Summary
Background
Tissue factor (TF) is frequently overexpressed in cancer cells and correlated with more aggressive tumor phenotypes and poor prognosis. In addition to promoting coagulation-dependent metastasis and cancer-associated thrombosis, tumor cell-expressed TF mediates direct cell signaling involving the protease activated receptor (PAR) 2. Ixolaris is a tick-derived inhibitor of the TF-FVIIa-Xa coagulation initiation complex which blocks primary tumor growth and angiogenesis in glioblastoma and melanoma models.
Methods
Here we address the anti-tumor effects of Ixolaris in TF-VIIa-PAR2 signaling-dependent breast cancer models, a xenograft model of highly aggressive human MDA-MB-231mfp cells and a syngeneic model of PAR2-deficient and replete PyMT mouse mammary carcinoma cells.
Results
Ixolaris potently inhibited the procoagulant activity of human MDA-MB-231mfp or murine PyMT breast cancer cells. Ixolaris blocked signaling by the ternary TF-FVIIa-FXa complex, and, surprisingly, at higher concentrations also the binary TF-FVIIa complex on MDA-MB-231 cells. We show that Ixolaris interacts with certain residues in the human VIIa protease domain that are involved in PAR2 cleavage. In contrast to human VIIa, Ixolaris was a poor inhibitor of murine TF-FVIIa signaling and did not attenuate PAR2-dependent tumor growth in a syngeneic mouse model of breast cancer progression.
Conclusion
These data show that Ixolaris inhibits PAR2 cleavage specifically by human TF signaling complexes and suggest that Ixolaris may block tumor growth of human cell models with ectopic FVIIa expression through inhibition of direct TF-FVIIa-PAR2 signaling as well as its anticoagulant activity.
Introduction
Tissue factor (TF) serves as the cofactor for coagulation factor VIIa (FVIIa) to initiate the extrinsic coagulation pathway, leading to the generation of thrombin, fibrin formation and platelet activation [1, 2]. TF is constitutively expressed in a cell-type specific manner and upregulated in a number of pathological processes [3, 4]. TF is induced in several tumor types by oncogenic transformation or hypoxia [5, 6] and TF expression is correlated with more aggressive tumor phenotypes and poor prognosis [7–9]. Therefore, the TF-FVIIa-initiated coagulation pathway plays important roles in cancer progression, cancer-associated thrombosis and metastasis [6, 10].
In addition to triggering thrombin generation and thrombosis, TF-dependent signaling contributes to primary tumor growth [11–13]. The TF-FVIIa binary complex evokes a number of tumor cell responses through activation of the protease activated receptor (PAR) 2, including production of proangiogenic chemokines and growth factors (IL8, CXCL-1) and growth factors mediating recruitment and maturation of macrophages [14, 15]. Remarkably, studies employing specific monoclonal antibodies against TF as well as site-directed mutagenesis on TF or FVIIa have shown that TF-FVIIa-mediated coagulation and signaling are essentially non-overlapping processes [11, 16, 17]. In this context, it has been demonstrated that blockade of TF signaling but not the TF procoagulant response attenuates primary tumor growth in a human breast cancer model [11]. On the other hand, targeting TF-mediated coagulation, but not signaling decreases metastasis in the same tumor model.
Independent evidence for the participation of PAR2 in tumor progression was obtained by employing an oncogene-driven model of spontaneous breast cancer development in mice [12, 18]. PAR2 deficiency reduced the appearance and growth of invasive breast cancer in mice that express the polyoma middle T antigen specifically in the mammary gland epithelium (PyMT mice). Remarkably, deletion of the cytoplasmic tail of TF recapitulated the delayed tumor development observed in PAR2-deficient PyMT mice, demonstrating that a crosstalk between TF and PAR2 contributes to primary tumor growth [12].
The relative contributions of coagulation and signaling functions of TF to tumor progression are incompletely understood. Additional insights into mechanisms of action of TF-specific inhibitors will enable appropriate targeting of this important tumor promoting pathway in cancer therapy. Ixolaris, a tick salivary 140 amino acid protein containing 2 Kunitz-like domains, binds to FXa or FX that serve as scaffolds for inhibition of the TF-FVIIa complex. Ixolaris is a direct inhibitor of the FVIIa catalytic site [19], but in contrast to TF pathway inhibitor (TFPI) [20] and similarly to the nematode anticoagulant protein C2 (NAPc2) [21], Ixolaris does not bind to the active site cleft of FXa. Instead, complex formation is mediated by the FXa heparin-binding exosite [22]. In addition, Ixolaris interacts with high affinity with FX through a precursor state of the heparin-binding exosite [23]. This interaction with zymogen FX is crucial for the long half-life of the inhibitor in vivo [24]. It has been demonstrated that Ixolaris blocks primary growth of human glioblastoma (U87-MG) and melanoma cells in a xenograft model and this effect is accompanied by a significant decrease in VEGF expression as well as diminished tumor angiogenesis [25, 26].
In this study, we demonstrate that Ixolaris is a potent anticoagulant and in parallel inhibits signaling of the TF coagulation initiation complex on human breast cancer cells. Unexpectedly, Ixolaris also blocks signaling of the TF-FVIIa binary complex through PAR2, independent of the FX scaffold that increases affinity. We map critical human FVIIa residues involved in the interaction with Ixolaris and show considerable overlap with the binding site for PAR2. In contrast, Ixolaris is a poor direct inhibitor of mouse FVIIa and does not inhibit TF-PAR2 signaling dependent tumor growth of murine models in vivo. Thus, we provide new insight into the inhibitory profile of this TF inhibitor in vitro and in vivo.
Methods
Proteins
Human or mouse soluble TF (sTF) [27, 28], mouse FVIIa (mFVIIa) and human FVIIa variants [17, 29], and anti-PAR2 polyclonal antibody [16] were produced as described. PAR2 agonist peptide (SLIGRL) was synthesized and HPLC purified in house. Recombinant Ixolaris was produced in High Five insect cells (Invitrogen) [19], and further purified and quantified [24]. We used anti-ERK1/2 and phospho-ERK1/2 antibodies (Cell Signaling Technology), FX (Haematology Technologies) and hirudin (Sigma). Recombinant nematode anticoagulant protein c2 (NAPc2) was kindly provided by Dr. G. Vlasuk (Corvas).
Cell culture
MDA-MB-231mfp [30] and PAR2-deficient murine PyMT breast cancer cells were cultured in L15 medium (Lonza), 10% FBS, glutamine, and insulin [11]. Cells were transduced with empty retroviral vector (mock) or murine PAR2 retrovirus as described [12].
Signaling assays
MDA-MB-231mfp, mock and PAR2 transduced PyMT cells were serum-deprived for 24 hours and stimulated for 90 minutes for mRNA induction in the presence of 200 nM hirudin to prevent thrombin-mediated effects. CXCL-1 and IL8 mRNA levels were quantified by Taqman real time PCR [11]. MDA-MB-231mfp cells were grown overnight in serum-free medium and stimulated for 24 hours for ELISA measurements (R&D Systems) of secreted IL-8. ERK phosphorylation in 24 hours serum-starved MDA-MB-231mfp cells stimulated for 60 minutes was measured by Western blotting and quantified by densitometry.
FXa generation assay
FXa generation was measured in HBS (10 mM HEPES, pH 7.4, 137 mM NaCl, 5.38 mM KCl, 1.5 mM CaCl2, 5.5 mM Glucose, 1% BSA). Ixolaris (0.1–100 nM) was preincubated with 100 nM FX for 10 min and then added to the cell supernatants containing 10 nM FVIIa. After defined times, FXa activation was quenched by the addition of EDTA and FXa was quantified with Spectrozyme FXa (American Diagnostica).
TF-FVIIa catalytic activity towards small substrates
Inhibition of human TF-FVIIa (1 µM/20 nM) amidolytic activity by Ixolaris was measured with 1 mM Spectrozyme FXa at 405 nm in a kinetic plate reader. Inhibition of mouse TF-FVIIa (250/50 nM) amidolytic activity by Ixolaris was measured in 100 mM NaCl, 50 mM Tris/HCl, 5 mM CaCl2, 0.1% BSA, pH 7.4 [29] with 1 mM S-2288.
