Abstract
Hepatitis C virus (HCV) infection has been associated with reduced bone mineral density, but its association with fracture rates is unknown, particularly in the setting of human immunodeficiency virus (HIV) coinfection. Our objectives were to determine whether persons with HCV infection alone are at increased risk for hip fracture compared to uninfected individuals and to examine if the risk of hip fracture is higher among HCV/HIV-coinfected persons compared to those with HCV alone, those with HIV alone, and those uninfected with either virus. We conducted a cohort study in 36,950 HCV/HIV-coinfected, 276,901HCV-monoinfected, 95,827 HIV-monoinfected, and 3,110,904 HCV/HIV-uninfected persons within the U.S. Medicaid populations of California, Florida, New York, Ohio, and Pennsylvania (1999–2005). Incidence rates of hip fracture were lowest among uninfected persons (1.29 events/1000 person-years), increased with the presence of either HIV infection (1.95 events/1000 person-years) or HCV infection (2.69 events/1000 person-years), and were highest among HCV/HIV-coinfected individuals (3.06 events/1000 person-years). HCV/HIV coinfection was associated with an increased relative hazard (adjusted hazard ratio [95% confidence interval]) of hip fracture compared to HCV-monoinfected (1.38 [1.25–1.53]), HIV-monoinfected (females: 1.76 [1.44–2.16]; males: 1.36 [1.20–1.55]), and uninfected persons (females: 2.65 [2.21–3.17]; males: 2.20 [1.97–2.47]). HCV monoinfection was associated with an increased risk of hip fracture compared to uninfected individuals, and the relative increase was highest in the youngest age groups (females, 18–39 years: 3.56 [2.93–4.32]; males, 18–39 years: 2.40 [2.02–2.84]).
Conclusion
Among Medicaid enrollees, HCV/HIV coinfection was associated with increased rates of hip fracture compared to HCV-monoinfected, HIV-monoinfected, and HCV/HIV-uninfected persons. HCV-monoinfected patients had an increased risk of hip fracture compared to uninfected individuals.
Keywords: Hepatitis C virus, HCV, HIV, fracture, coinfection
INTRODUCTION
Hepatitis C virus (HCV) infection exerts its main effects on the liver, inducing inflammation that leads to progressive liver fibrosis and ultimately cirrhosis in about 20% of chronic infections (1). However, HCV infection can also affect organ systems outside of the liver (2), particularly the skeletal system (termed “hepatic osteodystrophy”). Cross-sectional studies have shown that HCV infection is associated with reduced bone mineral density (3–7). The mechanisms for HCV-induced reductions in bone mineral density remain unclear, but chronic inflammation and liver dysfunction in the setting of HCV-associated hepatic decompensation might contribute to hepatic osteodystrophy (4, 8–10).
Although chronic HCV infection is associated with reduced bone mineral density (3–7), no longitudinal study has been performed to evaluate incidence rates of fracture. In addition, since low bone mineral density is a recognized metabolic complication of human immunodeficiency virus (HIV) infection (11), HCV/HIV coinfection (6), and antiretroviral therapy (12), HCV/HIV coinfection might increase fracture risk beyond that of HIV or chronic HCV alone. Evaluating the risk of fracture associated with HCV infection and HCV/HIV coinfection is important since these conditions are prevalent worldwide and because fractures, particularly those at the hip, adversely affect survival, with an effect on mortality similar to that of cardiovascular disease (13). Further, hip fractures cause significant pain and disability and typically require an emergency department visit, hospitalization, surgery, and rehabilitation stay, resulting in substantial health care costs.
This study sought to determine whether the reduced bone mineral density that has been reported to be associated with HCV infection and HCV/HIV coinfection translates into clinically important increases in fracture risk. We first evaluated the incidence of hip fracture among patients with HCV infection alone compared to HCV- and HIV-uninfected persons. We hypothesized that the risk of hip fracture was higher among patients with HCV monoinfection compared to uninfected individuals. We then examined hip fracture incidence among HCV/HIV-coinfected patients compared to those with HCV alone, those with HIV alone, and those uninfected with either virus. Our rationale for evaluating these three comparisons was to allow a more complete understanding of the hip fracture risk associated with HCV/HIV coinfection. We hypothesized that dual infection further increased the fracture risk compared to HCV-monoinfected, HIV-monoinfected, and uninfected individuals.
METHODS
Study Design and Data Source
We performed a retrospective cohort study among HCV/HIV-coinfected, HCV-monoinfected, HIV-monoinfected, and HCV/HIV-uninfected persons within the Medicaid populations of California, Florida, New York, Ohio, and Pennsylvania from 1999 to 2005. The Medicaid program consists of state-run programs with joint federal and state funding for hospital, medical, and outpatient care and drug benefits for low-income and special-needs individuals (14). The states included in this study were selected because they represent five of the largest Medicaid programs in the U.S., comprising approximately 22 million active enrollees, or almost 40% of the U.S. Medicaid population (15, 16). Medicaid claims report demographic information, inpatient and outpatient medical diagnoses (recorded by using International Classification of Diseases, Ninth Revision, diagnosis codes), and medications dispensed. Death dates were ascertained using Centers for Medicare and Medicaid Services data supplemented with mortality information from the Social Security Administration Death Master File. Since 17% of Medicaid beneficiaries are co-enrolled in Medicare, we obtained Medicare data on dually-eligible persons (17). Prior analyses of the linked Medicaid and Medicare claims indicate that the data are of high quality (18). The study was approved by the University of Pennsylvania Institutional Review Board, and a data use agreement was obtained from the Centers for Medicare and Medicaid Services.
