Abstract
In the present study, we used the GTP cyclohydrolase I-deficient mice, ie., hph-1 mice, to test the hypothesis that the loss of tetrahydrobiopterin (BH4) in cerebral microvessels causes endothelial nitric oxide synthase (eNOS) uncoupling resulting in increased superoxide anion production and inhibition of endothelial nitric oxide (NO) signaling. Both homozygous mutant (hph-1-/-) and heterozygous mutant (hph-1+/- mice) demonstrated reduction in GTP cyclohydrolase I activity and reduced bioavailability of BH4. In the cerebral microvessels of hph-1+/- and hph-1-/- mice, increased superoxide anion production was inhibited by supplementation of BH4 or NOS inhibitor - L-NAME, indicative of eNOS uncoupling. Expression of 3-nitrotyrosine was significantly increased, while NO production and cGMP levels were significantly reduced. Expressions of antioxidant enzymes namely CuZnSOD, MnSOD and catalase were not affected by uncoupling of eNOS. Reduced levels of BH4, increased superoxide anion production as well as inhibition of NO signaling were not different between the microvessels of male and female mice. The results of our study are the first to demonstrate that, regardless of gender, reduced BH4 bioavailability causes eNOS uncoupling, increases superoxide anion production, inhibits eNOS/cGMP signaling, and imposes significant oxidative stress in the cerebral microvasculature.
Keywords: oxidative stress, endothelial dysfunction, cerebral microvasculature, GTP cyclohydrolase I
Introduction
Under physiological conditions, reduction of molecular oxygen results in electron transfer required for oxidation of L-arginine at the reductase domain of endothelial nitric oxide synthase (eNOS). This electron transfer (from NADPH) is facilitated by essential co-factor of eNOS, tetrahydrobiopterin (BH4). When concentration of BH4 becomes suboptimal, electron transfer does not couple with production of NO. Under these conditions, eNOS becomes uncoupled to generate superoxide rather than NO (Alp & Channon 2004, Katusic et al. 2009, Moens & Kass 2006, Vasquez-Vivar 2009). Oxidative stress, characterized by elevated production of superoxide anions and/or impaired activation of antioxidant enzymes, has emerged as a primary mediator of endothelial dysfunction observed in various cerebrovascular disorders (Chrissobolis & Faraci 2008). While numerous studies, including ours, have examined the role of eNOS uncoupling in oxidative stress in the peripheral vasculature (Forstermann & Li 2011, Landmesser et al. 2003, Meininger et al. 2000, Schulz et al. 2008, Stroes et al. 1997), effects of eNOS uncoupling on cerebral microvasculature has not been studied.
The hyperphenylalaninemic mutant, hph-1 mice, reported to be deficient in GTP cyclohydrolase I, the rate-limiting enzyme in BH4 biosynthesis (Bode et al. 1988, Canevari et al. 1999, McDonald et al. 1988, Shimoji et al. 1999), has been widely used to study the effects of eNOS uncoupling on vascular function (Cosentino et al. 2001, d'Uscio et al. 2011, Khoo et al. 2005). In fact, Cosentino and colleagues reported that reactive oxygen species derived from uncoupling of eNOS mediate endothelium-dependent relaxations in aorta of hph-1 mice (Cosentino et al. 2001). However, in a recent study on hph-1 mice, we demonstrated that aorta and mesenteric arteries varied in response to oxidative stress caused by eNOS uncoupling (d'Uscio et al. 2011), suggestive of differential tolerance to oxidative stress induced by eNOS uncoupling between large conduit and small resistance arteries (d'Uscio et al. 2011).
In the present study, we aimed to determine the effects of GTP cyclohydrolase I deficiency on cerebral microvasculature. We hypothesized that, irrespective of gender, the cerebral microvasculature is highly sensitive to loss of BH4, thereby exhibiting augmented superoxide anion production, loss of NO and oxidative stress.