Quantification of PAR2 cleavage
PAR2 cleavage was quantified in Chinese hamster ovary cells stably transfected with TF and the endothelial protein C receptor (EPCR). Cells were transiently transfected with FLAG-tagged PAR2 as described [17, 31] and exposed under serum free conditions to the indicated proteases and inhibitors. A cell surface ELISA with horse radish peroxidase conjugated antibody was used to determine residual exposure of the amino-terminal FLAG-epitope and to calculate PAR2 cleavage relative to untreated controls and baseline values of untransfected cells.
Orthotopic tumor growth assay
All animal experiments were performed under approved protocols of The Scripps Research Institute IACUC. MDA-MB 231 mfp cells (2 X 106) in 50 µl of serum free medium were injected into the second left mammary gland of C.B-17 SCID mice. Mice were injected subcutaneously with 250 µg/kg Ixolaris daily from day 5 after tumor cell implantation. PyMT PAR2−/− breast carcinoma cells were transduced with control retrovirus or virus encoding wild-type murine PAR2, as described [12]. 5 × 105 cells in 50 µl of serum free medium were injected into the second left mammary gland of female C57BL/6J mice. Mice received 250 µg/kg Ixolaris subcutaneously daily starting at day 5 until day 27. Tumors were measured with calipers and tumor volumes (v) were calculated using the formula v=0.5* (length × width2). When tumors reached the maximally allowed sizes, all cohorts were sacrificed and the tumors were fixed in formalin for histological analysis.
Histology
Sections (5 µm) from paraffin-embedded tumors were blocked with 5% BSA/0.2% Tween-20 for 1 hour and stained overnight in the cold with anti CD34 (Abcam) or anti F4/80 (Catlag) antibodies. Slides were incubated for 30 min with secondary antibody goat anti-rat Alexa546 and counterstained with DAPI and mounted with fluorescent medium. Macrophages and vessel counts were quantified with Imaris software from at least 4 randomly chosen fields.
Statistical Analysis
Graphpad Prism Software (version 5) was used for one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparison test to assess differences within groups.
Results
Ixolaris blocks the TF coagulation initiation complex on breast cancer cells
Ixolaris is a potent inhibitor of TF by forming a quaternary TF-FVIIa-FXa-Ixolaris complex similar to the physiological inhibitor TFPI [32] or the nematode-derived inhibitor NAPc2 [21]. Unlike TFPI and similarly to NAPc2, Ixolaris inhibits the catalytic site of FVIIa, but does not directly interact with the FXa catalytic cleft [19]. We determined whether Ixolaris inhibits the extrinsic tenase complex on the aggressive breast cancer cell line MDA-MB-231mfp. As shown in Fig. 1A, Ixolaris dose dependently attenuated the generation of FXa measured in the supernatant of this cell-based system.
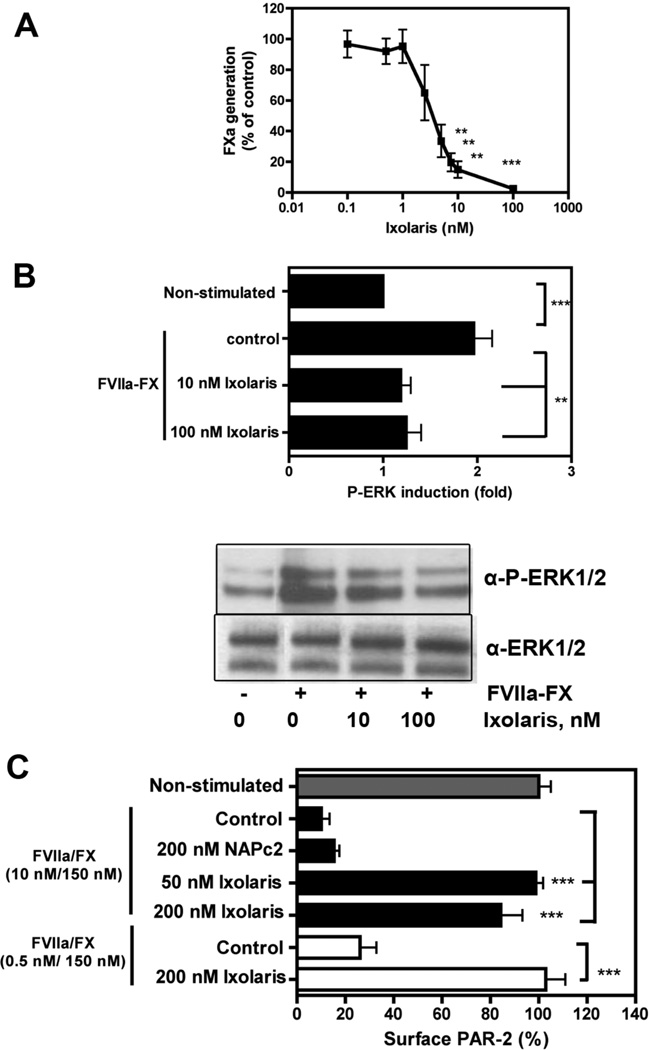
Figure 1. Ixolaris blocks the ternary TF-VIIa-X complex on MDA-MB- 231mfp human breast cancer cells.
(A) FXa generation on MDA-MB-231mfp cells was analyzed with 10 nM VIIa, 100 nM FX and varying amounts of Ixolaris (0–100 nM). The amount of FXa generated after 10 min was quantified with Spectrozyme FXa (mean ± S.D. n= 3, ** p< 0.01 and *** p<0.001, one-way ANOVA followed by Bonferroni post test, different from control) (B) MDA-MB-231mfp cells were stimulated with 0.5 nM/100 nM FVIIa/FX in the absence or presence of Ixolaris (10nM or 100 nM) for 60 min followed by Western blotting to quantify phosphorylated ERK1/2 using total ERK1/2 as loading control. FX was premixed with Ixolaris prior to stimulation. A typical experiment and quantification of 3 independent assays are shown (mean ± S.D. of fold induction over untreated controls, n=3 **, p<0.01, one-way ANOVA followed by Bonferroni post test). (C) Effect of Ixolaris on PAR-2 cleavage by the coagulation initiation complex was analyzed in CHO-TF cells transiently expressing FLAG-hPAR-2. Cells were incubated with FVIIa/FX and Ixolaris or NAPc2, as indicated, and residual PAR-2 surface expression was measured after 1 h (mean ± S.D. n=3, ** p<0.01, *** p<0.001, unpaired t test, versus control).
MDA-MB-231mfp cells are an established model for TF-PAR2 signaling [11]. We next analyzed whether Ixolaris blocked signaling of the TF-FVIIa-Xa coagulation initiation complex by measuring MAP kinase ERK1/2 phosphorylation in serum-starved MDA-MB-231mfp cells. Representative Western blots for ERK1/2 phosphorylation are shown in Fig. 1B. Ixolaris at 10 nM (p<0.01) or 100 nM (p<0.01) reduced ERK1/2 phosphorylation induced by TF-FVIIa-FXa stimulation for 60 minutes. Thus, Ixolaris inhibits both TF-initiated coagulation and signaling of the ternary coagulation initiation complex in breast cancer cells.
In order to directly show that PAR2 cleavage by the ternary complex is inhibited by Ixolaris, we used a Chinese hamster ovary (CHO) cell model for ternary complex and EPCR-dependent cell surface PAR2 cleavage. As previously shown, highly efficient PAR2 cleavage in the presence of 10 nM FVIIa and 150 nM FX was not blocked by another ternary complex inhibitor, NAPc2, known for its inability to inhibit the catalytic site of FXa (Fig. 1C). In contrast, Ixolaris at 50 (p<0.001) and 200 nM (p<0.001) was highly efficient to inhibit ternary complex-mediated PAR2 cleavage. Ixolaris also blocked ternary complex-mediated PAR2 cleavage (p<0.01) when a physiologically more relevant concentration of 0.5 nM FVIIa together with 100 nM FX was used.
