Abstract
The multi-ligand receptor RAGE was discovered on account of its ability to bind and transduce the cell stress-provoking signals of advanced glycation endproducts (AGEs). The finding that RAGE also bound pro-inflammatory molecules set the stage for linking RAGE and inflammation to the pathogenesis of diabetic macro- and microvascular complications. In this review, we focus on the roles of RAGE and its ligands in diabetes complications. We recount the findings from mice, rats, swine and human subjects suggesting that RAGE action potently contributes to vascular, inflammatory and end-organ stress and damage in types 1 and 2 diabetes. We detail the efforts to track ligands and RAGE in human subjects with diabetes to address if this axis may be a biomarker reflective of the state of the diabetic complications. Lastly, we suggest specific strategies to tackle AGE-ligand-RAGE interactions as potential therapeutic targets for diabetes and its complications.
Introduction
Diabetes is a highly prevalent metabolic disorder that is rapidly rising in incidence throughout the world [1]. Diabetes affects both small and large blood vessels and is a leading cause of morbidity and mortality. Multiple mechanisms have been suggested to contribute to the pathogenesis of diabetic complications; glucose causes both acute and chronic consequences [2]. One of the chronic complications of high levels of blood glucose is the irreversible glycation and oxidation of proteins and lipids leading to the formation of the advanced glycation endproducts or AGEs. AGEs exert their actions at least in part via the receptor for AGE (RAGE). The key discovery that RAGE bound non-AGE ligands, particularly members of the S100/calgranulin family, high mobility group box 1 (HMGB1) and Mac-1, linked RAGE directly to inflammatory responses [3]. There is a plethora of evidence linking inflammation to the pathogenesis of both macro- and microvascular diabetic complications. In this review, we will focus on the ligand-RAGE axis and evidence for its involvement in the complications of diabetes.
Advanced Glycation Endproducts, Non-AGE Ligands of RAGE
AGEs are the products of the Maillard reaction and accumulate to increased degrees in the tissues and plasma/serum of subjects with diabetes, particularly in the extracellular matrix. For example, the basement membranes of proteins in long-term diabetes are often glycated and demonstrate thickening (such as the basement membranes in the kidney and eye) [4]. Other targets of glycation include long-lived proteins of the lens and plasma. RAGE was discovered for its ability to bind AGEs [5]. When AGEs were formed by incubating proteins in reducing sugars such as ribose, glucose or glucose-6-phosphate, or when AGEs were retrieved from human diabetic bodily fluids by immunoprecipitation with anti-AGE antibodies, it was shown that such AGEs bound in a dose-dependent and saturable manner to RAGE [6]. In vivo, AGEs may form via multiple biochemical mechanism incited by high levels of glucose; a major precursor of AGE is methylglyoxal (MG) [7]. MG is largely formed by the nonenzymatic degradation of the glycolytic intermediates, including dihydoxyacetone phosphate and glyceraldehyde-3-phosphate. Once MG is formed, action of the enzyme glyoxalase-1 (Glo1) is the chief pathway by which it is detoxified, thereby blocking the development of AGEs from the MG precursor [8]. It is important to note that RAGE is not the only receptor for AGEs; molecules such as CD36 and macrophage scavenger receptors bind AGE as well [9–11]. Certainly, it is plausible that the heterogeneous nature of AGEs within diabetic tissue impacts AGE interaction with specific receptors in distinct ways. At this time, it is not established if specific forms of AGEs preferentially bind RAGE or other AGE receptors, and if these processes are tissue-specific.
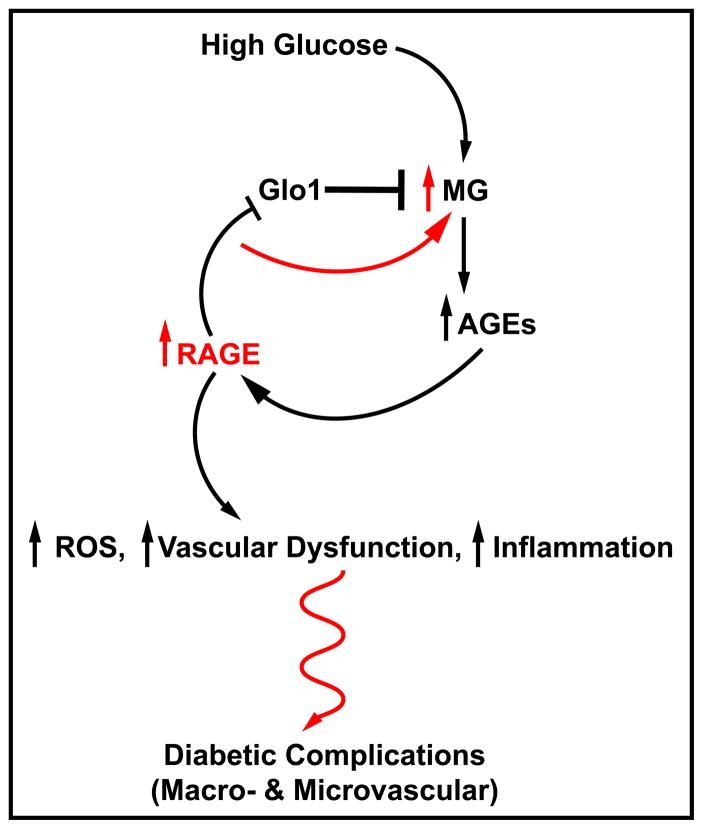
RAGE impacts on the biology of AGEs in multiple ways. First, one of the specific AGEs that binds RAGE is carboxy(methyl)lysine AGE, or CML-AGE [12]. CML is a highly prevalent AGE in diabetic tissues and plasma/serum. In cultured endothelial cells and mononuclear phagocytes, CML-AGE-RAGE interaction activates signal transduction pathways and results in upregulation of genes such as Vascular Cell Adhesion Molecule-1 (VCAM-1) that propagate cellular stress and contribute to complications. Administration of soluble RAGE (extracellular RAGE ligand decoy), antibodies to RAGE or deletion of RAGE blocks the impact of CML-AGE on signaling and alteration of cellular properties [12]. Second, RAGE contributes to regulation of Glo1; in the kidneys of diabetic OVE26 mice devoid of RAGE, levels of MG and AGEs were significantly lower than those observed in RAGE-expressing OVE26 mice [13]. Investigation revealed that RAGE deficient mice displayed significantly higher levels of Glo1 mRNA, protein and activity compared to their RAGE-expressing littermates [13]. Thus, the finding that RAGE downregulates Glo1 suggests that RAGE both transduces the actions of MG and AGEs that adversely impact the blood vessel, and blocks the detoxification of a major key AGE precursor, MG. Hence, a vicious cycle of RAGE-dependent activity in diabetic tissues may account, at least in part, for vascular dysfunction and, ultimately, diabetic complications (Figure 1).