Methods
Mice
Three months old male and female, homozygous mutant (hph-1-/-) and heterozygous mutant (hph-1+/-) mice and sex-matched wild-type (C57BL/6) littermates were maintained on standard chow with free access to drinking water. Male hph-1-/- mice (on C57BL/6 background) provided to us by Dr. Keith M. Channon (University of Oxford, Oxford, UK) were bred with commercially available female C57BL/6 mice (Jackson Laboratories). Genotyping was performed as previously reported (Khoo et al. 2004). Housing facilities and all the experimental protocols were performed as per the guidelines laid by the Institutional Animal Care and Use Committee of the Mayo Clinic College of Medicine. Mice were killed by injecting an overdose of pentobarbital. In some experiments, female wild-type, hph-1-/- and hph-1+/- mice were injected subcutaneously with 100 μmol/kg (b.w.) of BH4 [(6R)-5,6,7,8-tetrahydro-L-biopterin dihydrochloride; Schircks Laboratories, Jona, Switzerland]. Three hours later, mice were killed and brain was isolated and studied.
Blood vessel isolation
Brain was removed and placed in cold (4° C) modified Krebs-Ringer bicarbonate solution (in mmol/l: NaCl 118.6; KCl 4.7; CaCl2 2.5; MgSO4 1.2; KH2PO4 1.2; NaHCO3 25.1; glucose 10.1; EDTA 0.026). Under a surgical microscope, anterior and posterior cerebral, middle cerebral and basilar arteries were separated away from the brain. Cerebral microvessels were subsequently isolated from the brain by Dextran centrifugation, as described earlier (Austin et al. 2010). Briefly, brain was homogenized in ice cold PBS with Dounce homogenizer and centrifuged at 2000g at 4°C. The supernatant was discarded and the pellet was suspended and layered over 15% Dextran (in PBS) (Sigma, St. Louis, MO) and centrifuged at 4000g for 20 minutes at 4°C. The non-vascular fraction remained suspended on the top and was removed, while the pellet was re-suspended in 1% bovine serum albumin (BSA) and was then passed through a 40 μm nylon mesh (BD Falcon). Microvessels retained on the mesh were washed with BSA/PBS and collected by centrifugation at 1000g for 10 minutes at 4°C.
Measurement of biopterin levels and GTP cyclohydrolase I activity
Cerebral microvessels were homogenized in extraction buffer containing 50 mmol/l Tris (pH 7.4), 1 mmol/L dithiothreitol, and 1 mmol/L EDTA at 4°C and were centrifuged at 10,000g (8 min at 4°C). Biopterin levels were determined after differential oxidation in acid (which converts both BH4 and its oxidized derivative 7,8-BH2 to biopterin) and base (which converts only 7,8-BH2 to biopterin) conditions by reversed-phase HPLC, as reported previously (d'Uscio et al. 2003, d'Uscio et al. 2011).
For determination of GTP cyclohydrolase I activity, cerebral microvessel homogenates were filtered using a Sephadex G25M column (GE Healthcare) to remove endogenous neopterin, BH4 and phenylalanine. An incubation solution of 0.1 mmol/l Tris-HCl (pH 7.4), 100 μmol/l PMSF, 10 mg/ml BSA and 10 μl of GTP was added to 100 μl homogenates and incubated for 2 hours at 37°C. Reaction was terminated at the end of incubation by the addition of 1 mol/l HCl. An iodine solution (1% I2/2%KI, 1:1 wt/vol) was added and the samples were incubated for 1 hour at room temperature in the dark. 10 μl of ascorbic acid (20%) was subsequently added followed by 1 mol/l NaOH. Neopterin triphosphate thus formed was dephosphorylated by incubation with alkaline phosphatase (250 mU/μl, Fisher) for one hour at 37°C. Samples were then centrifuged at 11,000 rpm for 10 minutes. Neopterin was then measured by reversed-phase HPLC with fluorescence detection, as reported earlier (d'Uscio et al. 2003, d'Uscio et al. 2011).
Detection of intracellular superoxide anion
Intracellular superoxide anion levels in cerebral microvessels were quantified using a HPLC-based fluorescence detection of the oxidation of dihydroethidium. Briefly, microvessels were incubated in Krebs-HEPES buffer containing 50 μmol/L of dihydroethidium (Molecular Probes) at 37°C for 15 minutes. In some experiments, blood vessels were either incubated with L-NAME (30 μmol/L) for 30 minutes prior to addition of dihydroethidium (d'Uscio et al. 2010, d'Uscio et al. 2011). The samples were washed to remove the free dihydroethidium and incubated in Krebs-HEPES buffer for one hour at 37°C. The blood vessels were homogenized in cold methanol and centrifuged at 9,000g. The supernatant was analyzed by fluorescence detection by HPLC (Beckman Coulter) in 37% acetonitrile in 0.1% trifluoroacetic acid aqueous solution. Data were quantified using 2-hydroxyethidium standard from the reaction between dihydroethidium and Fremy's salt and normalized against tissue protein levels (d'Uscio et al. 2010, d'Uscio et al. 2011).
