Abstract
Background
The low level of response (LR) or sensitivity to alcohol is genetically influenced and predicts heavy drinking and alcohol problems. Functional magnetic resonance imaging (fMRI) studies using cognitive tasks suggest that subjects with a low LR process cognitive information differently after placebo and alcohol than those with a high LR, but no studies have evaluated if similar LR group differences are seen during an emotional processing task.
Methods
fMRI data were gathered from 116 non-alcoholic subjects (60 women) following oral placebo or ~0.7 ml/kg of ethanol while performing a modified emotional faces processing task. These included 58 low- and high-LR pairs matched on demography and aspects of substance use.
Results
Blood alcohol levels and task performance were similar across LR groups, but low LR subjects consumed ~ 0.8 drinks more per occasion. Thirteen brain regions (mostly the middle and inferior frontal gyri, cingulate, and insula) showed significant LR group or LR by placebo/alcohol condition interactions for emotional (mostly happy) faces relative to non-face trials. Low LR subjects generally showed decreasing BOLD response contrasts across placebo to alcohol, while high LR showed increasing contrasts from placebo to alcohol, even after controlling for drinking quantities and alcohol-related changes in cerebral blood flow.
Conclusions
Thus, LR group fMRI differences are as prominent during an emotional face task as during cognitive paradigms. Low LR individuals processed both types of information in a manner that might contribute to an impaired ability to recognize modest levels of alcohol intoxication in a range of life situations.
Keywords: fMRI, alcohol sensitivity, emotional stimuli, fear, Hariri, alcoholism
Introduction
Risk factors for heavy drinking operate primarily through intermediate characteristics that relate to environmental events and genetic influences to impact on the development of heavy drinking and alcohol use disorders (AUDs) [1–5]. One such preexisting phenotype is the low level of response (LR) to alcohol, where individuals who require higher doses of alcohol to achieve an effect (reflecting a lower sensitivity or more rapid intra-session tolerance) are more likely to develop AUDs than those who react more intensely to ethanol [6–9]. This trait can be measured as less alcohol-related change in positive (e.g., feeling high) and negative (e.g., feeling dizzy) subjective feelings of intoxication, hormones, and EEG measures at a given blood alcohol concentration (BAC), or as less effect per drink on a retrospective questionnaire [4, 10–16]. A low LR is seen in ~40% of the children of alcoholics, but only ~10% of controls, and the heritability of this trait is 40%-60% [3, 5, 10]. Low LR predicts later heavy drinking, but has little overlap with other genetically-influenced risk factors (e.g., alcohol metabolizing enzymes and impulsivity [3,11].
Until recently, little has been known about how people with lower and higher LR differ on neuronal activity with and without alcohol. A previous functional magnetic resonance imaging (fMRI) study examined the relationship between LR status and neural activation during a visual working memory (VWM) task in the absence of a beverage challenge [17]. Using the retrospective Self-Report of the Effects of Alcohol (SRE) questionnaire to determine LR, despite similar task performance subjects with lower LRs had greater blood-oxygen level-dependent (BOLD) responses to the task, which remained significant after controlling for demography and recent alcohol consumption. Two subsequent studies with two new samples, one with 60 subjects and the other a pilot study with 10 subjects, following both placebo and alcohol corroborated both the similar task performance across LR matched groups and the greater BOLD response contrast to high working memory demands after placebo for those with low LR [18, 19]. Here, alcohol (~0.70 ml/kg) either greatly diminished or reversed the LR group differences seen after placebo. The pattern of neural effects was consistent with the cognitive demands of the visual working memory task (e.g., in the right frontal gyri, cingulate, and parietal lobe).
These findings, suggesting the LR status may relate to differential brain processing across placebo (or non-drinking) and alcohol conditions, were supported by data from 49 high/low matched pairs while performing a stop signal task [20]. Despite similar task performance, the low LR group again evidenced more BOLD response contrast after placebo, but the after-alcohol low LR subjects had lower while higher LR subjects had greater contrast. For these non-alcoholic subjects, LR group by placebo/alcohol condition interactions were most prominent in posterior brain regions that might reflect perception of auditory and visual stimuli, not in the more frontal regions more likely to reflect disinhibition. Finally, an additional analysis revealed that low LR subjects had significantly less increase in cerebral blood flow after alcohol than those with higher LR [21].
The studies cited above focused primarily on cognitive tasks, and, despite the impact of alcohol on affect [22], the relationship of these effects to LR has not been evaluated. The insula, anterior cingulate gyrus, prefrontal cortex, and amygdala are important in the detection of emotionally salient stimuli, affective responses, and regulation of affect [23–29]. The amygdala is involved in fear conditioning [30], reward-related processing [31], encoding of emotionally salient stimuli [32], risk taking [33], processing positively valenced stimuli [34], as well as in the pathophysiology of anxiety disorders [35, 36]. Thus, these regions appear to be a confluent point of emotion, cognition and physiology, and appropriate regions of interest to consider for fMRI analyses of emotion-related tasks.
