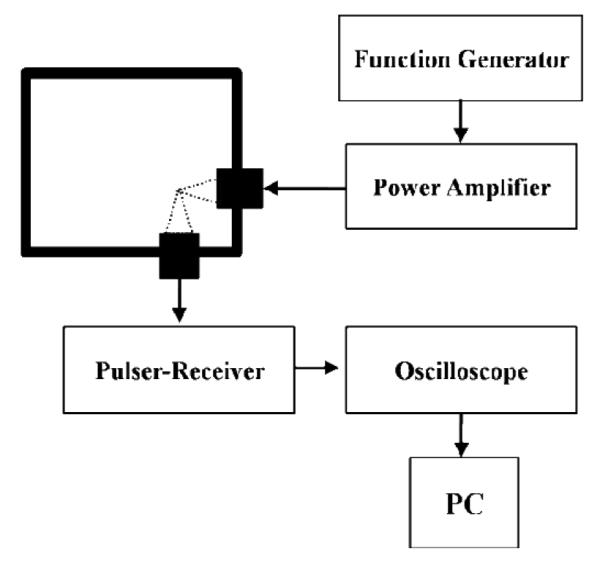
Schematic of the experimental setup for in vitro measurement of scattering
2.9 Culture of cancer cells and harvesting of conditioned media
All cell lines were obtained from American Type Culture Collection (Manassas, VA). PANC-1 (Human pancreatic cancer), PC-3 (Human prostate cancer), MCF-7 (Human breast cancer), 22Rv-1 (Human prostate cancer), HeLa (Human cervical cancer) and bEnd-3 (immortalized rat brain endothelial cells) were cultured in clear (without added phenol red) RPMI media supplemented with 5% antibiotics (penicillin, streptomycin), 10% (by volume) fetal bovine serum and grown in incubator at 37 °C in humidified atmosphere containing 5% CO2. After three generations, confluent cells were centrifuged (200 g) and the supernatant media collected and frozen until use.
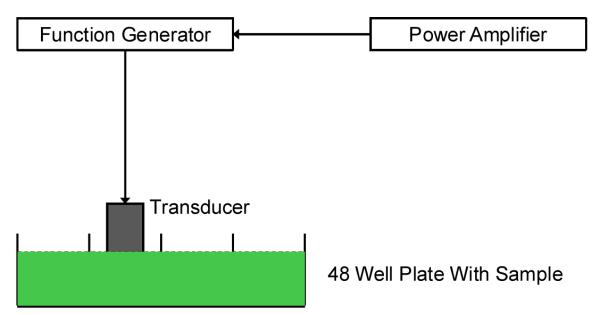
2.10 Ultrasound mediated release studies
The in vitro setup for ultrasound enhanced release studies employed a single element unfocused immersion transducer (Model IP301HP; Valpey Fisher Corporation, Hopkinton, MA). The active element of the transducer has a diameter of 0.3175 mm and a nominal center frequency of 3.5 MHz with a −6 dB bandwidth of 85%. An arbitrary function generator was used to generate a continuous sinusoidal wave with desired parameters (Model 33250A; Agilent, Santa Clara, CA) which was then amplified with a power amplifier (Model A-150; ENI, Rochester, NY) and fed to the transducer. The release studies were carried out in a 48 well plate filled with 500 μL sample with a total lipid concentration of 0.02 mg/mL. The transducer face was always kept immersed in the sample volume at a distance of 20 mm from the base (Figure 2). The homogeneity of the sample was maintained using a small magnetic stirrer. The entire plate was kept on an ice bath to minimize the temperature changes. Most release studies were performed with 3 MHz continuous wave at 1 MPa pressure for an exposure period of 180 seconds. We note that although the current setup has often been used to subject biological cells or liposomes to ultrasound 30, it allows reflections from air water interface. This effect has been studied in detail recently 31 to show that the reflection creates a standing wave pattern giving rise to a spatially varying acoustic field. Following these studies, we are currently developing a setup that would address this effect, and will be used to determine optimal ultrasound excitation parameters. For the limited goal of demonstrating ultrasound mediated release here, current setup is adequate. Also note that less than 1% energy transfer due to stimulation from a transducer positioned in one well was measured at a neighboring well indicating negligible inter well interference. All experiments were repeated three times to ensure the reproducibility of results obtained.
Figure 2.
Schematic of the in vitro experimental setup for ultrasound mediated release studies
2.11 Ultrasound enhanced MMP-9 triggered release
This study was carried out in a 48 well plate, with 500 μL of 0.02 mg/mL of liposomes (prior to freeze drying) in each well using an ice bath. Three different procedures were followed for these experiments. In one set of experiments, we incubated 0.02 mg/mL of ELIPs with 2 QM MMP-9 for an hour and then applied the ultrasound (3 MHz, 1 MPa) for 180 seconds. In second set, we applied the ultrasound first and then allowed ELIPs to incubate with MMP-9. In third set we added MMP-9 to ELIPs and immediately applied the ultrasound to get the simultaneous exposure result. Control samples were also acquired for each of these set of experiments. All the experiments were conducted in triplicates in order to ensure repeatability of the results obtained.
For studies with conditioned media harvested from different metastatic cancer cell lines, we added 50 μL of media to 450 μL of ELIPs (lipid concentration: 0.02 mg/mL) and after one hour we applied the ultrasound. Two different negative control measurements were performed: one without the cell culture media (RPMI) and ultrasound and other with RPMI media as well as ultrasound whereas bEnd 3 cell media was treated as a positive control.
2.12 Ultrasonic imaging of the echogenic liposomes
The Terason t3200™ Diagnostic Ultrasound (MedCorp LLC., Tampa, FL) was utilized to image the reflection of the echogenic liposomes. A layer of Aquasonic® 100 (Parker Laboratories, Inc., Fairfield, New Jersey) ultrasound gel was applied to the 15L4 Linear (4.0-15.0 MHz) (MedCorp LLC., Tampa, FL) ultrasound transducer sound plate. The transducer with gel was placed over the parafilm covering the wells containing ELIPs (0.2 mg/mL in all four wells) in a 96 well plate. The ultrasound scan properties of the echogenic liposomes were set at 0.7 Mechanical Index (MI), 0.6 Thermal Index (TIS), Omni Beam activated, level C Image Map, level 3 Persistence, high (H) frequency, level 3 TeraVision, level 51 2D Gain, level 60 Dynamic Range (DR), 3 cm scan depth, and 22 Hz frame rate. The images were labeled and saved.
