Abstract
The presence of many salts, such as sodium chloride, can adversely affect the performance of native electrospray ionization mass spectrometry for the analysis of proteins and protein complexes by reducing the overall molecular ion abundances and distributing signal for any given charge state into many cationized forms with various numbers of adducts attached. Several solution additives, such as ammonium bromide, ammonium iodide, and NaSbF6, can significantly lower the extent of sodium ion adduction to the molecular ions of proteins and protein complexes. For ubiquitin, addition of 25 mM ammonium bromide or ammonium iodide into aqueous solutions also containing 1.0 mM NaCl results in a factor of 72 and 56 increase, respectively, in the relative abundances of the fully protonated molecular ions compared to when these additives are not present. The effectiveness of this method for reducing sodium ion adduction is related to the low proton affinity (PA) values of the anions. Anions with very low PA also have a propensity to adduct as an acid molecule, but these adducts can be readily dissociated from the molecular ions either by activation in the source or subsequently by collisional activation in the mass spectrometer. This method of reducing sodium ion adduction to proteins is simple and requires no experimental modifications, making it an attractive alternative to other methods for desalting proteins prior to mass spectrometry analysis.
Introduction
Electrospray ionization (ESI) mass spectrometry (MS) is widely used to gently ionize and measure the masses of intact large molecules and complexes with high sensitivity (less than a femtomole of sample is possible).1–13 However, the sensitivity of ESI-MS can be significantly lower when some salts, such as sodium chloride, are also present in the sample solution.13–21 Even low millimolar concentrations of some metal ion salts can cause severe ion suppression and peak broadening due to cluster and adduct formation.13–19 For example, addition of 10 mM CsCl to an aqueous solution containing 1 μM lysozyme resulted in a 330-fold reduction in protein ion abundance.18 Sodium is ubiquitous in nature, and its presence in many samples can significantly degrade analysis by ESI-MS. Extensive sodium ion adduction to protein ions often occurs even for proteins that have little or no metal ion binding affinity in solution (nonspecific adduction). The adverse effects of some salts can be especially challenging for some biological samples that require essential salts or high ionic strength to assemble and maintain their functional forms in solution.11–13
There are several strategies to overcome the adverse effects of metal ion salts. The most common approach is to remove the salt from solution prior to ESI-MS, and this can be done using a variety of techniques, such as dialysis,22,23 liquid chromatography,23,24 and ion exchange.25,26 However, removal of salts can also adversely affect the structures of some molecules and can affect binding of molecular complexes.11–13 Sodium ion adduction in ESI depends on a number of factors, including ion polarity,27 the pI of the protein, and the pH of the solution. More adduction typically occurs for low charge state ions.14–16 McLuckey and coworkers found that sodium adduction to gaseous positively charged protein ions can be significantly reduced when the solution pH is ~3 units lower than the pI of the protein.15 High concentrations of ammonium acetate can reduce sodium adduction to protein ions and can be used to improve the mass measuring accuracy of large protein complexes where adducts to molecular ions are not resolved.12–14 Addition of 7 M ammonium acetate to aqueous solutions containing 20 mM sodium chloride, and either cytochrome c or ubiquitin, resulted in a ~7- and ~11-fold improvement in signal-to-noise ratios (S/N) for the molecular ions of these respective proteins.14
Some anions, such as L-tartrate and citrate, can also reduce the extent of nonspecific metal ion adduction to biomolecules.28–32 Konermann and coworkers found that nonspecific calcium adduction to proteins was significantly lower with calcium tartrate compared to calcium chloride or calcium acetate when these salts were added to the ESI solution, a result attributed to L-tartrate acting as a solution-phase chelator of calcium.28 Gas-phase ion/ion reactions between DNA anions and several chelating ions, such as citrate, in a dual nanospray setup have also been shown to significantly reduce nonspecific metal ion adduction to DNA anions.29 Metal ion adduction to oligonucleotides can also be reduced by adding acid vapors into the drying gas.33
Ions in solution can also affect the conformation and stability of protein and protein complex ions in the gas phase.34–37 Attachment of acid molecules of select Hofmeister anions, ClO4−, I−, and SO42−, to ubiquitin, cytochrome c, and α-lactalbumin can induce compact conformations in the resulting gas-phase protein ions generated by ESI.34 Ruotolo and coworkers found that anions with high gas-phase acidities, such as nitrate and chloride, bind to protein complexes in solution or during ESI and resulted in significant gas-phase stabilization of the protein complex ions.35
The extents of sodium ion and acid molecule adduction to positively charged protein ions formed by ESI from aqueous solutions that contain millimolar concentrations of sodium salts of various anions depend to a significant extent on the proton affinity (PA) of the anion.38 The PA of an anion (A−) is the negative enthalpy change for the gas-phase reaction:
For eleven sodium salts of anions that have PA values ranging from 260 to 371 kcal•mol−1, the extent of sodium ion and acid molecule adduction to four proteins was inversely related. For anions with a high PA, deprotonation of acidic sites (Asp, Glu, and the C-terminus) can be favorable depending on the pI of the protein.38 Deprotonation of acidic sites in the protein by the anion in the late stages of droplet evaporation makes possible strongly favorable interactions between the deprotonated site and sodium cations.38 This results in little nonspecific sodium ion adduction to protein ions for anions with PA values below ~315 kcal•mol−1 and increasing adduction with increasing PA of the anion.38 For anions with low PA values (< ~315 kcal•mol−1), deprotonation of acidic sites is less favored, and acid molecule adduction to basic sites (Arg, Lys, His, N-terminus) occurs.38 The number of basic sites in peptides and proteins can be accurately determined from the number of adducts.38–40
Here, we report that solution additives with anions that have low PA values can significantly reduce the extent of nonspecific sodium ion adduction and improve the abundances of gaseous protein and protein complex ions generated by ESI. Ammonium bromide and iodide are particularly effective at reducing nonspecific sodium adduction to protein ions at a significantly lower concentration than ammonium acetate, and acid molecule adduction, which depends on ion source conditions, is minimized.