The current study examined the relationship of LR to activity in these affect-related brain areas during an emotion face processing task. Based on prior fMRI results using two cognitive tasks, we hypothesized that low LR subjects will demonstrate higher BOLD response contrast with placebo and lower values after alcohol, while the high LR individuals will show the opposite pattern. This may reflect a general pattern in low LR subjects of how they process information that also extends to emotional-based tasks, even after controlling for alcohol effects on CBF [37]. We also predicted that this low LR/high LR pattern would be seen in one or more brain regions related to recognition and processing of emotional faces, including those implicated in primary emotion processing (e.g., amygdala and insula) and in top-down control of emotional processing (e.g., anterior and perigenual cingulate and medial prefrontal cortex). Support for these predictions would provide evidence that a person’s LR status may be an important correlate not only of how they process cognitive information but also for the manner in which they process emotional stimuli.
Materials and Methods
Participants
Using methods approved by the University of California, San Diego (UCSD) Human Research committee, a questionnaire was distributed to randomly selected 18–25-year-old Anglo and white Hispanic students. The instrument gathered information on demography and substance use and current DSM-IV major psychiatric disorders through questions from the SSAGA (Semi-Structured Assessment for the Genetics of Alcoholism) interview [38–40]. The answers were used to identify right-handed, healthy students who: 1) had experience with alcohol; 2) no history of brain trauma, epilepsy, alcohol or drug dependence; 3) had no current Axis I or II psychiatric disorder; 4) were not pregnant; and 5) had no irremovable body metal.
To establish the subject’s LR, they were scheduled for a laboratory-based alcohol challenge (>90% of those invited agreed to participate). Here, the subjects were required to have zero baseline breath alcohol concentrations (BrACs), after which they drank alcohol (0.75 ml/kg for males and 0.70 for females) given as a 20% by volume solution in a room temperature carbonated beverage and consumed from a closed thermos over 10 minutes to produce approximately equivalent BrACs across genders [41–43]. The initial alcohol challenge and fMRI sessions were scheduled on non-consecutive days, but within a week of each other whenever possible. During the initial alcohol challenge session, at 15 minutes, and every 30 minutes over 180 minutes participants completed the Subjective High Assessment Scale (SHAS) and BrAC evaluations, but reflecting restrictions in both testing time and finances, no other biological components were evaluated. The SHAS consists of 13 items graded on a 39-point scale to evaluate alcohol effects [43]. Subjects then participated in two fMRI sessions, receiving in random order alcohol (the same dose as in the prior session) or placebo, entering the scanner 20 minutes later, and participating in the Hariri task at 70 minutes after drinking. Here, the subject and experimenters were blind to the beverage during alcohol and placebo sessions, which used the same beverage administration closed container [42] as in the non-fMRI prior session, with the beverage consumed orally over 10 minutes in a carbonated soda. For both conditions, the straw had a reservoir with 1 mL 95% alcohol that was the first liquid to reach the subject’s mouth.
Task
A modified version of the Hariri emotional face-processing task [44–46] was used where in each 5-second trial subjects were presented with a target face and two probe faces, with instructions to match the probe and emotional expression of the target by pressing a button. Each block had 6 consecutive trials where faces were angry, fearful, or happy in which the three separate emotional face-matching blocks were given for each emotion and different people were pictured for each emotion. The 5-second sensorimotor control tasks used vertical or horizontal ovals or circles with instructions to match the shape of the probe to the target. Each block of faces and of the control task was presented three times in a pseudo-randomized order. The task began with an 8-second fixation period and was comprised of interspersed 12-second fixation periods between each face or sensorimotor control task. For each of the 18 trials (3 blocks of 6 trials), response accuracy and reaction times were obtained, and the entire task lasted 512 seconds.
Analysis
Acquisition of images
Testing was carried out using a 3T GE CXK4 Magnet (General Electric Medical. Systems, Milwaukee, WI) at the UCSD Keck Imaging Center equipped with 8 high-bandwidth receivers that allow for shorter readout times and reduced signal distortions and ventromedial signal dropout. Each session lasted one hour and encompassed: a 3-plane scout scan (10 seconds), as well as a standard anatomical protocol (i.e., a sagittally acquired spoiled gradient recalled sequence) (FOV = 25 cm, matrix = 192 × 256, 172 sagittally acquired slices 1-mm thick, TR = 8 ms, TE = 3 ms, flip angle = 12°). An 8-channel brain array coil was used to axially acquire T2*-weighted echo-planar images (EPIs) with: FOV = 23 cm, matrix = 64 X 64, 30 slices 2.6-mm thick, gap = 1.4 mm, TR = 2000 ms, TE = 32 ms, flip angle = 90°.
Image analysis pathway
The Analysis of Functional NeuroImages (AFNI) software package [47] was used for processing the basic structural and functional image. Changes in echo planar images (EPI) intensity in relation to task characteristics [48] were evaluated using the multivariate regressor approach detailed below. A 3D-coregistration algorithm [49] developed to minimize the amount of image translation and rotation relative to all other images used Echoplanar images that were co-registered. Each subject had six motion parameters obtained, three of which were used as regressors to adjust for EPI intensity changes due to motion artifacts. To ensure that different relationships with the regressors were not due to the acquisition of different slices at different times during the repetition interval, all slices of the EPI scans were temporally aligned following registration.