In some experiments, female mice were injected subcutaneously with 100 μmol/kg BH4. The dose and duration of BH4 treatment has been previously demonstrated to elevate BH4 levels as well as cGMP concentration in the brain (Canevari et al. 1999). Three hours later, brains were isolated and superoxide anion production were measured in the absence and presence of L-NAME (30 μmol/l).
Western blot analysis
Cerebral microvessels were homogenized in lysis buffer containing [50 mmol/L NaCl, 50 mmol/L NaF, 50 mmol/L sodium pyrophosphate, 5 mmol/L EDTA, 5 mmol/L EGTA, 0.1 mmol/L Na3VO4, 1% Triton X-100, 10 mmol/L HEPES, pH 7.4, and protease inhibitor cocktail (Sigma)]. The experimental methods for protein expression studies are published elsewhere (Santhanam et al. 2007). Monoclonal antibodies against eNOS (1:500), nNOS (1:500), phospho S1177eNOS (1:500) [BD Transduction], catalase (1:1000), β-actin (1:5000) [Sigma], 3-nitrotyrosine [Stressgen] and polyclonal antibodies (1:1000 dilution) against CuZn superoxide dismutase (CuZnSOD), manganese superoxide dismutase (MnSOD), extracellular superoxide dismutase (EC-SOD) [Stressgen], Neuron Specific Nuclear Protein [NeuN, Millipore] were used and bands were visualized by enhanced chemiluminescence (Super Signal West Pico Chemiluminescence, Thermo Scientific, IL).
Measurement of nitrite/nitrate
Nitric oxide in the cerebral arteries and cerebral microvessels were measured as total nitrite/nitrate (NO3 + NO2) using a commercially available fluorometric nitrite/nitrate assay kit according to manufacturer's instructions (Cayman Chemical Co.) (Austin et al. 2010).
Measurement of cGMP levels
Cerebral microvessels were homogenized in lysis buffer [containing 50 mmol/L NaCl, 50 mmol/L NaF, 50 mmol/L sodium pyrophosphate, 5 mmol/L EDTA, 5 mmol/L EGTA, 0.1 mmol/L Na3VO4, 1% Triton X-100, 10 mmol/L HEPES, pH 7.4, and protease inhibitor cocktail (Sigma)]. Basal cGMP content in the lysates were determined by enzyme immunoassay according to manufacturer's instructions (Cell Bio Labs, Inc., San Diego, CA).
Statistical analysis
Data are represented as mean ± SEM, ‘n’ represents the number of mice used in each group. Un-paired students ‘t’ test was used to determine statistical difference between two groups, and multiple comparisons were performed by one-way ANOVA followed by Bonferroni's post-hoc test. A value of P<0.05 was considered statistically significant.
Results
Characterization of cerebral microvessels
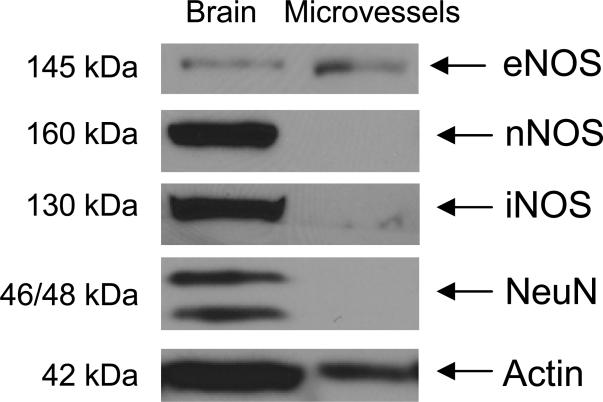
Expression of vascular and neuronal markers was determined in cerebral microvessels. Expressions of neuronal NOS and NeuN could be detected only in the brain, whereas microvessels did not express these neuronal markers (Figure 1). Expression of eNOS could be detected in the microvessels as well as in brain, while inducible NOS was predominantly expressed in brain (Figure 1).
Figure 1.
