Abstract
The α9α10 nicotinic acetylcholine receptor (nAChR) may be a potential target in pathophysiology of the auditory system, chronic pain and breast and lung cancers. Alpha-conotoxins, from the predatory marine snail Conus, are potent nicotinic antagonists, some of which are selective for the α9α10 nAChR. Here we report a two-order of magnitude species difference in the potency of α-conotoxin RgIA for the rat vs. human α9α10 nAChR. We investigated the molecular mechanism of this difference. Heterologous expression of the rat α9 with the human α10 subunit in Xenopus oocytes resulted in a receptor that was blocked by RgIA with potency similar to that of the rat α9α10 nAChR. Conversely, expression of the human α9 with that of the rat α10 subunit resulted in a receptor that was blocked by RgIA with potency approaching that of the human α9α10 receptor. Systematic substitution of residues found in the human α9 subunit into the homologous position in the rat α9 subunit revealed that a single point mutation, Thr56 to Ile56, primarily accounts for this species difference. Remarkably, although the α9 nAChR subunit has previously been reported to provide the principal (+) binding face for binding of RgIA, Thr56 is located in the (−) complementary binding face.
Keywords: nicotinic, conotoxin, structure-activity, acetylcholine, point-mutant, species difference
Introduction
Nicotinic acetylcholine receptors (nAChRs) are members of the Cys-loop family of ligand gated ion channels, whose other members include 5HT3, GABAA, GABAC and glycine receptors (Lester et al. 2004; Azam and McIntosh 2006). Pentameric nAChRs are found both at the neuromuscular junction as well as in central and peripheral neurons. The neuronal nAChRs are composed of combination of α and/or β subunits; nine non-muscle α (α2–α10) and three β (β2–β4) subunits have been identified (Albuquerque et al. 2009). Alpha subunits may combine as homopentameric, or different αand β subunits may combine to form heteromeric, nAChRs. These various nAChR subtypes have distinct pharmacological functions and physiological roles and have distinct yet overlapping expression patterns in central and peripheral neurons (Gotti et al. 2006; Gotti et al. 2007; Albuquerque et al. 2009; Millar and Gotti 2009; Wu and Lukas 2011).
α9 and α10 subunits were originally characterized in cochlear hair cells (Elgoyhen et al. 1994; Elgoyhen et al. 2001). These subunits assemble to form the nAChR that mediates synaptic transmission between efferent cholinergic fibers that descend from the brainstem and hair cells of the cochlea. The nAChRs of outer hair cells not only regulate hair cell innervation but also are part of the molecular mechanism that may protect the inner ear from damage resulting from excessively loud noise (Elgoyhen et al. 1994; Vetter et al. 1999; Elgoyhen and Katz 2011).
In addition to the auditory system, transcripts and/or protein for the α9 and α10 subunits have been reported in lymphocytes, skin keratinocytes, sperm, dorsal root ganglion, sympathetic neurons and immune cells (Lustig et al. 2001; Lips et al. 2002; Haberberger et al. 2004; Kurzen et al. 2004; Peng et al. 2004; Kumar and Meizel 2005). The physiological roles of the α9α10 nAChR in non-auditory systems are not well understood. However, recent studies have suggested that α9-containing receptors may be important in pathophysiological states. α9α10 antagonists are analgesic in neuropathic pain models (Vincler et al. 2006; McIntosh et al. 2009). The analgesic effects of α9α10 blockade may be the result of an immunomodulatory effect. Chronic constriction injury (CCI) of the sciatic nerve increases the number of ACh-producing lymphocytes at the site of nerve injury providing a localized source of ACh (Vincler et al. 2006). The administration of α9α10 antagonists significantly reduced the number of lymphocytes and macrophages, including choline acetyltransferase-positive lymphocytes that occur after CCI (Vincler et al. 2006). This α9α10-mediated decrease in the number of immune cells correlates with the analgesic effects observed and is in agreement with the dependence of injury-induced behavioral hypersensitivity on the presence of lymphocytes and macrophages (but see (Klimis et al. 2011; Lewis et al. 2012)). Small molecule α9α10 nAChR antagonists have also recently been developed. These antagonists are active in several animal models of chronic pain (Holtman et al. 2011; Zheng et al. 2011). The α9α10 nAChR has also been implicated in wound healing, human breast and lung cancer (Lee et al. 2010; Chikova and Grando 2011; Lee et al. 2011; Wu et al. 2011; Chikova et al. 2012). Thus, there is an urgent need for understanding the molecular pharmacology of the human α9α10 receptor. α-conotoxins, small, disulfide-rich peptides, have become standard ligands for structural and functional studies of nAChRs. The most potent and selective ligand known for α9α10 nAChR is α-conotoxin (α-CTx) RgIA, isolated from the carnivorous marine snail Conus regius (Ellison et al. 2006). Here we show that α-CTx RgIA is 300-fold less potent on the human vs. rat α9α10 receptor. Mutational studies indicate that the primary determinant of this disparity is a single amino acid difference between the rat and human α9 nAChR subunit. Nicotinic ligands bind at the subunit interface between receptor subunits. Although the (+) face of the α9 subunit has been considered the principal binding site for α9;α10 nAChRs (Perez et al. 2009), surprisingly, the point mutation identified in the present study lies on the (−) face of α9. The present findings indicate a critical role for the complementary face of the α9 subunit and provide mechanistic insight into α-CTx binding to the α9α10 nAChR.
