Abstract
The goals of the present study were to compare coronary resistance microvessel (CRM) remodeling between type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) mice, and to determine the impact of aerobic exercise training on CRM remodeling in diabetes. Eight week old male mice were divided into T1DM: control sedentary (Control-SD), T1DM sedentary (T1DM-SD) induced by streptozotocin, and T1DM exercise trained (T1DM-TR); T2DM: control sedentary (Db/db-SD), T2DM sedentary (db/db-SD), and T2DM trained (db/db-TR). Aerobic exercise training (TR) was performed on a mouse treadmill for 8 weeks. CRMs were isolated and mounted on a pressure myograph to measure and record vascular remodeling and mechanics. CRM diameters, wall thickness, stress-strain, incremental modulus remained unchanged in T1DM-SD mice compared to control, and exercise training showed no effect. In contrast, CRMs isolated from db/db-SD mice exhibited decreased luminal diameter with thicker microvascular walls, which significantly increased the wall:lumen ratio (Db/db-SD: 5.8 ± 0.3 vs. db/db-SD: 8.9 ± 0.7, p < 0.001). Compared to db/db-SD mice, coronary arterioles isolated from db/db-TR mice had similar internal diameter and wall thickness, while wall:lumen ratio (6.8 ± 0.2, p < 0.05) and growth index (db/db-SD: 16.2 vs. db/db-TR: 4.3, % over Db/db) were reduced. These data show that CRMs undergo adverse inward hypertrophic remodeling only in T2DM, but not T1DM, and that aerobic exercise training can partially mitigate this process.
Keywords: coronary remodeling, exercise, vascular, type 2 diabetes, type 1 diabetes, myocardial infarction
1. INTRODUCTION
Both type 1 diabetes mellitus (T1DM), and type 2 diabetes mellitus (T2DM) are increasing in prevalence in both adults and adolescent populations (CDC, 2011; Hotu et al., 2004; Ma and Chan 2009). Furthermore, both T1DM and T2DM are associated with vascular endothelial cell dysfunction, contributing to vasculopathies such as retinopathy, peripheral vascular disease, and coronary artery disease (CAD) (Brindisi et al., 2010; Buse et al., 2007), putting both T1DM and T2DM patients at an increased risk for myocardial infarction and ischemia (Roger et al., 2011; Soedamah-Muthu et al., 2006).
The adverse coronary vascular events associated with T1DM and T2DM are typically attributed to impaired endothelial function (Koltai et al., 1997; Park et al., 2011) and/or atherosclerosis (Larsen et al., 2007; Wong et al., 2005; Zeiher et al., 1991). For example, coronary arteries isolated from experimentally-induced T1DM rats showed impairments to acetylcholine-induced vasodilation, indicating endothelial dysfunction (Romero et al., 2008), and other studies showed that coronary arterioles isolated from T2DM db/db mice elicited depressed vasodilatory response to acetylcholine, but not sodium nitroprusside, indicating endothelial dysfunction but not smooth muscle dysfunction (Park et al., 2011). However, a recent study from our laboratory revealed that coronary resistance microvessels (CRMs) isolated from T2DM db/db mice undergo adverse inward hypertrophic remodeling independent of endothelial dysfunction and atherosclerosis (Katz et al., 2011). These findings were also associated with a significant reduction in coronary flow reserve (CFR), suggesting that the passive structural properties of the coronary microvasculature also contribute to adverse coronary function. Given that coronary blood flow is tightly matched with myocardial oxygen consumption (Tune et al., 2002), a chronic impairment in CFR can lead to myocardial ischemia. Whether adverse CRM remodeling occurs in T1DM remains to be determined.
There is no question that a sedentary lifestyle and lack of exercise contributes to the high incidence of T2DM (Hu 2003). Indeed, exercise training improves cardiovascular dysfunction in T2DM, including improved coronary endothelial function (Lee et al., 2011b; Moien-Afshari et al., 2008; Sixt et al., 2010) and atherosclerosis (Schuler et al., 1992; Sturek 2011), but the effects of exercise training on CRM remodeling is unknown. Furthermore, there is little evidence to date that exercise training is effective in mitigating cardiovascular dysfunction in T1DM. Therefore, the goals of the present study were to compare CRM remodeling between T1DM and T2DM mice, and to determine whether aerobic exercise training could mitigate the deleterious effects of CRM remodeling in diabetes.