Study Patients
Patients aged 18 years or older with diagnoses of HCV and/or HIV infections were identified using previously validated algorithms (19–21). HCV-monoinfected patients were defined by: 1) a diagnosis of HCV infection, and 2) no diagnosis of HIV infection or prescriptions for antiretroviral medications. HCV/HIV-coinfected patients had: 1) a diagnosis of HCV infection, 2) a diagnosis of HIV infection, and 3) claims for antiretroviral medications on two separate occasions. We included only antiretroviral-treated HCV/HIV patients because current management guidelines recommend antiretroviral therapy in all HCV/HIV-coinfected patients regardless of CD4 T lymphocyte count (22). The overall number of antiretroviral-untreated HCV/HIV-coinfected patients was too small to permit evaluation. HIV-monoinfected patients had: 1) a diagnosis of HIV infection, 2) antiretroviral claims on two occasions, and 3) no diagnosis of HCV infection. HCV/HIV-uninfected patients had no HCV or HIV diagnoses and no antiretroviral prescriptions.
Patients were excluded if they had: 1) only one Medicaid claim (i.e., no follow-up), 2) a hip fracture diagnosed prior to start of follow-up (defined below), 3) diagnosis of hepatitis B virus infection (to isolate the effect of HCV infection), or 4) received standard or pegylated interferon (since this therapy affects bone mineral density (23)).
We matched each HCV-monoinfected and HCV/HIV-coinfected patient on age (within one year), sex, and state with up to ten randomly selected uninfected persons. Matching on these variables reduced to workable proportions the number of uninfected individuals for data analysis while maintaining balance between cohorts on these variables. Ninety-two percent of HCV-monoinfected patients were matched with 10 uninfected patients (minimum number of matches, 6). Ninety-three percent of HCV/HIV-coinfected patients were matched with 10 uninfected patients (minimum number of matches, 7). We did not match HCV/HIV-coinfected patients with HCV-monoinfected and HIV-monoinfected patients because the sample sizes of the two monoinfected cohorts were substantially smaller than the cohort of HCV/HIV-uninfected persons, thus making statistical analysis feasible.
The 90 days prior to the start of follow-up represented the baseline period for all cohorts, during which baseline comorbidities and therapies were determined. Follow-up for HCV-monoinfected, HCV/HIV-coinfected, and HIV-monoinfected patients began on the date of their initial HIV and/or HCV diagnosis. However, if the first claim in the database was an HIV and/or HCV diagnosis, follow-up began 90 days after their initial diagnosis. Follow-up for uninfected persons began 90 days after their initial claim. Follow-up continued until a hip fracture, death, or last claim before December 31, 2005.
Main Study Outcome
The primary outcome was a diagnosis of fracture of the proximal femur (hip). Diagnoses of hip fracture (Appendix A) from Centers for Medicare and Medicaid Services claims were found to be highly valid in a previous survey, with 94% of cases confirmed via medical records (24).
Data Collection
Demographic data collected included: age; sex; race; and state of residence. Diagnoses associated with osteoporosis or risk of falling (Table 1) were recorded prior to or at the start of follow-up. Hepatic decompensation was defined by a diagnosis of ascites, spontaneous bacterial peritonitis, and/or variceal hemorrhage (25). We also examined use of medications that impact bone metabolism (Table 1). Patients were considered exposed to a medication if a drug claim was recorded within 90 days prior to the start of follow-up.
Table 1.
Baseline characteristics of study patients, by cohort.
| Characteristic | HCV/HIV Coinfected (n=36,950) | HCV Monoinfected (n=276,901) | HIV Monoinfected (n=95,827) | HCV/HIV Uninfected Matched to HCV/HIV Coinfected Cohort* (n=366,829) | HCV/HIV Uninfected Matched to HCV Monoinfected Cohort‡ (n=2,744,075) |
|---|---|---|---|---|---|
|
| |||||
| Median age (yrs, IQR) | 42 (37 – 48) | 47 (40 – 56) | 39 (33 – 46) | 42 (37 – 48) | 48 (40 – 56) |
|
| |||||
| Female sex (no., %) | 10,820 (29.3%) | 128,453 (46.4%) | 35,313 (36.9%) | 107,607 (29.3%) | 1,275,098 (46.5%) |
|
| |||||
| Race/ethnicity (no., %) | |||||
| White | 10,266 (27.8%) | 127,626 (46.1%) | 26,146 (27.3%) | 141,100 (38.5%) | 1,110,107 (40.5%) |
| Black | 14,700 (39.8%) | 58,868 (21.3%) | 42,554 (44.4%) | 72,017 (19.6%) | 456,909 (16.7%) |
| Hispanic (no., %) | 2,939 (8.0%) | 38,950 (14.1%) | 8,100 (8.5%) | 61,606 (16.8%) | 476,099 (17.4%) |
| Other | 9,045 (24.5%) | 51,457 (18.6%) | 19,027 (19.9%) | 92,106 (25.1%) | 700,960 (25.5%) |
|
| |||||
| State (no., %) | |||||
| California | 7,872 (21.3%) | 117,495 (42.4%) | 21,901 (22.9%) | 78,213 (21.3%) | 1,165,817 (42.5%) |