Figure 1. RAGE – right in the middle of a vicious cycle of glycation and inflammation – potential links to diabetic complications.
In hyperglycemia characteristic of types 1 and 2 diabetes, a major result is excess production of methylglyoxal (MG). MG, through a series of subsequent reactions, may form the irreversible AGEs. When AGEs bind to their chief cell surface signaling receptor, a diverse set of consequences ensues – generation of oxidative stress, vascular dysfunction and inflammation – all of which synergize to trigger diabetic complications. The surprising finding that AGE levels were lower in hyperglycemic RAGE null mice led to the observation that RAGE downregulates Glo1, the chief enzyme responsible to detoxify MG. Hence, RAGE action contributes to AGE action, but, also to the perpetuation of AGE generation. We propose that a vicious cycle of AGE-RAGE stress is a key inciting factor in diabetes complications.
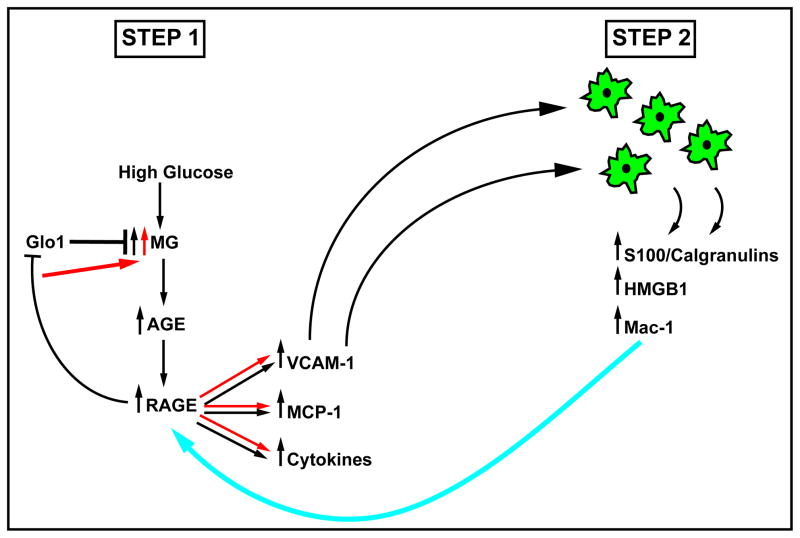
Beyond AGEs, RAGE also binds certain members of the S100/calgranulin family (such as S100A12, S100B, S100P, S100A4, S100A8/A9); HMGB1; and Mac-1 [14–16]. Key signatures of these molecules include inflammation and induction of cellular migration. Indeed, both processes are intimately linked, as inflammatory mechanisms depend on the influx and activation of monocytes/macrophages, lymphocytes and neutrophils in foci of cellular stress. RAGE is expressed on each of these cell types and RAGE mediates the actions of AGEs, certain of the S100/calgranulins and HMGB1 on recruitment of immune cells and their production and release of cytokines and matrix metalloproteinases (MMPs) [3]. We predict that in hyperglycemia, AGE formation ultimately reaches a critical threshold to activate RAGE. Once RAGE is activated, an early and seminal event is attraction of immune cells to AGE-laden foci; such immune cells become activated and during that process release RAGE ligands such as the S100/calgranulins and HMGB1. Vascular-immune cell interactions via Mac-1 further propagate inflammation, leading to vascular perturbation and, ultimately, end-organ damage (Figure 2).
Figure 2. RAGE and its ligands – a self-perpetuating axis of glucose and inflammatory stress in diabetic tissues.
In diabetic tissues vulnerable to complications, we propose that hyperglycemia-mediated formation of AGEs is an early step; the slow but inexorable accumulation of AGEs ultimately achieves a critical threshold of ligand capable of activation of RAGE. Once RAGE is activated, a seminal event is attraction of immune cells to AGE-laden foci. We predict that these immune cells become activated and during that process release pro-inflammatory RAGE ligands such as S100/calgranulins and HMGB1. Then, vascular-immune cell interactions via mac-1 further propagate inflammation, leading to vascular perturbation and, ultimately, end-organ damage in diabetes.
Extensive evidence illustrates that AGEs and non-AGE ligands accumulate in the plasma/serum of human subjects with diabetes and in fact, may be biomarkers of the status of complications [17–18]. Beisswenger and colleagues showed that AGE accumulation in tissues targeted for complications in diabetes (such as retina and kidney) preceded the development of overt complications, suggesting that AGEs may be participants in the pathogenesis of complications [17]. Furthermore, clinical studies have demonstrated higher levels of S100A12 in type 2 diabetic subjects vs. controls [19], and higher levels of HMGB1 in subjects with type 1 diabetes are associated with greater prevalence of microalbuminuria and retinopathy, but not cardiovascular disease [20].
Finally, in addition to measurement of AGEs and non-AGE ligands in diabetic subjects, it is possible to measure soluble forms of RAGE in the plasma/serum of human subjects [21]. There are at least two mechanisms by which sRAGEs may be produced; first, total extracellular RAGE may be cleaved from the cell surface by the action of MMPs or ADAM10 [22]. Second, endogenous secretory or esRAGE is a RAGE splice variant that may be released; a novel 18 amino acid sequence in the C2-like extracellular domain of esRAGE has resulted in the generation of novel epitopes for which a unique ELISA system has been generated [23]. Many studies have now reported that total and/or esRAGEs display distinct patterns in diabetic subjects vs. those without diabetes. Furthermore, in diabetic populations, sRAGE and esRAGE levels may be related to the degree of complications, or to protection from diabetic complications. Interestingly, suggestive of the premise that sRAGEs and esRAGEs may be regulated, studies in human subjects have shown that treatment with therapeutic agents, such as perindopril or statins may alter these levels in human subjects [24–26]. Indeed, in our own work, we first tested sRAGE as a ligand decoy and showed, as described below, that administration of sRAGE to diabetic animals was at least partially protective against macro- and microvascular complications. Yet, the studies reporting sRAGE levels in human subjects have revealed some discordant findings. It is important to note that most published studies are small and cross-sectional in design. It is likely, however, that further sRAGE studies in human subjects with diabetes is a worthwhile effort, as, to date, no specific biomarker or panel of biomarkers has been identified to accurately track the onset and progression (or resistance) to complications. In the sections to follow, we detail the evidence suggesting that blockade or deletion of RAGE is beneficial in micro- and macrovascular complications of diabetes.