2. MATERIALS AND METHODS
2.1 Animals
T1DM was induced in 8-week-old male wild-type C57BLKS/J mice (The Jackson Laboratories) injected intraperitoneally with either 20 mM sodium citrate buffer (Control) or a single 180 mg/kg dose of streptozotocin (STZ) at a final volume of 300 μL as previously described (Denroche et al., 2011; Kivela et al., 2006; Lehti et al., 2006). Only mice that exhibited fasting blood glucose of >200 mg/dL 2 weeks after STZ injection were included in the study. T2DM experiments were conducted on male control, nondiabetic heterozygous Db/db and T2DM db/db mice that were obtained from The Jackson Laboratories. All mice were housed under a 12-hour light/dark cycle at 22°C and 60% humidity and were allowed ad libitum access to standard low-fat laboratory chow and water. This study was conducted in accordance with the National Institutes of Health Guidelines, and it was approved by the institutional Animal Care and Use Committee at Nationwide Children’s Hospital.
2.2 Aerobic Exercise Protocol
T1DM and T2DM db/db mice were exercise trained (TR) on a small-animal treadmill with individual lanes and no electric stimulation (Columbus Instruments, Columbus, OH). Sedentary (SD) mice were not subjected to the treadmill training. Because one of the goals of our study was to determine whether exercise training was effective in mitigating deleterious CRM remodeling in diabetes, we did not include control-TR groups to assess the effect of exercise training in the absence of diabetes. To reduce the stress effects of the treadmill protocol, 7-week-old mice were initially submitted to an adaptation period prior to commencing the exercise training protocol at 8 weeks of age. This adaptation consisted of daily runs for 1 week, 0% grade, and beginning at 5–6 m/min for 30 min on the first day and was progressively increased to 5–6 m/min for 60 min by the fifth day. Only the animals that successfully completed the adaptation period were included in trained group.
After the adaptation period, the 8-week training program began. The duration and the speed started at 6 m/min for 60 min and 0% grade for the first week of training. During the second week, the animals were submitted to a critical velocity test to assess aerobic capacity and to establish intensity of treadmill training as previously described (Billat et al., 2005). Accordingly, the speed was gradually increased to 10 m/min for 60 min and 0% grade during the last 6 weeks of training for a total of 8 weeks of aerobic exercise training. All animals were trained 5 consecutive days/week between 6:00 a.m. and 9:00 a.m.
Finally, to evaluate the effectiveness of the training program, sedentary and trained mice were submitted to an acute incremental exercise testing on the treadmill during the last week of the study. The intensity of exercise was increased by 3 m/min (3–30 m/min) every 3 min at 0% grade until exhaustion, defined as an inability to maintain running speed. This test provided the total distance, total time, and the maximal speed run for each animal.
2.3 Fasting Glucose Measurements
Mice were fasted for 8 hours during the light cycle, after which blood was drawn from the tail vein at baseline and weekly until then end of the 8-week exercise training protocol. Blood glucose concentration was measured using the Accu-Chek Advantage meter (Roche, Indianapolis, IN).
2.4 Preparation of Coronary Resistance Microvessels
After the 8-week exercise training protocol, 16–17-week-old mice were anesthetized using 2% isoflurane, vaporized with 100% oxygen. The heart was excised and dissected in 4°C physiologic salt solution (PSS) composed of the following (in mM): 130 NaCl, 4 KCl, 1.2 MgSO4, 4 NaHCO3, 10 HEPES, 1.2 KH2PO4, 5 glucose, and 2.5 CaCl2 at pH 7.4. The right ventricle was carefully removed, and septal coronary resistance microvessels (CRMs, <150 μm internal diameter) were carefully isolated from the ventricular septum at the level of the papillary muscle. CRMs were excised and mounted onto 2 glass microcannulas within a pressure myograph chamber (Living Systems, Burlington, VT). Prior to any measurements, vessels were equilibrated for 30 minutes under constant intraluminal pressure (50 mmHg) at 37°C in PSS. Internal diameter, left and right wall thickness were measured using a video dimension analyzer (Living Systems Instrumentation, Burlington, VT).
2.5 Measurements of Coronary Arteriole Structure and Passive Mechanical Properties
All experiments were performed in Ca2+-free PSS in the presence of 2mM EGTA and 100μM sodium nitroprusside. A passive pressure-diameter curve was generated by increasing intraluminal pressure from a minimum of 0 mmHg to a maximum of 125 mmHg, and left and right wall thickness (WT) and internal diameters (Di) were recorded at each pressure; 0, 10, 25, 50, 75, 100 and 125 mmHg. The following structural and mechanical parameters were calculated as previously described (Katz et al., 2011):
External diameter (De) = Di + (2 × WT)
Wall/lumen ratio = (WT / Di) × 100
Cross Sectional Area (CSA) = π (De 2 − Di 2)/4, where De is the external diameter.