Experimental
Mass Spectrometry
Mass spectra were acquired using either a LTQ-orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) or a 9.4 T Fourier-transform ion cyclotron resonance (FT-ICR) mass spectrometer equipped with an external ESI source that is described elsewhere.41 Ions are generated by nanoESI from borosilicate capillaries that are pulled so that the tips have a ~2 μm inner diameter (model P-87 capillary puller, Sutter Instruments, Novato, CA). The capillary is loaded with a small volume (~2–10 μL) of analyte solution, and a platinum wire is inserted into the solution. The borosilicate capillary is positioned ~2 mm away from the source inlet capillary. Ions are generated by applying a potential difference of 800 to 1200 V between the platinum wire and the inlet capillary. Total ion abundances can vary by a factor of up to five when different borosilicate capillaries are used. Due to the significant variability in the S/N between ESI capillaries, the average and standard deviation of the abundance of the protonated molecular ion and most abundant ion relative to the total ion abundance for each charge state are reported. To compare the effect of additives on absolute S/N, aqueous solutions containing 10 μM protein and 1.0 mM NaCl with or without an ammonium additive were analyzed using a single ESI capillary, which was washed between samples with water to reduce effects of cross contamination. This procedure was repeated using five different capillaries.
Bacillus amyloliquefaciens barstar and barnase were obtained by methods described previously.42 Bovine ubiquitin, bovine cytochrome c, sodium chloride, ammonium acetate, ammonium bromide, ammonium iodide, ammonium tartrate, ammonium citrate, and NaSbF6 were obtained from Sigma Aldrich (St. Louis, MO). The ammonium salt of SbF6 is not commercially available, so NaSbF6 was used instead.
Computational Chemistry
Initial geometries for the neutral and singly deprotonated forms of citric and L-tartaric acid were generated by a Monte Carlo conformational search using Macromodel 9.3 (Schrödinger, Inc., Portland, OR, U.S.A.). A selection of the low-energy conformers was used to create isomer geometries that represent different hydrogen bonding patterns, and geometry optimization at the B3LYP/6–31+G** level of theory was done using Q-Chem 4.043 (Q-Chem, Inc., Pittsburgh, PA, U.S.A). The geometries were further optimized with B3LYP/6–311++G** prior to vibrational frequency calculations at the same level of theory. Zero-point energies, enthalpy, and entropy corrections at 298 K were calculated using unscaled harmonic oscillator vibrational frequencies. Proton affinities and gas-phase basicities were calculated from −ΔH° and −ΔG°, respectively, for the protonation of singly deprotonated citric and L-tartaric acid. These values were obtained from the lowest energy neutral and singly deprotonated structures (298 K).
Results and Discussion
Effects of Anions on Sodium Ion Adduction to Ubiquitin
The use of a new nanoESI capillary for each sample eliminates cross contamination of samples but makes it more challenging to accurately determine the extent to which various solution additives affect absolute signal owing primarily to the reproducibility of ion signal obtained from different capillaries. To determine how various additives affect ion signal, mass spectra of ubiquitin from solutions with and without different additives were obtained with a single ESI capillary that was cleaned prior to loading the capillary with a new sample, and this procedure was repeated using five different capillaries. The effects of the different additives on the relative abundances of both the fully protonated molecular ion and the most abundant ion relative to the total ion abundances of the 5+ and 6+ charge states of ubiquitin are given in Table 1, and representative ESI mass spectra obtained with a single ESI capillary that gave S/N values closest to the average of the five capillaries are shown in Figure 1.
Table 1.
Relative abundances of the fully protonated and most abundant molecular ion and average # of sodium adducts for ubiquitin 5+ and 6+.