TF-FVIIa and TF-FVIIa-FXa signaling induce mRNA expression of proangiogenic IL-8 and CXCL-1 [11, 15, 33]. To determine whether Ixolaris inhibits these downstream targets of PAR2 signaling, mRNA levels were determined in serum-starved MDA-MB-231mfp cells stimulated by TF-FVIIa- FXa for 90 minutes. Ixolaris at 10 nM and 100 nM decreased CXCL-1 (p<0.05 and p<0.01, respectivily) and 100 nM reduced IL-8 (p<0.05) with similar efficiency as a cleavage-blocking anti-PAR2 antibody (Fig. 2A). In addition, Ixolaris blocked IL-8 release from TF-FVIIa-FXa stimulated cells. MDA-MB-231mfp cells were stimulated by TF-FVIIa-FXa in the absence or presence of Ixolaris for 24 hours. As shown in Fig. 2B, Ixolaris at 10 nM (p<0.01) and 100 nM (p<0.01) inhibited IL-8 release from stimulated MDA-MB-231mfp cells.
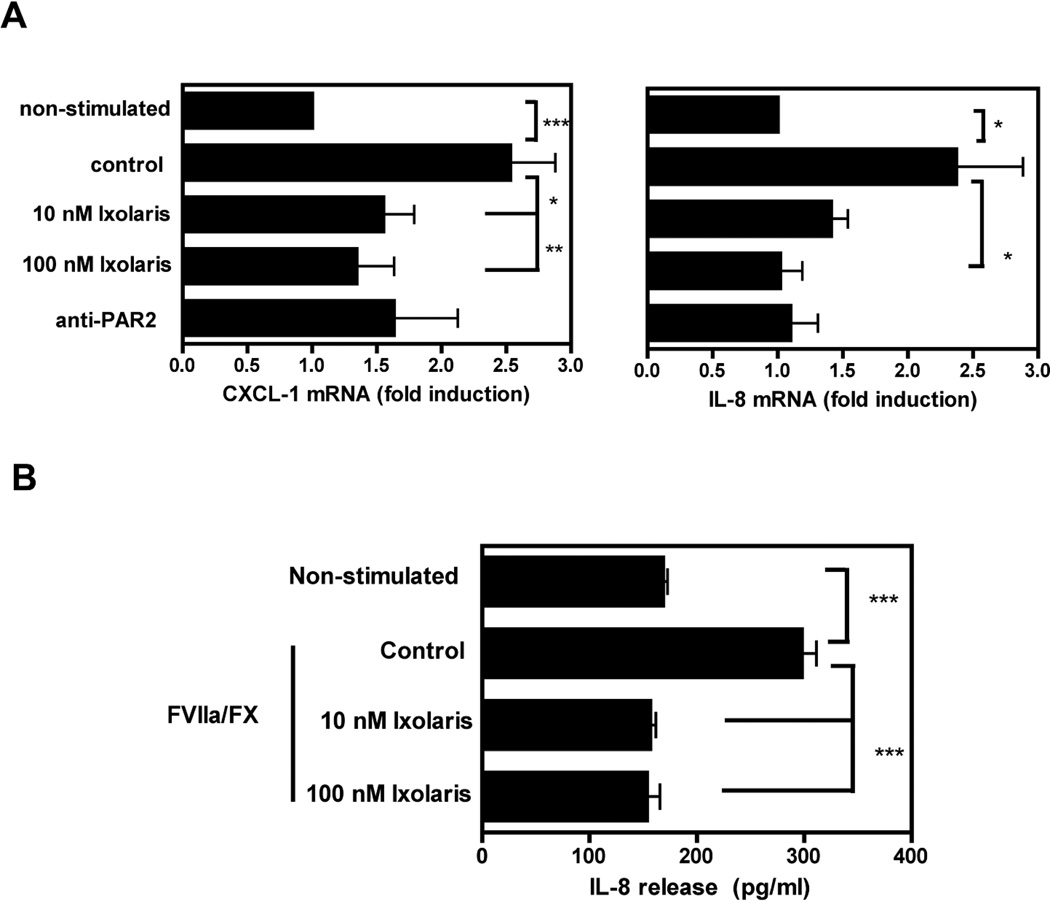
Figure 2. Ixolaris blocks ternary TF-VIIa-Xa complex mediated cytokine production by MDA-MB-231mfp cells.
(A) FX (100 nM) was incubated for 10 min with Ixolaris (10 nM or 100 nM) before addition to MDA-MB-231mfp cells in the presence of FVIIa (0.5 nM). Cells were preincubated for 15 minutes with PAR-2-cleavage-blocking antibody (anti-PAR2), prior to stimulation by FVIIa/FX. IL-8 and CXCL-1 mRNA induction over control after 90 min was determined by quantitative PCR (mean ± S.D. n≥6 *, p<0.05 **, p<0.01, one-way ANOVA followed by Bonferroni post test). (B) IL-8 release from MDA-MB-231mfp cells upon stimulation by FVIIa/FX (0.5 nM/100 nM) in the absence or in the presence of 10 nM or 100 nM Ixolaris was determined after 24 h of incubation (mean ± S.D. n=3, *** p<0.001 one-way ANOVA followed by Bonferroni post test).
Ixolaris is a direct inhibitor of the binary TF-FVIIa signaling complex
Because the cleavage assay (Fig. 1C) indicated that Ixolaris inhibited FXa-independent PAR2 cleavage at high concentrations of FVIIa, we determined whether Ixolaris inhibited IL-8 and CXCL-1 induction by the TF-FVIIa binary complex. Indeed, Ixolaris dose dependently prevented IL-8 and CXCL-1 upregulation by TF-FVIIa in MDA-MB-231mfp cells (Fig. 3A). To better understand the biochemical basis for the interaction of Ixolaris with the FVIIa protease domain, we studied 6 FVIIa variants [17] with mutations in the FVIIa protease domain at or near the active site cleft, as depicted in the model of Fig 3B. We first analyzed the dose dependent inhibition of FXa generation mediated by these variants on human MDA-MB-231mfp breast cancer cells. No major decrease in inhibitory potency was observed (Fig. 3C), excluding that these mutations in the FVIIa protease domain caused steric hindrance to prevent the interaction of FX/Xa-bound Ixolaris with FVIIa.
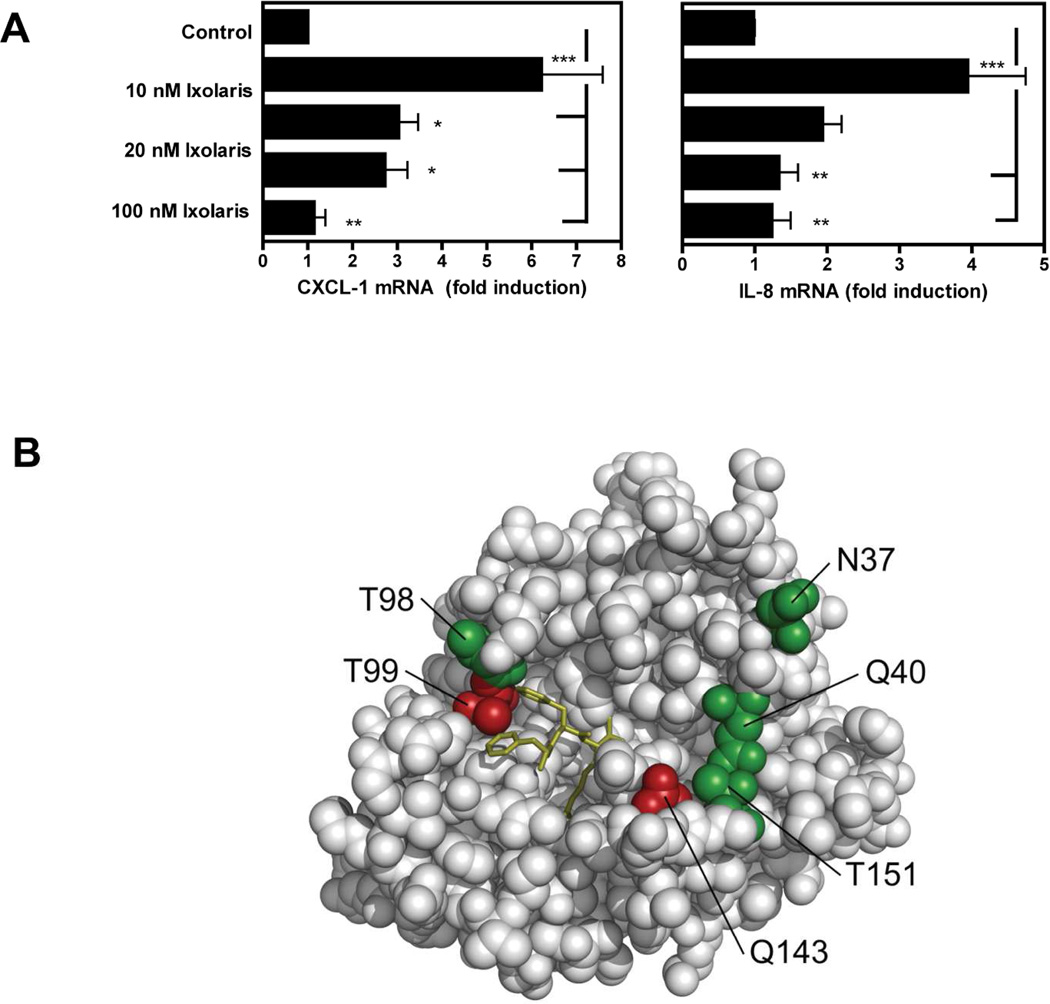
Figure 3. Ixolaris directly blocks FVIIa signaling and catalytic activities.