Growth index = (CSAC − CSAD) / CSAC, where c is control and D is diabetic.
Circumferential Stress (σ) = (P × Di) / (2WT), where P is pressure in dynes/cm2, Di is the internal diameter for a given intraluminal pressure and WT is medial thickness for a given intraluminal pressure.
Circumferential Strain (ε) = (Di − D0) / D0, where Di is the internal diameter for a given intraluminal pressure and D0 is the original diameter measured at 0 mmHg of intraluminal pressure.
Young’s elastic modulus (E) = stress (σ) / strain (ε) is used to determine arterial stiffness. However, since the stress-strain relationship was non-linear we obtained the tangential or incremental elastic modulus (Einc), or the slope of the stress-strain relationship (Δσ/Δε) over a range of pressures.
2.6 Statistical Analysis
All data are expressed as mean ± SEM with a probability of p < 0.05 used to denote statistical significance using GraphPad Prism 5.0 (GraphPad Software, LaJolla, CA). One-way ANOVA followed by a Bonferroni’s post-hoc test was performed on incremental exercise test results. Two-way repeated measures ANOVA followed by a Bonferroni’s post-hoc test was performed on pressure myography data. All other measurements were analyzed using an unpaired student’s t-test.
3. RESULTS
3.1 Exercise Training Effectiveness
Using three acute measures, our data show that both sedentary T1DM and T2DM mice have significant impairments in physical performance (Table 1). Compared to sedentary (SD) control mice, T1DM-SD and T2DM-SD mice displayed significant reductions in total time to perform the incremental test, total distance run during the incremental test, and maximal speed during the incremental test. The 8-week aerobic exercise training program for T1DM and T2DM mice employed in our study promoted a significant improvement in all three parameters of physical performance measured during the incremental test (Table 1).
Table 1.
Incremental Exercise Test in both sedentary (SD) and exercise trained (TR) T1DM and T2DM mice.
| T1DM
|
T2DM
|
|||||
|---|---|---|---|---|---|---|
| Control-SD | T1DM-SD | T1DM-TR | Db/db-SD | db/db-SD | db/db-TR | |
|
|
|
|||||
| Total Time (min) | 21.5 ± 0.6 | 9.8 ± 0.6 *** | 18.4 ± 0.9 ††† | 23.4 ± 1.1 | 10.5 ± 0.5 *** | 16.3 ± 0.6 ††† |
| Total Distance (m) | 265 ± 13 | 65 ± 7 *** | 193 ± 16 ††† | 311 ± 29 | 74 ± 6 *** | 162 ± 12 ††† |
| Maximal Speed (m/min) | 23 ± 0.7 | 11 ± 0.8 *** | 20 ± 0.9 ††† | 26 ± 1.1 | 12 ± 0.6*** | 18 ± 0.6 ††† |
p<0.0001 vs. respective control;
p<0.0001 vs. diabetic sedentary mice;
n = 7–10 per group.
3.2 Final Body Weight, Blood Glucose
At the end of the study, T1DM-SD mice exhibited significantly lower body weights than Control-SD mice injected with only citrate buffer (Table 2), which was unaffected by exercise training. Conversely, fasting blood glucose was significantly higher in T1DM-SD animals, which was maintained in the T1DM-TR group. Both body weight and blood glucose were significantly augmented in the T2DM db/db-SD mice when compared with heterozygous sedentary mice, which remained unchanged during exercise training.
Table 2.
Final body weight and fasting blood glucose measured at the end of the 8-week exercise training protocol.
| T1DM
|
T2DM
|
|||||
|---|---|---|---|---|---|---|
| Control-SD | T1DM-SD | T1DM-TR | Db/db-SD | db/db-SD | db/db-TR | |
|
|
|
|||||
| Body Weight (kg) | 26.9 ± 0.6 | 20.5 ± 0.8 *** | 17.8 ± 0.6 *** | 29.1 ± 0.4 | 45.4 ± 1.2 *** | 42.1 ± 1.6 *** |
| Fasting Blood Glucose (mg/dL) | 115 ± 4 | 393 ± 49 *** | 370 ± 50 *** | 117 ± 4 | 536 ± 10 *** | 496 ± 17*** |
p<0.0001 vs. respective control;
n = 9 per group.