Methods
Materials
Acetylcholine chloride, atropine, and bovine serum albumin (BSA) were obtained from Sigma. α-CTx RgIA was synthesized as described previously (Ellison et al. 2006). Clones of rat α9 and α10 cDNAs in pGEMHe and pSGEM vectors, respectively, were kindly provided by A. Belen Elgoyhen (Universidad de Buenos Aires, Buenos Aires, Argentina). Clones for the human α9 and α10 subunits were kindly provided by Lawrence Lustig (Johns Hopkins University, Baltimore, MD). The human subunits were subcloned into the pSGEM vector for Xenopus oocyte expression.
Methods
Construction of point mutations
Point mutants were made by PCR. Primers containing the desired point mutation flanked by at least 15 bases on either side were synthesized. Using the non-strand displacing action of Pfu Turbo DNA polymerase, the mutagenic primers were extended and incorporated by PCR. The methylated, nonmutated parental cDNA was digested with Dpn I. The mutated DNA was transformed into DH10B or DH5α competent cells and isolated using the Qiaprep mini prep kit (Qiagen, Valencia, CA) and sequenced to ascertain the incorporation of the desired mutation.
cRNA preparation and injection
Capped cRNA for the various subunits were made using the mMessage mMachine in vitro transcription kit (Ambion, Austin, TX) following linearization of the plasmid. The cRNA was purified using the Qiagen RNeasy kit (Qiagen, Valencia, CA). The concentration of cRNA was determined by absorbance at 260 nm. cRNA of either wildtype α9 or mutant α9 subunit was mixed at a 1:1 ratio with wildtype α10 for a final concentration of at least 500 ng/µl for each subunit cRNA. One hundred to 150 nl of this mixture was injected into each Xenopus oocyte with a Drummond microdispenser (Drummond Scientific, Broomall, PA), as described previously (Cartier et al. 1996), and incubated at 17°C. Oocytes were injected within one day of harvesting and recordings were made 2–4 days post-injection.
Voltage-clamp Recording
Oocytes were voltage-clamped and exposed to ACh and peptide as described previously (Cartier et al. 1996). Briefly, the oocyte chamber consisting of a cylindrical well (~30 µl in volume) was gravity perfused at a rate of ~2 ml/min with ND-96 buffer (96.0 mM NaCl, 2.0 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, 5 mM HEPES, pH 7.1–7.5, supplemented with 0.1 mg/ml BSA). The oocyte was subjected once a minute to a 1 sec pulse of 100 µM ACh. For toxin concentrations of 1 µM and lower, once a stable baseline was achieved, either ND-96 alone or ND-96 containing varying concentrations of the α-conotoxins was perfusion-applied, during which 1-sec pulses of 100 µM ACh were applied every 90 sec until a constant level of block was achieved. For toxin concentrations of 10 µM and higher, the buffer flow was stopped and the toxin bath-applied and allowed to incubate with the oocyte for 5 min, after which the ACh pulse was resumed.
Data Analysis
For the baseline response, at least three ACh responses were averaged. To determine the percent block induced by toxin, 2–3 ACh responses, obtained after a steady state block had been achieved, were averaged and the value divided by the pre-toxin baseline value to yield a % response. The dose-response data were fit to the equation, Y = 100/(1 + 10^((LogEC50 – Log[Toxin])×nH)), where nH is the Hill coefficient, by non-linear regression analysis using GraphPad Prism (GraphPad Software, San Diego, CA). Each data point is mean ± SEM from at least 3 oocytes.