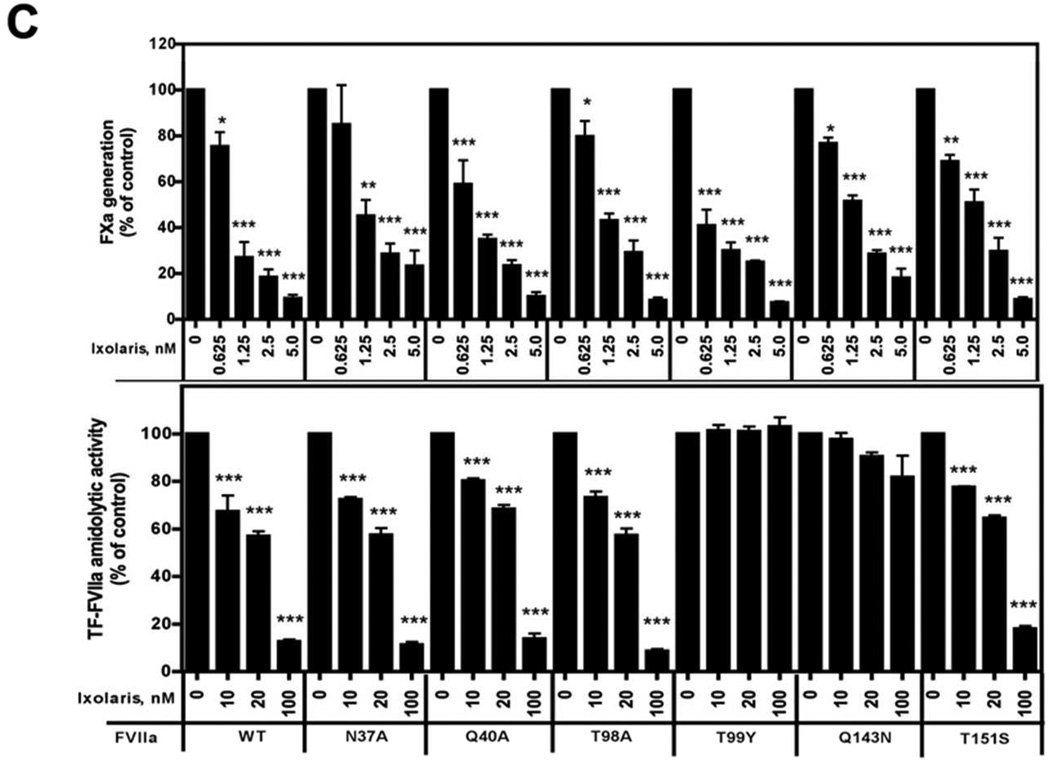
(A) MDA-MB-231mfp cells were stimulated with FVIIa (10 nM) in the presence of increasing concentrations of Ixolaris (0–100 nM). IL-8 and CXCL-1 mRNA induction over control after 90 min was determined by quantitative PCR (mean ± S.D., n≥6, * p<0.05; ** p<0.01, one-way ANOVA followed by Bonferroni post test). (B) Model of the protease domain of FVIIa based on the crystal structure of sTF-FVIIa by Banner et al. [47]. The position of mutated residues (in green and red) and the crucial contacts modulating Ixolaris binding (T99 and Q143 in red) are shown. Q40, Q143 and T151 are the key contacts for FVIIa-mediated PAR-2 cleavage. (C) Identification of contact areas of Ixolaris with the FVIIa protease domain. Top panel: Effect of mutation in the FVIIa protease domain on Ixolaris inhibition of FXa generation was analyzed with FVIIa variant (10 nM) in the presence of 100 nM FX on MDA-MB-231mfp cells incubated for 10 min (mean ± S.D. n=3). Botton panel: Inhibition of amidolytic activity of FVIIa wt or variants towards Spectrozyme FXa was measured in the presence of 1 µM human sTF and increasing concentrations of Ixolaris (mean ± S.D. n=3., * p<0.05, ** p<0.01, *** p<0.001, one-way ANOVA followed by Bonferroni post test).
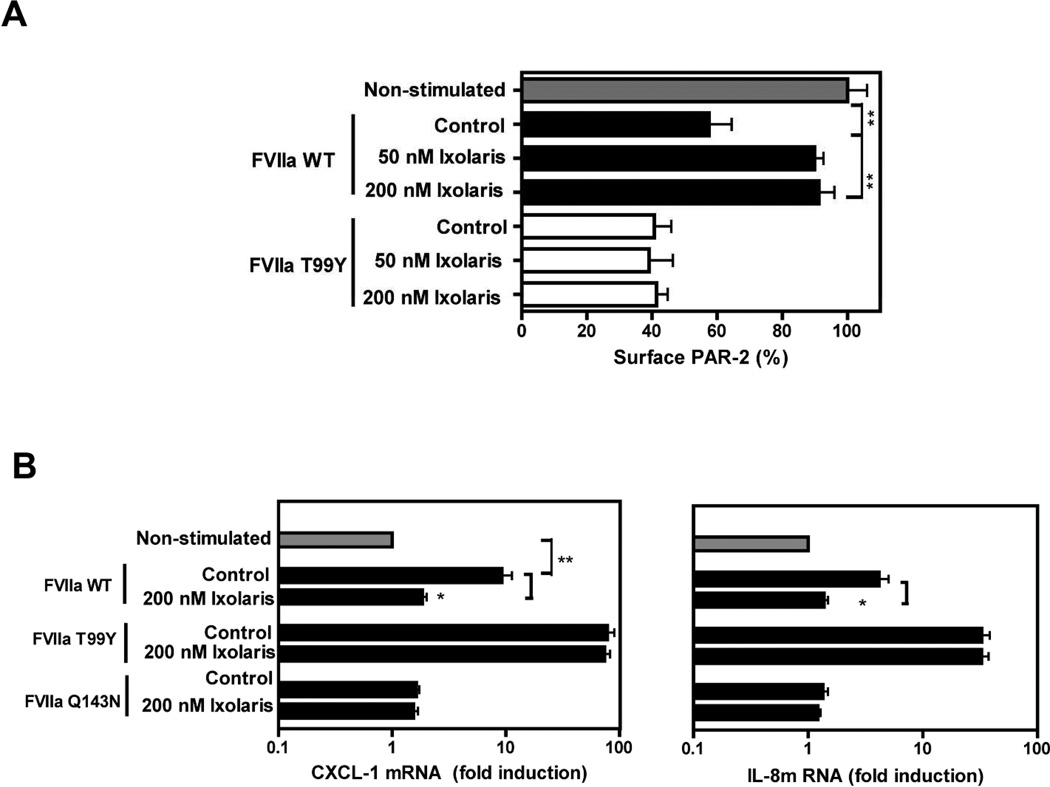
However, the tight binding of the quaternary TF-FVIIa-FXa-Ixolaris complex might have masked more subtle mutational effects on Ixolaris affinity. We therefore also analyzed the direct interaction of Ixolaris with TF-FVIIa in the absence of FX/FXa, using as readout the amidolytic activity of FVIIa. As shown in Fig. 3C, this approach identified 2 mutants, T99Y and Q143N (residue numbering according to the chymotrypsin numbering system), with markedly reduced susceptibility to inhibition by Ixolaris. Both residue positions have been implicated in the recognition of PAR2 by FVIIa, providing evidence that Ixolaris directly interferes with PAR2 docking to the FVIIa protease domain.