Multiple regressor analyses
The orthogonal regressors of interest were the four images (happy, angry, fearful, and circle/oval [i.e., shape]). These 0–1 regressors were convolved with a gamma variate function [50] modeling a prototypical hemodynamic response (6–8 second delay [51]) and to account for the temporal dynamics of the hemodynamic response (typically 12–16 seconds) [52], with each convolved time series normalized as a regressor of interest. These regressors, along with motion regressors, were entered into the AFNI program 3dDeconvolve to determine the height of each regressor for each subject. The main dependent measure was the voxel-wise normalized percent signal change created by dividing the regressor coefficient by the zero-order regressor. A Gaussian filter (FWHM 4mm) was used to account for variations in anatomy for individuals, and we did not use a high-pass filter. Spatially smoothed percent signal change data were transformed into anatomical magnetic resonance image Talairach coordinates, followed by manual transformation in AFNI.
Anatomically-constrained functional regions of Interest
Our anatomically-constrained functional region of interest (ROI) approach used a previously developed anatomical mask for the ROIs to test the proposed hypothesis about the involvement of low versus high LR status regarding insular cortex, medial prefrontal cortex (MPFC), and amygdala based on the Talairach Atlas [53]. The mPFC included primarily the ventral and dorsal anterior cingulate (ACC; including BA 32, 24), spreading into ventromedial PFC regions (BA 10). This medial PFC region was further partitioned into dorsal and ventral extents at z=0, and the insula was partitioned into anterior and posterior portions using a linear interpolation for the location of the middle insular gyrus. Thus, in total there were eight a priori ROIs (bilateral amygdala, dorsal and ventral medial PFC, bilateral anterior and posterior insula). Amygdala regions consisted of 2752 ul (43 voxels) each, anterior insula regions consisted of 9920 ul (155 voxels) each, posterior insula regions consisted of 8512 ul (133 voxels) each, dorsal medial PFC consisted of 21824 ul (341 voxels), ventral medial PFC consisted of 17984 ul (281 voxels), and whole brain consisted of 2,334,464 ul (36476 voxels) (see Figure S1 in the Supplement). A threshold adjustment based on Monte Carlo simulations (conducted via AFNIs in Alpha Sim, a threshold p of .05, two-tailed) was used to guard against identifying false positive areas of activation. The minimum volumes for a significant cluster were: 192 μL for amygdala; 256 μL for insula; 320 μL for medial PFC; and 832 μL for whole brain. Corrected voxel-wise probabilities using Alpha Sim were p: <.0021 for amygdala; <.0015 for anterior insula; <.0016 for posterior insula; <.0008 for ventromedial and for dorsomedial PFC; and <.0001 for whole brain. Center of mass Talairach coordinates (x,y,z) were used and labeled based on visual observation and Talairach Daemon software [53]. The cerebellum was not considered in the analyses. Note that Table S1 in the Supplement presents the results of the whole brain analyses.
Statistics
Second-level analyses were conducted based on the statistical programming language R (http://cran.r-project.org/) (i.e., a mixed-model analysis used the R program lme). Specifically, a voxel-wise model was used to examine the fixed effects consisting of emotion type, group, education, and response latency, and the random effects were subjects (i.e., an individual intercept was fitted for each subject). These were performed within the constraints of ROIs to reduce Type 1 errors. Voxel-wise multiple linear regression analyses were conducted with latency to respond to angry, fearful, or happy faces as independent measures, and the percent signal change between faces and the sensorimotor control condition as the dependent measure using the lm program of R. Subsequently, clusters of activation within the anatomically-constrained functional regions of interest were considered significant only if the clusters were larger than the above-mentioned volume thresholds. The average of each cluster was tabulated across the different experimental conditions. All analyses covaried for both CBF (overall blood flow differences in ml/100g/min for placebo vs. alcohol sessions) and for usual drinks per occasion.
Results
Population Characteristics
In an extension of our prior work [19–21], data were available on 116 individuals comprised of 58 matched pairs of low and high LR subjects. The sample generated 232 fMRI sessions with alcohol and placebo runs, along with 116 alcohol challenges carried out prior to imaging sessions. As shown in Table 1, the two LR groups were well matched on demography, drinking frequency, as well as tobacco and cannabis use, with resulting similar BACs at 60 minutes, a time close to administration of the Hariri task. Low LR subjects also reported a higher number of drinks per occasion, which could have contributed to the low LR through acquisition of intersession tolerance, thus confounding the determination of a low LR. Therefore, this variable was used as a covariate in all fMRI analyses.
Table 1.