3.3 T1DM Septal Coronary Resistance Microvessel Remodeling and Mechanics
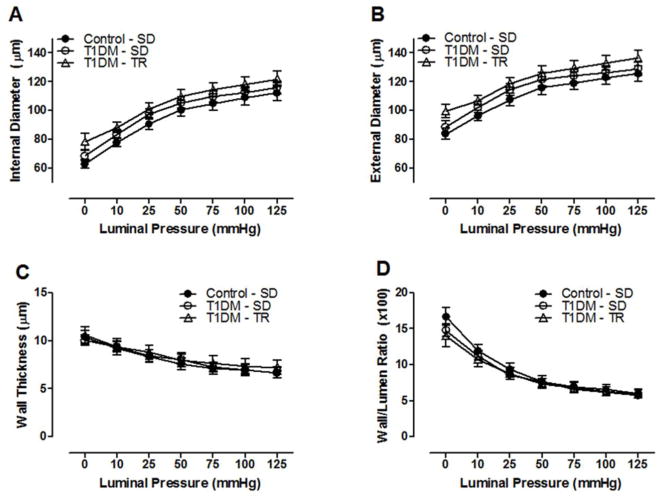
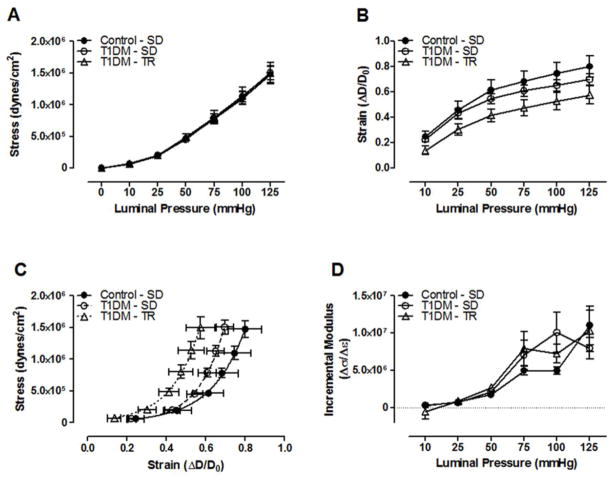
Septal CRMs isolated from T1DM-SD mice did not undergo any significant structural remodeling 8 weeks after the induction of T1DM using streptozotocin as exhibited by a lack of alteration in luminal and external diameters, wall thickness, or the wall/lumen ratio (Figure 1A–D). Furthermore, aerobic exercise training of T1DM mice had no effect on coronary remodeling. The mechanical properties of septal CRMs, including circumferential stress was unaltered, although a slight but not significant decrease in strain resulted in a leftward shift in the stress-strain curve. However, the incremental modulus of elasticity remained unaltered among T1DM groups either in the presence or absence of exercise training (Figure 2A–D), suggesting no significant effect of T1DM on vessel wall elasticity in the presence or absence of exercise.
Figure 1.
T1DM coronary resistance microvessel passive structural measurements. (A) internal luminal diameter, (B) external diameter, (C) wall thickness, and (D) wall/lumen ratio over a range of pressures. n=8–10 per group.
Figure 2.
T1DM coronary resistance microvessel passive mechanical properties. (A) Circumferential stress and (B) strain over a range of pressures. (C) CRM stress-strain curves (D) and incremental modulus of elasticity. n=8–10 per group.