3. RESULTS AND DISCUSSION
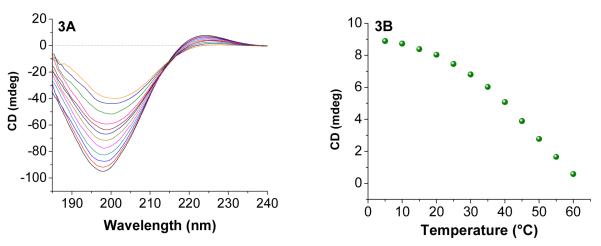
Nonfibrillar collagens (types IV and V) are the principal substrates of MMP-932. The collagens contain high amounts of the amino acid triad Glycine-Proline-Hydroxyproline (GPO) and this contributes to the triplehelical structure33. We have previously synthesized triple helical substrate lipopeptides for MMP-9 and optimized the contents release from liposomes in the presence of this enzyme10a. For these studies, we decided to synthesize the same lipopeptide [LP4, amino acid sequence: CH3(CH2)16CONH-GPQGIAGQR(GPO)4GG-COOH, where MMP-9 cleavage site is between Glycine and Isoleucine] for preparing the ELIPs. The lipopeptide LP4 was synthesized employing a microwave assisted peptide synthesizer, purified by reverse phase HPLC and the purity was confirmed by MALDI-TOF mass spectrometry (Calculated MH+: 2333.26; Observed: 2333.28). Circular dichorism (CD) spectroscopic studies confirmed triple helical structure for LP4 in phosphate buffer (pH = 4.0; Figure 3A). Poor solubility of the lipopeptide in buffer of higher pH prevented us from conducting these studies at physiological pH (7.4). Temperature-dependent CD spectra (5 – 60 °C) indicated the presence of an isosbestic point (Figure 3A) and the melting temperature was determined to be 49 °C (Figure 3B). This melting temperature is similar to that observed for human collagen (48 °C)34. The isosbestic point in Figure 3A indicates that the triple helical lipopeptide is melting to monomeric species without going through any intermediates35.
Figure 3.
(A) Temperature dependent CD spectra (5-60 °C) of LP4 (0.5 mg/mL) in 4 mM phosphate buffer (pH = 4). The presence of positive peak at 225 nm and negative peak at 198 nm indicates the triple helical nature of LP4. (B) The melting curve for LP4 monitored at 225 nm is shown.
After confirming triple helical structure of lipopeptide LP4 in solution, we prepared ELIPs incorporating 30 mol% of LP4 and 70 mol% of POPC in phosphate buffer (pH = 8.0) employing a reported protocol21b, c. This liposomal composition was based on our previous mechanistic and optimization studies on MMP-9 triggered release of liposomal contents10. We observed that LP4 retains the triple helical structure when incorporated into the liposomes (Figure 4, black trace). It is hypothesized that the hydrolysis of triple helical substrate peptides by MMP-9 requires unwinding of the triple helix by the enzyme followed by hydrolysis36. We observed that upon incubation of these liposomes with 2 μM recombinant human MMP-9, the triple helicity decreased substantially (Figure 4, red trace). These results demonstrate that MMP-9 is able to unwind and cleave the triple helical LP4 even when incorporated in the lipid bilayer of the ELIPs. We also observed that 2 μM of MMP-7, MMP-10 or Trypsin released less than 5% of the encapsulated contents from the liposomes. This is likely due to the inability of these enzymes to unwind the triple helix2b, 37.
Figure 4.
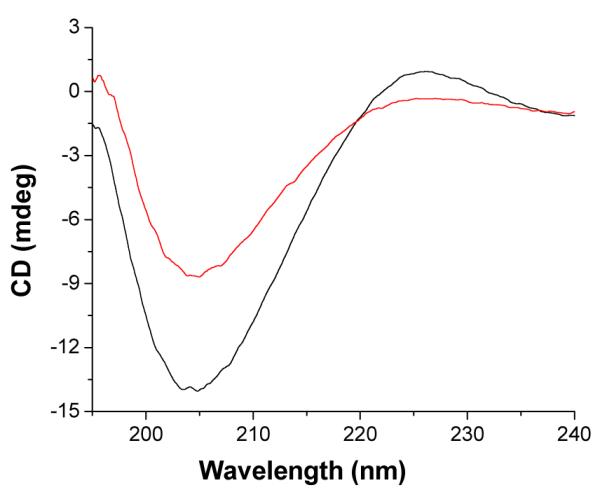
CD spectra of liposome incorporated LP4 is shown before (black trace) and after (red trace) incubation with 2 QM MMP-9 for an hour. These experiments were conducted in 25 mM phosphate buffer, pH = 8.0.
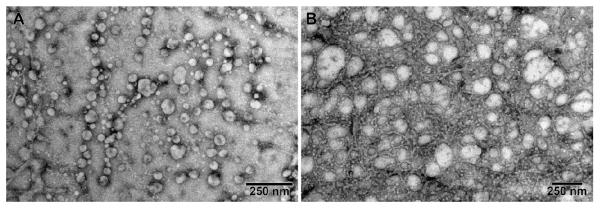
Next, we prepared ELIPs with carboxyfluorescein encapsulated and the liposomes were subsequently freeze-dried for long-term storage and shipment38. We determined the average diameters of the ELIPs before and after freeze-drying employing dynamic light scattering. We observed that average diameter of the liposomes in the reconstituted powder was larger (190 ± 35 nm; Figure 5D) compared to that prior to freeze-drying (116 ± 22 nm, Figure 5C). The polydispersity index also increased to 0.85 from 0.3 – indicating a large distribution of size in the reconstituted liposomes39. Transmission electron microscopy corroborated these observations. TEM images show that the reconstituted ELIPs were heterogeneous (50 nm – 1 Qm) with median size between 100 – 200 nm (Figures 5A and B). Using an optical microscope, we observed micron sized liposomes also (Supporting Information, Figure S1). During the freeze drying and subsequent reconstitution steps, some of the liposomes fuse with each other – leading to a more heterogeneous size distribution. We have used mannitol40 during the ELIPs preparation which plays a crucial role in ensuring echogenicity by aiding air entrapment.23c Also note that the relatively high polydispersity indicates presence of larger liposomes (also seen in the inset of Figure 5D and Supporting Information Figure S1). These larger liposomes with air pockets of size around a micrometer are primarily responsible for the echogenicity of ELIPs. Consistent with this, we also observed that the liposome solution prior to freeze drying was not echogenic.