| Ubiquitin 5+ | |||
|---|---|---|---|
| Ammonium Additive | Relative Abundance of Protonated Molecular Ion | Relative Abundance of Most Abundant Ion | Average # of Sodium Adducts |
| No Additive | 1 ± 1% (25)a | 11 ± 1% (364)a | 6.3 ± 1.4 |
| 1 M Ammonium Acetate | 12 ± 3% (342)a | 13 ± 5% (387)a | 4.8 ± 1.4 |
| 25 mM Ammonium Iodide | 48 ± 7% (1749)a | 48 ± 7% (1749)a | 1.5 ± 0.2 |
| 25 mM Ammonium Bromide | 70 ± 12% (3211)a | 70 ± 12% (3211)a | 0.5 ± 0.2 |
| Ubiquitin 6+ | |||
|---|---|---|---|
| Ammonium Additive | Relative Abundance of Protonated Molecular Ion | Relative Abundance of Most Abundant Ion | Average # of Sodium Adducts |
| No Additive | 2 ± 1% (17)a | 13 ± 3% (100)a | 5.8 ± 0.8 |
| 1 M Ammonium Acetate | 44 ± 5% (443)a | 44 ± 5% (443)a | 1.3 ± 0.1 |
| 25 mM Ammonium Iodide | 64 ± 3% (3044)a | 64 ± 3% (3044)a | 0.4 ± 0.2 |
| 25 mM Ammonium Bromide | 74% ± 9% (6578)a | 74 ± 9% (6578)a | 0.4 ± 0.1 |
Values in parentheses are the absolute S/N obtained from a single ESI capillary.
Figure 1.
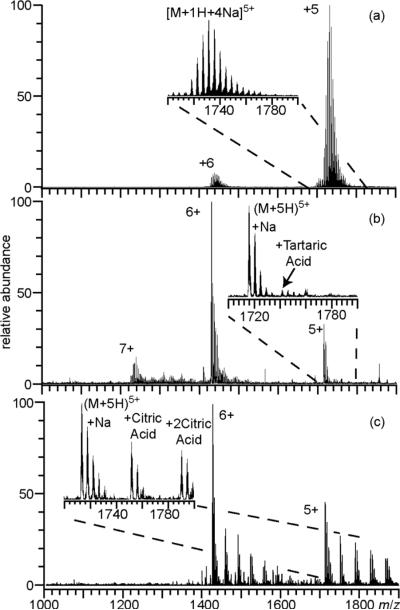
ESI mass spectra obtained from aqueous solutions containing 10 μM ubiquitin with 1.0 mM NaCI and (a) no additional additive, (b) 1.0 M ammonium acetate, (c) 25 mM ammonium bromide, (d) 25 mM ammonium iodide, or (e) 1.0 mM NaSbF6. Insets show an expanded region of the 5+ charge state.
ESI of an aqueous solution containing 1.0 mM NaCl and 10 μM ubiquitin results in molecular ions with extensive sodium ion adduction (Figure 1a). Both the 5+ and 6+ charge states have an average of six sodium ions adducted, and up to 17 sodium ions adduct to both charge states. Less than 3% of the total molecular ion abundance is from exclusively protonated molecular ions. The most abundant adducted form of the 5+ and 6+ ions account for only 11% and 13% of the total ion abundance for these respective charge states. Sodium ion adducts distribute the protein ion abundance into multiple cationized forms with various numbers of adducts, which lowers the S/N for each form of the protein ion in each charge state. With 1.0 M ammonium acetate added to this solution, the charge state distribution shifts to slightly higher charge, and less sodium ion adduction occurs, particularly to the higher charge state ion (Figure 1b). The average number of sodium ions adducted to the 5+ and 6+ charge states of ubiquitin is 4.8 and 1.3, respectively, and the fully protonated molecular ions account for approximately 28% of the total ion abundance.
A more substantial reduction in the extent of sodium ion adduction to ubiquitin occurs when either 25 mM ammonium bromide or ammonium iodide is added to solutions containing 1.0 mM NaCl (Figures 1c and 1d, respectively). With both ammonium additives, the charge state distribution is centered at the 6+, and the average number of sodium ions adducted to this charge state is 0.4. A more significant reduction in the average number of sodium ion adducts occurs to the 5+ charge state with ammonium bromide (0.4) compared to ammonium iodide (1.5), but both additives are significantly more effective at reducing sodium ion adduction to ubiquitin than ammonium acetate even when ammonium acetate is at a much higher concentration. With either ammonium iodide or ammonium bromide, the exclusively protonated molecular ions are the most abundant form of ubiquitin and account for 56% and 72% of the total ion abundance, respectively. With ammonium iodide, adduction of HI molecules occurs, accounting for approximately 33% of the total ion abundance, whereas virtually no HBr adduction is observed under these conditions.
It was previously postulated that ammonium acetate reduces nonspecific sodium adduction to protein ions as a result of precipitation of sodium acetate in the ESI droplet as solvent preferentially evaporates and the salt is enriched.14 However, the sodium salts of bromide and iodide are approximately a factor of 1.5 and 1.9 more soluble, respectively, than sodium acetate, indicating the effectiveness of bromide and iodide is not a result of the solubility of the sodium salts of these anions. The more significant reduction in sodium ion adducts with bromide and iodide compared to acetate is likely due to the 25 and 34 kcal•mol−1 lower PA values of these respective anions (PA of acetate = 348 kcal•mol−1).38 The lower PA values for iodide and bromide makes deprotonation of acidic sites on ubiquitin less favorable compared to acetate, thus fewer locations are available on the protein where sodium ion adduction is favorable. HI adducts are observed, whereas no HBr adduction occurs, consistent with the 9 kcal•mol−1 lower PA of iodide compared to bromide.38
To determine the extent to which anions with even lower PA values can reduce sodium ion adduction, an ESI mass spectrum of ubiquitin was obtained from an aqueous solution containing 1.0 mM NaCl and 1.0 mM of the sodium salt of SbF6−, which has a PA value that is 88 kcal•mol−1 lower than that of acetate (Figure 1e). Only 1.0 mM NaSbF6 was used because higher concentrations result in poor ubiquitin ion abundance due to substantial formation of Na+(NaSbF6)n clusters. The charge state distribution is centered at 7+, and the average charge is ~2.8 charges higher than the solution without NaSbF6. The average number of sodium ion adducts to the fully protonated 5+ to 8+ charge states is less than 0.3, and the exclusively protonated molecular ions account for 57% of the total ubiquitin ion abundance. This is the lowest sodium ion adduction observed for any of the additives investigated despite the factor of two higher concentration of sodium in this solution. As observed previously for salts with anions that have low PA values,38,40 substantial HSbF6 adduction occurs to ubiquitin ions, and accounts for approximately 33% of the total ubiquitin ion abundance.