Protein expression studies demonstrating expression of NO synthase in the microvessels and brain. Expression of neuronal markers, nNOS and NeuN were not detected in purified microvessels, while nNOS and NeuN could be detected in the brain. Protein expression of iNOS was significantly lower in purified microvessels as compared to brain. Protein expression studies were performed by SDS-PAGE on 25 μg total protein in brain and microvessels, and data are representative of expression from three different wild-type (C57BL/6) mice.
Bioavailability of BH4 in GTP cyclohydrolase I-deficient cerebral microvessels
Enzymatic activity of GTP cyclohydrolase I, the rate-limiting enzyme in BH4 biosynthesis, was significantly reduced in cerebral microvessels of hph-1-/- mice. Interestingly, GTP cyclohydrolase I activity was also significantly reduced in cerebral microvessels of hph-1+/- mice as compared to wild-type controls (Figure 2A). There were no differences in GTP cyclohydrolase I activity between male and female mice (Figure 2A).
Figure 2.
Enzymatic activity of GTP cyclohydrolase I (A) and levels of tetrahydrobiopterin (BH4, B) were significantly decreased in the cerebral microvessels of hph-1-/- and hph-1+/- mice as compared to their sex-matched controls (* P<0.05, n=6 in comparison to wild-type male mice; #P<0.05, n=8 in comparison to wild-type female mice). Levels of 7,8-dihydrobiopterin (BH2), oxidized product of BH4 did not significantly increase in the cerebral microvessels of either hph-1-/- or hph-1+/- mice (C). In the cerebral microvessels of both male and female mice, ratio of BH4 to BH2, indicator of BH4 bioavailable for eNOS activation, were significantly decreased in the cerebral microvessels of hph-1-/- and hph-1+/- mice (* P<0.05, n=6 in comparison to wild-type male mice; #P<0.05, n=8 in comparison to wild-type female mice).
In wild-type mice, levels of BH4 in the cerebral microvessels were not significantly different between male and female animals (Figure 2B). We observed significant attenuation of BH4 in the hph1-/- mice of both gender (Figure 2B). Consistent with reduced GTP cyclohydrolase I activity in hph-1+/- mice irrespective of gender, BH4 levels were also significantly reduced, and the attenuated values were comparable to those from hph-1-/- mice (Figure 2B). The levels of 7,8- BH2 in the cerebral microvasculature of hph-1-/- and hph-1+/- mice were similar to 7,8-BH2 levels in wild-type mice (Figure 2C). However, the ratio of BH4 to 7,8-BH2 were significantly decreased in cerebral microvessels of hph-1-/- and hph-1+/- mice, and this decrease in ratio of BH4 to 7,8-BH2 ratio was independent of gender (Figure 2D).
Protein expression studies on the cerebral microvasculature of hph-1-/- and hph-1+/- mice demonstrated that expressions of eNOS remained unchanged (Figure 3).
Figure 3.
Representative Western blots and densitometric analysis demonstrating expression of eNOS in the cerebral microvessels of male and female, hph-1-/- and hph-1+/- mice. Expression of eNOS did not differ between these three experimental groups (n=6).
Endothelial NOS uncoupling and superoxide anion production
In the cerebral microvasculature of hph-1-/- mice, levels of superoxide anion were significantly increased in male as well as female mice. In addition, we observed a similar increase in superoxide anion production in the cerebral microvessels of hph-1+/- mice (Figure 4A).
Figure 4.
(A) Superoxide anion production in the cerebral microvessels of hph-1-/- and hph-1+/- mice. Basal superoxide production between wild-type male and female mice was not statistically significant. Superoxide anion production remained significantly increased in male and female cerebral microvessels in both hph-1-/- and hph-1+/- groups (* P<0.05, in comparison to their wild-type controls, n=8). Treatment with L-NAME (30 μmol/l) significantly reduced the superoxide anion production in cerebral microvessels of both hph-1-/- and hph-1+/- mice (# P<0.05 in L-NAME treated group as compared to corresponding sex-matched vehicle (Krebs-HEPES buffer) treatment, n=8). (B) Superoxide anion production in microvessels of female wild-type, hph-1-/- and hph-1+/-mice injected with BH4 (100 μmol/kg, 3hours). Treatment with BH4 abolished the increase in superoxide anion production observed in cerebral microvessels of hph-1-/-and hph-1+/- mice. Treatment with L-NAME (30 μmol/l) did not have any further effect on the superoxide anion production (P=ns, n=4).