Figure 5.
TEM images (A: before freeze drying B: after freeze drying) and DLS size distributions (by number) of carboxyfluorescein encapsulated ELIPs (C: before freeze drying; D: after freeze drying). The inset plot of panel D shows the presence of liposomes in the size range 700 – 1100 nm. The TEM images of the samples were obtained using a LaB6 emitter at low magnifications and with the beam spread to reduce the amount of electron beam interaction per unit area and hence beam damage to sample.
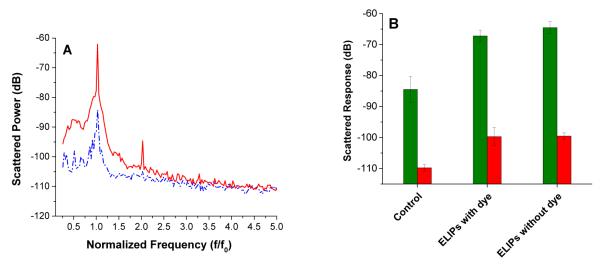
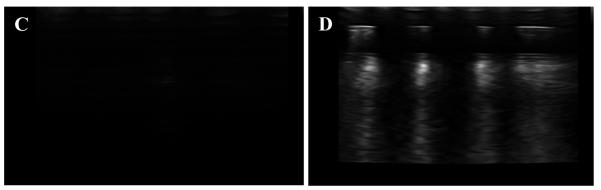
Echogenicity of the ELIPs (reconstituted from freeze dried powders) with and without encapsulated carboxyfluorescein was investigated using the acoustic setup for scattering measurements shown in Figure 1. We observed that the liposomes showed echogenicity only when freeze dried and subsequently reconstituted. However, this leads to some loss of the encapsulated dye. As mentioned above, the echogenicity is likely due to the presence of larger size liposomes with encapsulated gas in the reconstituted samples (see Figures 5B and 5D). The ELIPs were excited at an acoustic pressure amplitude of 500 kPa. Figure 6A shows the FFT of the scattered response from suspension with and without any ELIPs. At an acoustic pressure of 500 kPa, we observed distinct peaks at both fundamental frequency (i.e., at excitation frequency of 3.5 MHz) and second harmonic frequency (i.e., at twice the excitation frequency, 7 MHz) in presence of the ELIPs. However, no distinct subharmonic peak (i.e. response at half the excitation frequency, 1.75 MHz) was observed. The nominal central frequency (5.54 MHz) of the receiving transducer is rather high compared to the subharmonic frequency (1.75 MHz). However, using a receiving transducer with a lower central frequency (2.25 MHz) did not change the result. We conclude that these ELIPs do not generate any significant subharmonic response at this excitation frequency. Figure 6B shows the experimentally measured echogenic response from the ELIPs (the scattered fundamental and second harmonic components). Control measurements are acquired without any ELIPs in the sample solution. Each set of experiments was repeated five times. The mean of the five different runs is plotted along with the corresponding standard deviation. We also imaged the ELIPs (in a well plate) employing a Terason t3200 ultrasonic medical imaging system using a 4 – 15 MHz transducer (Figure 6C, D).
Figure 6.
(A) FFT of the scattered signal from a suspension with (red trace) and without (blue trace) liposomes at an acoustic pressure of 500 kPa. (B) Fundamental (green columns) and second harmonic (red columns) of ultrasound scattered response from ELIPs prepared with or without dye loading and reconstituted in PBS and PBS-BSA solutions. (C, D): Ultrasound images (5 – 10 MHz transducer) of 4 wells of a 96 well plate containing buffer (C) and echogenic liposomes (0.2 mg/mL in each well) incorporating LP4 peptide and encapsulating the dye carboxyfluorescein (D).
We observed that both ELIP formulations with and without encapsulated dye show echogenicity with nearly 20 dB enhancement of the fundamental response when reconstituted in PBS-BSA. The preparation procedure includes addition of mannitol, a weak cryoprotectant. It has been hypothesized that lyophilization in presence of mannitol creates defects in the bilayer that serves as nucleation sites for air entrapment 23a, b, 25a, 41. The presence of air creates a mismatch in acoustic impedance rendering the liposomes echogenic i.e., capable of reflecting ultrasound waves 42. It has been reported that these air pockets are created during the rehydration step in the PBS-BSA/PBS solution42a. Creation of air pockets is facilitated by the use of mannitol during the lyophilization 23a, 25a, 42a. Mannitol thus plays a critical role in the echogenicity of the ELIPs. Recently, we have shown that a small but finite amount of mannitol is required for echogenicity. 23c Although echogenicity of the ELIPs have been attributed to the presence of these air pockets, the exact location of these air pockets still remains uncertain43. Hypothesized structure of ELIPs considers existence of trapped air within the bilayer in between hydrophobic tails of the lipid molecules24, 44. Air can also be trapped as a small lipid monolayer coated bubble freely floating within the aqueous core of the liposomes23b, 24. Recently, TEM image have shown the existence of such individual lipid coated bubbles45. The lipid coating in both situations—in the bilayer or for individual bubbles—can render the air pocket stable against Laplace pressure driven dissolution by drastically decreasing the effective surface tension at the air liquid interface46. The lipid shell also modifies the echogenicity of the air pocket and thereby of the emulsion29, 47. Notably, there is no difference in the echogenicity of the ELIPs due to dye-loading. The ELIPs also show non linear response with around 10 dB enhancement for second harmonic response.
We conducted the enzymatic release studies employing an optimized concentration of freshly-prepared liposomes (0.01 mg/mL of total lipid). We used the self-quenching property of the encapsulated dye (carboxyfluorescein) in determining the contents release from the liposomes10b. In order to determine if the presence of the entrapped air pockets in the echogenic emulsion has any effect in the fluorescence of the dye, we recorded the emission spectra of carboxyfluorescein (5 μM in 25 mM HEPES buffer, pH = 8.0) in the presence of ELIPs (without any encapsulated dye) and regular liposomes. We observed that these emission spectra were superimposable – indicating that the entrapped air in the ELIPs is not affecting the emission from carboxyfluorescein (Supporting Information, Figures S2 – S4).