FVIIa Q143N is completely defective in mediating PAR2 cleavage and signaling, whereas FVIIa T99Y shows improved activation and signaling [17]. We asked whether Ixolaris no longer inhibits PAR2 cleavage of the T99Y mutant in our established cleavage assay. As shown in Fig. 4A, Ixolaris blocked TF-FVIIa mediated cleavage of PAR2. In contrast, Ixolaris failed to inhibit the cleavage of PAR2 by FVIIa T99Y, consistent with the markedly reduced affinity for this mutant (Fig. 3C). We further evaluated the effect of Ixolaris on cytokine production by FVIIa variants. IL-8 (p<0.05) and CXCL-1 (p<0.05) induction by WT FVIIa was decreased in the presence of Ixolaris and showed similar levels to what was observed with the signaling deficient FVIIa Q143N (Fig. 4B). In accordance with data obtained for PAR2 cleavage, Ixolaris had no effect on signaling of FVIIa T99Y, measured by two independent readouts of induction of IL-8 and CXCL-1 mRNA levels. These data show that a single point mutation in the FVIIa protease domain effectively abolished the signaling inhibitory activity of Ixolaris towards the TF-FVIIa binary complex.
Figure 4. Mutation of FVIIa residue T99 prevents Ixolaris inhibition of TF-FVIIa mediated PAR-2 cleavage and signaling.
(A) CHO-TF cells transiently expressing FLAG-hPAR-2 were incubated with 20 nM FVIIa WT or T99Y in the presence of 50 nM or 200 nM Ixolaris. Residual surface PAR-2 was measured after 1 h of incubation (mean ± S.D., n=3, ** p<0.01, unpaired t test). (B) MDA-MB-231mfp cells were stimulated with 10 nM FVIIa wt, the signaling-defective variant Q143N, or T99Y with enhanced PAR-2 signaling in the absence or in the presence of 200 nM Ixolaris for 90 min, and PAR-2 dependent IL-8 and CXCL-1 mRNA induction was quantified by RT-PCR (mean ± S.D., n=3, * p<0.05, unpaired t test, different from control).
Effect of Ixolaris on murine models of TF-FVIIa signaling
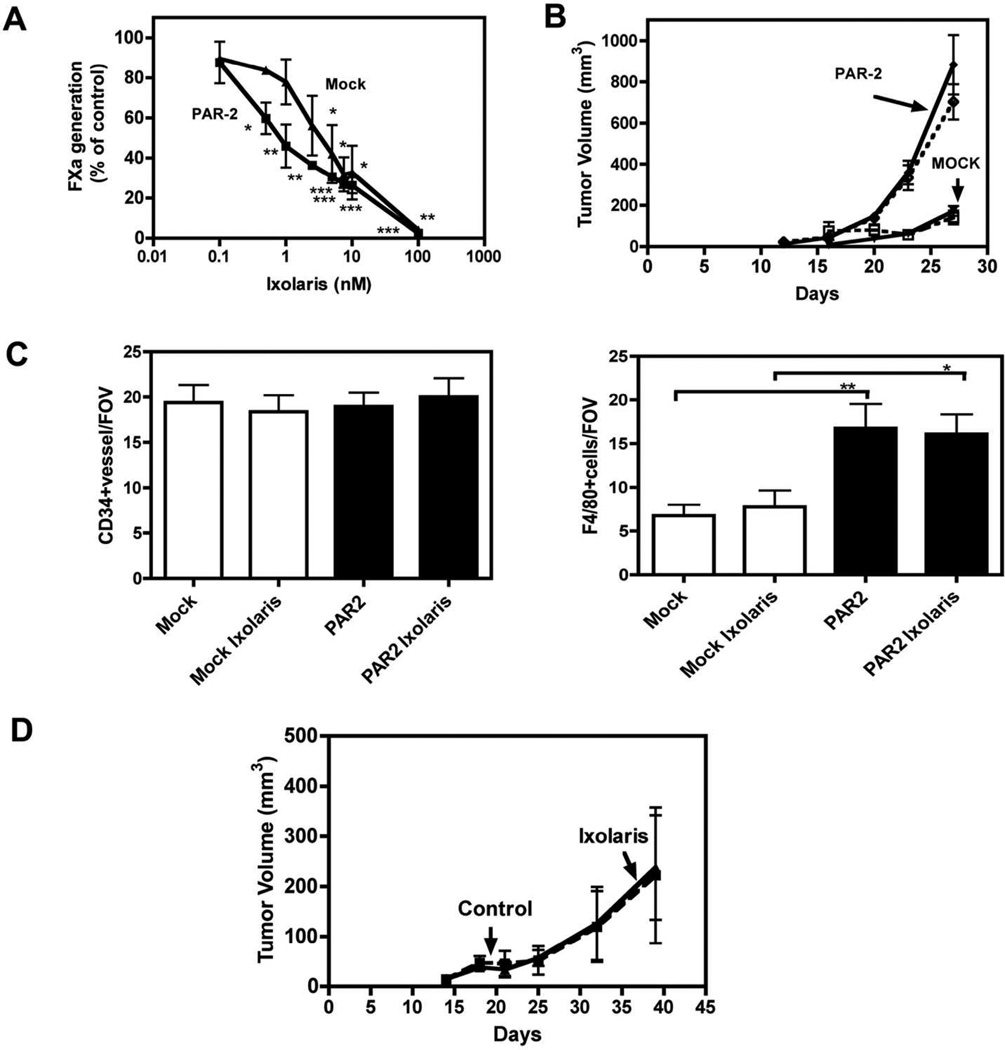
In order to evaluate the effects of Ixolaris on TF-FVIIa binary complex signaling in vivo, we characterized TF inhibition by Ixolaris in murine PyMT breast cancer cells [12]. PAR2-deficient breast cancer cells were either mock-transduced or reconstituted with murine PAR2 and inhibition of the extrinsic tenase complex by Ixolaris was characterized. As shown in Fig 5A, Ixolaris dose dependently attenuated the generation of FXa measured in the supernatant of mock and PAR2- transduced PyMT PAR2−/− breast cancer cells.
Figure 5. Effect of Ixolaris on mouse breast cancer cells.
(A) FXa generation on mock (▼) and PAR2 (■) transduced murine breast cancer cells was analyzed with 10 nM murine FVIIa, 100 nM human FX and varying concentrations of Ixolaris (0–100 nM). The amount of FXa generated after 10 min was quantified with Spectrozyme FXa (mean ± S.D.; n= 3) (B) Typical experiment of orthotopic tumor growth of C57BL/6 mammary fat pad implanted with mock or PAR-2 transduced PYMT PAR2−/− breast cancer cells (5x105/mouse). Animals were treated daily by subcutaneous administration with PBS (open symbols) or Ixolaris (250 µg/kg) (closed symbols). Tumor volumes are mean ± S.D., n = 8 mice/group, *** p<0.001, ANOVA Kruskal-Wallis, different from mock transduced tumors. (C) Vessel density of mock and PAR2 tumors were determined on fixed sections stained with anti-CD34. Tumor macrophages density of mock and PAR2 tumors were determined on fixed sections stained with anti F4/80. Tumor vessels and tumor macrophages were quantified with the IMARIS software and at least 4 different areas per tumor were analyzed (mean ± S.D. n=3., * p<0.05, ** p<0.01, ANOVA). (D) Typical experiment showing orthotopic tumor growth of mammary fat pad implanted MDA-MB-231 mfp breast cancer cells (2 X 106/mouse) in female C.B-17 SCID mice. Tumor volume are mean ± S.D., n=10 mice/group. Animals were treated daily by subcutaneous administration with PBS (open symbols) or Ixolaris (250 µg/kg) (closed symbols).