To determine if the acid molecule adducts that occur with anions that have low PA values (i.e. HSbF6 or HI) can be readily dissociated from ubiquitin using more energetic ion source conditions, ESI mass spectra of ubiquitin in aqueous solutions with 1.0 mM NaCl and 25 mM ammonium iodide or 1.0 mM NaSbF6 were obtained on a LTQ-Orbitrap instrument using capillary temperatures of 100 °C or 300 °C (Figure S-1). At 100 °C, minimal sodium ion adduction is observed to ubiquitin ions with either additive, but substantial HI or HSbF6 adduction occurs. At 300 °C, HI adducts are eliminated without a significant increase (less than 1%) in the amount of sodium ion adduction to ubiquitin. An insignificant increase in the amount of sodium ion adduction is also observed at 300 °C with NaSbF, but only a small decrease (~14%) in the amount of HSbF6 adduction to ubiquitin ions occurs compared to that at 100 °C, indicating that HSbF6 adducts are more tightly bound to the protein ions than HI. However, HSbF6 adducts can be readily removed by collisionally activating the ubiquitin ions after introduction into the mass spectrometer (Figure S-2). These results indicate that the extent of acid molecule adduction that occurs to protein ions depends significantly on the ion source conditions, and acid molecule adducts can readily be dissociated from protein ions by activating the ions either in the source or after introduction into the mass spectrometer.
Effects of Anions on Sodium Adduction to Other Proteins and Complexes
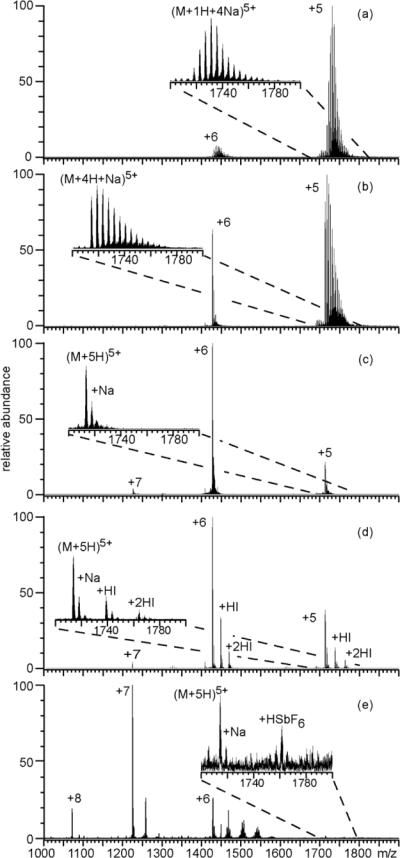
Remarkably similar results on how anions affect the extent of sodium ion adduction to molecular ions of ubiquitin are obtained with cytochrome c. ESI of an aqueous solution containing 10 μM cytochrome c and 1.0 mM NaCl results in a charge state distribution centered at 7+ with an average and maximum number of 6.5 and 18 sodium ions adducted, respectively, to this ion. The fully protonated molecular ion is only 3% of the total ion abundance of the 7+ charge state. The most abundant form of the 7+ ions, (M + 5H + 2Na)7+, is only 10% of the total ion abundance for this charge state, as a result of cytochrome c ion signal being distributed into multiple forms with various numbers of sodium adducts. Sodium adduction is lower for solutions with 1.0 mM NaCl and 1.0 M ammonium acetate, with an average and maximum number of sodium ion adducts of 3.4 and 9, respectively, to the 7+ charge state (Figure 2b). (cytochrome c + 7H)7+ is significantly more abundant with ammonium acetate, accounting for 21% of the total ion abundance of this charge state. A dramatic reduction in sodium ion attachment occurs for solutions with 1.0 mM NaCl and 25 mM of either ammonium bromide or ammonium iodide (Figure 2c and 2d, respectively). The most abundant charge state is the 7+, and the average and maximum number of sodium ion adducts is 0.5 and 3, respectively, with ammonium bromide and 0.8 and 5, respectively, with ammonium iodide. (cytochrome c + 7H)7+ is the most abundant ion in the 7+ charge state with ammonium iodide or ammonium bromide, and accounts for 61% and 69% of the total ion abundance, respectively, for this charge state. For solutions with 1.0 mM NaCl and 1.0 mM NaSbF6, the charge state distribution is shifted to higher charge, and the average number of sodium ion adducts to all charge states of cytochrome c is less than 0.3. There is more extensive adduction of HSbF6 (78% of total ion abundance) to cytochrome c than ubiquitin, whereas less adduction of HI occurs (6% of total ion abundance). This indicates that the extent of molecular adduction depends on both the ion source conditions as well as the physical properties of the protein.