RAGE & Cardiovascular Disease
Accelerated Atherosclerosis and Diabetes
Epidemiological data in type 1 and type 2 diabetic subjects implicate diabetes as a major risk factor for the development of accelerated atherosclerosis and the key manifestations of heart attacks and strokes. Long-term data in type 1 diabetic populations [27] and in type 2 diabetic populations [28] suggested that strict vs. standard control of hyperglycemia was associated with partial protection against complications, including those in the cardiovasculature. Experiments in animal models had been hindered by the fact that diabetic mice or rats – even when fed high-fat, cholesterol rich diets, failed to develop advanced atherosclerosis typical of that observed in long-term diabetic subjects. In the mid-1990s, a major advance in the field was the development of mice vulnerable to atherosclerosis by deletion of apolipoprotein E or deletion of the low density lipoprotein (LDL) receptor. In both mouse models, induction of diabetes using the pancreatic beta cell toxin streptozotocin, resulted in accelerated atherosclerosis lesion area and complexity [29–30]. Most notably, hyperglycemia accelerated inflammatory cell influx into the lesions and increased multiple markers of inflammation, such as adhesion molecules, chemokines, inflammatory cytokines and MMPs – even in the areas of the vascular tree (aorta) that did not demonstrate frank atherosclerotic lesions [31]. These data confirmed that diabetes was indeed associated with increased inflammation in the macrovessels. In parallel with studies in mouse models that revealed increased expression of RAGE ligands and RAGE in the diabetic blood vessels, Virmani and colleagues reported that in human subjects with diabetes, lesions were both larger and expressed greater levels of RAGE, RAGE ligands such as AGEs and S100A12, and displayed more monocytes/lesion area vs. age-matched non-diabetic controls [32].
Hence, to test the potential role of RAGE in atherosclerosis, particularly in diabetes, multiple distinct strategies have been used. In apoE null mice or in high fat diet-fed LDL receptor null mice, administration of sRAGE or genetic deletion of RAGE blocked accelerated atherosclerosis in the diabetic mice in a manner independent of changes in levels of glucose or lipids, suggesting that the primary risk factors for atherosclerosis were RAGE-independent [29, 31, 33]. In other studies, introduction of cytoplasmic domain-deleted RAGE (which binds ligand but fails to transduce signals) reduced atherosclerosis compared to control mice (of note, these experiments were performed in non-diabetic apoE null mice) [34]. Finally, in studies in non-diabetic mice, lethal irradiation and reconstitution of apoE null mice with RAGE null vs. wild-type bone marrow resulted in reduced progression of disease compared with wild-type bone marrow recipients [35]. It is important to note that even in non-diabetic mice, AGEs (in oxidized forms of LDL for example [34]), S100/calgranulins and HMGB1 also increase on account of the inflammatory response observed in the lesions.
In the case of macrovascular disease, diabetes is also linked to a failure of regression. Parathath and colleagues, using Reversa mice, showed that even when lipid levels were normalized, diabetes was associated with impaired regression [36].
Finally, vascular repair mechanisms are attenuated in diabetes and RAGE may also participate in these processes. It has been shown that C-reactive protein (CRP) upregulates RAGE in rat endothelial progenitor cells (EPC) and this results in increased vulnerability to oxidative stress and EPC apoptosis [37]. Other studies have shown that CRP reduces endothelial nitric oxides synthase (eNOS), a RAGE-dependent process in EPCs that results in apoptosis [38]. Taken together, these considerations implicate key roles for RAGE ligands and RAGE in enhancing vascular inflammation in diabetes and, potentially, in reducing repair mechanisms.
Cardiac Dysfunction – Ischemia/Reperfusion Injury
A prominent cardiovascular complication of diabetes is increased vulnerability to ischemia/reperfusion (I/R), as evidenced by more frequent and larger heart attacks and strokes. In experimental animals, we and others have shown that in diabetic rodent (rats and mice) hearts, diabetes is associated with increased damage after I/R. In the isolated perfused heart, we found that diabetic hearts subjected to no-flow ischemia followed by reperfusion displayed increased release of lactate dehydrogenase, more dysfunction (increased left ventricular end diastolic pressure) and reduced levels of cardiac ATP compared to non-diabetic hearts. Key roles for RAGE were identified, as administration of sRAGE or genetic deletion of RAGE greatly reduced I/R injury in the diabetic heart, in parallel with improved function and preservation of ATP [39]. A key mechanism by which RAGE exerts its benefit is via blocking pathological signaling that causes cardiomyocyte apoptosis. By preserving cardiomyocytes in this setting, RAGE antagonism prevents loss of this critical cell type in the heart [40].
Innate cardiac dysfunction also accompanies long-term diabetes in human subjects and in mice; these processes are independent of macrovascular disease. Ma and colleagues showed that induction of diabetes in mice with streptozotocin resulted in decreased left ventricular contractility that was prevented at least in part via blockade of RAGE [41]. In isolated murine cardiomyocytes, AGEs induced prolongation of time to peak shortening (TPS) and time to 90% relengthening in a manner that was RAGE dependent, as illustrated by blockade of these effects by anti-RAGE IgG. Nielsen and co-workers studied type 2 diabetic db/db mice and assessed the effects of a blocking RAGE antibody. RAGE blockade prevented the increase in left ventricular diastolic chamber stiffness and blocked the reduction in cardiac systolic function. In parallel, fibrosis (collagen (col1a1)) was reduced by the RAGE antibody [42].
Taken together, these data suggest that even in the microvascular tissues of the heart, AGEs and diabetes exert maladaptive effects on function and the development of fibrosis, at least in part, via RAGE.
RAGE and Diabetic Nephropathy
The diabetic kidney was one of the first tissues in which extensive AGE accumulation was observed – throughout the glomeruli and the tubular interstitium. Tanji and colleagues [43] were the first to localize RAGE expression in the human and murine diabetic kidney to the podocyte and glomerular endothelial cells in the kidney. In studies comparing diabetic and non-diabetic human kidney, the expression of RAGE was greater in the diabetic state. Similar findings were observed in the type 2 diabetic db/db mouse model.