Characteristics of Participants with High and low Levels of Response (LR) to Alcohol (Mean and Standard Deviations)
| Low LR (n=58) M (SD) |
High LR (n=58) M (SD) |
t-statistic or χ2 | |
|---|---|---|---|
| Age (years) | 19.8 (1.47) | 20.1(1.51) | −1.0 (p=0.31) |
| % Female (n) | 52.6% | 52.6% | 0 (p=1.0) |
| Years of education completed | 13.6 (1.1) | 13.6 (1.2) | −0.08 (p=0.93) |
| Height (inches) | 68.3 (4.0) | 68.3 (4.1) | 0.0 (p=1.0) |
| Weight (pounds) | 156.2 (26.2) | 155.3 (25.6) | 0.18 (p=0.86) |
| Days/month used alcohol a | 4.5 (4.6) | 3.5 (3.7) | 1.31 (p=0.19) |
| Usual drinks per occasion past 6 mo *a | 4.1 (1.8) | 3.3 (1.8) | 2.31 (p=0.02) |
| Days/month of tobacco use past 6 mo a | 0.8 (1.3) | 2.2 (4.2) | −0.70 (p=0.48) |
| Tobacco units/occasion past 6 mo a | 0.3 (0.5) | 0.2 (0.5) | 0.56 (p=0.58) |
| % Ever used cannabis | 59.2% | 46.9% | 1.48 (p=0.23) |
| Lifetime cannabis use occasions | 29.1 (77.9) | 22.6 (84.7) | 0.42 (p=0.67) |
| BAC at 60 minutes (mg/dL) | 0.06 (0.02) | 0.06 (0.02) | −0.51 (p=0.60) |
p < 0.05
= data for prior 6 months
Behavioral Analyses and BACs
During the Hariri task, the low and high LR groups had similar reaction times after placebo and following alcohol for angry, fearful and happy faces, and for the sensorimotor control oval image after placebo. The only significant difference was that low LR subjects responded faster than high LR subjects to the oval after alcohol (905.8 [166.03] vs. 994.5 ms [211.54], t = −2.51, p = .01). Remaining p values for reaction time across LR groups ranged from .15 to .79. Groups showed no significant differences for the percent correct for any condition of the task, where the largest group differences across low LR and high LR subjects, respectively, were seen after alcohol for happy faces (98.8 [2.82]% correct vs. 97.3 [5.12]%, t = 1.97, p = .055) and for fearful faces after alcohol (96.5 [5.46]% correct vs. 93.9 [9.09]%, t = 1.88, p = .06). The remaining p values for percent correct ranged from .19 to .94.
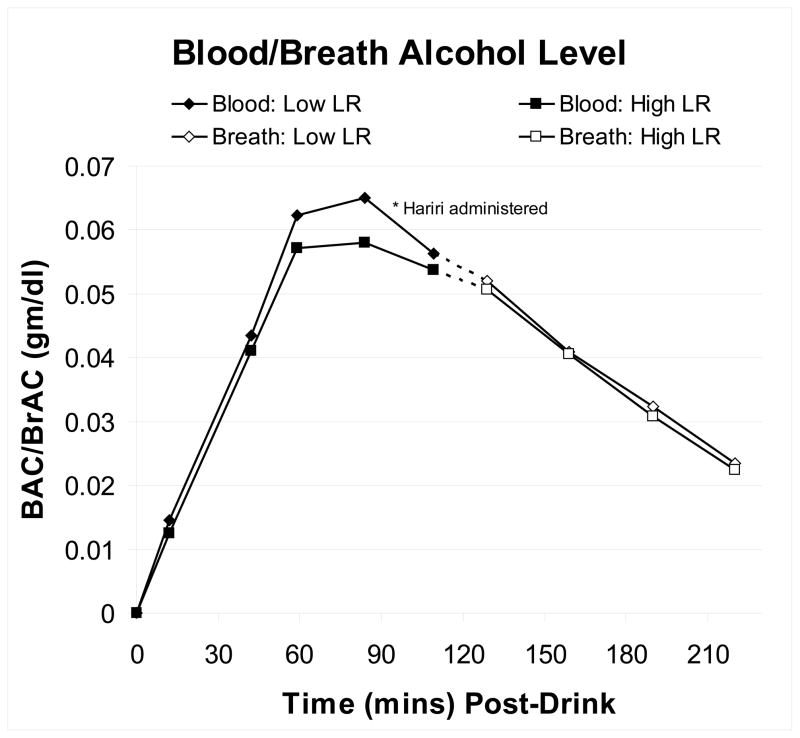
Figure 1 presents the BAC (time zero to 90 minutes) and BrAC (for time 120-0 minutes) values for the two LR groups during the fMRI alcohol challenges. The figure also notes that the 8 minute, 32 second Hariri task began ~70 minutes into the protocol. Focusing on the three BAC time points most relevant to the Hariri task (30–90 minutes) and the 98 subjects who had valid BAC values, the LR group (F = 2.09, p = .15) and LR x time (F = 1.44, p = .24) effects were not significant, but there was a significant BAC time effect (F = 101.60, p <.001).
Figure 1.
Blood alcohol values (BACs) were obtained from time zero through90 minutes (closed diamonds and squares), and breath alcohol values (BrACs) were then used to monitor when subjects would be allowed to go home (open diamond and squares). The time between BAC and BrAC values is indicated by dots. Data are given for the 98 subjects who had complete BAC values during the time just prior to, during, and after the Hariri task; 30–90 minutes.