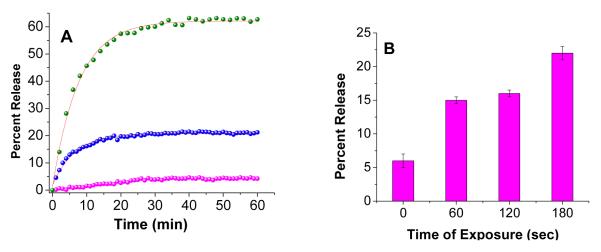
In the absence of any added enzyme, less than 10% of the encapsulated dye was released in 60 minutes (Figure 7, magenta spheres). Upon incubation with 1 μM of recombinant human MMP-9, we observed 25% release of the encapsulated dye in 20 minutes (Figure 7A, blue spheres). After this time, no further release was observed from the liposomes (Figure 7A, blue spheres). Increasing the concentration of MMP-9 to 2 μM leads to 62% release in 50 minutes (Figure 7A, green spheres). These release profiles can be fitted with a single exponential rate equation (Figure 7A, red lines through the observed data points) with rate constants of (12.9 ± 0.4) × 10−2 s−1 (1 μM MMP-9) and (15.1 ± 0.1) × 10−2 s−1 (2 μM MMP-9).
Figure 7.
(A) MMP-9 triggered release profile for encapsulated carboxyfluorescein from ELIPs after incubation for an hour with 1 QM (blue spheres) and 2 QM (green spheres) recombinant MMP-9. The magenta spheres indicate the release from the liposomes in the absence of any added enzyme. The red lines indicate the fitted curves through these data points using a single exponential rate equation. (B) High frequency ultrasound (3 MHz, continuous wave, 1 MPa) triggered release from ELIPs with varying exposure time.
MMP-9 cleaves the triple helical peptide exposed on the liposome surface, leading to destabilization of the lipid bilayer and release of the encapsulated content48. As the bilayer “heals” itself, the release from the liposomes slows down. We also observed that the recombinant MMP-9 used in these studies undergoes self-hydrolysis and resulting in the formation of catalytically inactive enzyme. The reduction in the concentration of the active enzyme will also contribute to reducing the rate of release from the liposomes. It should be noted that for healthy individuals, the serum concentration of MMP-9 is less than 10 nM. For gastric, colorectal and pancreatic cancer patients the serum concentration of MMP-9 can be as high as 1 μM2a. We also observed that exposing the liposomes to diagnostic frequency ultrasound (3 MHz, 1 MPa) for 3 minutes led to around 25% release of the encapsulated dye (Figure 7B); ultrasound destabilizes the ELIPs49
The lipopeptide LP4 adopts triple helical conformation in the liposomes (Figure 4). The fibronectin domain of MMP-9 unwinds the triple helix and subsequently, the catalytic domain hydrolyzes the peptide bond50. We hypothesized that enzymes incapable of unwinding the triple helix should not release the encapsulated dye from the liposomes. In accordance with this, we observed that MMP-7, MMP-10 and trypsin (2 μM each) failed to release the encapsulated dye from the liposomes (data not shown).
After ensuring release by MMP-9 and diagnostic frequency ultrasound separately, we proceeded to determine the combined effect of these two triggers on contents release from the ELIPs. In this endeavor, we first incubated the ELIPs with MMP-9 (2 μM) for an hour and observed 62% release of the encapsulated dye. Subsequent application of ultrasound (3 MHz, 3 minutes) increased the release to 71% (Table 1). As MMP-9 releases the encapsulated dye by disturbing the lipid bilayer of the liposomes, it is likely that the entrapped air is also escaping – making the resultant liposomes less responsive to ultrasound trigger. When we applied the ultrasound first for 3 minutes, we observed 15% release of the contents from the liposomes. This indicates that a majority of the liposomes are not releasing the contents in the presence of ultrasound alone. However, upon subsequent treatment with MMP-9 (2 μM) for an hour, the release increased to 65%. When we incubated the liposomes with MMP-9 (2 μM) and applied ultrasound at the same time, the release decreased to 30%. A probable reason for this may be the local increase in temperature during the ultrasonic excitation51. The local heating will deactivate MMP-9, leading to a decrease in the contents release from the liposomes. The control in Table 1 represents release when the transducer was inserted in the liposomal solution without sending any ultrasound waves (for 3 minutes).
Table 1.
Ultrasound (US) enhanced recombinant MMP-9 triggered contents release from ELIPs.
| Conditions | Release (% ± SD) with MMP-9 |
Release (% ± SD) with US |
Total Release (% ± SD) |
|---|---|---|---|
| MMP-9 followed by US | 62 ± 10 | 9 ± 1 | 71 ± 5 |
| US followed by MMP-9 | 50 ± 12 | 15 ± 4 | 65 ± 10 |
| Simultaneous | 30 ± 2 | ||
| Control (no US) | 4 ± 4 |
Various cancer cells are known to secrete varying amounts of MMP-9 in the extracellular matrix52. We decided to determine if the conditioned media from cancer cells can release the encapsulated dye from the ELIPs and if this release can be further enhanced by the application of diagnostic frequency ultrasound. In this endeavor, we cultured the cells HeLa (cervical cancer), PC 3 (prostate cancer), 22Rv1 (prostate cancer), MCF-7 (breast cancer) and PANC 1 (pancreatic cancer) in dye-free RPMI. After reaching confluency, the cells were pelleted and the media was harvested. The immortalized mouse brain endothelial cell line bEnd-3 (which does not secrete MMP-9) was taken as control53. Based on our results with recombinant MMP-9, we incubated the ELIPs with the conditioned media for an hour, followed by the application of ultrasound pulses (3 MHz) for 3 minutes.