The increased production of superoxide anions in hph-1-/- mice and hph-1+/- mice were abolished by NOS inhibitor L-NAME (30 μmol/l, Figure 4A), confirming the role of NOS in increased superoxide generation. Furthermore, superoxide anion production in wild-type mice was not affected by L-NAME, demonstrating selectivity of L-NAME in reversing superoxide generated by eNOS uncoupling. In addition, treatment with BH4 abolished the increase in superoxide anion production observed in cerebral microvessels of both hph-1-/- and hph-1+/- mice (Figure 4B). Subsequent treatment with L-NAME did not have any further effect on the superoxide anion production (Figure 4B).
To further confirm the selective contribution of eNOS uncoupling toward increased superoxide anion production in the cerebral microvasculature of hph-1-/- and hph-1+/- mice, we studied the expression of antioxidants proteins. Genetic inactivation of GTP cyclohydrolase I did not affect protein expression of CuZnSOD, MnSOD, and catalase (Figure 5) in the cerebral microvasculature. Protein expressions of extracellular SOD (ECSOD) or glutathione peroxidase1 could not be detected in the cerebral microvessels (data not shown).
Figure 5.
Representative Western blots and densitometric analysis demonstrating expression of antioxidant proteins in the cerebral microvessels of both male and female, hph-1-/- and hph-1+/- mice. Expressions of CuZn superoxide dismutase (CuZnSOD), MnSOD and catalase did not differ in cerebral microvessels of hph-1-/- and hph-1+/- mice, as compared to their sex-matched wild-type controls (n=6).
Effect of eNOS uncoupling on NO signaling
The levels of 3-nitrotyrosine, marker of peroxynitrite formation, was significantly increased in the cerebral microvessels of both hph-1-/- and hph-1+/- mice in comparison to their sex-matched wild-type control mice (Figure 6).
Figure 6.
Expression of 3-nitrotyrosine in the cerebral microvessels of both male and female, hph-1-/- and hph-1+/- mice. Densitometric analysis showed that 3-nitrotyrosine expression was significantly increased in both hph1-/- and hph1+/- mice (*P<0.05, n=4).
Levels of nitrite/nitrate, an indicator of NO production, tended to be higher in female mice as compared to male wild-type mice (Figure 7). In comparison to wild-type controls, levels of nitrite/nitrate were significantly reduced in cerebral microvessels of hph-1-/- mice. Similar to the reduction seen in hph-1-/- mice, levels of nitrite/nitrate were significantly reduced in cerebral microvessels of hph-1+/- mice (Figure 7).
Figure 7.
Levels of nitrite/nitrate, indicator of NO production, was significantly reduced in cerebral microvessels of hph-1-/- and hph-1+/- mice as compared to their sex-matched wild-type control mice (* P<0.05, n=6 in comparison to wild-type male mice; #P<0.05, n=6 in comparison to wild-type female mice).
Consistent with reduced NO production, basal levels of cGMP were significantly reduced in cerebral microvessels of male and female hph-1-/- mice (Figure 8). Similar reductions in basal levels of cGMP were also observed in hph-1+/- mice, irrespective of gender (Figure 8).
Figure 8.
Bar diagram depicting significantly reduced levels of cGMP, second messenger of NO, in cerebral microvessels of hph-1-/- and hph-1+/- mice as compared to their sex-matched wild-type control mice (* P<0.05, n=6 in comparison to wild-type male mice; #P<0.05, n=6 in comparison to wild-type female mice).
Discussion
We report several novel findings: a) significant reductions in BH4 levels were detected in the cerebral microvessels of homozygous or heterozygous hph-1 mice, b) reduced bioavailability of BH4 in cerebral microvessels of hph-1 mice resulted in increased production of superoxide anion, c) the increased superoxide anion production was selectively inhibited by either supplementation with BH4 or treatment with NOS inhibitor - L-NAME, demonstrating that eNOS is the source of superoxide anion, d) uncoupling of eNOS did not alter expression of antioxidant proteins in the cerebral microvessels, e) 3-nitrotyrosine formation was significantly increased in the cerebral microvessels of hph-1 mice, and f) NO production and basal cGMP levels were significantly reduced, indicative of endothelial dysfunction in BH4-deficient cerebral microvessels. Finally, no major difference was detected between the cerebral microvessels of male and female hph-1 mice. To the best of our knowledge, this is the first study to determine the effects of GTP cyclohydrolase I and BH4 deficiency on cerebral microvasculature.