| Florida | 6,261 (16.9%) | 32,549 (11.8%) | 25,688 (26.8%) | 62,073 (16.9%) | 320,888 (11.7%) |
| New York | 20,306 (55.0%) | 86,487 (31.2%) | 40,609 (42.4%) | 201,584 (55.0%) | 857,488 (31.2%) |
| Ohio | 1,003 (2.7%) | 20,673 (7.5%) | 4,066 (4.2%) | 9,957 (2.7%) | 204,459 (7.5%) |
| Pennsylvania | 1,508 (4.1%) | 19,697 (7.1%) | 3,563 (3.7%) | 15,002 (4.1%) | 195,423 (7.1%) |
|
| |||||
| Incident hip fractures (no., %) | 643 (1.7%) | 3,943 (1.4%) | 795 (0.8%) | 1,653 (0.5%) | 31,352 (1.1%) |
|
| |||||
| Median follow-up (years, IQR) | 5.2 (2.9 – 6.7) | 2.3 (0.9 – 4.2) | 3.7 (1.6 – 6.4) | 2.2 (0.7 – 5.3) | 2.7 (0.9 – 6.0) |
|
| |||||
| Total follow-up time (person-years) | 171,217 | 737,759 | 358,898 | 1,068,634 | 8,849,719 |
|
| |||||
| Baseline health condition (no., %)† | |||||
| Alcoholism | 3,747 (10.1%) | 32,076 (11.6%) | 4,414 (4.6%) | 16,350 (4.5%) | 72,476 (2.6%) |
| Asthma or COPD | 3,254 (8.8%) | 34,915 (12.6%) | 6,462 (6.7%) | 16,846 (4.6%) | 173,662 (6.3%) |
| Cancer | 1,106 (3.0%) | 12,575 (4.5%) | 3,619 (3.8%) | 6,755 (1.8%) | 82,448 (3.0%) |
| Chronic kidney disease | 2,081 (5.6%) | 30,903 (11.2%) | 4,187 (4.4%) | 9,214 (2.5%) | 87,679 (3.2%) |
| Dementia | 609 (1.6%) | 6,032 (2.2%) | 1,390 (1.5%) | 1,962 (0.5%) | 35,791 (1.3%) |
| Diabetes mellitus | 2,007 (5.4%) | 46,350 (16.7%) | 3,997 (4.2%) | 23,840 (6.5%) | 260,727 (9.5%) |
| Congestive heart failure | 804 (2.2%) | 18,577 (6.7%) | 1,795 (1.9%) | 6,257 (1.7%) | 88,884 (3.2%) |
| Hepatic decompensation | 366 (1.0%) | 12,176 (4.4%) | 483 (0.5%) | 1,195 (0.3%) | 10,404 (0.4%) |
| Myocardial infarction | 875 (2.4%) | 22,418 (8.1%) | 1,963 (2.0%) | 12,124 (3.3%) | 154,313 (5.6%) |
| Peptic ulcer disease | 410 (1.1%) | 6,861 (2.5%) | 863 (0.9%) | 3,001 (0.8%) | 29,726 (1.1%) |
| Peripheral vascular disease | 346 (0.9%) | 9,068 (3.3%) | 635 (0.7%) | 2,904 (0.8%) | 50,622 (1.8%) |
| Rheumatoid arthritis/non-specific arthropathy | 604 (1.6%) | 12,308 (4.4%) | 1,017 (1.1%) | 5,086 (1.4%) | 62,344 (2.3%) |
| Seizure disorder | 1,096 (3.0%) | 11,484 (4.1%) | 2,221 (2.3%) | 7,424 (2.0%) | 53,981 (2.0%) |
| Stroke | 599 (1.6%) | 10,695 (3.9%) | 1,499 (1.6%) | 5,306 (1.4%) | 78,788 (2.9%) |
|
| |||||
| Baseline medication use (no., %)^ | |||||
| Anxiolytic | 4,050 (11.0%) | 33,220 (12.0%) | 7,346 (7.7%) | 15,970 (4.4%) | 129,691 (4.7%) |
| Antidepressant | 9,801 (26.5%) | 71,173 (25.7) | 18,734 (19.5%) | 33,672 (9.2%) | 275421 (10.0%) |
| Anticonvulsant/gabapentin | 4,697 (12.7%) | 42,979 (15.5%) | 9,790 (10.2%) | 21,762 (5.9%) | 165,637 (6.0%) |
| Antiparkinsonian | 532 (1.4%) | 7,278 (2.6%) | 1,115 (1.2%) | 6,479 (1.8%) | 46,878 (1.7%) |
| Antipsychotic | 3,344 (9.1%) | 33,558 (12.1%) | 7,212 (7.5%) | 20,707 (5.6%) | 143,678 (5.2%) |
| Calcium supplementation | 433 (1.2%) | 6,009 (2.2%) | 738 (0.8%) | 1,683 (0.5%) | 26,383 (1.0%) |
| Corticosteroid (inhaled) | 1,208 (3.3%) | 11,148 (4.0%) | 2,473 (2.6%) | 5,713 (1.6%) | 49,886 (1.8%) |
| Corticosteroids (oral) | 1,208 (3.3%) | 10,810 (3.9%) | 3,641 (3.8%) | 6,740 (1.8%) | 59,219 (2.2%) |
| Hormone therapy (estrogen) | 594 (1.6%) | 8,335 (3.0%) | 1,220 (1.3%) | 2,864 (0.8%) | 57,296 (2.1%) |
| NSAID/aspirin | 6,442 (17.4%) | 39,704 (14.3%) | 13,264 (13.8%) | 34,920 (9.5%) | 270,594 (9.9%) |
| Proton pump inhibitor | 2,991 (8.1%) | 40,131 (14.5%) | 6,906 (7.2%) | 14,791 (4.0%) | 126,757 (4.6%) |
| Statin | 545 (1.5%) | 15,003 (5.4%) | 2,495 (2.6%) | 13,013 (3.5%) | 140,876 (5.1%) |
| Tenofovir | 599 (1.6%) | 18 (0.0%) | 3,538 (3.7%) | 0 (0.0%) | 0 (0.0%) |
| Testosterone | 909 (2.5%) | 600 (0.2%) | 2,707 (2.8%) | 268 (0.1%) | 2,371 (0.1%) |
| Thiazide diuretic | 868 (2.3%) | 17,730 (6.4%) | 2,030 (2.1%) | 9,492 (2.6%) | 103,024 (3.8%) |
| Thyroxine | 320 (0.9%) | 9,543 (3.4%) | 813 (0.8%) | 4,633 (1.3%) | 56,915 (2.1%) |
Abbreviations: IQR, interquartile range; COPD, chronic obstructive pulmonary disease; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NSAID, nonsteroidal anti-inflammatory drug
Up to 10 HCV/HIV-uninfected patients were matched on age, sex, and state with each HCV/HIV-coinfected subject
Up to 10 HCV/HIV-uninfected patients were matched on age, sex, and state with each HCV-monoinfected subject
Cohorts were also evaluated for celiac disease, Cushing’s disease, hyperparathyroidism, inflammatory bowel disease, obesity, osteomalacia, Paget’s disease, and systemic lupus erythematosus, but the prevalence was <2% within each group. Please see Appendix C for the prevalence of these additional health conditions in each cohort.
Cohorts were also evaluated for use of bisphosphonates, calcitonin, cholestyramine, and vitamin D, but the prevalence of use of these medications was <2% within each group. Please see Appendix C for the prevalence of baseline use of these medications in each cohort.