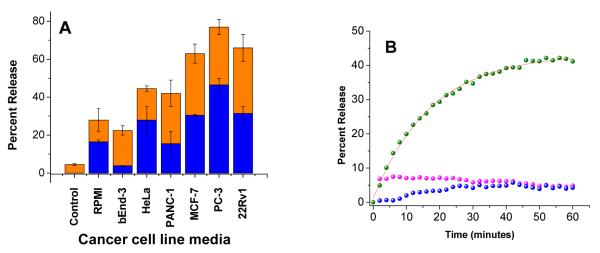
We observed that the culture media (RPMI containing 10% by volume of fetal bovine serum) released 16% of the liposome encapsulated dye (Figure 8A). As cells become confluent and consume fetal bovine serum, the conditioned media from bEnd-3 cells showed less release (<5%) compared to fresh RPMI (Figure 8A). The release also decreases slightly over time. Since the release was very low (< 5%), we did not conduct any further studies to determine the reasons for this time-dependent decrease. The conditioned media from the cancer cells released varying amounts of encapsulated dye (16 – 50%, Figure 8A). We observed that the release was highest in the presence of the conditioned media from the PC-3 cells (47%, Figure 8A and 8B). The time course of the dye release in the presence of conditioned media from PC 3 cells can be fitted with a single exponential rate equation with rate constant of (57.4 ± 1.6) × 10−2 s−1. However, we observed that the amount of contents release does not correlate with the total amount of MMP-9 present in the conditioned media (as determined by ELISA). ELISA determines the total amount of MMP-9, which includes both catalytically active and inactive enzymes. It is also possible that besides MMP-9, other triple helicase secreted in the conditioned media (i.e., MMP-2, MMP-14, ADAM-10 etc.) are contributing to the release of the dye13b, 54. However, we were pleased to find that upon application of ultrasound for 3 minutes, the amounts of dye release increased to 25 – 75% (Figure 8A). Currently we are testing the effects of ultrasound frequency, incorporation of PEG lipid and cholesterol on the contents release from these liposomes and these results will be reported later.
Figure 8.
(A) Release of liposomal contents in presence of conditioned cell culture media from metastatic cancer cells (blue bars) and upon subsequent application of 3 minutes of 3 MHz ultrasound pulse (orange bars) are shown. Control represent release in absence of media as well as ultrasound (B) The kinetic profiles of the contents release in the presence of conditioned media of PC-3 (Green spheres) and bEnd-3 (Magenta spheres) cells and in the absence of any media ie. Control (Blue spheres) are shown. The red line shows the fitted curve using a single exponential rate equation.
4. CONCLUSION
We have demonstrated that echogenic liposomes can be prepared incorporating a substrate lipopeptide for MMP-9 in the lipid bilayer. The liposomes retain echogenicity after encapsulation of a hydrophilic dye and can be imaged by ultrasound. The encapsulated contents from the liposomes are released in presence of recombinant as well as cancer cell secreted MMP-9. The contents release can be further increased by the application of diagnostic frequency ultrasound pulses. This study demonstrates that diagnostic frequency ultrasound can be used simultaneously with enzymes as an additional method to trigger contents release from suitably constructed liposomes. We employed continuous excitations for the release study, which deliver more energy than a pulsed excitation used in ultrasound imaging. Future studies are required systematically varying the intensity, frequency and duty cycles of the ultrasound stimulation to determine the optimum excitation parameters. With further developments, these liposomes have the potential to serve as multimodal carriers for triggered release and simultaneous ultrasound imaging.
Supplementary Material
ACKNOWLEDGEMENT
This research was supported by NIH grants 1R01 CA 113746, 1R01 CA 132034 to SM and DKS, NSF grant DMR 1005011to SM and DMR 1005283, NIH grant P20RR016472 to KS. The Proteomics Core Facility is supported by grants from the National Center for Research Resources (5P20RR016471-12) and the National Institute of General Medical Sciences (8 P20 GM103442-12) from the National Institutes of Health. We thank Prof. Christy K. Holland and Jonathan Kopechek (Department of Internal Medicine, University of Cincinnati) for helpful discussions.
ABBREVIATIONS
- ELIPs
Echogenic Liposomes
- MMP-9
Matrix metalloproteinase-9
- LP4
Lipopeptide 4
Footnotes
Optical microscopic images of the ELIPs and the fluorescence spectra of carboxyfluorescein in the presence of regular and echogenic liposomes. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Lian T, Ho RJY. Trends and developments in liposome drug delivery systems. Journal of Pharmaceutical Sciences. 2001;90(6):667–680. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]; (b) Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein binding properties. Prog Lipid Res. 2003;42(6):463–478. doi: 10.1016/s0163-7827(03)00033-x. [DOI] [PubMed] [Google Scholar]; (c) Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]; (d) Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: Therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 2.(a) Ding N, Lu Y, Lee RJ, Yang C, Huang L, Liu J, Xiang G. Folate receptor targeted fluorescent paramagnetic bimodal liposomes for tumor imaging. International journal of nanomedicine. 2011;6:2513–20. doi: 10.2147/IJN.S23934. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Soares DC, Cardoso VN, de Barros AL, de Souza CM, Cassali GD, de Oliveira MC, Ramaldes GA. Antitumoral activity and toxicity of PEG coated and PEG folate coated pH sensitive liposomes containing (1)(5)(9)Gd DTPA BMA in Ehrlich tumor bearing mice. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2012;45(1 2):58–64. doi: 10.1016/j.ejps.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Andresen TL, Thompson DH, Kaasgaard T. Enzyme triggered nanomedicine: drug release strategies in cancer therapy. Molecular membrane biology. 2010;27(7):353–63. doi: 10.3109/09687688.2010.515950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagami T, Ernsting MJ, Li SD. Efficient tumor regression by a single and low dose treatment with a novel and enhanced formulation of thermosensitive liposomal doxorubicin. J Control Release. 2011;152(2):303–9. doi: 10.1016/j.jconrel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Torres E, Mainini F, Napolitano R, Fedeli F, Cavalli R, Aime S, Terreno E. Improved paramagnetic liposomes for MRI visualization of pH triggered release. J Control Release. 2011;154(2):196–202. doi: 10.1016/j.jconrel.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Paasonen L, Laaksonen T, Johans C, Yliperttula M, Kontturi K, Urtti A. Gold nanoparticles enable selective light induced contents release from liposomes. J Control Release. 2007;122(1):86–93. doi: 10.1016/j.jconrel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Huang SL, MacDonald RC. Acoustically active liposomes for drug encapsulation and ultrasound triggered release. Biochim Biophys Acta. 2004;1665(1 2):134–41. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhigaltsev IV, Maurer N, Wong KF, Cullis PR. Triggered release of doxorubicin following mixing of cationic and anionic liposomes. Biochim Biophys Acta. 2002;1565(1):129–35. doi: 10.1016/s0005-2736(02)00543-6. [DOI] [PubMed] [Google Scholar]