We next evaluated whether Ixolaris inhibited PAR2-dependent breast cancer growth in vivo. Mock or PAR2-reconstitued PyMT cells were injected into the mammary fat pads to study orthotopic tumor growth. Mice were treated with 250 µg/kg Ixolaris from day 5 to day 27 and tumor growth was monitored. As shown in Fig. 5B, reconstitution of PAR-2 significantly enhanced tumor growth, as previously demonstrated [12]. However, the dosing scheme of Ixolaris that was highly effective to block glioblastoma and melanoma cell growth [25, 26] had no effect on tumor growth of these breast cancer populations. In our previous experiments, we found increased vessel density with no change in macrophage counts following PAR2 reconstitution of PyMT-PAR2−/− cells [12]. Here, PAR2 reconstitution increased macrophage recruitment without significant changes is vessel density (Fig 5C), consistent with in vitro data that TF-FVIIa-PAR2 signaling not only induces regulators of angiogenesis, but also macrophage growth factors [15]. The lack of inhibition of orthotopic murine breast cancer growth by Ixolaris was confirmed in an independent experiment with the same inhibitor dosing and tumor model. We also tested the effect of Ixolaris in the MDA-MB-231mfp xenograft model that is dependent on TF-PAR2 signaling [11]. As seen with the mouse PyMT breast cancer cells, treatment with Ixolaris had no effect on MDA-MB-231mfp tumor growth in vivo.
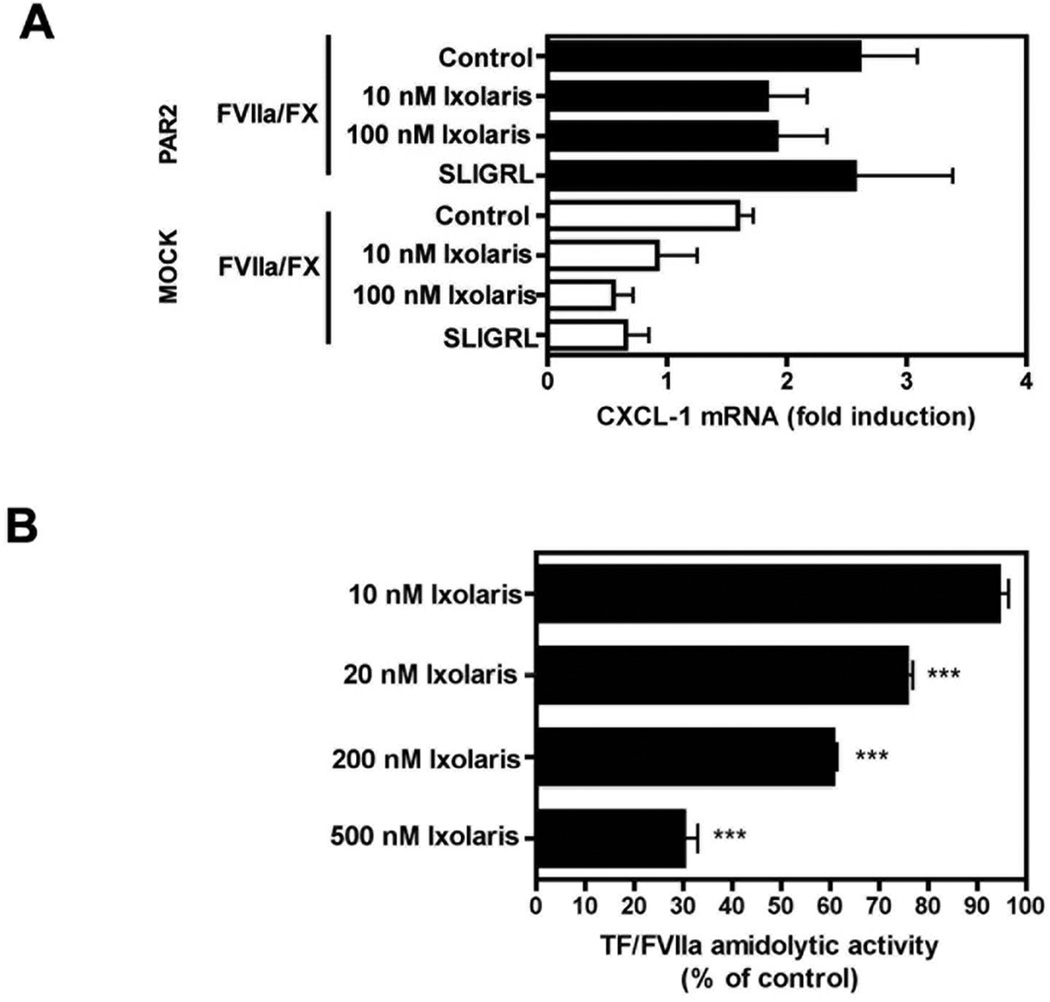
To resolve the lack of tumor growth inhibition and inability to reduce macrophage counts in the PyMT model by Ixolaris, we addressed the potency of Ixolaris to inhibit TF signaling in murine breast cancer cells. Induction of CXCL-1 mRNA levels was determined in serum-starved mock or PAR2- reconsituted PyMT PAR2−/− cells stimulated by TF/FVIIa/FX for 90 minutes. In contrast to the potent inhibition of TF-dependent coagulation on PyMT cells (Fig. 5A) and effective inhibition of ternary and binary complex signaling on human breast cancer cells (Fig. 1, 2), Ixolaris did not significantly reduce signaling of murine breast cancer cells stimulated with physiological concentrations of FVIIa and FX (Fig. 6A). Furthermore, unlike the ~90% inhibition of human TF-FVIIa amidolytic activity by 100 nM Ixolaris (Fig. 3C), Ixolaris at 500 nM produced <80% inhibition of murine TF-FVIIa (Fig. 6B), demonstrating significantly reduced affinity for the murine binary TF-FVIIa complex when compared to the human counterpart. These data provide an explanation why previously established effective in vivo doses of Ixolaris failed to inhibit the growth of a TF-PAR2 signaling driven tumor model in the mouse.
Figure 6. Ixolaris inefficiently inhibits murine TF-FVIIa.
(A) Mock and PAR2 murine breast cancer cells were stimulated with the PAR2 agonist peptide SLIGRL or 10 nM/100 nM FVIIa/FX for 90 minutes. FX (100 nM) was incubated for 10 min with Ixolaris (10 nM or 100 nM) before addition to mock and PAR-2 cells. CXCL-1 mRNA induction over control after 90 min was determined by quantitative PCR (mean ± S.D., n≥3). (B) Amidolytic activity of mFVIIa/soluble mTF (50 nM, 250 nM) was measured in the presence of increasing concentrations (0–500 nM) of Ixolaris using the chromogenic substrate S-2288 (mean ± S.D. n= 3, *** p<0.001, one-way ANOVA followed by Bonferroni post test, different from control).
Discussion
TF promotes tumor progression, metastasis, and angiogenesis [34–36] through direct signaling mediated by PAR2 [5, 11] and in part through the activation of coagulation and thrombin signaling [37, 38]. Here, we demonstrate that Ixolaris, a potent tick-derived inhibitor of TF has anticoagulant and anti-signaling activities in human breast cancer cells. We furthermore identify a novel function of Ixolaris to inhibit directly TF-FVIIa-mediated PAR2 signaling at higher concentrations of the inhibitor.