Statistical Analysis
The number of fractures and person-time of observation was determined for each cohort. We estimated incidence rates (in events/1000 person-years) and 95% confidence intervals (CIs) of hip fractures for each cohort using direct standardization by age, sex, and state to the characteristics of uninfected persons (26).
The primary analysis examined the time to fracture. Rates of incident fractures were compared among the following cohorts: 1) HCV-monoinfected and uninfected; 2) HCV/HIV-coinfected and uninfected; 3) HCV/HIV-coinfected and HIV-monoinfected (to examine the effect of HCV infection on hip fracture risk in the setting of treated HIV infection); and 4) HCV/HIV-coinfected and HCV-monoinfected (to examine the effect of treated HIV on hip fracture risk in the setting of HCV infection). Major differences in the prevalence of medical comorbidities and baseline usage of medications associated with osteoporosis were observed between the cohorts (Table 1). Because of the many potential confounding variables relative to the number of hip fractures, we used propensity scores to balance, or control for, these confounders between the groups (27, 28). Separate propensity score models were developed using logistic regression for each of the four comparisons of interest (HCV-monoinfected versus uninfected; HCV/HIV-coinfected versus uninfected; HCV/HIV-coinfected versus HIV-monoinfected; and HCV/HIV-coinfected versus HCV-monoinfected). All variables in Table 1 were included in propensity score models, except for age, sex, and state (which were included as covariates in final multivariable models) and hepatic decompensation (since this condition might be in the causal pathway to fracture). Cox proportional hazards analyses yielded hazard ratios (HRs) and 95% CIs of hip fracture, stratified by quintiles of propensity score and state (29). We examined interactions between cohort, sex, and age groups (18–39; 40–49; 50–59; 60–69; ≥ 70 years).
We next estimated cumulative incidences of hip fracture over the maximum period of observation (6.75 years) among HCV-monoinfected and HCV/HIV-coinfected patients compared to uninfected patients matched to have characteristics similar to each of the HCV-infected cohorts using propensity score-weighted Cox regression models (30). Details appear in Appendix B.
Since cross-sectional studies suggest that HCV-infected patients with hepatic decompensation have lower bone mineral density than HCV-infected persons with normal liver function (3, 4), we conducted an exploratory analysis to evaluate whether HCV-monoinfected patients with hepatic decompensation had an increased risk of hip fracture compared to similar monoinfected patients without this diagnosis. Propensity score models estimated the probability of decompensation. Each HCV-monoinfected patient with hepatic decompensation was matched 1:1 on propensity score (nearest-neighbor matching within 0.02 of the propensity score), sex, and state to a monoinfected patient without decompensation (31). Cox models evaluated relative hazards of fracture associated with hepatic decompensation.
Finally, given that HCV/HIV-coinfected patients may have had their HCV and HIV diagnoses recorded on different dates and since the earlier diagnosis date represented the start of follow-up, we conducted sensitivity analyses to evaluate HRs of fractures using the later date of these patients’ diagnoses as the start of follow-up. We also conducted a sensitivity analysis to examine the effect of potentially unmeasured confounders on the relative hazard of hip fracture (32).
We estimated that 5,632 HCV-infected patients would provide 90% power to detect a relative hazard of hip fracture of 1.5 between HCV-infected and uninfected persons, assuming a two-tailed alpha of 0.05 and a 1% rate of fracture among the uninfected cohort (33). Data were analyzed using SAS 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
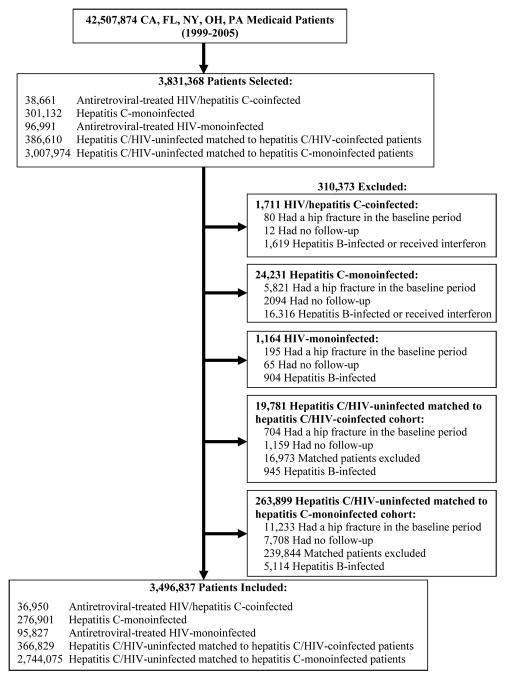
Among 42,507,874 patients enrolled in the five states between 1999 and 2005 (Figure 1), we identified 301,132 (0.7%) HCV-monoinfected, 38,661 (0.09%) HCV/HIV-coinfected, and 96,991 (0.2%) HIV-monoinfected individuals. We matched 3,007,974 HCV/HIV-uninfected persons on age, sex, and state to the HCV-monoinfected cohort and similarly matched 386,610 uninfected individuals to the coinfected cohort. After exclusions (Figure 1), the final sample included 276,901 HCV-monoinfected; 36,950 HCV/HIV-coinfected; 95,827 HIV-monoinfected; 2,744,075 uninfected patients matched to the HCV-monoinfected cohort; and 366,829 uninfected patients matched to the coinfected cohort.
Figure 1.
Selection of hepatitis C virus (HCV)/human immunodeficiency virus (HIV)-coinfected, HCV-monoinfected, HIV-monoinfected, and HCV/HIV-uninfected subjects in the study.
Table 1 summarizes the demographic and clinical characteristics of the infected cohorts and the characteristics of age-, sex-, and state-matched uninfected patients. HCV-monoinfected patients were older, more frequently female, and more commonly of white race than HCV/HIV-coinfected and HIV-monoinfected patients. In addition, compared to coinfected and HIV-monoinfected patients, HCV-monoinfected patients more commonly had medical diagnoses that had known associations with osteoporosis or risk of falling, including alcoholism, asthma, cardiovascular disease, diabetes mellitus, chronic kidney disease, hyperparathyroidism, and rheumatoid arthritis. HCV-monoinfected patients also more frequently received medications associated with osteoporosis, particularly corticosteroids and proton pump inhibitors.