- 9.Aili D, Mager M, Roche D, Stevens MM. Hybrid nanoparticle liposome detection of phospholipase activity. Nano Lett. 2011;11(4):1401–5. doi: 10.1021/nl1024062. [DOI] [PubMed] [Google Scholar]
- 10.(a) Banerjee J, Hanson AJ, Gadam B, Elegbede AI, Tobwala S, Ganguly B, Wagh AV, Muhonen WW, Law B, Shabb JB, Srivastava DK, Mallik S. Release of liposomal contents by cell secreted matrix metalloproteinase 9. Bioconjug Chem. 2009;20(7):1332–9. doi: 10.1021/bc9000646. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Elegbede AI, Banerjee J, Hanson AJ, Tobwala S, Ganguli B, Wang R, Lu X, Srivastava DK, Mallik S. Mechanistic studies of the triggered release of liposomal contents by matrix metalloproteinase 9. Journal of the American Chemical Society. 2008;130(32):10633–42. doi: 10.1021/ja801548g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Beijnum JR, Petersen K, Griffioen AW. Tumor endothelium is characterized by a matrix remodeling signature. Frontiers in bioscience (Scholar edition) 2009;1:216–25. doi: 10.2741/S21. [DOI] [PubMed] [Google Scholar]
- 12.Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 13.(a) Bauvois B. New facets of matrix metalloproteinases MMP 2 and MMP 9 as cell surface transducers: outside in signaling and relationship to tumor progression. Biochimica et biophysica acta. 2012;1825(1):29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]; (b) Fisher JF, Mobashery S. Mechanism based profiling of MMPs. Methods in molecular biology (Clifton, N.J.) 2010;622:471–87. doi: 10.1007/978-1-60327-299-5_27. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue type plasminogen activator. Neurobiology of disease. 2010;38(3):376–85. doi: 10.1016/j.nbd.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Busti C, Falcinelli E, Momi S, Gresele P. Matrix metalloproteinases and peripheral arterial disease. Internal and emergency medicine. 2010;5(1):13–25. doi: 10.1007/s11739-009-0283-y. [DOI] [PubMed] [Google Scholar]
- 14.Konstantino Y, Nguyen TT, Wolk R, Aiello RJ, Terra SG, Fryburg DA. Potential implications of matrix metalloproteinase 9 in assessment and treatment of coronary artery disease. Biomarkers. 2009;14(2):118–29. doi: 10.1080/13547500902765140. [DOI] [PubMed] [Google Scholar]
- 15.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287–97. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6(6):480–98. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 17.(a) Shaw GJ, Meunier JM, Huang SL, Lindsell CJ, McPherson DD, Holland CK. Ultrasound enhanced thrombolysis with tPA loaded echogenic liposomes. Thromb Res. 2009;124(3):306–10. doi: 10.1016/j.thromres.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Schroeder A, Honen R, Turjeman K, Gabizon A, Kost J, Barenholz Y. Ultrasound triggered release of cisplatin from liposomes in murine tumors. J Control Release. 2009;137(1):63–8. doi: 10.1016/j.jconrel.2009.03.007. [DOI] [PubMed] [Google Scholar]; (c) Evjen TJ, Nilssen EA, Rognvaldsson S, Brandl M, Fossheim SL. Distearoylphosphatidylethanolamine based liposomes for ultrasound mediated drug delivery. Eur J Pharm Biopharm. 2010;75(3):327–33. doi: 10.1016/j.ejpb.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 18.(a) Hoskins P. Diagnostic ultrasound: physics and equipment. Greenwich Medical Media: Distributed … in the USA by Jamco Distribution: London; San Francisco: 2003. p. ix.p. 233. [Google Scholar]; (b) Leighton TG. In: The acoustic bubble. First printing pbk, editor. Academic Press; San Diego; London: 1997. p. xxvi.p. 613. [Google Scholar]
- 19.(a) Lin HY, Thomas JL. PEG Lipids and oligo(ethylene glycol) surfactants enhance the ultrasonic permeabilizability of liposomes. Langmuir. 2003;19(4):1098–1105. [Google Scholar]; (b) Lin HY, Thomas JL. Factors affecting responsivity of unilamellar liposomes to 20 kHz ultrasound. Langmuir. 2004;20(15):6100–6106. doi: 10.1021/la049866z. [DOI] [PubMed] [Google Scholar]; (c) Schroeder A, Avnir Y, Weisman S, Najajreh Y, Gabizon A, Talmon Y, Kost J, Barenholz Y. Controlling liposomal drug release with low frequency ultrasound: Mechanism and feasibility. Langmuir. 2007;23(7):4019–4025. doi: 10.1021/la0631668. [DOI] [PubMed] [Google Scholar]
- 20.(a) Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;162(1 2):1–16. doi: 10.1016/j.chemphyslip.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 21.(a) Kono K, Nakashima S, Kokuryo D, Aoki I, Shimomoto H, Aoshima S, Maruyama K, Yuba E, Kojima C, Harada A, Ishizaka Y. Multi functional liposomes having temperature triggered release and magnetic resonance imaging for tumor specific chemotherapy. Biomaterials. 2011;32(5):1387–95. doi: 10.1016/j.biomaterials.2010.10.050. [DOI] [PubMed] [Google Scholar]; (b) Britton GL, Kim H, Kee PH, Aronowski J, Holland CK, McPherson DD, Huang SL. In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes. Circulation. 2010;122(16):1578–87. doi: 10.1161/CIRCULATIONAHA.109.879338. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kopechek JA, Haworth KJ, Raymond JL, Douglas Mast T, Perrin SR, Klegerman ME, Huang S, Porter TM, McPherson DD, Holland CK. Acoustic characterization of echogenic liposomes: frequency dependent attenuation and backscatter. The Journal of the Acoustical Society of America. 2011;130(5):3472–81. doi: 10.1121/1.3626124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo T, Zhang W, Shi B, Cheng X, Zhang Y. Enhanced bone regeneration around dental implant with bone morphogenetic protein 2 gene and vascular endothelial growth factor protein delivery. Clinical oral implants research. 2012;23(4):467–73. doi: 10.1111/j.1600-0501.2011.02164.x. [DOI] [PubMed] [Google Scholar]