TF-FVIIa signaling induces the expression of diverse angiogenic regulators, chemokines, and anti-apoptotic genes, including VEGF, CSF1, CSF2, IL-8 and CXCL-1, which promote angiogenesis directly or indirectly through macrophage recruitment [14, 15, 39, 40]. Our results demonstrate that Ixolaris decreases ternary TF-FVIIa-FXa complex-induced expression of IL-8 and CXCL-1 in MDAMB- 231 breast cancer cells. In addition, we show that Ixolaris blocks PAR2 cleavage by the TF-FVIIa-Xa ternary complex in a heterologous expression system. Ixolaris binds to the heparin exosite on FXa and this interaction with FXa appears to be sufficient to prevent the protease from cleaving PAR2. This is in striking contrast to NAPc2 which uses a different exosite in FXa to inhibit TF-FVIIa [41]. NAPc2 blocks the active site of FVIIa, while locking in FXa in a signaling active conformation on the ternary TF-FVIIa-FXa complex [42]. Thus, Ixolaris binds with high affinity to the ternary TF complex to inactivate FVIIa [19] and either imposes steric hindrance on FXa in this complex to prevent cleavage of PAR2 or, alternatively, inhibits activation of the docked substrate more efficiently than NAPc2.
Our results demonstrate that higher concentrations of Ixolaris inhibit PAR2 signaling by the TF-FVIIa binary complex. The ability of Ixolaris to inhibit FVIIa directly may be particularly important for human tumor cells that upregulate FVIIa under hypoxic conditions [43]. Hypoxia induces TF in glioblastoma cells [44] and furthermore triggers the release of TF+ microvesicles to induce TF-FVIIa-dependent endothelial cell crossactivation and angiogenesis [45]. Oncogenic transformation of glioblastoma cells also upregulates both TF and FVIIa [46] and blocking TF-FVIIa-PAR2 signaling inhibits gliomablastoma growth [13]. The human breast cancer model used here does not ectopically synthesize FVII. We therefore propose that previously shown tumor growth inhibition in FVIIa expressing glioblastoma models [25] could be, in part, dependent on the newly identified ability of Ixolaris to directly block human TF-FVIIa-mediated activation of PAR2.
We mapped critical residues for Ixolaris binding using FVIIa protease domain mutants that have altered PAR2 recognition while minimally affecting the recognition of macromolecular substrates [17]. Ixolaris and PAR2 both interact with FVIIa residue Q143. Mutation of T99 to the larger residue Tyr improves cleavage of PAR2, but filling the fairly open S2 subsite with this hydrophobic side chain severely impaired inhibition of TF-FVIIa amidolytic and signaling activity by Ixolaris. Notably, both mutations of the FVIIa protease domain had no measurable effect on Ixolaris inhibition of TF procoagulant activity in the presence of FX. This is consistent with the scaffolding functions of FX/FXa that increase affinity of Ixolaris for TF-FVIIa and possibly may alter slightly the docking of Ixolaris with the FVIIa catalytic cleft.
Studies employing the PyMT model demonstrated a critical cooperation between PAR2 and TF cytoplasmic domain signaling in promoting angiogenesis in breast cancer. In addition, macrophage recruitment is less abundant in early tumors of PAR2−/− mice, providing initial evidence that the recruitment of proangiogenic immune cells is also dependent on PAR2 signaling [12]. Using the same dosing as previously applied in glioblastoma and melanoma models [25, 26], Ixolaris showed no effect on in vivo tumor growth, vessel density or macrophage recruitment in the PAR2-signaling dependent PyMT tumor model. In exploring this puzzling observation, we found that Ixolaris has markedly reduced affinity for the murine TF-FVIIa complex, as demonstrated by diminished potency to inhibit its amidolytic and signaling activity. Since the anticoagulant activity of Ixolaris towards murine and human FVIIa was similar (Figs. 1, 5), Ixolaris acts mainly as an anti-coagulant in most tumor models that involve mouse FVIIa as the primary ligand for TF. Supporting this hypothesis, Ixolaris also showed no effect on tumor growth of MDA-MB-231 mfp cells without ectopic FVIIa synthesis that rely on mouse FVIIa for tumor growth in vivo. In conclusion, we have shown that Ixolaris blocks human ternary and binary TF-FVIIa-PAR2 signaling and it will be of interest to further evaluate the antitumor effects of Ixolaris in human xenograft models that rely on tumor cell autonomous production of human FVIIa for TF-PAR2 signaling.
Acknowledgments
This work was supported by NIH grant HL-60742 (WR), a fellowship from the Brazilian funding agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (TCC-L), and by Novo Nordisk (JD). This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Because Ivo MB Francischetti is a government employee and this is a government work, the work is in the public domain in the United States.
References
- 1.Ruf W, Edgington TS. Structural biology of tissue factor, the initiator of thrombogenesis in vivo. FASEB J. 1994;8:385–390. [PubMed] [Google Scholar]
- 2.Gomez K, McVey JH. Tissue factor initiated blood coagulation. Front Biosci. 2006;11:1349–1359. doi: 10.2741/1888. [DOI] [PubMed] [Google Scholar]
- 3.Osterud B, Bjørklid E. Sources of tissue factor. Semin Thromb Hemost. 2006;32:11–23. doi: 10.1055/s-2006-933336. [DOI] [PubMed] [Google Scholar]
- 4.Francischetti IM, Seydel KB, Monteiro RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15:81–107. doi: 10.1080/10739680701451516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruf W, Disse W, Carneiro-Lobo TC, Yokota N, Schaffner F. Tissue factor and cell signaling in cancer progression and thrombosis. J Thromb Haemost. 2011;9:306–315. doi: 10.1111/j.1538-7836.2011.04318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rak J, Milsom C, May L, Klement P, Yu J. Tissue factor in cancer and angiogenesis: the molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin Thromb Hemost. 2006;32:54–70. doi: 10.1055/s-2006-933341. [DOI] [PubMed] [Google Scholar]
- 7.Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RC. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg. 1995;82:1101–1104. doi: 10.1002/bjs.1800820831. [DOI] [PubMed] [Google Scholar]
- 8.Nakasaki T, Wada H, Shigemori C, Miki C, Gabazza EC, Nobori T, Nakamura S, Shiku H. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol. 2002;69:247–254. doi: 10.1002/ajh.10061. [DOI] [PubMed] [Google Scholar]
- 9.Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, Hostetter G, Harvey J, Taubman MB. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–2875. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 10.Rickles FR, Shoji M, Abe K. The role of the hemostatic system in tumor growth, metastasis, and angiogenesis: tissue factor is a bifunctional molecule capable of inducing both fibrin deposition and angiogenesis in cancer. Int J Hematol. 2001;73:145–150. doi: 10.1007/BF02981930. [DOI] [PubMed] [Google Scholar]
- 11.Versteeg HH, Schaffner F, Kerver M, Petersen HH, Ahamed J, Felding-Habermann B, Takada Y, Mueller BM, Ruf W. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111:190–199. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaffner F, Versteeg HH, Schillert A, Yokota N, Petersen LC, Mueller BM, Ruf W. Cooperation of tissue factor cytoplasmic domain and PAR2 signaling in breast cancer development. Blood. 2010;116:6106–6113. doi: 10.1182/blood-2010-06-289314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gessler F, Voss V, Dutzmann S, Seifert V, Gerlach R, Kogel D. Inhibition of tissue factor/protease-activated receptor-2 signaling limits proliferation, migration and invasion of malignant glioma cells. Neuroscience. 2010;165:1312–1322. doi: 10.1016/j.neuroscience.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 14.Hjortoe GM, Petersen LC, Albrektesen T, Sorensen BB, Norby PL, Mandal SK, Pendurthi UR, Rao LV. Tissue factor-factor VIIa specific upregulation of IL-8 expression in MDA-MB-231 cells is mediated via PAR-2 and results in increased cell migration. Blood. 2004;103:3029–3037. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albrektsen T, Sorensen BB, Hjortoe GM, Fleckner J, Rao LVM, Petersen LC. Transcriptional program induced by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J Thromb Haemost. 2007;5:1588–1597. doi: 10.1111/j.1538-7836.2007.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci USA. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen KS, Ostergaard H, Olsen OH, Bjelke JR, Ruf W, Petersen LC. Engineering of substrate selectivity for tissue factor-factor VIIa complex signaling through protease activated receptor 2. J Biol Chem. 2010;285:19959–19966. doi: 10.1074/jbc.M110.101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Versteeg HH, Schaffner F, Kerver M, Ellies GL, Andrade-Gordon P, Mueller MB, Ruf W. Protease- Activated Receptor (PAR) 2, but not PAR- 1, signaling promotes the development of Mammary Adenocarcinoma in Polyoma Middle T Mice. Cancer Res. 2008;68:7219–7227. doi: 10.1158/0008-5472.CAN-08-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francischetti IM, Valenzuela JG, Andersen JF, Mather TN, Ribeiro JM. Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood. 2002;99:3602–3612. doi: 10.1182/blood-2001-12-0237. [DOI] [PubMed] [Google Scholar]