Image Analyses
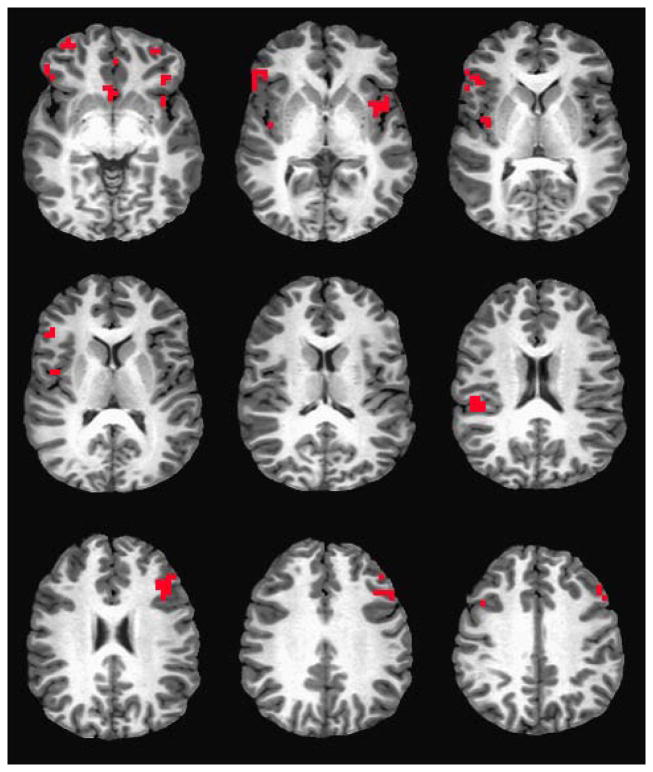
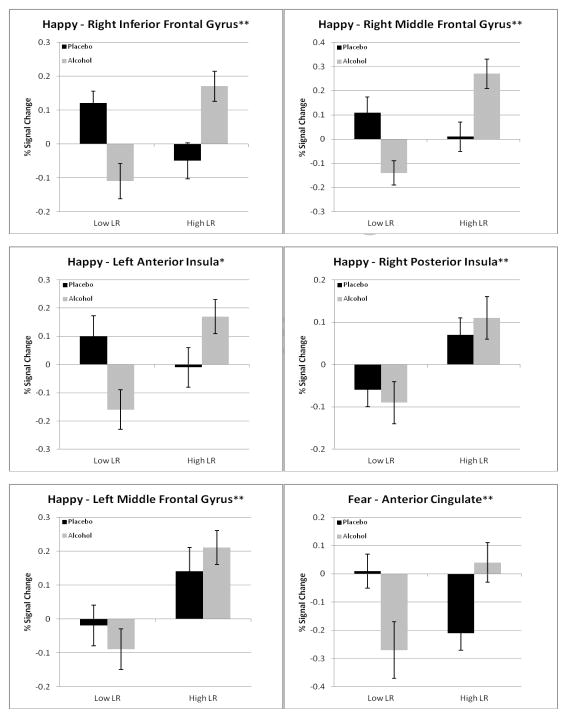
Details about the regions that demonstrated significant LR main effects or LR group by placebo/alcohol condition interactions in BOLD response contrast for emotional faces relative to oval shape conditions are shown in Table 2 and Figure 2. Graphic examples of some prominent patterns from Table 2 are offered in Figure 3. In the table, for each region of interest, the first set of columns presents the anatomic region, the Brodmann areas, the volume, and the Talairach Atlas coordinates. These are followed by the BOLD response contrast values for low and high LR subjects after receiving placebo and alcohol.
Table 2.
Regions Showing Significant LR Group or Group by Placebo/Alcohol Condition Interaction Effects in BOLD Response Contrast for Emotional Faces Relative to Oval (Non-Face) Trials
| Anatomic Region x | Brodmann Area(s) | Volume (μl) | Talairach x | Low LR | High LR | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Placebo Mean | Alcohol Man | Placebo Mean | Alcohol Mean | |||
| Happy Faces | |||||||||
| R inferior frontal gyrus z | 13,45,47 | 1792 | 54 | 32 | 0 | 0.12 | −0.11 a | −0.05 | 0.17 b |
| R middle frontal gyrus z | 10 | 640 | 34 | 48 | −8 | 0.11 | −0.14 a | 0.01 | 0.27 b |
| L anterior insula z | 13 | 576 | −46 | 12 | 0 | 0.10 | −0.16 a | −0.01 | 0.17 b |
| Anterior cingulate z | 25,32 | 960 | 2 | 8 | −8 | 0.18 | 0.06 a | 0.07 | 0.26 b |
| R posterior insula y | 13,40,41 | 512 | 50 | −24 | 20 | −0.06 | −0.09 a | 0.07 | 0.11 b |
| L inferior frontal gyrus y | 47 | 576 | −42 | 28 | −4 | 0.03 | −0.03 | 0.17 | 0.21 b |
| L middle frontal gyrus y | 11 | 960 | −34 | 48 | −12 | −0.02 | −0.09 a | 0.14 | 0.21 b |
| R middle insula y | 13 | 576 | 42 | −4 | 8 | 0.001 | −0.02 a | 0.13 | 0.11 a |
| L middle insula y | 13 | 576 | −42 | 0 | 4 | 0.04 | −0.09 a | 0.11 | 0.16 b |
| Angry Faces | |||||||||
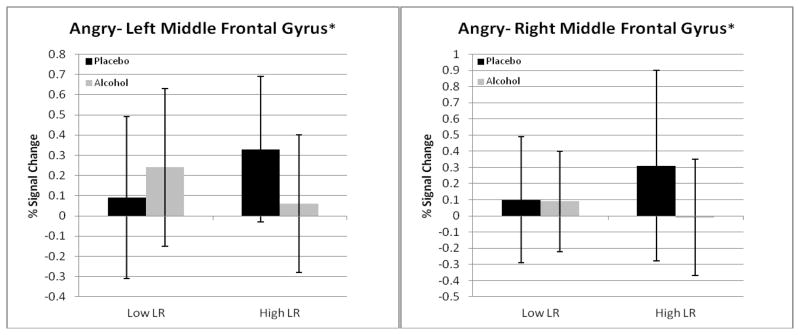
| L middle frontal gyrus z | 9 | 1280 | −50 | 20 | 32 | 0.09 | 0.24 b | 0.33 | 0.06 a |
| R middle frontal gyrus z | 8 | 704 | 46 | 12 | 40 | 0.10 | 0.09 a | 0.31 | −0.01a |
| Fear Faces | |||||||||
| Anterior cingulate z | 32 | 576 | −2 | 40 | −8 | 0.01 | −0.27 a | −0.21 | 0.04 b |
| All Faces | |||||||||
| L middle frontal gyrus y | 11 | 512 | −34 | 52 | −8 | −0.04 | −0.07 a | 0.18 | 0.11a |
= Talairach coordinates refer to peak effect group difference within the cluster. Abbreviations: R right; L left.