We observed similar reductions in GTP cyclohydrolase I activity between hph-1-/- and hph-1+/- mice. Consistent with reduced GTP cyclohydrolase I activity, we also detected similar reductions in levels of BH4 between hph-1-/- and hph-1+/- mice. While we do not have an exact explanation for this observation, we also detected similar reductions in BH4 levels in small mesenteric arteries from hph-1-/- and hph-1+/- mice (d'Uscio LV, Smith LA, Katusic ZS, unpublished observations). These results suggest that mutation in single gene copy is sufficient to significantly reduce levels of BH4 in small resistant arteries in cerebral and peripheral circulation. Interestingly, and in contrast to BH4, deficiency of GTP cyclohydrolase I activity did not reduce the levels of 7,8-BH2 in the cerebral microvessels. This may be explained by potential oxidation of BH4 to 7,8-BH2 by peroxynitrite (Milstien & Katusic 1999), thereby increasing 7,8-BH2 to levels comparable with 7,8-BH2 levels detected in wild-type mice. As a result, ratio of BH4 to 7,8-BH2 was significantly reduced in vessels obtained from hph-1 mice.
We previously reported that eNOS is uncoupled in peripheral and conduit resistance arteries derived from hph-1 mice (d'Uscio et al. 2011). Conceptually, results of the present study regarding metabolism of BH4 in cerebral microvessels are consistent with findings obtained on peripheral blood vessels. However, eNOS-derived production of superoxide anion appears to be significantly higher in cerebral microvessels as compared to previously detected superoxide anion formation in aorta and mesenteric arteries. The exact reasons for higher production of superoxide anion by uncoupled eNOS in cerebral circulation are unclear. Interestingly, despite relatively high levels of superoxide anion in cerebral microvessels, we did not detect any alteration in antioxidant capacity or expression of eNOS, thereby suggesting that in the cerebral microvessels, adaptive responses to oxidative stress are absent. We ruled out the adaptive up-regulation of superoxide dismutase(s) and catalase, the enzymes that were previously reported to be increased in peripheral resistant vessels of hph-1 mice (d'Uscio et al. 2011). However, our studies do not rule out involvement of other antioxidants, including glutathione, in eliciting adaptive responses to oxidative stress. Furthermore, our conclusion is in agreement with reported inability of cerebral arterioles (but not aorta or large cerebral arteries) to develop adaptive response to increased superoxide anion production caused by deficiency of MnSOD (Andresen et al. 2004, Faraci et al. 2006b). Similar to our findings, availability of NO in cerebral arterioles was reduced as a result of increased production of superoxide anion in MnSOD-deficient mice (Faraci et al. 2006b). In addition, peroxynitrite formation, demonstrated by increased 3-nitrotyrosine levels provide additional evidence supporting the concept that oxidative stress is present in BH4-deficient cerebral microvasculature. In aggregate, our results suggest that eNOS uncoupling has more pronounced detrimental effect on availability of NO and formation of cyclic GMP in cerebral than in peripheral circulation (d'Uscio et al. 2011). Moreover, we provide evidence that uncoupling of eNOS causes oxidative stress in the cerebral microvessels.
Our findings may help to explain observations reported by number of previous studies demonstrating higher susceptibility of cerebral circulation to endothelial dysfunction under pathological conditions including hypertension (Didion et al. 2002, Didion et al. 2000, Faraci et al. 2006a), diabetes (Kitayama et al. 2006), hyperhomocysteinemia (Dayal et al. 2004), feeding with high fat diet (Beyer et al. 2008) as well as aging (Brown et al. 2007, Modrick et al. 2009). Of note, all of these conditions are associated with increased risk of stroke, dementia and Alzheimer's disease. Indeed, our previous study demonstrated that eNOS activity and production of NO in cerebral microvessels modulate expression and processing of amyloid precursor protein (Austin et al. 2010). In addition, our studies may also have important implications for understanding the pathogenesis of cerebral vasospasm. In fact, recent studies documented uncoupling of eNOS during development of cerebral vasospasm induced by subarachnoid hemorrhage (Sabri et al. 2011a), and demonstrated the ability of simvastatin to re-couple eNOS and alleviate cerebral vasospasm and neuronal injury (Sabri et al. 2011b). Further studies are obviously required to establish exact contribution and relevance of eNOS uncoupling in pathogenesis of endothelial dysfunction in the cerebral circulation.