3.4 T2DM Septal Coronary Resistance Microvessel Remodeling and Mechanics
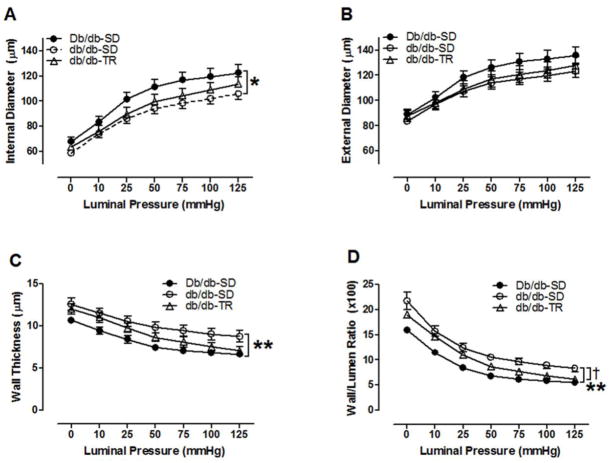
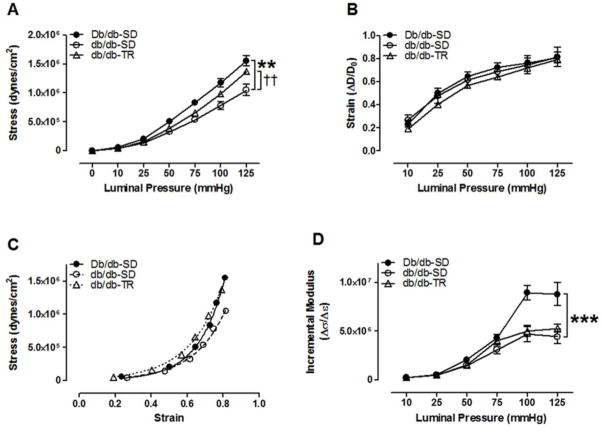
T2DM imparted inward remodeling of septal CRMs as evidenced by a reduction in lumen diameter (Figure 3A) and increased wall thickness (Figure 3C) and wall/lumen ratio (Figure 3D), and a growth index of 16.2% in 16–17-week-old db/db mice. External diameters remained unchanged among groups (Figure 3B). This adverse CRM remodeling was partially reversed by 8 weeks of aerobic exercise training (Figure 3A–D), including a reduction in the growth index to 4.3%. We further investigated the passive mechanical properties of CRMs isolated from both sedentary and exercise trained T2DM mice. Circumferential wall stress was significantly reduced in the db/db- SD CRMs when compared to Db/db-SD (Figure 4A). This was partially reversed by exercise training. Circumferential wall strain remained unchanged among groups (Figure 4B). db/db-SD CRMs exhibited reduced slope and rightward shift in the stress-strain curve (Figure 4C), which contributed to the reduction in CRM stiffness in the incremental modulus (Figure 4D). CRMs isolated from db/db-TR mice remained less stiff as calculated by incremental modulus of elasticity (Figure 4D).
Figure 3.
T2DM coronary resistance microvessel passive structural measurements. (A) internal diameter, (B) external diameter, (C) wall thickness, (D) wall:lumen ratio. *p<0.05 and **p<0.01 vs. Db/db-SD; †p<0.05 vs. db/db-SD; n=8–10 per group.
Figure 4.
T2DM coronary resistance microvessel passive mechanical properties. (A) Circumferential stress and (B) strain over a range of pressures. (C) CRM stress-strain curves (D) and incremental modulus of elasticity. **p<0.01 and ***p<0.001 vs. Db/db- SD; ††p<0.01 vs. db/db-SD; n=8–10 per group.
4. DISCUSSION
Diabetes is a cardiovascular disease (Grundy et al., 1999) that has been associated with increased risk of myocardial infarction, which is related to the severity of coronary artery disease. However, studies on diabetic coronary artery disease in diabetes have focused mainly on endothelial function and atherosclerosis, and no studies to date have compared CRM remodeling between T1DM and T2DM or evaluated the effectiveness of exercise training in improving CRM remodeling. In our current study, we compared structural remodeling and mechanics of CRMs in both T1DM and T2DM in the presence and absence of chronic aerobic exercise training. Surprisingly, our study revealed no overt alterations in structural remodeling or mechanics of CRMs isolated from T1DM mice, and exercise training remained ineffective in altering that status. In contrast, our data indicate that exercise training was effective in mitigating the deleterious inward hypertrophic structural remodeling of CRMs isolated from T2DM db/db mice. Finally, we show that exercise training was effective in improving exercise tolerance of both T1DM and T2DM mice as evidenced by increased speed, distance traveled, and time spent during an acute incremental exercise test conducted at the end of the 8-week exercise training period.