= Significant group effect, p<0.01
= Significant group by condition effect, p<0.01
= BOLD response contrast decreased from placebo to alcohol conditions
= BOLD response contrast increased from placebo to alcohol conditions
Figure 2.
Brain regions demonstrating LR group or group by placebo/alcohol interactions in BOLD response contrast for emotional faces relative to oval shape conditions described in Table 2 and Figure 3.
Figure 3.
Graphs, including standard deviations for eight of the 13 brain regions in Table 2 as examples of LR group differences. The asterisks at the top of the figures expand the information on statistical significance from Table 2 (all 13 are p <.01) to demonstrate the p values for LR group effects and group by condition interactions (* = p <.01; ** = p <.001).
Two patterns of results emerged from the 13 regions that had significant LR group or LR by placebo/alcohol condition effects in Table 2, with most of the findings observed for happy faces. First, in 12 of the 13 regions in Table 2, low LR subjects showed a decreasing BOLD response contrast intensity or little change from placebo to alcohol conditions in the inferior and middle frontal gyri, insula, and anterior cingulate. However, for high LR, in nine of the 13 regions the BOLD response contrast increased from placebo to alcohol. These included four of the nine regions with effects for happy faces and the single region for fearful faces. Therefore, while low LR individuals tended to demonstrate little change or a diminished brain response after alcohol relative to placebo, the high LR group showed an increasing response after alcohol compared to placebo. Second, comparisons of low versus high LR groups after placebo revealed that in eight of the 13 regions in Table 2, lower BOLD response contrast was seen for those with low LRs.
Reflecting the LR group differences on usual drinks per occasion, as well as past differences in cerebral blood flow changes after alcohol [21], the analyses reported in Table 2 were repeated after covarying for these two values. Results remained consistent with those reported above. In addition, LR group and group by condition results using whole-brain analyses also followed a similar pattern, as shown in Table S1 in the Supplement
Discussion
The focus of this study was on examining whether individuals with a low LR to alcohol demonstrated unique emotion-related brain processing compared to subjects with high LR, thus extending previous fMRI findings with high and low LR subjects that had used cognitive tasks. Prior papers evaluated BOLD contrasts across placebo and alcohol for low and high LR groups during a VWM task and in the context of a stop signal task, with no clear evidence of hemispheric lateralization [17–20]. The most prominent pattern of prior results was that low LR subjects were likely to decrease BOLD response contrast from placebo to alcohol, while the high LR subjects demonstrated the opposite pattern. However, no prior study evaluated results when the focus was on emotional responding rather than executive functions.
This question is important because acute administration of alcohol impacts on emotion and affect along with cognitive performance, and alcohol-dependent persons are likely to evidence both impaired judgment and problems with emotional aspects of their lives [22, 54]. As predicted, LR group or LR group by placebo/alcohol condition differences were found in frontal, insula, and anterior cingulate regions. Findings were seen in both right and left regions, with a possible over-representation on the right, a result that will require additional study to evaluate further. However, there were no significant LR group or group by placebo/alcohol condition interactions in the amygdala in these non-alcoholic young subjects. Perhaps the differences from some prior studies with depressants [44] reflect dose effects, different expectations of drug effects, or different receptor effects across alcohol and benzodiazepines, speculations that will require additional studies to address.
The pattern in the high LR subjects of increasing BOLD response contrast from placebo to alcohol may indicate that these individuals require greater cognitive and affective effort to process information after alcohol than following placebo. This result may be more in tune with what an individual expects to happen when consuming ethanol, and may contribute to the greater ability of high LR subjects to recognize intoxication. Such perturbations of CNS functioning by alcohol in individuals with high LRs may contribute to their more intense reaction to the effects of this drug as evidenced by greater alcohol-related increases in ACTH and prolactin, increased latency of the P300 wave of event-related potentials, and more prominent alcohol effects regarding changes in alpha rhythm on background cortical electroencephalograms [4, 12–16]. Those physiological changes might further enhance a person’s ability to recognize the effects of alcohol at the moderate doses used in our alcohol challenge paradigms.