Unadjusted Incidence Rates of Hip Fracture
The median follow-up time ranged from 2.3 years for HCV-monoinfected patients to 5.2 years for HCV/HIV-coinfected patients (Table 1). Unadjusted incidence rates of hip fractures were lowest among uninfected persons (1.29 [95% CI, 0.85–1.94] events/1000 person-years), increased with the presence of either HIV (1.95 [95% CI, 1.33–2.85] events/1000 person-years) or HCV infection (2.69 [95% CI, 1.96–3.70] events/1000 person-years), and were highest among HCV/HIV-coinfected patients (3.06 [95% CI, 1.96–4.80] events/1000 person-years).
Risk of Hip Fracture in HCV-Monoinfected Patients Compared to Uninfected Persons
After adjusting for age, sex, state, propensity score, and the interaction between sex and age, HCV monoinfection was associated with an increased rate of hip fracture compared to uninfected persons (adjusted HR, 1.47; 95% CI, 1.42–1.52). However, relative hazards of hip fractures associated with HCV monoinfection varied by sex and age groups (p<0.001 for all interactions). Below the age of 70 years, HCV infection was associated with an increased relative hazard of hip fracture compared to uninfected individuals, regardless of sex (Table 2). For both women and men, hazard ratios were highest for persons 18–39 years and lowest for those aged 60–69 years. Among persons 70 years and older, no association between HCV infection and hip fracture was observed. Relative hazards of hip fracture were higher for women than men among those below age 50 years.
Table 2.
Estimated hip fracture rates and relative hazards of hip fracture (with 95% confidence intervals) for hepatitis C virus-monoinfected patients compared to those uninfected with either human immunodeficiency virus or hepatitis C virus infections, by sex and age group.
| Sex, Age Group (Years) | HCV Status | No. Patients | Hip Fracture Rate Per 1,000 | Additional Hip Fractures Per 1,000 HCV Patients | Adjusted Hazard Ratio of Hip Fracture (95% CI)* |
|---|---|---|---|---|---|
|
| |||||
| Females | |||||
| 18 – 39 | HCV-Infected | 33,060 | 10.8 | 6.2 | 3.56 (2.93 – 4.32) |
| Uninfected | 330,161 | 4.6 | Ref | ||
|
| |||||
| 40 – 49 | HCV-Infected | 41,736 | 21.5 | 8.0 | 2.04 (1.83 – 2.28) |
| Uninfected | 409,461 | 13.5 | Ref | ||
|
| |||||
| 50 – 59 | HCV-Infected | 25,575 | 39.1 | 12.0 | 1.56 (1.40 – 1.73) |
| Uninfected | 256,917 | 27.1 | Ref | ||
|
| |||||
| 60 – 69 | HCV-Infected | 14,457 | 64.9 | 18.2 | 1.41 (1.27 – 1.57) |
| Uninfected | 144,787 | 46.7 | Ref | ||
|
| |||||
| ≥70 | HCV-Infected | 13,625 | 128.2 | 15.9 | 0.96 (0.89 – 1.04) |
| Uninfected | 133,772 | 112.3 | Ref | ||
|
| |||||
| Males | |||||
| 18 – 39 | HCV-Infected | 28,909 | 16.2 | 7.8 | 2.40 (2.02 – 2.84) |
| Uninfected | 284,389 | 8.3 | Ref | ||
|
| |||||
| 40 – 49 | HCV-Infected | 55,462 | 26.2 | 10.6 | 1.93 (1.76 – 2.11) |
| Uninfected | 519,230 | 15.7 | Ref | ||
|
| |||||
| 50 – 59 | HCV-Infected | 39,867 | 37.7 | 12.7 | 1.60 (1.46 – 1.76) |
| Uninfected | 417,434 | 24.9 | Ref | ||
|
| |||||
| 60 – 69 | HCV-Infected | 15,231 | 60.8 | 25.1 | 1.70 (1.52 – 1.91) |
| Uninfected | 158,546 | 35.7 | Ref | ||
|
| |||||
| ≥70 | HCV-Infected | 8,979 | 89.8 | 19.3 | 1.10 (0.98 – 1.24) |
| Uninfected | 89,378 | 70.6 | Ref | ||
CI=confidence interval; HCV, hepatitis C virus
Final model included terms for cohort; age; sex; state; propensity score; and interactions between cohort and sex, cohort and age, age and sex, and age, sex, and cohort.
Cumulative incidences of hip fractures for HCV-monoinfected and uninfected persons, grouped by sex and age, are shown in Table 2. Among women, HCV monoinfection was associated with 6.2 additional hip fractures per 1000 for the youngest age group (18–39 years) and 15.9 additional hip fractures per 1000 for the oldest group (≥70 years). Among men, HCV monoinfection resulted in an estimated 7.8 additional hip fractures per 1000 for the youngest age group (18–39 years) and 19.3 additional fractures per 1000 for the oldest group (≥70 years).
Among HCV-monoinfected patients, hepatic decompensation was associated with an increased rate of hip fractures (HR, 1.22; 1.02–1.46).
Risk of Hip Fracture in HCV/HIV-Coinfected Patients Compared to Uninfected Persons
Hazard ratios of hip fractures associated with HCV/HIV coinfection varied by sex in comparisons with uninfected persons (p<0.001 for interaction) but not by age group. Antiretroviral-treated HCV/HIV-coinfected patients had higher rates of hip fracture compared to persons uninfected with either virus (Table 3). Hazard ratios for fracture appeared higher among women than men. Similar results were observed when the later date of HCV or HIV diagnosis was used as the start of follow-up.
Table 3.