T1DM patients are at an increased risk of ischemic events such as myocardial infarction, yet there have been few studies on the coronary blood supply to the heart in T1DM. Clinical evidence indicates increased coronary atherosclerosis and endothelial dysfunction in these patients (Grundy et al., 1999). For example, endothelial expression of gap junctions Cx-37 and Cx-40 were reduced in STZ (T1DM) rats, which impacted intercellular communication and permeability, as well as endothelial dysfunction in T1DM (Makino et al., 2008). There are only a few studies that have investigated vascular remodeling in T1DM, none of which have looked at remodeling of the coronary circulation. Crijns et al. reported eutrophic outward remodeling of large mesenteric arteries in diabetic rats induced by streptozotocin (Crijns et al., 1999), similar to our study in T2DM where we reported eutrophic outward remodeling of mesenteric arterioles in T2DM db/db mice (Souza-Smith et al., 2011). Our data showing no overt remodeling of CRMs isolated from T1DM mice suggest that CRM remodeling may not contribute to adverse myocardial ischemia and that the increased incidence of myocardial infarction in T1DM patients may be primarily due to endothelial dysfunction and/or atherosclerosis as others have reported. We cannot rule out the possibility that changes in CRM stiffness may also contribute to impaired coronary blood flow since we observed a trend toward increased CRM stiffness as evidenced by a leftward shift in the stress-strain curve (Figure 2C) in T1DM that did not achieve statistical significance. It is tempting to speculate that this observation may be attributable to elevated extracellular matrix cross-linking induced by increased advanced glycation end product formation in chronic high-glucose states (Yan et al., 2008). Further studies are warranted to investigate these mechanisms.
In contrast to T1DM, we observed the expected pattern of inward hypertrophic remodeling of CRMs isolated from T2DM db/db mice that was associated with a reduction in stiffness, as we previously reported (Katz et al., 2011). Importantly, this remodeling was the result of VSMC proliferation and an imbalance in the collagen/elastin ratio. Furthermore, adverse CRM remodeling in T2DM was associated with a significant impairment in both basal and hyperemic coronary flow, which limited the capacity of coronary flow reserve. Our current data are in keeping with our previous studies. It is important to note that our pressure myography studies were performed under passive Ca2+-free conditions in the presence of EGTA and SNP, and therefore, it would reason that the adverse CRM remodeling observed in our current study was independent of endothelial dysfunction. However, given that coronary endothelial dysfunction has been reported in vivo in the db/db mouse model of T2DM (Park et al., 2011), we cannot rule out the contribution of the endothelium to the remodeling process as it chronically occurs in vivo. Another interesting finding in comparing CRM remodeling between T1DM and T2DM is that high glucose alone does not appear to promote the adverse inward hypertrophic coronary resistance microvessel remodeling as it was not observed in the T1DM mice. As high glucose, dyslipidemia, inflammation are common to both T1DM and T2DM patients, whether adverse remodeling is the cumulative result of these common cardiovascular insults combined with insulin resistance central to T2DM is an important question that remains to be investigated. This point that stems from our study also highlights the importance of using an appropriate diabetic animal model because there were obvious differences in CRM remodeling between T1DM and T2DM, even though both had similar degrees of hyperglycemia. The ease of use of beta cell toxins such as streptozotocin to induce diabetes cannot be superimposed as a type 2 or a pseudo-type 2 diabetic model.
Exercise training has long been a preventive and therapeutic measure to combat the deleterious effects of diabetes, especially T2DM. One of the long-term challenges is exercise tolerance in T1DM patients, since increased physical activity demands more energy utilization by the muscles, which is impaired in T1DM due to the lack of insulin to activate the GLUT transporters for glucose uptake. Our current data show that both sedentary T1DM and T2DM mice exhibit exercise intolerance that is improved with chronic exercise training, although the T1DM mice experienced profound weight loss by the end of the 8-week training program.
The cardiovascular consequences of regular aerobic exercise training are numerous in benefit. In addition to significantly improved glycemic control, insulin resistance, and dyslipidemia (Marwick et al., 2009), exercise training has been shown to improve mild heart failure, oxidative stress, blood pressure (Kim et al., 2006), and endothelial dysfunction (Maiorana et al., 2001) in T2DM patients. Only a few studies have investigated the effects of exercise training on vascular remodeling. For example, Kim et al. showed that type 2 diabetic patients subjected to 6 months of exercise training reduced carotid artery intima-media thickness (Kim et al., 2006). Indeed, our current data showing improved CRM remodeling in exercise trained T2DM db/db mice is in keeping with other improved cardiovascular benefits, such as improved coronary artery function (Moien-Afshari et al., 2008), reduced inflammation (Leon et al., 2005), improved coronary endothelial function (Lee et al., 2011b), reduced oxidative stress (Nojima et al., 2008), and increased adiponectin (Lee et al., 2011a). Our data suggest that lifestyle modifications including exercise training in patients may be beneficial in mitigating CRM remodeling only in T2DM, whereas there may be little to no therapeutic benefit in T1DM.