In contrast, across studies [17–20] low LR subjects demonstrated less neural activity during a range of cognitive and emotional tasks after consuming alcohol compared to a no-alcohol condition. This decrease in brain activation with alcohol compared to placebo might be more difficult to perceive as an effect of alcohol, and might contribute to less intense subjective feelings of intoxication. The ability of low LR subjects to execute an affective response in the current protocol using less effort when consuming modest doses of alcohol might also be perceived as rewarding. This could contribute to an enhanced likelihood of continuing alcohol consumption during an evening.
It is important to recognize that the brain response to alcohol in low LR subjects was not uniform. Specifically, for angry faces two regions had significant LR by condition interactions, but here, in the left and right middle frontal gyri, low LR subjects demonstrated increasing BOLD contrasts or small decreases across placebo to alcohol, while decreasing activation was seen for those with high LR. Using a no-alcohol paradigm, Marinkovic et al. [55] reported that individuals with AUDs demonstrated deficient activation in the amygdala and hippocampus when processing emotional faces, a finding that was hypothesized to potentially relate to difficulties they might have in recognizing emotional situations. It is important to keep in mind that the focus of the current work is on relatively highly functional individuals from a nonclinical sample, some of whom carry an enhanced risk for future alcohol problems through a low LR to alcohol, but none of whom have yet developed alcohol dependence.
There are several limitations in our research protocol. The subjects were all university students, predominantly Caucasian, and demonstrated no current substance use disorder or major psychiatric condition. The evaluation of emotional faces was carried out with only a single protocol, the Hariri task, and it is not clear whether similar results would be observed with alternative paradigms. The alcohol challenge involved a modest ethanol dose (peak BAC ~.06 mg/dl), and it would be interesting to determine the fMRI correlates of the LR group differential if higher alcohol doses had been administered. In addition, the alcohol was given as an oral beverage, and did not include approaches such as the intravenous alcohol clamp that might generate additional interesting data [56]. Furthermore, in order to diminish spurious findings, we limited the analyses to brain regions with known effects for this task, and it is possible additional regions would have been identified in a whole brain analyses. Also, our focus on expanding our understanding of mechanisms contributing to the lower feelings of intoxication in low LR subjects, along with restrictions in both finances and testing time, resulted in the absence of more biologically-based hormone and EEG measures. While subjects were blind to the alcohol versus placebo fMRI beverage, it is possible participants could tell the difference between the two conditions. Future work will evaluate whether such imaging characteristics themselves predict future alcohol problems, if the fMRI results add significantly to the LR measures in predicting outcomes, and if they might help identify young subjects early in their drinking careers who might be more amenable to earlier education and prevention efforts [57].
In summary, the LR group differences during an emotion-related task were in the same direction and as prominent as had been seen during cognitive paradigms. The results may contribute to an impaired ability to recognize modest alcohol intoxication in low LR subjects.
Supplementary Material
Acknowledgments
The funding for this research project was provided by NIAAA/NIH grant 5R01AA15760. Dr. Scott Matthews’ VA salary is supported by a CDA-2 from VA CS R&D
Footnotes
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGue M, Slutske W, Iacono WG. Personality and substance use disorders: II. Alcoholism versus drug use disorders. J Consult Clin Psychol. 1999;67:394–404. doi: 10.1037//0022-006x.67.3.394. [DOI] [PubMed] [Google Scholar]
- 2.Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 3.Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- 4.Ehlers CL, Havstad J, Prichard D, Theiler J. Low doses of ethanol reduce evidence for nonlinear structure in brain activity. J Neurosci. 1998;18:7474–7486. doi: 10.1523/JNEUROSCI.18-18-07474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luczak SE, Elvine-Kreis B, Shea SH, Carr LG, Wall TL. Genetic risk for alcoholism relates to level of response to alcohol in Asian-American men and women. J Stud Alcohol. 2002;63:74–82. [PubMed] [Google Scholar]
- 6.Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- 7.Gilman JM, Ramchandani VA, Crouss T, Hommer DW. Subjective and neural responses to intravenous alcohol in young adults with light and heavy drinking patterns. Neuropsychopharmacology. 2012;37:467–477. doi: 10.1038/npp.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fillmore MT, Weafer J. Acute tolerance to alcohol in at-risk binge drinkers. Psychol Addict Behav. 2011 Oct 24; doi: 10.1037/a0026110. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweizer TA, Volge-Sprott M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Exp Clin Psycholpharmacol. 2008;16:240–250. doi: 10.1037/1064-1297.16.3.240. [DOI] [PubMed] [Google Scholar]
- 10.Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, et al. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res. 2001;25:323–329. [PubMed] [Google Scholar]
- 11.Schuckit MA, Smith TL, Trim RS. Letter to the Editor. Alcohol Clin Exp Res. 2010;34:203–205. [Google Scholar]
- 12.Schuckit MA, Gold EO. A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Arch Gen Psychiatry. 1988;45:211–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- 13.Polich J, Burns T, Bloom FE. P300 and the risk for alcoholism: family history, task difficulty, and gender. Alcohol Clin Exp Res. 1988;12:248–254. doi: 10.1111/j.1530-0277.1988.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 14.Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Auditory P3 findings in mission Indian youth. J Stud Alcohol. 2001;62:562–570. doi: 10.15288/jsa.2001.62.562. [DOI] [PubMed] [Google Scholar]