In the context of the AGE-RAGE axis, administration of sRAGE to db/db mice treated for >6 months was protective against microalbuminuria, thickening of the glomerular basement membrane (GBM), mesangial matrix expansion, and renal cortex expression of pro-fibrotic genes [44]. sRAGE had no effect on the levels of the glycemia suggesting that the glucose-derived factors that propelled kidney dysfunction were not attenuated by lack of RAGE. Multiple studies have been performed in RAGE null mice; in every case, these studies have revealed at least partial protection from the adverse effects of diabetes in the murine kidney, as detailed below.
Myint and colleagues studied RAGE-expressing and RAGE null mice; diabetes was induced by inter-breeding with a transgenic mouse in which iNOS was over-expressed on the rat insulin promoter, thereby causing beta cell loss and type 1 diabetes. Compared to diabetic wild-type RAGE-expressing mice, diabetic RAGE null mice were at least partially protected against glomerular hypertrophy and increased glomerular number, mesangial expansion, advanced glomerulosclerosis and albuminuria [45]. In a second study, Tan and co-workers showed similar findings in diabetic RAGE null mice (streptozotocin) vs. diabetic wild-type controls; these authors implicated oxidative stress in the mechanisms of RAGE action in this model, as they reported that both mitochondrial and cytosolic oxidative stress were reduced in the kidney in the mice devoid of RAGE [46]. Interestingly, when Tan and colleagues treated diabetic mice with alagebrium (an AGE cross-link breaker), they reported that pathological features of nephropathy were attenuated, but there was no beneficial effect on urinary albumin excretion. Both RAGE deletion and alagebrium resulted in reduced CML AGE levels in the diabetic kidney and in the urine [46]. Finally, Reiniger and colleagues bred the type 1 OVE26 mouse (FVB genetic background) into the homozygous RAGE null background (also FVB) and found that there was a dramatic reduction in pathological markers of mesangial thickening, thickening of the GBM and podocyte effacement [13]. In diabetic OVE26 mice, striking albuminuria results, which was significantly reduced by deletion of RAGE. Further, inulin clearance (at 7 months of age) in the OVE26 mice was reduced by approximately 30%; in RAGE null OVE26 mice, there was no loss of glomerular filtration rate [13].
Studies were performed in these animals in glomeruli isolated from RAGE expressing and RAGE null OVE26 mice shortly after the first development of albuminuria but months before the development of glomerulosclerosis. Affymetrix gene arrays showed that PAI-1 and Tgf-beta were significantly impacted by diabetes; in RAGE null glomeruli, however, levels of PAI-1 and Tgf-beta transcripts were greatly reduced, in parallel with decreased collagen mRNA transcripts [13].
Successful fulfillment of the AGE hypothesis in the diabetic kidney was not met in diabetic human subjects treated with aminoguanidine, an AGE inhibitor, due to toxicity and lack of prevention of loss of glomerular function (p value was 0.05) [47]. Together with the lack of statistical significance and the toxic effects of the agent, this approach was abandoned. However, the partial effectiveness, at least, of aminoguanidine in this population might suggest that future AGE targets should be developed – with an effort to improved safety as a first step.
Lastly, soluble RAGEs have been measured in human diabetic subjects; it has been concluded that renal function itself might impact sRAGE levels (that is, decreased renal function may cause a rise in sRAGE levels) [48–49]. Thus, it is possible that levels of sRAGE – if studied in large populations in a prospective manner with repeated measures – might be biomarkers of the state of the human diabetic kidney.
RAGE and Diabetic Neuropathy
AGEs and RAGE have been demonstrated in human and experimental models of diabetic neuropathy. In human subjects, AGEs were identified in the perineurium, endothelial cells, and pericytes of endoneurial microvessels and in myelinated and unmyelinated fibers in the sural, peroneal and saphenous nerves [50]. In human subjects, in the sural nerve, and in diabetic rats, in the sciatic nerve, the specific AGE pentosidine was found to be increased in cytoskeletal and myelin protein extracts. RAGE expression was identified in peripheral neurons, Schwann cells, and in infiltrating immune cells [50].
Recently, our group published findings on the domestic pig (Sus scrofa). After six months of streptozotocin-induced diabetes, analysis revealed significantly lower numbers of small diameter myelinated fibers in parallel with increased immunoreactivity for RAGE and S100B. MG levels were increased in the nerve tissue compared to controls [51]. Thus, in rodents, swine and human subjects, the ligand-RAGE axis displays increased expression, thereby suggesting that RAGE might contribute to the pathogenesis of diabetic neuropathy.
Studies using RAGE null mice have now shown that after long-term diabetes (>6 months), deletion of RAGE is protective against loss of myelinated fibers and against loss of sensory and motor conduction velocities and pain perception [52–53]. Molecular mechanisms believed to underlie these findings in RAGE null mice include reduced activation of NF-κB and reduced activation of Protein Kinase C betaII isoform.
In human subjects, effort has been made to determine if levels of sRAGEs are biomarkers of nerve and foot involvement in diabetes. To date, three studies have been published in this area. Humpert and colleagues studied type 2 diabetic subjects and measured total sRAGE and esRAGE at one time point. They found no correlation between the levels of either form of sRAGE and neurodisability score or symptom score. As a measure of autonomic neuropathy, these authors tested inspiration/expiration heart rate variability and found no relationship with levels of sRAGEs [54]. Witzke and colleagues studied 30 healthy controls, 30 subjects with type 2 diabetes and Charcot complication and 20 subjects with type 2 diabetes and stage 2 (nonacute) Charcot neuroarthropathy (CNA). The patients with CNA had lower levels of total sRAGE compared to healthy controls and compared to diabetic subjects without CNA. Hence, in these studies, lower levels of sRAGE were associated with greater disease [55]. El-Mesallamy studied type 2 diabetic subjects with and without diabetic foot disease, and non-diabetic obese, and non-obese non-diabetic healthy controls. Whereas the type 2 diabetic subjects without diabetic foot disease had higher levels of sRAGE (total) vs. the healthy controls, sRAGE levels in the diabetic foot disease group were the lowest among all the groups [56].