- 20.Broze GJ., Jr Tissue factor pathway inhibitor and the revised theory of coagulation. Annu Rev Med. 1995;46:103–112. doi: 10.1146/annurev.med.46.1.103. [DOI] [PubMed] [Google Scholar]
- 21.Bergum PW, Cruikshank A, Maki S, Kelly CR, Ruf W, Vlasuk G. Role of zymogen and activated factor X as scaffolds for the inhibition of the blood coagulation factor VIIa-tissue factor complex by recombinant nematode anticoagulant protein c2. J Biol Chem. 2001;276:10063–10071. doi: 10.1074/jbc.M009116200. [DOI] [PubMed] [Google Scholar]
- 22.Monteiro RQ, Rezaie AR, Ribeiro JM, Francischetti IM. Ixolaris: a factor Xa heparin-binding exosite inhibitor. Biochem J. 2005;387:871–877. doi: 10.1042/BJ20041738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monteiro RQ, Rezaie AR, Bae JS, Calvo E, Andersen JF, Francischetti IM. Ixolaris binding to factor X reveals a precursor state of factor Xa heparin-binding exosite. Protein Sci. 2008;17:146–153. doi: 10.1110/ps.073016308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nazareth RA, Tomaz LS, Ortiz-Costa S, Atella GC, Ribeiro JM, Francischetti IM, Monteiro RQ. Antithrombotic properties of Ixolaris, a potent inhibitor of the extrinsic pathway of the coagulation cascade. Thromb Haemost. 2006;96:7–13. doi: 10.1160/TH06-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carneiro-Lobo TC, Konig S, Machado DE, Nasciutti LE, Forni MF, Francischetti IM, Sogayar MC, Monteiro RQ. Ixolaris, a tissue factor inhibitor, blocks primary tumor growth and angiogenesis in a glioblastoma model. J Thromb Haemost. 2009;7:1855–1864. doi: 10.1111/j.1538-7836.2009.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Oliveira AD, Lima LG, Mariano-Oliveira A, Machado DE, Nasciutti LE, Andersen JF, Petersen LC, Francischetti IM, Monteiro RQ. Inhibition of tissue factor by Ixolaris reduces primary tumor growth and metastasis in a murine model of melanoma. Thrombosis Res. 2012 doi: 10.1016/j.thromres.2012.05.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone MJ, Ruf W, Milles DJ, Edgington TS, Wright PE. Recombinant soluble human tissue factor secreted by Saccharomyces cerevisiae and refolded from Escherichia coli inclusion bodies: glycosylation of mutants, activity and physical characterization. Biochem J. 1995;310:605–614. doi: 10.1042/bj3100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;35:25416–25424. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- 29.Petersen LC, Norby PL, Branner S, Sorensen BB, Elm T, Stennicke HR, Persson E, Bjorn SE. Characterization of recombinant murine factor VIIa and recombinant murine tissue factor: a human-murine species compatibility study. Thromb Res. 2005;116:75–85. doi: 10.1016/j.thromres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Jessani N, Humphrey M, McDonald WH, Niessen S, Masuda K, Gangadharan B, Yates JR, 3rd, Muller BM, Cravatt BF. Carcinoma and stromal enzyme activity prolifes associated with breast tumor growth in vivo. Proc Nat Acad Sci USA. 2004;38:13756–13761. doi: 10.1073/pnas.0404727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Disse J, Petersen HH, Larsen KS, Persson E, Esmon CT, Teyton L, Peterson LC, Ruf W. The endothelial protein C receptor supports tissue factor ternary coagulation initiation complex signaling through protease-activated receptors. J Biol Chem. 2011;7:5756–5767. doi: 10.1074/jbc.M110.201228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girard TJ, Warren LA, Novotny WF, Likert KM, Brown SG, Miletich JP, Broze GJ., Jr Functional significance of the Kunitz-type inhibitory domains of lipoprotein-associated coagulation inhibitor. Nature. 1989;338:518–520. doi: 10.1038/338518a0. [DOI] [PubMed] [Google Scholar]
- 33.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 34.Contrino J, Hair C, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209–215. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 35.Seto S, Onodera H, Kaido T, Yoshikawa A, Ishigami S, Arii S, Imamura M. Tissue factor expression in human colorectal carcinoma: correlation with hepatic metastasis and impact on prognosis. Cancer. 2000;88:295–301. doi: 10.1002/(sici)1097-0142(20000115)88:2<295::aid-cncr8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 36.Brat DJ, Van Meir EG. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest. 2004;84:397–405. doi: 10.1038/labinvest.3700070. [DOI] [PubMed] [Google Scholar]
- 37.Ruf W, Mueller BM. Thrombin generation and the pathogenesis of cancer. Semin Thromb Hemost. 2006;32:61–68. doi: 10.1055/s-2006-939555. [DOI] [PubMed] [Google Scholar]
- 38.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–362. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease Activated Receptor-2 Is Essential for Factor VIIa and Xa–Induced Signaling, Migration, and Invasion of Breast Cancer Cells. Cancer Res. 2006;66:307–314. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- 40.Gil-Bernabé AM, Ferjancic S, Tlalka M, Zhao L, Allen PD, Im JH, Watson K, Hill SA, Armirkhosravi A, Francis JL, Pollard JW, Ruf W, Muschel RJ. Recruitment of monocytes/macrophages by tissue factor- mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119:3164–3175. doi: 10.1182/blood-2011-08-376426. [DOI] [PubMed] [Google Scholar]
- 41.Murakami MT, Rios-Steiner J, Weaver SE, Tulinsky A, Geiger JH, Arni RK. Intermolecular interactions and characterization of the novel factor Xa exosite involved in macromolecular recognition and inhibition: crystal structure of human Gla-domainless factor Xa complexed with the anticoagulant protein NAPc2 from the hematophagous nematode Ancylostoma caninum. J Mol Biol. 2007;366:602–610. doi: 10.1016/j.jmb.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 42.Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci USA. 2001;98:7742–7747. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koizume S, Jin M-S, Miyagi E, Hirahara F, Nakamura Y, Piao J-H, Asai A, Yoshida A, Tsuchiya E, Ruf W, Miyagi Y. Activation of cancer cell migration and invasion by ectopic synthesis of coagulation factor VIIa. Cancer Res. 2006;66:9453–9460. doi: 10.1158/0008-5472.CAN-06-1803. [DOI] [PubMed] [Google Scholar]
- 44.Rong Y, Post DE, Pieper RO, Durden DL, Van Meir EG, Brat DJ. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res. 2005;65:1406–1413. doi: 10.1158/0008-5472.CAN-04-3376. [DOI] [PubMed] [Google Scholar]
- 45.Svensson KJ, Hucharzewka P, Christianson HC, Skold S, Lofstedt T, Johansson MC, Morgelin M, Bengzon J, Ruf W, Belting M. Hypoxia triggers a procoagulant pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Nat Acad Sci USA. 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magnus N, Garnier D, Rak J. Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood. 2010;116:815–818. doi: 10.1182/blood-2009-10-250639. [DOI] [PubMed] [Google Scholar]
- 47.Banner DW, D'Arcy A, Chéne C, Winkler FK, Guha A, Konigsberg WH, Nemerson Y, Kirchhofer D. The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature. 1996;380:41–46. doi: 10.1038/380041a0. [DOI] [PubMed] [Google Scholar]