5. CONCLUSIONS
Our data show that there are obvious different remodeling patterns that occur in the coronary microcirculation between type 1 and type 2 diabetes mellitus, and further shows that aerobic exercise training significantly improves adverse CRM remodeling only in T2DM. Hyperglycemia in the setting of impaired insulin production/availability (T1DM) does not impart alterations in coronary structure, and therefore, adverse coronary events in these patient populations likely reflect a combination of endothelial dysfunction and atherosclerosis as has been previously established. Moreover, our data suggests that exercise training is ineffective in altering CRM structure in T1DM. In contrast, hyperglycemia in the setting of elevated insulin and insulin resistance (T2DM) imparts adverse CRM remodeling can be improved by aerobic exercise training. We postulate that at least part of the coronary, and therefore cardiac, benefit of regular aerobic exercise training in T2DM is due to improved coronary resistance microvessel remodeling.
Acknowledgments
This research was supported by National Institutes of Health (NIH) 2R01HL056046 and 2R01HL63318 (to PAL), T32HL098039 (to AJT), and The Heart Center at Nationwide Children’s Hospital. Maria A. Delbin was supported in part by FAPESP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol. 2005;98:1258–63. doi: 10.1152/japplphysiol.00991.2004. [DOI] [PubMed] [Google Scholar]
- Brindisi MC, Bouillet B, Verges B, Halimi S. Cardiovascular complications in type 1 diabetes mellitus. Diabetes Metab. 2010;36:341–4. doi: 10.1016/j.diabet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115:114–26. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- CDC. National Diabetes Fact Sheet: national estimates and general information on diabetes and prediabetes in the United States. Vol. 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- Crijns FR, Wolffenbuttel BH, De Mey JG, Struijker Boudier HA. Mechanical properties of mesenteric arteries in diabetic rats: consequences of outward remodeling. Am J Physiol. 1999;276:H1672–7. doi: 10.1152/ajpheart.1999.276.5.H1672. [DOI] [PubMed] [Google Scholar]
- Denroche HC, Levi J, Wideman RD, Sequeira RM, Huynh FK, Covey SD, Kieffer TJ. Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes. 2011;60:1414–23. doi: 10.2337/db10-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–46. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- Hotu S, Carter B, Watson PD, Cutfield WS, Cundy T. Increasing prevalence of type 2 diabetes in adolescents. J Paediatr Child Health. 2004;40:201–4. doi: 10.1111/j.1440-1754.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- Hu FB. Sedentary lifestyle and risk of obesity and type 2 diabetes. Lipids. 2003;38:103–8. doi: 10.1007/s11745-003-1038-4. [DOI] [PubMed] [Google Scholar]
- Katz PS, Trask AJ, Souza-Smith FM, Hutchinson KR, Galantowicz ML, Lord KC, Stewart JA, Jr, Cismowski MJ, Varner KJ, Lucchesi PA. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res Cardiol. 2011;106:1123–34. doi: 10.1007/s00395-011-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Lee SJ, Kang ES, Kang S, Hur KY, Lee HJ, Ahn CW, Cha BS, Yoo JS, Lee HC. Effects of lifestyle modification on metabolic parameters and carotid intima-media thickness in patients with type 2 diabetes mellitus. Metabolism. 2006;55:1053–9. doi: 10.1016/j.metabol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Kivela R, Silvennoinen M, Touvra AM, Lehti TM, Kainulainen H, Vihko V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J. 2006;20:1570–2. doi: 10.1096/fj.05-4780fje. [DOI] [PubMed] [Google Scholar]
- Koltai MZ, Hadhazy P, Posa I, Kocsis E, Winkler G, Rosen P, Pogatsa G. Characteristics of coronary endothelial dysfunction in experimental diabetes. Cardiovasc Res. 1997;34:157–63. doi: 10.1016/s0008-6363(97)00050-3. [DOI] [PubMed] [Google Scholar]
- Larsen JR, Tsunoda T, Tuzcu EM, Schoenhagen P, Brekke M, Arnesen H, Hanssen KF, Nissen SE, Dahl-Jorgensen K. Intracoronary ultrasound examinations reveal significantly more advanced coronary atherosclerosis in people with type 1 diabetes than in age- and sex-matched non-diabetic controls. Diab Vasc Dis Res. 2007;4:62–5. doi: 10.3132/dvdr.2007.009. [DOI] [PubMed] [Google Scholar]