- 23.(a) Huang SL, Hamilton AJ, Nagaraj A, Tiukinhoy SD, Klegerman ME, McPherson DD, MacDonald RC. Improving ultrasound reflectivity and stability of echogenic liposomal dispersions for use as targeted ultrasound contrast agents. Journal of Pharmaceutical Sciences. 2001;90(12):1917–1926. doi: 10.1002/jps.1142. [DOI] [PubMed] [Google Scholar]; (b) Huang SL, McPherson DD, Macdonald RC. A method to co encapsulate gas and drugs in liposomes for ultrasound controlled drug delivery. Ultrasound Med Biol. 2008;34(8):1272–80. doi: 10.1016/j.ultrasmedbio.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Paul S, Russakow D, Nahire R, Nandy T, Ambre AH, Katti K, Mallik S, Sarkar K. In vitro measurement of attenuation and nonlinear scattering from echogenic liposomes. Ultrasonics. 2012 doi: 10.1016/j.ultras.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang SL. Liposomes in ultrasonic drug and gene delivery. Advanced Drug Delivery Reviews. 2008;60(10):1167–1176. doi: 10.1016/j.addr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 25.(a) Huang SL, MacDonald RC. Acoustically active liposomes for drug encapsulation and ultrasound triggered release. Biochimica Et Biophysica ActaCBiomembranes. 2004;1665(1 2):134–141. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]; (b) Kopechek JA, Abruzzo TM, Wang B, Chrzanowski SM, Smith DAB, Kee PH, Huang S, Collier JH, McPherson DD, Holland CK. Ultrasound Mediated Release of Hydrophilic and Lipophilic Agents From Echogenic Liposomes. Journal of Ultrasound in Medicine. 2008;27(11):1597–1606. doi: 10.7863/jum.2008.27.11.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Smith DAB, Vaidya SS, Kopechek JA, Huang SL, Klegerman ME, Mcpherson DD, Holland CK. Ultrasound Triggered Release of Recombinant Tissue Type Plasminogen Activator from Echogenic Liposomes. Ultrasound in Medicine and Biology. 2010;36(1):145–157. doi: 10.1016/j.ultrasmedbio.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopechek JA, Abruzzo TM, Wang B, Chrzanowski SM, Smith DA, Kee PH, Huang S, Collier JH, McPherson DD, Holland CK. Ultrasound mediated release of hydrophilic and lipophilic agents from echogenic liposomes. J Ultrasound Med. 2008;27(11):1597–606. doi: 10.7863/jum.2008.27.11.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee J, Hanson AJ, Muhonen WW, Shabb JB, Mallik S. Microwave assisted synthesis of triple helical, collagen mimetic lipopeptides. Nat Protoc. 2010;5(1):39–50. doi: 10.1038/nprot.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi WT, Forsberg F. Ultrasonic characterization of the nonlinear properties of contrast microbubbles. Ultrasound in Medicine and Biology. 2000;26(1):93–104. doi: 10.1016/s0301-5629(99)00117-9. [DOI] [PubMed] [Google Scholar]
- 29.Sarkar K, Shi WT, Chatterjee D, Forsberg F. Characterization of ultrasound contrast microbubbles using in vitro experiments and viscous and viscoelastic interface models for encapsulation. Journal of the Acoustical Society of America. 2005;118(1):539–550. doi: 10.1121/1.1923367. [DOI] [PubMed] [Google Scholar]
- 30.(a) Omata D, Negishi Y, Yamamura S, Hagiwara S, Endo Takahashi Y, Suzuki R, Maruyama K, Nomizu M, Aramaki Y. Involvement of Ca(2)(+) and ATP in enhanced gene delivery by bubble liposomes and ultrasound exposure. Mol Pharm. 2012;9(4):1017–23. doi: 10.1021/mp200606d. [DOI] [PubMed] [Google Scholar]; (b) Omata D, Negishi Y, Hagiwara S, Yamamura S, Endo Takahashi Y, Suzuki R, Maruyama K, Aramaki Y. Enhanced gene delivery using Bubble liposomes and ultrasound for folate PEG liposomes. Journal of drug targeting. 2012;20(4):355–63. doi: 10.3109/1061186X.2012.660162. [DOI] [PubMed] [Google Scholar]; (c) Omata D, Negishi Y, Hagiwara S, Yamamura S, Endo Takahashi Y, Suzuki R, Maruyama K, Nomizu M, Aramaki Y. Bubble liposomes and ultrasound promoted endosomal escape of TAT PEG liposomes as gene delivery carriers. Mol Pharm. 2011;8(6):2416–23. doi: 10.1021/mp200353m. [DOI] [PubMed] [Google Scholar]; (d) Negishi Y, Omata D, Iijima H, Takabayashi Y, Suzuki K, Endo Y, Suzuki R, Maruyama K, Nomizu M, Aramaki Y. Enhanced laminin derived peptide AG73 mediated liposomal gene transfer by bubble liposomes and ultrasound. Mol Pharm. 2010;7(1):217–26. doi: 10.1021/mp900214s. [DOI] [PubMed] [Google Scholar]; (e) Negishi Y, Endo Y, Fukuyama T, Suzuki R, Takizawa T, Omata D, Maruyama K, Aramaki Y. Delivery of siRNA into the cytoplasm by liposomal bubbles and ultrasound. J Control Release. 2008;132(2):124–30. doi: 10.1016/j.jconrel.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 31.(a) Hensel K, Mienkina MP, Schmitz G. Analysis of ultrasound fields in cell culture wells for in vitro ultrasound therapy experiments. Ultrasound Med Biol. 2011;37(12):2105–15. doi: 10.1016/j.ultrasmedbio.2011.09.007. [DOI] [PubMed] [Google Scholar]; (b) Kopechek JA, Kim H, McPherson DD, Holland CK. Calibration of the 1 MHz Sonitron ultrasound system. Ultrasound Med Biol. 2010;36(10):1762–6. doi: 10.1016/j.ultrasmedbio.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birkedal Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 33.Motooka D, Kawahara K, Nakamura S, Doi M, Nishi Y, Nishiuchi Y, Kee Kang Y, Nakazawa T, Uchiyama S, Yoshida T, Ohkubo T, Kobayashi Y. The triple helical structure and stability of collagen model peptide with 4(S) hydroxyprolyl pro gly units. Biopolymers. 2011 doi: 10.1002/bip.21730. [DOI] [PubMed] [Google Scholar]
- 34.Raabe HM, Molsen H, Mlinaric SM, Acil Y, Sinnecker GH, Notbohm H, Kruse K, Muller PK. Biochemical alterations in collagen IV induced by in vitro glycation. Biochem J. 1996;319(Pt 3):699–704. doi: 10.1042/bj3190699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basel MT, Shrestha TB, Troyer DL, Bossmann SH. Protease sensitive, polymer caged liposomes: a method for making highly targeted liposomes using triggered release. ACS nano. 2011;5(3):2162–75. doi: 10.1021/nn103362n. [DOI] [PubMed] [Google Scholar]