- 15.Reese C, Polich J. Alcoholism risk and the P300 event-related brain potential: modality, task, and gender effects. Brain Cogn. 2003;53:46–57. doi: 10.1016/s0278-2626(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 16.Mick I, Spring K, Uhr M, Zimmermann US. Alcohol administration attenuates hypothalamic-pituitary-adrenal (HPA) activity in healthy men at low genetic risk for alcoholism, but not in high-risk subjects. Addict Biol. 2012 Jan 29; doi: 10.1111/j.1369-1600.2011.00420.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. J Stud Alcohol. 2004;65:692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- 18.Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol Attenuates Load-related Activation During a Working Memory Task: Relation to Level of Response to Alcohol. Alcohol Clin Exp Res. 2006;30:1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trim RS, Simmons AN, Tolentino NJ, Hall SA, Matthews SC, Robinson SK, et al. Acute Ethanol Effects on Brain Activation in Low- and High-Level Responders to Alcohol. Alcohol Clin Exp Res. 2010;34:1162–1170. doi: 10.1111/j.1530-0277.2010.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuckit MA, Tapert S, Matthews SC, Paulus MP, Tolentino NJ, Smith TL, et al. fMRI differences between subjects with low and high responses to alcohol during a stop signal task. Alcohol Clin Exp Res. 2011;36:130–140. doi: 10.1111/j.1530-0277.2011.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolentino NJ, Wierenga CE, Hall S, Tapert SF, Paulus MP, Liu TT, et al. Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin Exp Res. 2011;35:1034–1040. doi: 10.1111/j.1530-0277.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howland J, Rohsenow DJ, Greece JA, Littlefield CA, Almeida A, Heeren T, et al. The effects of binge drinking on college students’ next-day academic test-taking performance and mood state. Addiction. 2010;105:655–665. doi: 10.1111/j.1360-0443.2009.02880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 24.Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proc Biol Sci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 26.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmons A, Matthews SC, Stein MB, Paulus MP. Anticipation of emotionally aversive visual stimuli activates right insula. Neuroreport. 2004;15:2261–2265. doi: 10.1097/00001756-200410050-00024. [DOI] [PubMed] [Google Scholar]
- 29.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 30.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 31.Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- 32.Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, et al. Decision-making in a Risk-taking Task: a PET Study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- 34.Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12:2779–2783. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- 35.Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl. 2003;417:38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- 36.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 37.Rickenbacher E, Greve DN, Azma S, Pfeuffer J, Marinkovic K. Effects of alcohol intoxication and gender on cerebral perfusion: an arterial spin labeling study. Alcohol. 2011;45:725–737. doi: 10.1016/j.alcohol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 39.Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC Press; 1994. [Google Scholar]
- 41.Breslin FC, Kapur BM, Sobell MB, Cappell H. Gender and alcohol dosing: a procedure for producing comparable breath alcohol curves for men and women. Alcohol Clin Exp Res. 1997;21:928–930. doi: 10.1111/j.1530-0277.1997.tb03860.x. [DOI] [PubMed] [Google Scholar]
- 42.Mendelson JH, McGuire M, Mello NK. A new device for administering placebo alcohol. Alcohol. 1984;1:417–419. doi: 10.1016/0741-8329(84)90014-4. [DOI] [PubMed] [Google Scholar]
- 43.Eng MY, Schuckit MA, Smith TL. The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Depend. 2005;79:83–93. doi: 10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by Lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- 45.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 46.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 47.Cox RW. Software for analysis and visualization of functional magnetic neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 48.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 49.Eddy WF, Fitzgerald M, Noll DC. Improved image registration by using Fourier interpolation. Magn Reson Med. 1996;36:923–931. doi: 10.1002/mrm.1910360615. [DOI] [PubMed] [Google Scholar]
- 50.Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. NeuroImage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- 52.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 53.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuckit MA, Smith TL, Danko GP, Pierson J, Trim R, Nurnberger JI, Jr, et al. A comparison of factors associated with substance-induced versus independent depressions. J Stud Alcohol Drugs. 2007;68:805–812. doi: 10.15288/jsad.2007.68.805. [DOI] [PubMed] [Google Scholar]
- 55.Marinkovic K, Oscar-Berman M, Urban T, O’Reilly CE, Howard JA, Sawyer K, et al. Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res. 2009;33:1880–1892. doi: 10.1111/j.1530-0277.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramchandani VA, O’Connor S, Neumark Y, Zimmermann US, Morzorati SL, de Wit H. The alcohol clamp: applications, challenges, and new directions – an RSA 2004 symposium summary. Alcohol Clin Exp Res. 2006;30:155–164. doi: 10.1111/j.1530-0277.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- 57.Schuckit MA, Kalmijn JA, Smith TL, Saunders G, Fromme K. Structuring a college alcohol prevention program on the low level of response to alcohol model: a pilot study. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2011.01723.x. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.