- Lee S, Park Y, Dellsperger KC, Zhang C. Exercise training improves endothelial function via adiponectin-dependent and independent pathways in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2011a;301:H306–14. doi: 10.1152/ajpheart.01306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Park Y, Zhang C. Exercise Training Prevents Coronary Endothelial Dysfunction in Type 2 Diabetic Mice. Am J Biomed Sci. 2011b;3:241–252. doi: 10.5099/aj110400241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehti TM, Silvennoinen M, Kivela R, Kainulainen H, Komulainen J. Effects of streptozotocin-induced diabetes and physical training on gene expression of extracellular matrix proteins in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2006;290:E900–7. doi: 10.1152/ajpendo.00444.2005. [DOI] [PubMed] [Google Scholar]
- Leon AS, Franklin BA, Costa F, Balady GJ, Berra KA, Stewart KJ, Thompson PD, Williams MA, Lauer MS. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111:369–76. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- Ma RC, Chan JC. Diabetes: incidence of childhood type 1 diabetes: a worrying trend. Nat Rev Endocrinol. 2009;5:529–30. doi: 10.1038/nrendo.2009.180. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O’Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor R, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001;38:860–6. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am J Physiol Cell Physiol. 2008;295:C221–30. doi: 10.1152/ajpcell.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwick TH, Hordern MD, Miller T, Chyun DA, Bertoni AG, Blumenthal RS, Philippides G, Rocchini A. Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:3244–62. doi: 10.1161/CIRCULATIONAHA.109.192521. [DOI] [PubMed] [Google Scholar]
- Moien-Afshari F, Ghosh S, Elmi S, Khazaei M, Rahman MM, Sallam N, Laher I. Exercise restores coronary vascular function independent of myogenic tone or hyperglycemic status in db/db mice. Am J Physiol Heart Circ Physiol. 2008;295:H1470–80. doi: 10.1152/ajpheart.00016.2008. [DOI] [PubMed] [Google Scholar]
- Nojima H, Watanabe H, Yamane K, Kitahara Y, Sekikawa K, Yamamoto H, Yokoyama A, Inamizu T, Asahara T, Kohno N. Effect of aerobic exercise training on oxidative stress in patients with type 2 diabetes mellitus. Metabolism. 2008;57:170–6. doi: 10.1016/j.metabol.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Park Y, Yang J, Zhang H, Chen X, Zhang C. Effect of PAR2 in regulating TNF-alpha and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol. 2011;106:111–23. doi: 10.1007/s00395-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, Caldwell RB, Caldwell RW. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler G, Hambrecht R, Schlierf G, Niebauer J, Hauer K, Neumann J, Hoberg E, Drinkmann A, Bacher F, Grunze M, et al. Regular physical exercise and low-fat diet. Effects on progression of coronary artery disease. Circulation. 1992;86:1–11. doi: 10.1161/01.cir.86.1.1. [DOI] [PubMed] [Google Scholar]
- Sixt S, Beer S, Bluher M, Korff N, Peschel T, Sonnabend M, Teupser D, Thiery J, Adams V, Schuler G, et al. Long- but not short-term multifactorial intervention with focus on exercise training improves coronary endothelial dysfunction in diabetes mellitus type 2 and coronary artery disease. Eur Heart J. 2010;31:112–9. doi: 10.1093/eurheartj/ehp398. [DOI] [PubMed] [Google Scholar]
- Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care. 2006;29:798–804. doi: 10.2337/diacare.29.04.06.dc05-1433. [DOI] [PubMed] [Google Scholar]
- Souza-Smith FM, Katz PS, Trask AJ, Stewart JA, Jr, Lord KC, Varner KJ, Vassallo DV, Lucchesi PA. Mesenteric resistance arteries in type 2 diabetic db/db mice undergo outward remodeling. PLoS One. 2011;6:e23337. doi: 10.1371/journal.pone.0023337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturek M. Ca2+ regulatory mechanisms of exercise protection against coronary artery disease in metabolic syndrome and diabetes. J Appl Physiol. 2011;111:573–86. doi: 10.1152/japplphysiol.00373.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tune JD, Richmond KN, Gorman MW, Feigl EO. Control of coronary blood flow during exercise. Exp Biol Med (Maywood) 2002;227:238–50. doi: 10.1177/153537020222700404. [DOI] [PubMed] [Google Scholar]
- Wong ND, Rozanski A, Gransar H, Miranda-Peats R, Kang X, Hayes S, Shaw L, Friedman J, Polk D, Berman DS. Metabolic syndrome and diabetes are associated with an increased likelihood of inducible myocardial ischemia among patients with subclinical atherosclerosis. Diabetes Care. 2005;28:1445–50. doi: 10.2337/diacare.28.6.1445. [DOI] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285–93. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–92. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]