Estimated hip fracture rates and relative hazards of hip fracture (with 95% confidence intervals) for antiretroviral-treated human immunodeficiency virus (HIV)/hepatitis C virus-coinfected patients compared to persons uninfected with either virus, by sex and age group.
| Sex, Age Group (Years)* | HCV Status | No. Patients | Hip Fracture Rate Per 1,000 | Additional Hip Fractures Per 1,000 HCV/HIV Patients | Adjusted Hazard Ratio of Hip Fracture (95% CI)‡ |
|---|---|---|---|---|---|
|
| |||||
| Females | |||||
| 18 – 39 | HCV/HIV-Coinfected | 4,310 | 8.9 | 4.9 | 2.77 (1.76 – 4.37) |
| Uninfected | 43,175 | 4.0 | Ref | ||
|
| |||||
| 40 – 49 | HCV/HIV-Coinfected | 5,026 | 28.0 | 17.3 | 2.89 (2.26 – 3.71) |
| Uninfected | 49,491 | 10.7 | Ref | ||
|
| |||||
| ≥50 | HCV/HIV-Coinfected | 1,484 | 52.1 | 30.5 | 2.27 (1.65 – 3.11) |
| Uninfected | 14,941 | 21.6 | Ref | ||
|
| |||||
| Males | |||||
| 18 – 39 | HCV/HIV-Coinfected | 8,505 | 17.4 | 9.1 | 2.28 (1.80 – 2.89) |
| Uninfected | 84,386 | 8.3 | Ref | ||
|
| |||||
| 40 – 49 | HCV/HIV-Coinfected | 12,619 | 28.3 | 14.3 | 2.27 (1.94 – 2.67) |
| Uninfected | 124,650 | 14.0 | Ref | ||
|
| |||||
| ≥50 | HCV/HIV-Coinfected | 5,006 | 47.6 | 25.3 | 2.06 (1.70 – 2.50) |
| Uninfected | 50,186 | 22.3 | Ref | ||
CI=confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus
Due to the small number of HCV/HIV-coinfected patients above the age of 60 years, we were unable to evaluate comparisons in the 60–69 years and ≥70 years strata and have limited evaluation to the ≥50 year age range.
Final models for each comparison included terms for cohort; age; sex; state; propensity score; and interactions between cohort and sex, age and sex.
Cumulative incidences of hip fractures for HCV/HIV-coinfected and uninfected persons, grouped by sex and age, are shown in Table 3. Among women, coinfection was associated with 4.9 additional hip fractures per 1000 for the youngest age group (18–39 years) and 30.5 additional hip fractures per 1000 for the oldest group (≥50 years). Among men, coinfection resulted in an estimated 9.1 additional hip fractures per 1000 for the youngest age group (18–39 years) and 25.3 additional fractures per 1000 for the oldest group (≥50 years).
Risk of Hip Fracture in HCV/HIV-Coinfected Compared to HCV-Monoinfected and HIV-Monoinfected Persons
Rates of hip fractures associated with HCV/HIV coinfection varied by sex in comparisons with HIV-monoinfected (p<0.001 for interaction). Antiretroviral-treated HCV/HIV-coinfected patients had higher rates of hip fracture compared to antiretroviral-treated HIV-monoinfected patients (females: HR, 1.76 [95% CI, 1.44–2.16]; males: 1.36 [95% CI, 1.20–1.55]).
Hazard ratios of hip fractures were not different by age and sex for comparisons between HCV/HIV-coinfected and HCV-monoinfected patients. HCV/HIV coinfection was associated with a higher rate of hip fracture compared to HCV-monoinfected patients (adjusted HR, 1.38; 95% CI, 1.25–1.53).
Similar results were observed when the later date of HCV or HIV diagnosis was used as the start of follow-up.
Potential Impact of Unmeasured Confounders
Sensitivity analyses to evaluate the effect of unmeasured confounding determined that an unmeasured confounder would need to have >20% prevalence and be 3.5-times more likely to be present among HCV-uninfected than infected persons to make the results non-significant.
DISCUSSION
Among U.S. Medicaid enrollees, antiretroviral-treated HCV/HIV-coinfected patients experienced increased rates of hip fractures compared to HCV-monoinfected, antiretroviral-treated HIV-monoinfected, and HCV/HIV-uninfected persons. HCV monoinfection was associated with an increased rate of hip fractures compared to uninfected persons below age 70 years, and the relative increase was highest among younger persons. Additionally, HCV-monoinfected patients with hepatic decompensation had higher rates of hip fractures compared to those without decompensation. Finally, hazard ratios of hip fractures were higher among women than men.
The mechanisms for the association between HCV infection and hip fracture remain unclear. Elevated serum levels of inflammatory cytokines associated with chronic HCV infection (e.g., tumor necrosis factor-alpha, interleukin-1, interleukin-6) could increase receptor activator of nuclear factor kappa-B ligand (RANKL), promoting osteoclastogenesis and increasing bone resorption (4, 8). Moreover, tumor necrosis factor-alpha inhibits osteoblast differentiation, inhibits collagen synthesis in osteoblasts, and promotes osteoblast apoptosis (8). The net effect of this inflammatory cascade is reduced bone mineral density and increased fracture risk. Further, compensated cirrhosis and liver synthetic dysfunction in the setting of hepatic decompensation could increase the risk of hypogonadism (10), reduce hepatic hydroxylation of vitamin D (9), and impair hepatic production of insulin-like growth factor-1, which promotes bone formation (7), and these conditions could further reduce bone density and contribute to fractures. Our finding that hepatic decompensation was associated with increased rates of hip fractures suggests that liver dysfunction might also contribute to metabolic bone disease (3, 4). Finally, illicit drug use, alcohol abuse, poor nutrition, and fragility among HCV-infected patients might also contribute to increased fracture risk from trauma, irrespective of the impact of HCV infection on bone mineral density. Additional research is needed to determine the mechanisms by which chronic HCV and HCV/HIV coinfection affect bone mineral density and fracture incidence.