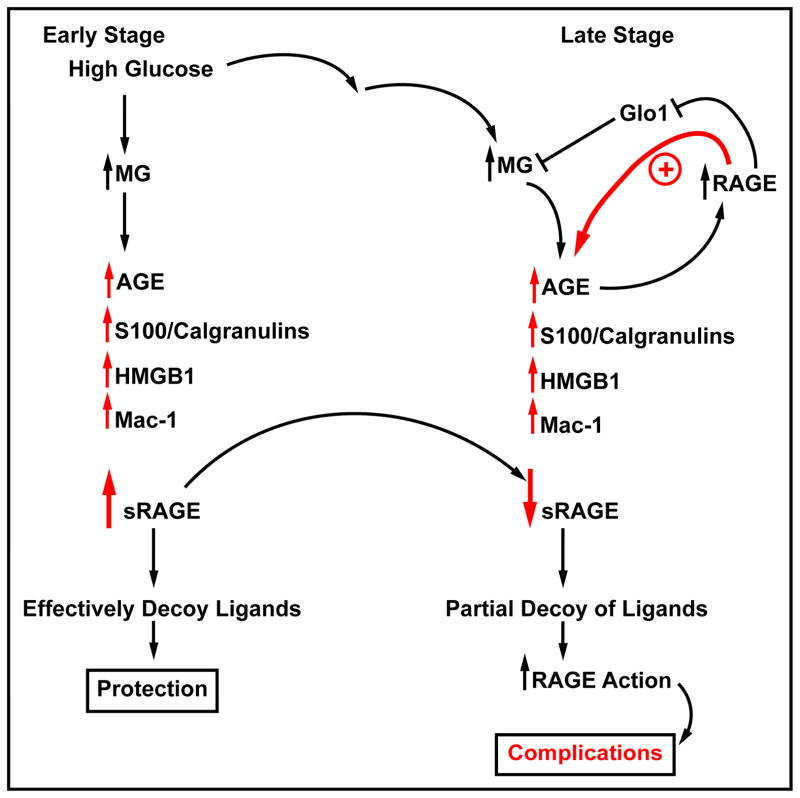
These intriguing findings suggest that perhaps in diabetes without complications levels of sRAGEs are higher than controls; perhaps shedding of the increased expression of cell surface RAGE is a protective mechanism to decoy and trap the accumulating deleterious ligands. However, later when quite advanced complications are in place, lower levels of sRAGEs may be associated with disease; perhaps mechanisms that endogenously release the sRAGEs are impaired, and/or that the increased ligand burden consumes all available sRAGEs (Figure 3). Also, it is possible that levels of sRAGE are genetically regulated; no evidence exists yet on this point.
Figure 3. Soluble RAGE – high vs. low & “good” or “bad?”.
To date, quite a number of human subject studies on sRAGE (total sRAGE or esRAGE) have been reported. Intriguingly, some of these studies suggest that “high” sRAGE may be maladaptive and others show that “low” levels of RAGE are most foreboding for diabetes. What may account for these differences in sRAGE values? We suggest that perhaps in early diabetes, without complications, that levels of sRAGEs are higher than controls without diabetes. Perhaps shedding of the increased expression of cell surface RAGE is a protective mechanism to decoy and trap the accumulating deleterious ligands, such as AGEs, S100/calgranulins, HMGB1 and mac-1. However, in late stages of the disease when quite advanced tissue damage has occurred, lower levels of sRAGEs may be associated with complications. At this stage, perhaps mechanisms that endogenously release the sRAGEs are impaired, and/or that the increased ligand burden consumes all available sRAGEs. Prospective, multi-time point studies, assessing both total sRAGE and esRAGE, are required to sort out the meaning of sRAGEs – are they solely biomarkers or in endogenous antagonists of ligand-RAGE damage?
RAGE and Diabetic Retinopathy
The complications of diabetes in the eye and retina may be devastating, as visual loss is a major complication. Although approximately 30% of adults in the United States with diabetes develop retinopathy, it is estimated that up to 4% of these subjects may progress to highly significant loss of vision [57]. RAGE expression in increased in the human diabetic retina and it is expressed in nearly all of the retina cell types [58]. Vascular cells, Muller cells, retinal neurons, and microglia strongly express RAGE in the human diabetic retina. Studies from cultured cells indicate that ligand-RAGE interaction in the cells causes inflammation, generation of oxidative stress, and in the case of retinal neurons, apoptosis [58]. In vivo, studies by Barile’s group employed long-term soluble RAGE decoy in hyperlipidemic apoE null diabetic db/db mice. Pathological and functional (electroretinography) measures were used to test the effects of hyperlipidemia, diabetes and the effects of sRAGE. Compared to all other groups, the hyperglycemic, hyperlipidemic mice demonstrated accelerated development of acellular capillaries and pericyte “ghosts.” ERG studies revealed prolonged latencies of the oscillatory potentials and b-wave in these mice. The highest retinal RAGE expression was observed in the hyperglycemic, hyperlipidemic mice, which was greater than that of all other groups [59]. Soluble RAGE treatment resulted in decreased neuroretinal and vascular damage in the hyperlipidemic, hyperglycemic mice [59].
Non-AGE ligands of RAGE have also been studied in diabetic retina. El-Asrar and colleagues examined levels of HMGB1 in diabetic mice and found that in the retinas, HMGB1 demonstrated higher expression compared to that in non-diabetic mice. Upon examination of vitreous fluid of human subjects with proliferative diabetic retinopathy (PDR) or in non-diabetic controls, these authors found that mean levels of HMGB1 in the vitreous fluid were 2–3x higher in the subjects with active PDR (neovascularization) vs. those with inactive disease or in the healthy non-diabetic subjects. The highest levels of HMGB1 were found in the diabetic subjects with PDR, active neovascularization and active hemorrhage. El-Asrar and colleagues found significant relationships between levels of HMGB1 and other markers of inflammation, including MCP-1 and ICAM-1 in vitreous fluids [60].
RAGE & Signal Transduction
Reports from multiple laboratories indicate that ligand-RAGE interaction stimulates signal transduction, and that such signaling is essential for RAGE-dependent modulation of gene expression and fundamental cellular properties. It has been shown that RAGE activates members of the mitogen activated protein (MAP) kinase family of signal transduction effectors, including p44/42 MAP kinase, JNK MAP kinase and p38 MAP kinase [15]. Furthermore, ligand-RAGE interaction stimulates activation of p21ras [61], Jak/STAT pathways [62], cdc42 and rac-1 [63], Akt, and GSK-3β signaling [64]. These signaling pathways converge on multiple transcription factors such as Egr-1 [65], NF-kB [63] and CREB [66], which mediate alterations in cell fate, migration and production of inflammatory and pro-fibrotic mediators. Indeed, RAGE is expressed on multiple types of cells and we hypothesize that the specific signaling pathway(s) activated by RAGE ligands is/are greatly dependent on the duration of ligand stimulation and the specific functions of each cell type. For example, RAGE ligands result in increased serine9 phosphorylation of GSK-3β in smooth muscle cells, processes which cause increased smooth muscle cell migration [64]. However, in contrast, in primary murine adult cardiomyocytes, RAGE ligands mediate decreased phosphorylation of serine 9 GSK-3β; this is linked to increased activation of cell death pathways in these cells [67]. These results strongly suggest that the actions of RAGE ligands may be unique in distinct cell types and are highly dependent on the properties, stress responses and fate of the impacted cells.