Figure 2.
ESI mass spectra obtained from aqueous solutions containing 10 μM cytochrome c with 1.0 mM NaCl and (a) no additional additive, (b) 1.0 M ammonium acetate, (c) 25 mM ammonium bromide, (d) 25 mM ammonium iodide, or (e) 1.0 mM NaSbF6. Insets show an expanded region of the 7+ charge state.
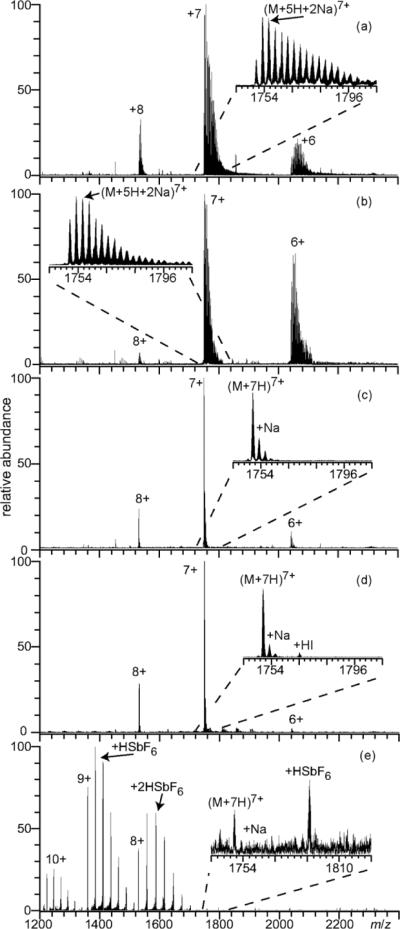
With large protein complexes, unresolved sodium and other metal ion adduction can result in significant broadening of charge states and an increase in the measured mass.12 Adduction can be so extensive that charge states are unresolved, making mass measurements challenging. Buffer loading with ammonium acetate can be used in some instances to reduce the effect of adduction but can also affect the protein binding at high concentrations.13,14 To determine whether the extent of sodium ion adduction to a protein complex can be reduced at a much lower additive concentration, ESI mass spectra of an aqueous solution of 5.0 μM barnase (bn) and 8.0 μM barstar (b*) containing 1.0 mM NaCl and either 1.0 M ammonium acetate (Figure 3a) or 25 mM ammonium bromide (Figure 3b) were obtained. The charge states of the bn/b* complex range from 8+ to 10+ and b*, the excess reagent, is also observed with 6+ and 5+ charges. More adduction to the lower charge states of the bn/b* complex occurs. The average number of sodium ion adducts to the 8+ and 9+ charge states are 3.6 and 2.3, respectively, with 1.0 M ammonium acetate, whereas these respective values are 0.3 and 0.2 with 25 mM ammonium bromide. The abundance of the fully protonated bn/b* complex with 9+ charges is ~25fold higher with ammonium bromide compared to ammonium acetate. These results indicate that ammonium bromide may be a useful additive to reduce the extent of sodium ion or other nonspecific metal ion adduction to protein complexes, as well as individual protein ions, formed by ESI from aqueous solutions with high ionic strength.
Figure 3.
ESI mass spectra obtained from an aqueous solution of 5 μM barnase (bn) and 8 μM barstar (b*) containing 1.0 mM NaCI and (a) 1.0 M ammonium acetate or (b) 25 mM ammonium bromide.
Effects of Salts on Absolute Ion Signal
To demonstrate the effect of different additives on absolute ion signal, the S/N for the protonated molecular ion and most abundant molecular ion of ubiquitin in the ESI mass spectra shown in Figure 1 is reported (Table 1, values in parentheses). With 1.0 mM NaCl, the total ion abundance of ubiquitin is poor, and the S/N of both the fully protonated molecular ion and the most abundant ion for each charge state are low. A low S/N of any single ion reduces the sensitivity with which tandem MS experiments can be made, and this is particularly a problem for the fully protonated molecular ion. With 1.0 mM ammonium acetate added to this solution, the S/N of the most abundant ion, (ubiquitin + 6H)6+, increases by a factor of 4.4, but there is only a small increase (~6%) for the most abundant ion in the 5+ charge state. Both 25 mM ammonium iodide and ammonium bromide are more effective than ammonium acetate at shifting both ubiquitin 5+ and 6+ to the fully protonated form. The S/N of the most abundant ion, (ubiquitin + 6H)6+, increases by a factor of 30 and 66 with ammonium iodide and ammonium bromide, respectively, compared to that obtained without an ammonium additive, and there is a substantial increase (>37%) for the most abundant ion in the 5+ charge state as well. In addition, the absolute S/N for the most abundant ion of ubiquitin 5+ and 6+ is higher by more than a factor of 4.5 with either of these additives compared to that obtained with ammonium acetate. These results show that ammonium iodide or ammonium bromide can improve sensitivity by shifting ion abundance for a given charge state from many adducted forms into predominantly the fully protonated form.