- 36.Lauer Fields JL, Sritharan T, Stack MS, Nagase H, Fields GB. Selective hydrolysis of triple helical substrates by matrix metalloproteinase 2 and 9. J Biol Chem. 2003;278(20):18140–5. doi: 10.1074/jbc.M211330200. [DOI] [PubMed] [Google Scholar]
- 37.de la Rica R, Aili D, Stevens MM. Enzyme responsive nanoparticles for drug release and diagnostics. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Chen C, Han D, Cai C, Tang X. An overview of liposome lyophilization and its future potential. J Control Release. 2010;142(3):299–311. doi: 10.1016/j.jconrel.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 39.Glavas Dodov M, Fredro Kumbaradzi E, Goracinova K, Simonoska M, Calis S, Trajkovic Jolevska S, Hincal AA. The effects of lyophilization on the stability of liposomes containing 5 FU. Int J Pharm. 2005;291(1 2):79–86. doi: 10.1016/j.ijpharm.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 40.Crowe JH, Crowe LM, Carpenter JF, Aurell Wistrom C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987;242(1):1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AlkanOnyuksel H, Demos SM, Lanza GM, Vonesh MJ, Klegerman ME, Kane BJ, Kuszak J, McPherson DD. Development of inherently echogenic liposomes as an ultrasonic contrast agent. Journal of Pharmaceutical Sciences. 1996;85(5):486–490. doi: 10.1021/js950407f. [DOI] [PubMed] [Google Scholar]
- 42.(a) Huang SL, Hamilton AJ, Pozharski E, Nagaraj A, Klegerman ME, McPherson DD, MacDonald RC. Physical correlates of the ultrasonic reflectivity of lipid dispersions suitable as diagnostic contrast agents. Ultrasound in Medicine and Biology. 2002;28(3):339–348. doi: 10.1016/s0301-5629(01)00512-9. [DOI] [PubMed] [Google Scholar]; (b) Kopechek JA, Haworth KJ, Raymond JL, Douglas Mast T, Perrin SR, Klegerman ME, Huang S, Porter TM, McPherson DD, Holland CK. Acoustic characterization of echogenic liposomes: Frequency dependent attenuation and backscatter. J Acoust Soc Am. 2011;130(5):3472. doi: 10.1121/1.3626124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.(a) Suzuki R, Takizawa T, Negishi Y, Utoguchi N, Sawamura K, Tanaka K, Namai E, Oda Y, Matsumura Y, Maruyama K. Tumor specific ultrasound enhanced gene transfer in vivo with novel liposomal bubbles. J Control Release. 2008;125(2):137–44. doi: 10.1016/j.jconrel.2007.08.025. [DOI] [PubMed] [Google Scholar]; (b) Pitt WG, Husseini GA, Maruyama K. Letter to the Editor:On Bubbles and Liposomes, Response by the authors, Letter to the Editor 2. Journal of Controlled Release. 2008;125:174–177. doi: 10.1016/j.jconrel.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang SL, Tiukinhoy S, Wang L, MacDonald R, Nagaraj A, McPherson D. Acoustically active liposomes of novel cationic anionic composition in conjunction with ultrasound for gene delivery into vascular smooth muscle cells. Molecular Therapy. 2003;7(5):S167–S167. [Google Scholar]
- 45.Kopechek JA, Haworth KJ, Raymond JL, Mast TD, Perrin SR, Klegerman ME, Huang SL, Porter TM, McPherson DD, Holland CK. Acoustic characterization of echogenic liposomes: Frequency dependent attenuation and backscatter. Journal of the Acoustical Society of America. 2011;130(5):3472–3481. doi: 10.1121/1.3626124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.(a) Sarkar K, Katiyar A, Jain P. Growth and dissolution of an encapsulated contrast microbubble. Ultrasound in Medicine and Biology. 2009;35(8):1385–1396. doi: 10.1016/j.ultrasmedbio.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Katiyar A, Sarkar K, Jain P. Effects of Encapsulation Elasticity on the stability of an Encapsulated Microbubble. Journal of Colloid and Interface Science. 2009;336:519–525. doi: 10.1016/j.jcis.2009.05.019. [DOI] [PubMed] [Google Scholar]; (c) Katiyar A, Sarkar K. Stability analysis of an encapsulated microbubble against gas diffusion. Journal of Colloid and Interface Science. 2010;343(1):42–47. doi: 10.1016/j.jcis.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.(a) Chatterjee D, Sarkar K. A Newtonian rheological model for the interface of microbubble contrast agents. Ultrasound in Medicine and Biology. 2003;29(12):1749–1757. doi: 10.1016/s0301-5629(03)01051-2. [DOI] [PubMed] [Google Scholar]; (b) Paul S, Katiyar A, Sarkar K, Chatterjee D, Shi WT, Forsberg F. Material characterization of the encapsulation of an ultrasound contrast microbubble and its subharmonic response: Strain softening interfacial elasticity model. Journal of the Acoustical Society of America. 2010;127(6):3846–3857. doi: 10.1121/1.3418685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarkar NR, Rosendahl T, Krueger AB, Banerjee AL, Benton K, Mallik S, Srivastava DK. “Uncorking” of liposomes by matrix metalloproteinase 9. Chem Commun (Camb) 2005;(8):999–1001. doi: 10.1039/b416827e. [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee D, Jain P, Sarkar K. Ultrasound mediated destruction of contrast microbubbles used for medical imaging and drug delivery. Physics of Fluids. 2005;17(10):100603. [Google Scholar]
- 50.Minond D, Lauer Fields JL, Cudic M, Overall CM, Pei D, Brew K, Visse R, Nagase H, Fields GB. The roles of substrate thermal stability and P2 and P1’ subsite identity on matrix metalloproteinase triple helical peptidase activity and collagen specificity. J Biol Chem. 2006;281(50):38302–13. doi: 10.1074/jbc.M606004200. [DOI] [PubMed] [Google Scholar]
- 51.Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Phys Ther. 2001;81(7):1351–8. [PubMed] [Google Scholar]
- 52.Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Patterns of MMP 2 and MMP 9 expression in human cancer cell lines. Oncol Rep. 2009;21(5):1323–33. doi: 10.3892/or_00000358. [DOI] [PubMed] [Google Scholar]
- 53.Chen F, Ohashi N, Li W, Eckman C, Nguyen JH. Disruptions of occludin and claudin 5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology. 2009;50(6):1914–23. doi: 10.1002/hep.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Molecular aspects of medicine. 2008;29(5):258–89. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.