Studies from our and other laboratories have indicated that the cytoplasmic domain of RAGE is essential for RAGE ligand-stimulated signal transduction. In cultured cells, introduction of a cytoplasmic domain-deleted RAGE exerts a dominant negative (DN) effect upon binding of RAGE ligands to the cells [14]. For example, in primary murine aortic endothelial cells retrieved from mice expressing DN RAGE, incubation with the prototypic RAGE ligand S100B reveals suppression of MAP kinase signaling and lack of upregulation of VCAM-1 and MMPs vs. the robust activation of these kinases and protein expression changes observed in endothelial cells retrieved from wild-type mice [34].
As the cytoplasmic domain of RAGE lacks endogenous tyrosine kinase activity, a critical question that arose was the identification of the precise intracellular mechanisms by which RAGE ligands stimulated RAGE-dependent signal transduction. Results from a yeast-two-hybrid experiment, verified through extensive experimentation, indicated that the cytoplasmic domain of RAGE bound diaphanous 1 or mDia1, a member of the formin family of actin cytoskeleton and Rho GTPase effector molecules [68]. RAGE binds the FH1 domain of mDia1 at least in part through the interaction of R5/Q6 amino acids of the human RAGE cytoplasmic tail (ctRAGE), as revealed by NMR studies and experiments using mutated R5Q6 in functional assays in primary murine aortic smooth muscle cells [69].
mDia1 is required for RAGE ligand (AGE)-mediated upregulation of Egr-1 in macrophages exposed to hypoxia [70]; it is required for RAGE ligand (AGE and S100B)-stimulated migration and activation of rac-1 and cdc42 in transformed cells [68], and mDia1 is required for S100B-mediated activation of rac1, Akt and GSK-3β, generation of oxidative stress, and cellular migration in primary murine aortic smooth muscle cells [64]. In vivo, mDia1 null mice reveal markedly reduced neointimal expansion consequent to guide wire-induced injury to the femoral artery [64]. It is important to note that homozygous RAGE null mice are also protected from neointimal expansion after femoral artery guide wire injury [71].
Experiments are underway to probe the impact of mDia1 in murine models of diabetic complications. If these studies identify that mDia1 is essential for macro- and microvascular complications, such data may highlight novel areas for therapeutic intervention in diabetes.
Summary & Perspectives – Prospects for the Clinic
At least four classes of AGE-RAGE direct strategies may be envisioned from the results of this work. Although non-specific efforts to reduce inflammation (statins, for example) may, in parallel, reduce RAGE expression, more specific strategies to block AGE-RAGE might be more efficacious as follows:
First, direct inhibition of AGEs or reduction of AGE cross-links (alagebrium, for example), is supported by experimental data. As discussed above, however, toxicity associated with aminoguanidine, together with its failure to meet the required endpoint in diabetic kidney disease in Phase III clinical trial, indicates that although aminoguanidine will not be further tested, the concept of AGE inhibition remains plausible [47] In this context, the polyol pathway may be one such key target to impact AGE formation. Increased glucose drives flux through this pathway, which includes most proximally aldose reductase (AR) and sorbitol dehydrogenase (SDH). Flux through this pathway generates AGE precursors, including MG and 3-deoxyglucosone [72]. It is conceivable that inhibitors of AR or SDH may be safe and targeted AGE inhibitors; it is notable that this pathway exerts other key effects in diabetes and hyperglycemia, as recently reviewed by Ramasamy and colleagues [72].
Second, the enzyme Glo1 reduces MG and therefore suppresses MG to AGE formation. Is Glo1 a reasonable target for therapeutic intervention? Possibly not; Distler and colleagues have recently published that transgenic mice which overexpress Glo1 display anxiety. In their fascinating studies, these authors show that MG is a GABAA receptor agonist and that if MG is extensively depleted, anxiety results [73]. Indeed, treatment of Glo1 mice with low doses of MG reduced anxiety. Hence, it remains to be determined if Glo1 is an acceptable target given that MG, indeed, has adaptive roles in the nervous system. In this context, perhaps local treatment at direct sites of diabetic complications outside the central nervous system may be both effective and safe. Further, in the setting of hyperglycemia, MG levels will be significantly higher than those observed in euglycemia. Perhaps even partial augmentation of Glo1 levels may be sufficient to reduce pathological but not homeostatic levels of MG. Extensive drug design and pharmacological testing will be required to address this point.
Third, sRAGE ligand decoys or direct antagonists of ligand binding to RAGE may be beneficial. Studies in animal models have clearly shown that RAGE is not involved in innate immune mechanisms; we recently showed that in mice subjected to massive liver injury (in which at least 50% of the wild-type mice succumb), although mice devoid of Myd88 die in greater numbers in the first 7 days post-surgery than the wild-type controls, mice devoid of RAGE display increased survival vs. all other groups over this time course [74]. Hence, we deduce that RAGE blockade will likely not suppress fundamental immune responses.
Fourth, is it conceivable that blockade of the RAGE cytoplasmic domain with mDia1 is a new target for development in diabetes complications? Based on experiments in which Shekhtman and colleagues have identified the amino acids of ctRAGE required for binding to mDia1, it is possible that therapies might emerge from this discovery. However, more study of mDia1 in diabetes complications is required and are underway at this time.
Finally, it is important to note that the studies reported and underway assessing levels of sRAGEs may yield, either alone, or in biomarker panels, formulas for predicting diabetes and the vulnerability to complications. Studies testing large populations over repeated measures and periods of time, matched to detailed clinical records and if possible, banked baseline samples at time of diabetes diagnosis, will be essential to accurately predict the value of sRAGE measurements. Indeed, if successful, then tracking sRAGEs may be a sensitive and specific means to determine the therapeutic efficacy of the aforementioned therapeutic possibilities.