The higher fracture rates observed among HCV/HIV-coinfected persons compared to HCV-monoinfected individuals might be due to the additive effects of HIV infection and antiretroviral therapy on bone mineral density. Uncontrolled HIV viremia upregulates cytokines that reduce osteoblast activity, promote osteoblast apoptosis, and activate osteoclasts to increase bone resorption (34, 35). HIV infection might increase the risk of other osteoporosis risk factors, such as poor nutrition, hypogonadism, lipoatrophy, and decreased muscle mass (36, 37). Moreover, initiation of antiretroviral therapy is associated with significant short-term bone loss in the range of 2–6% over 1–2 years (38). In particular, tenofovir use has been linked to decreased bone mineral density among HIV-infected adults (39), possibly because of phosphate wasting from tenofovir-induced proximal renal tubule dysfunction (40).
Comparisons between HCV-monoinfected and uninfected individuals demonstrated that hazard ratios were highest among younger persons and decreased with higher age. In terms of absolute differences, however, the increase was highest for older patients because of high absolute fracture rates in this group. These results are to be expected because many other factors contribute to fracture risk with advancing age and likely overwhelm the impact of HCV infection. The mechanism for the increased relative hazards of hip fracture at the younger ages remains unclear. While this finding could reflect the role of chronic HCV-associated inflammation, it also suggests that trauma might be contributing to fractures among younger patients. The data did not permit determination of fractures associated with trauma.
We observed that the relative hazards of hip fracture were higher for women than men when comparing HCV-monoinfected and uninfected persons below age 50 years, HCV/HIV-coinfected and HIV-monoinfected patients, and coinfected and uninfected persons. The pathogenesis responsible for these differences is unclear. It is unknown if there are clinically significant differences by sex in serum levels of hepatitis-associated cytokines, markers of bone turnover, or other factors that maintain bone balance (e.g., insulin-like growth factor-1) among HCV-infected patients. Moreover, the effect of HCV infection, particularly in the setting of hepatic decompensation, on ovarian function and estrogen production in women is also unknown. Future studies of serum levels of cytokines, markers of bone turnover, insulin-like growth factor-1, and sex hormones might elucidate the mechanisms for low bone density and fracture in HCV-infected women.
Although higher rates of fracture associated with HCV monoinfection and HCV/HIV-coinfection have potential implications for clinical management, neither of these conditions is considered to be associated with increased fracture risk by osteoporosis guidelines (41). Our study suggests that HCV monoinfection and HCV/HIV coinfection are associated with an increased risk of fracture. Future studies are needed to determine the relations between dual-energy x-ray absorptiometry estimates of bone mineral density and fracture risks associated with HCV monoinfection and HCV/HIV coinfection, and to develop guidelines for the identification of patients at risk. Determination of the mechanisms of reduced bone mineral density due to HCV and HCV/HIV coinfection is also needed to identify potential interventions that might prevent bone loss and mitigate fracture risk in these populations.
Our study had several limitations. We lacked laboratory data on HCV and HIV infections as well as radiographic confirmation of fracture diagnoses. However, diagnoses of HCV and HIV infections were identified using previously validated algorithms (19–21). Further, hip fracture diagnoses in Medicaid were shown to have 94% positive predictive value (24).
Second, we observed major differences in the prevalence of medical comorbidities and usage of medications associated with osteoporosis between the cohorts. However, we addressed this by developing separate propensity score models for each of the four comparisons of interest in order to balance, or control for, potential confounding variables across the comparison groups.
Third, our analyses only accounted for baseline use of antiviral treatment for chronic HCV infection, tenofovir use, and pharmacologic therapies for osteoporosis. We did not censor follow-up at the time of receipt of these medications since use of these drugs was uncommon among the cohorts studied and because it is unclear if hip fracture risk is affected by some of these therapies (i.e., tenofovir, interferon, ribavirin).
Fourth, the median follow-up among the HCV/HIV-coinfected cohort was longer than that for the age-, sex-, and state-matched HCV/HIV-uninfected cohort. If our study design had called for two cohorts followed over time from the same starting age, this longer observation time would limit possible comparisons. However, both coinfected and uninfected cohorts included patients among all age groups, produced risk sets of adequate size for all combinations of hip fracture risk factors across age groups, and therefore covered the spectrum of risks experienced by young, middle-aged, and older patients.
Fifth, residual confounding by unmeasured factors is possible in observational studies. We did not have information on body mass index, smoking, alcohol, illicit drug use, and duration of HCV and HIV infections. We also could not assess whether fractures were specifically trauma-related. However, to negate the positive associations observed in our study, such confounders would have to be strongly associated with fracture and related to infection but not related to the many risk factors already included in our models.
Finally, the study sample consisted of U.S. Medicaid enrollees, potentially limiting the generalizability of our results. However, Medicaid is the largest source of care for patients with HIV infection in the U.S. and provides coverage to a large proportion of patients with HCV infection (42). In addition, the study cohorts are demographically similar to U.S. HCV and HIV populations (43, 44).
Our study had a number of strengths. It evaluates the association between HCV infection and hip fracture, including among HIV-infected persons in a large population. The study also examined the risk of hip fracture associated with HCV-induced hepatic decompensation. Further, propensity score methods enabled us to adjust for many potential confounding variables that have not been considered in prior fracture studies.
In conclusion, this study provides evidence that HCV and HCV/HIV coinfection are associated with increased rates of hip fractures. Future studies should evaluate mechanisms for the increased fracture risks in these populations.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Allergy and Infectious Diseases (research grants K01-AI070001 and U01-AI069467), National Institute of Diabetes and Digestive and Kidney Diseases (research grant K24-DK076808), Clinical and Translational Science Award from the National Institutes of Health (grant #UL1-RR024134), and a Penn FOCUS Medical Student Fellowship in Women’s Health (Bertha Dagan Berman Award).
Results withstood Centers for Medicare and Medicaid Services privacy review and were approved for publication.