Sodium Ion Adduction with Ammonium Citrate or Tartrate
Both citrate and L-tartrate were reported to reduce the extent of nonspecific Ca2+ binding to protein ions formed by ESI, an effect attributed to these anions chelating ability of Ca2+ in solution.28 Ammonium salts of both anions also reduce metal ion adduction to protein and oligonucleotide ions formed by matrix assisted laser desorption/ionization (MALDI).30–32 To determine if citrate or L-tartrate can be effective additives for reducing sodium adduction to protein ions as well, ESI mass spectra of ubiquitin from aqueous solutions containing 1.0 mM NaCl and 25 mM of either ammonium tartrate or citrate were obtained under identical conditions as those used for the other ammonium additives (Figures 1 – 3), and these data are shown in Figure 4b and 4c, respectively. The charge state distribution is shifted slightly to higher charge and the extent of sodium ion adduction is significantly reduced compared to the solution with just 1.0 mM NaCl (Figure 4a). However, the fully protonated molecular ions of ubiquitin are 28% and 37% less abundant with ammonium tartrate and citrate compared to that observed with ammonium bromide. Substantial adduction of L-tartaric and citric acid to ubiquitin ions occurs and accounts for ~12% and 43% of the total ubiquitin ion abundance, respectively, whereas no HBr adducts occur. These results indicate that ammonium bromide is a better additive to reduce nonspecific adducts to protein ions compared to ammonium citrate or ammonium tartrate when relatively gentle ion source conditions are used.
Figure 4.
ESI mass spectra obtained from aqueous solutions containing 10 μM ubiquitin with 1.0 mM NaCI and (a) no additional additive, (b) 25 mM ammonium tartrate, or (c) 25 mM ammonium citrate.
In solution, neither citrate nor L-tartrate bind strongly to sodium ions (Kd ~ 1.6 and 2.0 M, respectively, for fully sodiated forms). This indicates that neither of these ions lowers the extent of sodium ion adduction to ubiquitin ions by sequestering Na+ in solution by chelation. The pH of ESI droplets can decrease as solvent evaporation occurs,44 and the stability of the different forms of these anions depends on solvation. Previous results indicate that the extent of sodium ion and acid molecule adduction best correlates to the PA of the singly deprotonated form of the anions.34 The PA and gas-phase basicity of the singly deprotonated citric or L-tartaric acid have not been previously reported, so these values were determined using computational chemistry.
The lowest energy structures of the neutral and singly deprotonated form of citric and L-tartaric acid are shown in Figure 5. Although only the structure of lowest energy for neutral citric and L-tartaric acid are shown, several other structures were found to be energetically competitive, and are shown in Figure S-3 and S-4, respectively. The most favorable deprotonation site on citric acid is at the central carboxylic acid (site 1), but deprotonation at the hydroxyl group (site 2) and terminal carboxylic acid (site 3) are only 2.2 and 3.2 kcal•mol−1 higher, respectively, in Gibbs free energy. Deprotonation of L-tartaric acid at the carboxylic acid (site 1) is energetically favored compared to deprotonation at the hydroxyl group (site 2), which is calculated to be 9.0 kcal•mol−1 higher in Gibbs free energy. From the computed 298 K enthalpies of the lowest energy structures, the PA values for singly deprotonated citric and L-tartaric acid were computed at the B3LYP/6–311++G** level of theory to be 303 and 312 kcal•mol−1, respectively, and the respective gas-phase basicity values were calculated to be 298 and 306 kcal•mol−1. These results indicate that the effectiveness of ammonium tartrate and ammonium citrate in desalting gaseous protein ions is likely a result of the low PA of these anions, and not their ability to sequester sodium ions in solution prior to ion formation.
Figure 5.
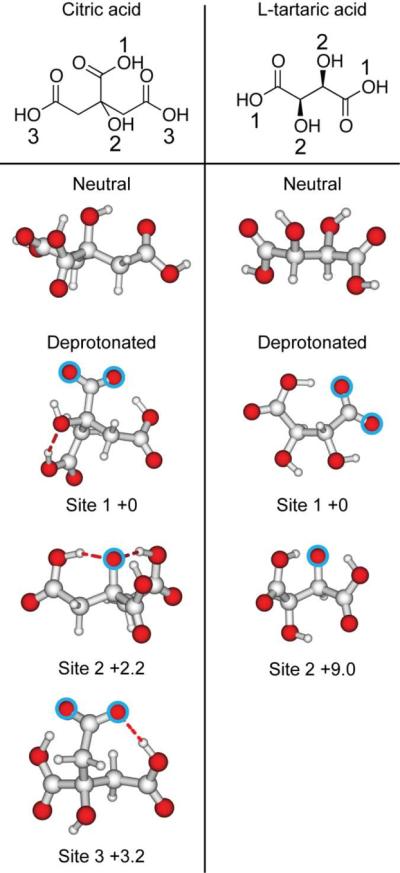
Calculated structures of lowest energy for citric acid and L-tartaric acid. Both the neutral and deprotonated forms are shown. The deprotonation site is indicated with a circle and relative Gibbs free energies are reported in kcal•mol−1.