Acknowledgments
The authors gratefully acknowledge grants from the US Public Health Service and the JDRF. We are grateful for the expert assistance of Ms. Latoya Woods in preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herman WH, Zimmet P. Type 2 diabetes: an epidemic requiring global attention and urgent action. Diabetes Care. 2012;35:943–944. doi: 10.2337/dc12-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8:417–429. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental axis signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monnier VM, et al. Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging and uremia. Diabetes. 1992;41(Suppl 2):36–41. doi: 10.2337/diab.41.2.s36. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt AM, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- 6.Schmidt AM, et al. Regulation of human mononuclear phagocyte migration by cell-surface binding proteins for advanced glycation end products. J Clin Invest. 91:2155–2168. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornalley PJ. Dicarbonyl intermediates in the Maillard reaction. Ann NY Acad Sci. 2005;1043:111–117.8. doi: 10.1196/annals.1333.014. [DOI] [PubMed] [Google Scholar]
- 8.Thornalley PJ. The glyoxalase system: new developments toward functional characterization of a metabolic pathway fundamental to life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohgami N, et al. CD36, a member of the class b scavenger receptor family, as a receptor for advanced glycation endproducts. J Biol Chem. 2001;276:3195–3202. doi: 10.1074/jbc.M006545200. [DOI] [PubMed] [Google Scholar]
- 10.Araki N, et al. Macrophage scavenger receptor mediates the endocytic uptake and degradation of advanced glycation endproducts of the Maillard reaction. Eur J Biochem. 1995;230:408–415. doi: 10.1111/j.1432-1033.1995.0408h.x. [DOI] [PubMed] [Google Scholar]
- 11.Rudijanto A. The expression and down stream effect of lectin-like oxidized low density lipoprotein (Lox-1) in hyperglycemic state. Acta Med Indones. 2007;39:36–43. [PubMed] [Google Scholar]
- 12.Kislinger TK, et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for advanced glycation end products that activate cell signaling pathways and modualte gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 13.Reiniger N, et al. Deletion of the receptor for advanced glycation endproducts reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse. Diabetes. 2010;59:2043–2054. doi: 10.2337/db09-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann MA, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 15.Taguchi A, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 16.Chavakis T, et al. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beisswenger PJ, et al. Formation of immunochemical advanced glycosylation end products precedes and correlates with early manifestations of renal and retinal disease in diabetes. Diabetes. 1995;44:824–829. doi: 10.2337/diab.44.7.824. [DOI] [PubMed] [Google Scholar]
- 18.Yanagisawa K, et al. Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metabolism. 1998;47:1348–1353. doi: 10.1016/s0026-0495(98)90303-1. [DOI] [PubMed] [Google Scholar]
- 19.Kosaki A, et al. Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5423–5428. doi: 10.1210/jc.2003-032223. [DOI] [PubMed] [Google Scholar]
- 20.Nin JW, et al. Serum high-mobility group box-1 levels are positively associated with micro- and macroalbuminuria but not with cardiovascular disease in type 1 diabetes: the EURODIAB Prospective Complications Study. Eur J Endocrinol. 2012;166:325–332. doi: 10.1530/EJE-11-0662. [DOI] [PubMed] [Google Scholar]
- 21.Vazzana N, Santilli F, Cuccurullo C, Davì G. Soluble forms of RAGE in internal medicine. Intern Emerg Med. 2009;4:389–401. doi: 10.1007/s11739-009-0300-1. [DOI] [PubMed] [Google Scholar]
- 22.Raucci A, et al. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 23.Yonekura H, Yamamoto Y, Sakurai S, Watanabe T, Yamamoto H. Roles of the receptor for advanced glycation endproducts in diabetes-induced vascular injury. J Pharmacol Sci. 2005;97:305–311. doi: 10.1254/jphs.cpj04005x. [DOI] [PubMed] [Google Scholar]
- 24.Forbes JM, et al. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J Am Soc Nephrol. 2005;16:2363–2372. doi: 10.1681/ASN.2005010062. [DOI] [PubMed] [Google Scholar]
- 25.Santilli F, et al. Decreased plasma soluble RAGE in patients with hypercholesterolemia: effects of statins. Free Radic Biol Med. 2007;43:1255–1262. doi: 10.1016/j.freeradbiomed.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Grossin N, Boulanger E, Wautier MP, Wautier JL. The different isoforms of the receptor for advanced glycation end products are modulated by pharmacological agents. Clin Hemorheol Microcirc. 2010;45:143–153. doi: 10.3233/CH-2010-1292. [DOI] [PubMed] [Google Scholar]
- 27.Nathan DM, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. New Engl J Med. 2003;348:2294–2303. doi: 10.1056/NEJMoa022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anand DV, et al. Determinants of progression of coronary artery calcification in type 2 diabetes role of glycemic control and inflammatory/vascular calcification markers. J Am Coll Cardiol. 2007;50:2218–2225. doi: 10.1016/j.jacc.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 29.Park L, et al. Suppression of accelerated diabetic atherosclerosis by soluble Receptor for AGE (sRAGE) Nature Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 30.Vikramadithyan RK. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bu DX, et al. Activation of the ROCK1 branch of the TGF-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE null mice. Circ Res. 2010;106:1040–1051. doi: 10.1161/CIRCRESAHA.109.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burke AP, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 33.Soro-Paavonen A, et al. RAGE deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harja E, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE in apo E deficient mice. J Clin Invest. 2008;1118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris-Rosenfeld S, et al. Deletion of bone marrow-derived receptor for advanced glycation end products inhibits atherosclerotic plaque progression. Eur J Clin Invest. 2011;41:1164–1171. doi: 10.1111/j.1365-2362.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- 36.Parathath S, et al. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60:1759–1769. doi: 10.2337/db10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, et al. Advanced glycation endproducts alter functions and promote apoptosis in endothelial progenitor cells through receptor for advanced glycation endproducts mediate overexpression of cell oxidant stress. Mol Cell Biochem. 2010;335:137–146. doi: 10.1007/s11010-009-0250-y. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Jin J, Song M, Dong H, Zhao G, Huang L. C-reactive protein down-regulates endothelial nitric oxide synthase expression and promotes apoptosis in endothelial progenitor cells through receptor for advanced glycation endproducts. Gene. 2012;496:128–135. doi: 10.1016/j.gene.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 39.Bucciarelli LG, et al. RAGE and modulation of ischemic injury in the diabetic myocardium. Diabetes. 2008;57:1941–1951. doi: 10.2337/db07-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shang L, et al. RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3beta signaling pathways. PLoS One. 2010;5:e10092. doi: 10.1371/journal.pone.0010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma H, et al. Advanced glycation endproduct (AGE) accumulation and AGE receptor (RAGE) upregulation contribute to the onset of diabetic cardiomyopathy. J Cell Mol Med. 2009;13:1751–1764. doi: 10.1111/j.1582-4934.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Nielsen JM, et al. Blockage of receptor for advanced glycation endproducts prevents development of cardiac dysfunction in db/db type 2 diabetic mice. Eur J Heart Fail. 11:638–647. doi: 10.1093/eurjhf/hfp070. [DOI] [PubMed] [Google Scholar]