List of Abbreviations
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
References
- 1.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439–1445. doi: 10.1053/jhep.2002.37191. [DOI] [PubMed] [Google Scholar]
- 3.Gallego-Rojo FJ, Gonzalez-Calvin JL, Munoz-Torres M, Mundi JL, Fernandez-Perez R, Rodrigo-Moreno D. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology. 1998;28:695–699. doi: 10.1002/hep.510280315. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Calvin JL, Gallego-Rojo F, Fernandez-Perez R, Casado-Caballero F, Ruiz-Escolano E, Olivares EG. Osteoporosis, mineral metabolism, and serum soluble tumor necrosis factor receptor p55 in viral cirrhosis. J Clin Endocrinol Metab. 2004;89:4325–4330. doi: 10.1210/jc.2004-0077. [DOI] [PubMed] [Google Scholar]
- 5.Leslie WD, Bernstein CN, Leboff MS. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941–966. doi: 10.1016/s0016-5085(03)01062-x. [DOI] [PubMed] [Google Scholar]
- 6.Lo Re V, 3rd, Guaraldi G, Leonard MB, Localio AR, Lin J, Orlando G, Zirilli L, et al. Viral hepatitis is associated with reduced bone mineral density in HIV-infected women but not men. AIDS. 2009;23:2191–2198. doi: 10.1097/QAD.0b013e32832ec258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouillard S, Lane NE. Hepatic osteodystrophy. Hepatology. 2001;33:301–307. doi: 10.1053/jhep.2001.20533. [DOI] [PubMed] [Google Scholar]
- 8.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, Lian JB, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 9.Hay JE. Bone disease in cholestatic liver disease. Gastroenterology. 1995;108:276–283. doi: 10.1016/0016-5085(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 10.Pignata S, Daniele B, Galati MG, Esposito G, Vallone P, Fiore F, Ricchi P, et al. Oestradiol and testosterone blood levels in patients with viral cirrhosis and hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1997;9:283–286. doi: 10.1097/00042737-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 11.McComsey GA, Tebas P, Shane E, Yin MT, Overton ET, Huang JS, Aldrovandi GM, et al. Bone Disease in HIV Infection: A Practical Review and Recommendations for HIV Care Providers. Clin Infect Dis. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 13.Mittalhenkle A, Gillen DL, Stehman-Breen CO. Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis. 2004;44:672–679. [PubMed] [Google Scholar]
- 14.Hennessy S, Carson JL, Ray WA, Strom BL. Medicaid databases. In: Strom BL, editor. Pharmacoepidemiology. 4. Chichester, UK: Wiley; 2005. [Google Scholar]
- 15.Centers for Medicare & Medicaid Services. [Accessed April 4, 2012];Medicaid Statistical Information System (MSIS) Tables. http://www.cms.gov/MedicaidDataSourcesGenInfo/MSIS/list.asp.
- 16.Centers for Medicare & Medicaid Services. [Accessed April 4, 2012];Medicaid Analytic eXtract (MAX) Validation Reports. http://www.cms.gov/MedicaidDataSourcesGenInfo/MVR/list.asp.
- 17.Medicare Payment Advisory Commission. Report to the Congress: New Approaches in Medicare. 2004. [Google Scholar]
- 18.Hennessy S, Leonard CE, Palumbo CM, Newcomb C, Bilker WB. Quality of Medicaid and Medicare data obtained through Centers for Medicare and Medicaid Services (CMS) Med Care. 2007;45:1216–1220. doi: 10.1097/MLR.0b013e318148435a. [DOI] [PubMed] [Google Scholar]
- 19.Keyes M, Andrews R, Mason ML. A methodology for building an AIDS research file using Medicaid claims and administrative data bases. J Acquir Immune Defic Syndr. 1991;4:1015–1024. [PubMed] [Google Scholar]
- 20.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 21.Markowitz JS, Gutterman EM, Hodes D, Klaskala W. Factors associated with the initiation of alpha-interferon treatment in Medicaid patients diagnosed with hepatitis C. J Viral Hepat. 2005;12:176–185. doi: 10.1111/j.1365-2893.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 22.Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, Telenti A, Gatell JM, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann WP, Kronenberger B, Bojunga J, Stamm B, Herrmann E, Bucker A, Mihm U, et al. Prospective study of bone mineral density and metabolism in patients with chronic hepatitis C during pegylated interferon alpha and ribavirin therapy. J Viral Hepat. 2008;15:790–796. doi: 10.1111/j.1365-2893.2008.01038.x. [DOI] [PubMed] [Google Scholar]
- 24.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 25.Lo Re V, 3rd, Lim JK, Goetz MB, Tate J, Bathulapalli H, Klein MB, Rimland D, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf. 2011;20:689–699. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little RJA. Direct standardization: A tool for teaching linear models for unbalanced data. Am Stat. 1982;36:38–43. [Google Scholar]
- 27.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137:693–695. doi: 10.7326/0003-4819-137-8-200210150-00015. [DOI] [PubMed] [Google Scholar]
- 28.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Collett D. Modeling survival data in medical research. New York: Chapman and Hall; 2003. [Google Scholar]
- 30.Lee BK, Lessler J, Stuart EA. Weight trimming and propensity score weighting. PLoS One. 2011;6:e18174. doi: 10.1371/journal.pone.0018174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39:33–38. [Google Scholar]
- 32.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54:948–963. [PubMed] [Google Scholar]
- 33.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem. 2003;278:48251–48258. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 35.Gibellini D, De Crignis E, Ponti C, Cimatti L, Borderi M, Tschon M, Giardino R, et al. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFalpha activation. J Med Virol. 2008;80:1507–1514. doi: 10.1002/jmv.21266. [DOI] [PubMed] [Google Scholar]
- 36.Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, Hoffmann M, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 37.Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, Yarasheski KE. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14:F63–67. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 39.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 40.Fux CA, Christen A, Zgraggen S, Mohaupt MG, Furrer H. Effect of tenofovir on renal glomerular and tubular function. AIDS. 2007;21:1483–1485. doi: 10.1097/QAD.0b013e328216f15b. [DOI] [PubMed] [Google Scholar]
- 41.National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. [Google Scholar]
- 42.Fleishman JA, Gebo KA, Reilly ED, Conviser R, Christopher Mathews W, Todd Korthuis P, Hellinger J, et al. Hospital and outpatient health services utilization among HIV-infected adults in care 2000–2002. Med Care. 2005;43:III40–52. doi: 10.1097/01.mlr.0000175621.65005.c6. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 44.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.