In summary, our findings support the concept that under conditions of GTP cyclohydrolase I deficiency in cerebral microvasculature, eNOS becomes uncoupled to generate superoxide anions thereby resulting in oxidative stress and impaired NO/cGMP signaling. We believe that the results of the present study regarding high production of eNOS-derived superoxide anion in cerebral microvessels of hph-1 mice may help to explain susceptibility of cerebral circulation to endothelial dysfunction.
Acknowledgments
No conflicts of interest, financial or otherwise, are declared by the author(s). This study was funded in part by American Heart Association Scientist Development Grant (08-35436N to AVS), National Institutes of Health Grants (HL 91867, HL111062 to ZSK), and the Mayo Foundation.
List of abbreviations used
- 7,8-BH2
7,8-dihydrobiopterin
- BH4
tetrahydrobiopterin
- cGMP
3,5-cyclic guanosine monophosphate
- CuZnSOD
Copper and Zinc superoxide dismutase
- ECSOD
extracellular superoxide dismutase
- eNOS
endothelial nitric oxide synthase
- hph-1
hyperphenylalaninemic
- iNOS
inducible nitric oxide synthase
- L-NAME
L- NG-nitro arginine-methyl ester
- MnSOD
Manganese superoxide dismutase
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
References
- Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- Andresen JJ, Faraci FM, Heistad DD. Vasomotor responses in MnSOD-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1141–1148. doi: 10.1152/ajpheart.01215.2003. [DOI] [PubMed] [Google Scholar]
- Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ. Res. 2010;107:1498–1502. doi: 10.1161/CIRCRESAHA.110.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor gamma causes cerebral vascular dysfunction in response to a high-fat diet. Circ. Res. 2008;103:654–661. doi: 10.1161/CIRCRESAHA.108.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode VC, McDonald JD, Guenet JL, Simon D. hph-1: a mouse mutant with hereditary hyperphenylalaninemia induced by ethylnitrosourea mutagenesis. Genetics. 1988;118:299–305. doi: 10.1093/genetics/118.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler. Thromb. Vasc. Biol. 2007;27:1941–1946. doi: 10.1161/ATVBAHA.107.146852. [DOI] [PubMed] [Google Scholar]
- Canevari L, Land JM, Clark JB, Heales SJ. Stimulation of the brain NO/cyclic GMP pathway by peripheral administration of tetrahydrobiopterin in the hph-1 mouse. J. Neurochem. 1999;73:2563–2568. doi: 10.1046/j.1471-4159.1999.0732563.x. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Faraci FM. The role of oxidative stress and NADPH oxidase in cerebrovascular disease. Trends Mol. Med. 2008;14:495–502. doi: 10.1016/j.molmed.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino F, Barker JE, Brand MP, et al. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Milstien S, Richardson D, Smith L, Katusic ZS. Long-term vitamin C treatment increases vascular tetrahydrobiopterin levels and nitric oxide synthase activity. Circ. Res. 2003;92:88–95. doi: 10.1161/01.res.0000049166.33035.62. [DOI] [PubMed] [Google Scholar]
- d'Uscio LV, Smith LA, Katusic ZS. Erythropoietin increases expression and function of vascular copper- and zinc-containing superoxide dismutase. Hypertension. 2010;55:998–1004. doi: 10.1161/HYPERTENSIONAHA.110.150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Uscio LV, Smith LA, Katusic ZS. Differential effects of eNOS uncoupling on conduit and small arteries in GTP-cyclohydrolase I-deficient hph-1 mice. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H2227–2234. doi: 10.1152/ajpheart.00588.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayal S, Arning E, Bottiglieri T, Boger RH, Sigmund CD, Faraci FM, Lentz SR. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke. 2004;35:1957–1962. doi: 10.1161/01.STR.0000131749.81508.18. [DOI] [PubMed] [Google Scholar]
- Didion SP, Ryan MJ, Baumbach GL, Sigmund CD, Faraci FM. Superoxide contributes to vascular dysfunction in mice that express human renin and angiotensinogen. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1569–1576. doi: 10.1152/ajpheart.00079.2002. [DOI] [PubMed] [Google Scholar]