Extensive adduction of citric and L-tartaric acid occurs under the relatively gentle ion source conditions used in these experiments. Results presented here and from earlier studies40 show that the extent of molecular adduction of acids to proteins can depend on ESI source conditions, and activation of these adducts through gas-phase collisions results in loss of the molecular acid.40 Repeating these experiments using a Thermo LTQ-orbitrap mass spectrometer with more energetic source conditions (300 °C capillary temperature) results in a spectrum with a similar number of sodium adducts, but no citric or L-tartaric acid molecules attached (Figure S-5). This indicates that the extent of molecular adduction, but not the amount of sodium ion adduction, observed in these experiments depends strongly on the extent to which the ions are activated in the ESI source or subsequently prior to mass analysis.
Conclusions
Sodium ion adduction to protein ions formed by ESI can adversely affect mass spectrometry measurements by reducing the overall ionization efficiency and distributing the existing ion abundance into many different adducted forms. Addition of some ammonium salts can reduce the amount of nonspecific sodium adduction to gaseous protein ions and significantly improve the ion abundances for proteins and protein complex ions formed by ESI. The effectiveness of these additives depends predominantly on the acidity of the anion. Ammonium bromide is particularly effective at lowering sodium adduction to gaseous protein ions without acid molecule adduction under relatively gentle source conditions. Anions, such as I− and SbF6−, that have lower PA values than Br− are also effective at reducing the extent of nonspecific sodium ion adduction, but attachment of the acid molecules can also occur. However, these molecular adducts can be readily dissociated from the protein ions by using harsher source conditions or by collisionally activating the ions after introduction into the mass spectrometer.
This method of desalting protein ions in native ESI is simple, very effective, requires no instrumental or other modifications, and can significantly increase the absolute abundances of the fully protonated forms of the molecular ions, which should result in improved detection limits, more accurate mass measurements, and improved tandem MS sensitivity. The method may be applicable to reducing interferences from nonspecific binding of many other metal ions as well. It may also be useful for measuring binding constants of proteins with low metal ion affinities by reducing the extent of nonspecific adduction that can occur in ESI-MS. These additives may also be effective for desalting biomolecular ions formed by MALDI.
Supplementary Material
Acknowledgements
The authors thank the National Institutes of Health (Grant no: R01GM096097) for generous financial support, Dr. Samuel I. Merenbloom for helpful discussions, and Dr. Harry J. Sterling for preparation of the barnase and barstar proteins.
References
- (1).Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- (2).Robinson CV, Chung EW, Kragelund BB, Knudsen J, Aplin RT, Poulsen FM, Dobson CM. J. Am. Chem. Soc. 1996;118:8646–8653. [Google Scholar]
- (3).Ruotolo BT, Giles K, Campuzano I, Sandercock AM, Bateman RH, Robinson CV. Science. 2005;310:1658–1661. doi: 10.1126/science.1120177. [DOI] [PubMed] [Google Scholar]
- (4).Sterling HJ, Kintzer AF, Feld GK, Cassou CA, Krantz BA, Williams ER. J. Am. Soc. Mass Spectrom. 2012;23:191–200. doi: 10.1007/s13361-011-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Lazar IM, Ramsey RS, Sundberg S, Ramsey JM. Anal. Chem. 1999;71:3627–3631. doi: 10.1021/ac990373m. [DOI] [PubMed] [Google Scholar]
- (6).Froehlich T, Arnold GJ. Methods in Mol. Biol. 2011;790:141–164. doi: 10.1007/978-1-61779-319-6_11. [DOI] [PubMed] [Google Scholar]
- (7).Valaskovic GA, Kelleher NL, McLafferty FW. Science. 1996;273:1199–1202. doi: 10.1126/science.273.5279.1199. [DOI] [PubMed] [Google Scholar]
- (8).Valaskovic GA, Kelleher NL, Little DP, Aaserud DJ, McLafferty FW. Anal. Chem. 1995;67:3802–3805. doi: 10.1021/ac00116a030. [DOI] [PubMed] [Google Scholar]
- (9).Belov ME, Gorshkov MV, Udseth HR, Anderson GA, Smith RD. Anal. Chem. 2000;72:2271–2279. doi: 10.1021/ac991360b. [DOI] [PubMed] [Google Scholar]
- (10).Emmett MR, Caprioli RM. J. Am. Soc. Mass Spectrom. 1994;5:605–613. doi: 10.1016/1044-0305(94)85001-1. [DOI] [PubMed] [Google Scholar]