- 43.Tanji N, et al. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11:1656–1666. doi: 10.1681/ASN.V1191656. [DOI] [PubMed] [Google Scholar]
- 44.Wendt TM, et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162:1123–1137. doi: 10.1016/S0002-9440(10)63909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myint KM, et al. RAGE control of diabetic nephropathy in a mouse model: effects of RAGE gene disruption and administration of low molecular weight heparin. Diabetes. 2006;55:2510–2522. doi: 10.2337/db06-0221. [DOI] [PubMed] [Google Scholar]
- 46.Tan AL, et al. Disparate effects on renal and oxidative parameters following RAGE deletion, AGE accumulation inhibition, or dietary AGE control in experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2010;298:F763–F770. doi: 10.1152/ajprenal.00591.2009. [DOI] [PubMed] [Google Scholar]
- 47.Bolton WK, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24:32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 48.Kalousová M, et al. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006;47:406–411. doi: 10.1053/j.ajkd.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura K, Adachi H, Matsui T, Kurita Y, Takeuchi M, Yamagishi S. Independent determinants of soluble form of receptor for advanced glycation end products in elderly hypertensive patients. Metabolism. 2009;58:421–425. doi: 10.1016/j.metabol.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 50.Thornalley PJ. Glycation in diabetic neuropathy: characteristics, consequences, causes and therapeutic options. Int Rev Neurobiol. 2002;50:37–57. doi: 10.1016/s0074-7742(02)50072-6. [DOI] [PubMed] [Google Scholar]
- 51.Juranek J, et al. Morphological changes and immunohistochemical expression of RAGE and its ligands in the sciatic nerve of hyperglycemic pig (Sus scrofa) Biochem Insights. 2010;2010:47–59. doi: 10.4137/BCI.S5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toth C, et al. Receptor for advanced glycation endproducts (RAGEs) and experimental diabetic neuropathy. Diabetes. 2008;57:1002–1017. doi: 10.2337/db07-0339. [DOI] [PubMed] [Google Scholar]
- 53.Bierhaus A, et al. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest. 2004;114:1741–1751. doi: 10.1172/JCI18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Humpert PM, et al. sRAGE and esRAGE are not associated with peripheral or autonomic neuropathy in type 2 diabetes. Horm Metab Res. 2007;39:899–902. doi: 10.1055/s-2007-993155. [DOI] [PubMed] [Google Scholar]
- 55.Witzke KA, et al. Loss of RAGE defense: a cause of Charcot neuroarthropathy? Diabetes Care. 2011;34:1617–1621. doi: 10.2337/dc10-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Mesallamy HO, et al. Levels of soluble advanced glycation end products receptors and other soluble serum markers as indicators of diabetic neuropathy in the foot. J Investig Med. 2011;59:1233–1238. doi: 10.2130/JIM.0b013e318231db64. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304:649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zong H, Ward M, Stitt AW. AGEs, RAGE and diabetic retinopathy. Curr Diab Rep. 2011;11:244–252. doi: 10.1007/s11892-011-0198-7. [DOI] [PubMed] [Google Scholar]
- 59.Barile GR, et al. The RAGE axis in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46:2916–2924. doi: 10.1167/iovs.04-1409. [DOI] [PubMed] [Google Scholar]
- 60.El-Asrar AM, et al. High mobility group box 1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis. 2011;17:1829–1838. [PMC free article] [PubMed] [Google Scholar]
- 61.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation endproducts triggers a p21(ras) dependent mitogen activated protein kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 62.Huang JS, Guh JY, Chen HC, Hung WC, Lai YH, Chuang LY. Role of receptor for advanced glycation endproduct (RAGE) and the Jak/STAT signaling pathway in AGE-induced collagen production in NRK-49F cells. J Cell Biochem. 2001;81:102–113. doi: 10.1002/1097-4644(20010401)81:1<102::aid-jcb1027>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 63.Huttunen HJ, Fages C, Rauvala H. Receptor for advanced glycation endproducts (RAGE)-mediated neurite outgrowth and activation of NF-kB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem. 1999;274:19919–19924. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- 64.Toure F, et al. Formin mDia1 Mediates Vascular Remodeling via Integration of Oxidative and Signal Transduction Pathways. Circ Res. 2012;110:1279–1293. doi: 10.1161/CIRCRESAHA.111.262519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang JS, et al. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation endproducts. Circ Res. 2008;102:905–913. doi: 10.1161/CIRCRESAHA.107.165308. [DOI] [PubMed] [Google Scholar]
- 66.Huttunen HJ, Kuja-Panula J, Rauvala H. Receptor for advanced glycation endproduct (RAGE) signaling induces CREB-dependent chromogranin expression during neuronal differentiation. J Biol Chem. 2002;277:38635–38646. doi: 10.1074/jbc.M202515200. [DOI] [PubMed] [Google Scholar]
- 67.Shang L, et al. RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3beta signaling pathways. PLoS One. 2010;5:e10092. doi: 10.1371/journal.pone.0010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hudson BI, et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457–34468. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rai V, et al. Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. J Biol Chem. 2012;287:5133–5144. doi: 10.1074/jbc.M111.277731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu Y, et al. Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J Biol Chem. 2010;285:23233–23240. doi: 10.1074/jbc.M110.117457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakaguchi T, et al. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–972. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramasamy R, Goldberg IJ. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ Res. 2010;106:1449–1458. doi: 10.1161/CIRCRESAHA.109.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Distler MG, et al. Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J Clin Invest. 2012 doi: 10.1172/JCI61319. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng S, et al. Opposing roles of RAGE and Myd88 signaling in extensive liver resection. FASEB J. 2012;26:882–893. doi: 10.1096/fj.11-192997. [DOI] [PMC free article] [PubMed] [Google Scholar]