- Didion SP, Sigmund CD, Faraci FM. Impaired endothelial function in transgenic mice expressing both human renin and human angiotensinogen. Stroke. 2000;31:760–764. doi: 10.1161/01.str.31.3.760. discussion 765. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Lamping KG, Modrick ML, Ryan MJ, Sigmund CD, Didion SP. Cerebral vascular effects of angiotensin II: new insights from genetic models. J. Cereb. Blood Flow Metab. 2006a;26:449–455. doi: 10.1038/sj.jcbfm.9600204. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J. Appl. Physiol. 2006b;100:2089–2093. doi: 10.1152/japplphysiol.00939.2005. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 2011;164:213–223. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic ZS, d'Uscio LV, Nath KA. Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol. Sci. 2009;30:48–54. doi: 10.1016/j.tips.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo JP, Nicoli T, Alp NJ, Fullerton J, Flint J, Channon KM. Congenic mapping and genotyping of the tetrahydrobiopterin-deficient hph-1 mouse. Mol. Genet. Metab. 2004;82:251–254. doi: 10.1016/j.ymgme.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, Wilkins MR, Channon KM. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111:2126–2133. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- Kitayama J, Faraci FM, Gunnett CA, Heistad DD. Impairment of dilator responses of cerebral arterioles during diabetes mellitus: role of inducible NO synthase. Stroke. 2006;37:2129–2133. doi: 10.1161/01.STR.0000231654.79017.df. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JD, Cotton RG, Jennings I, Ledley FD, Woo SL, Bode VC. Biochemical defect of the hph-1 mouse mutant is a deficiency in GTP-cyclohydrolase activity. J. Neurochem. 1988;50:655–657. doi: 10.1111/j.1471-4159.1988.tb02961.x. [DOI] [PubMed] [Google Scholar]
- Meininger CJ, Marinos RS, Hatakeyama K, Martinez-Zaguilan R, Rojas JD, Kelly KA, Wu G. Impaired nitric oxide production in coronary endothelial cells of the spontaneously diabetic BB rat is due to tetrahydrobiopterin deficiency. Biochem. J. 2000;349:353–356. doi: 10.1042/0264-6021:3490353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstien S, Katusic Z. Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem. Biophys. Res. Commun. 1999;263:681–684. doi: 10.1006/bbrc.1999.1422. [DOI] [PubMed] [Google Scholar]
- Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am. J Physiol. Heart Circ. Physiol. 2009;296:H1914–1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2006;26:2439–2444. doi: 10.1161/01.ATV.0000243924.00970.cb. [DOI] [PubMed] [Google Scholar]
- Sabri M, Ai J, Knight B, Tariq A, Jeon H, Shang X, Marsden PA, Loch Macdonald R. Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2011a;31:190–199. doi: 10.1038/jcbfm.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M, Ai J, Marsden PA, Macdonald RL. Simvastatin re-couples dysfunctional endothelial nitric oxide synthase in experimental subarachnoid hemorrhage. PLoS One. 2011b;6:e17062. doi: 10.1371/journal.pone.0017062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam AV, Smith LA, He T, Nath KA, Katusic ZS. Endothelial progenitor cells stimulate cerebrovascular production of prostacyclin by paracrine activation of cyclooxygenase-2. Circ. Res. 2007;100:1379–1388. doi: 10.1161/01.RES.0000265848.55035.5d. [DOI] [PubMed] [Google Scholar]
- Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- Shimoji M, Hirayama K, Hyland K, Kapatos G. GTP cyclohydrolase I gene expression in the brains of male and female hph-1 mice. J. Neurochem. 1999;72:757–764. doi: 10.1046/j.1471-4159.1999.0720757.x. [DOI] [PubMed] [Google Scholar]
- Stroes E, Kastelein J, Cosentino F, Erkelens W, Wever R, Koomans H, Luscher T, Rabelink T. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J. Clin. Invest. 1997;99:41–46. doi: 10.1172/JCI119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Vivar J. Tetrahydrobiopterin, superoxide, and vascular dysfunction. Free Radic. Biol. Med. 2009;47:1108–1119. doi: 10.1016/j.freeradbiomed.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]