- (11).Cohen SL, Ferredamare AR, Burley SK, Chait BT. Protein Sci. 1995;4:1088–1099. doi: 10.1002/pro.5560040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Hernandez H, Robinson CV. Nat. Protoc. 2007;2:715–726. doi: 10.1038/nprot.2007.73. [DOI] [PubMed] [Google Scholar]
- (13).Sterling HJ, Batchelor JD, Wemmer DE, Williams ER. J. Am. Soc. Mass Spectrom. 2010;21:1045–1049. doi: 10.1016/j.jasms.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Iavarone AT, Udekwu OA, Williams ER. Anal. Chem. 2004;76:3944–3950. doi: 10.1021/ac049724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Pan P, Gunawardena HP, Xia Y, McLuckey SA. Anal. Chem. 2004;76:11655–1174. doi: 10.1021/ac035209k. [DOI] [PubMed] [Google Scholar]
- (16).Pan P, McLuckey SA. Anal. Chem. 2003;75:5468–55474. doi: 10.1021/ac034344u. [DOI] [PubMed] [Google Scholar]
- (17).Tang L, Kebarle P. Anal. Chem. 1993;65:3654–3668. [Google Scholar]
- (18).Wang GD, Cole RB. Anal. Chem. 1994;66:3702–3708. [Google Scholar]
- (19).Juraschek R, Dulcks T, Karas M. J. Am. Soc. Mass Spectrom. 1999;10:300–308. doi: 10.1016/S1044-0305(98)00157-3. [DOI] [PubMed] [Google Scholar]
- (20).Ikonomou MG, Blades AT, Kebarle P. Anal. Chem. 1990;62:957–967. [Google Scholar]
- (21).Mirza UA, Chait BT. Anal. Chem. 1994;66:2898–2904. doi: 10.1021/ac00090a017. [DOI] [PubMed] [Google Scholar]
- (22).Liu CL, Wu QY, Harms AC, Smith RD. Anal. Chem. 1996;68:3295–3299. doi: 10.1021/ac960286j. [DOI] [PubMed] [Google Scholar]
- (23).Dalluge JJ. Fresenius J. Anal. Chem. 2000;366:701–711. doi: 10.1007/s002160051564. [DOI] [PubMed] [Google Scholar]
- (24).Bauer KH, Knepper TP, Maes A, Schatz V, Voihsel M. J. Chromatogr. A. 1999;837:117–128. doi: 10.1016/s0021-9673(99)00048-5. [DOI] [PubMed] [Google Scholar]
- (25).Huber CG, Buchmeiser MR. Anal. Chem. 1998;70:5288–5295. doi: 10.1021/ac980791b. [DOI] [PubMed] [Google Scholar]
- (26).Jiang Y, Hofstadler SA. Anal. Biochem. 2003;316:50–57. doi: 10.1016/s0003-2697(03)00024-1. [DOI] [PubMed] [Google Scholar]
- (27).Hu P, Ye Q, Loo JA. Anal. Chem. 1994;66:4190–4194. doi: 10.1021/ac00095a013. [DOI] [PubMed] [Google Scholar]
- (28).Pan JX, Xu K, Yang XD, Choy WY, Konermann L. Anal. Chem. 2009;81:5008–5015. doi: 10.1021/ac900423x. [DOI] [PubMed] [Google Scholar]
- (29).Turner KB, Monti SA, Fabris D. J. Am. Chem. Soc. 2008;130:13353–13363. doi: 10.1021/ja8045734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zhu YF, Taranenko NI, Allman SL, Martin SA, Chen CH. Rapid Commun. Mass Spectrom. 1996;10:1591–1596. doi: 10.1002/(SICI)1097-0231(199602)10:3<383::AID-RCM485>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- (31).Currie GJ, Yates JR. J. Am. Soc. Mass Spectrom. 1993;4:955–963. doi: 10.1016/1044-0305(93)80022-Q. [DOI] [PubMed] [Google Scholar]
- (32).Asara JM, Allison J. J. Am. Soc. Mass Spectrom. 1999;10:35–44. doi: 10.1016/S1044-0305(98)00129-9. [DOI] [PubMed] [Google Scholar]
- (33).Kharlamova A, Prentice BM, Huang TY, McLuckey SA. Int. J. Mass Spectrom. 2011;300:158–166. [Google Scholar]
- (34).Merenbloom SI, Flick TG, Daly MP, Williams ER. J. Am. Soc. Mass Spectrom. 2011;22:1978–1990. doi: 10.1007/s13361-011-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Han LJ, Hyung SJ, Mayers JJS, Ruotolo BT. J. Am. Chem. Soc. 2011;133:11358–11367. doi: 10.1021/ja203527a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Freeke J, Robinson CV, Ruotolo BT. Int. J. Mass Spectrom. 2010;298:91–98. [Google Scholar]
- (37).Freeke J, Bush MF, Robinson CV, Ruotolo BT. Chem. Phys. Lett. 2012;524:1–9. [Google Scholar]
- (38).Flick TG, Merenbloom SI, Williams ER. J. Am. Soc. Mass Spectrom. 2011;22:1968–1977. doi: 10.1007/s13361-011-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Stephenson JL, McLuckey SA. Anal. Chem. 1997;69:281–285. doi: 10.1021/ac961119m. [DOI] [PubMed] [Google Scholar]
- (40).Flick TG, Merenbloom SI, Williams ER. Anal. Chem. 2011;83:2210–2214. doi: 10.1021/ac1031012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Robinson EW, Williams ER. J. Am. Soc. Mass Spectrom. 2005;16:1427–1437. doi: 10.1016/j.jasms.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Krishnaswamy SR, Williams ER, Kirsch JF. Protein Sci. 2006;15:1465–1475. doi: 10.1110/ps.062083406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Shao Y, et al. Phys. Chem. Chem. Phys. 2006;8:3172–3191. doi: 10.1039/b517914a. [DOI] [PubMed] [Google Scholar]
- (44).Gatlin CL, Tureček F. Anal. Chem. 1994;66:712–718. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.