Results
Rat and human α9α10 nAChRs are differentially sensitive to α-CTx RgIA
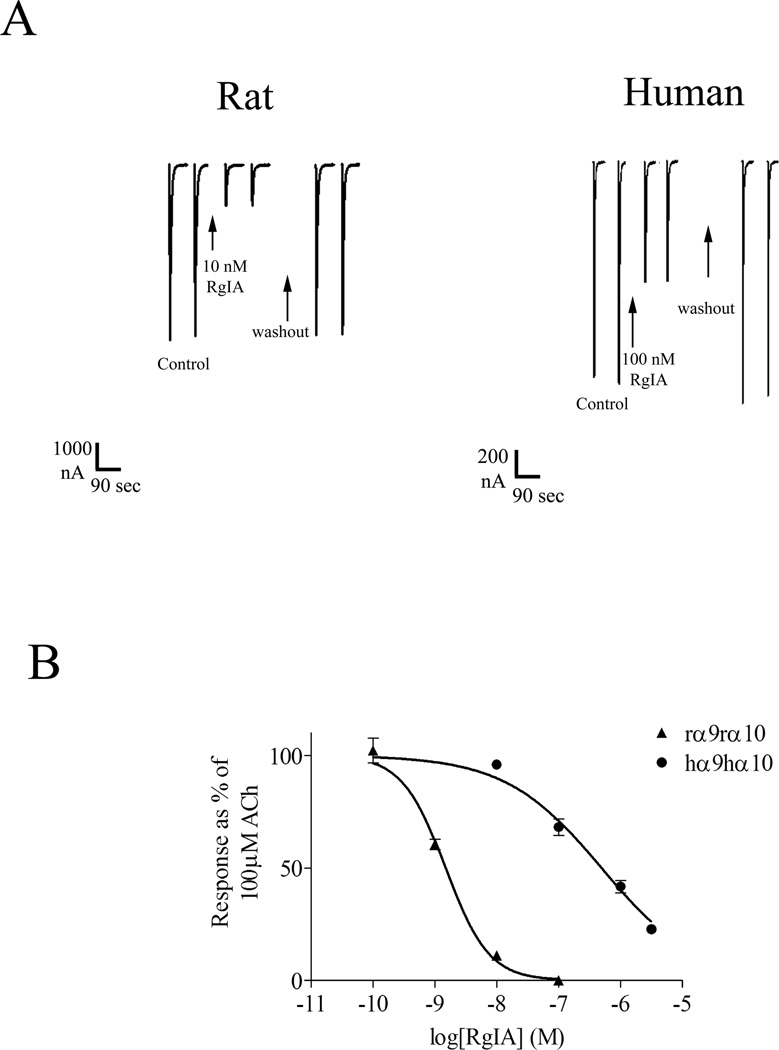
Disulfide-rich toxin antagonists from marine snails of the genus Conus have played pivotal roles in dissecting the structure and function of nAChRs (Millard et al. 2004; Janes 2005; Dutertre and Lewis 2006; Livett et al. 2006; Han et al. 2008; Azam and McIntosh 2009; Kasheverov et al. 2009; Armishaw 2010; Muttenthaler et al. 2011). The activity of α-CTx RgIA was originally characterized on heterologously expressed rat α9α10 nAChRs and native α9α10 nAChRs expressed in rat cochlear hair cells (Ellison et al. 2006). For a number of previously characterized α-conotoxins the affinity for rodent vs. human nAChRs has been similar (Dowell et al. 2003; Azam et al. 2005). In the present study, we compared the activity of α-CTx RgIA on the rat α9α10 receptor with that of the human nAChR (Figure 1). We note that current amplitudes of the oocyte-expressed human α9α10 nAChR were usually much smaller than those observed for the rat α9α10 nAChR (Fig. 1A). Lower expression of the human nAChR has also been observed by other investigators (Halai et al. 2009). In contrast to what has been reported for other α-conotoxins, there was a large, two-order of magnitude difference of α-CTx RgIA potency between the rat and human receptor. α-CTx RgIA blocked the rat α9α10 nAChR expressed in Xenopus oocytes with an IC50 of 1.5 nM (95% confidence interval (CI): 0.84–1.8 nM) (Tables 1 & 2; Fig. 1B). When tested on heterologously expressed human α9α10 nAChR, α-CTx RgIA was more than 300-fold less potent with an IC50 of 490 nM (95% CI: 350–700 nM) (Tables 1 & 2; Fig. 1B).
Figure 1.
Responses of rat and human α9α10 nAChRs to ACh and α-CTx RgIA. A) Rat and human α9α10 nAChRs were heterologously expressed in Xenopus oocytes as described in Methods. 100 µM ACh generally evoked larger currents in Xenopus oocytes expressing rat α9α10 nAChRs than in oocytes expressing human α9α10 nAChRs. Ten nM α-CTx RgIA caused substantial block of ACh-induced current of rat α9α10 nAChRs, whereas 100 nM α-CTx RgIA blocked less than 50% of the current of human α9α10 nAChRs. The toxin was perfusion-applied. Representative traces are shown. B) The rat α9α10 nAChR was 300-fold more sensitive to α-CTx RgIA than was the human α9α10 nAChR. Values are mean ± SEM from at least three oocytes and are shown in Table 1.
Table 1.
IC50s for α-CTx RgIA on mixed-species α9α10 nAChRs. Data are average from at least three different oocytes. r, rat; h, human. CI: confidence interval.
| nAChR | IC50 (nM) | 95% CI | Hill Coefficient |
|---|---|---|---|
| rα9r10 | 1.49 | 1.13–1.95 | 1.2 ± 0.19 |
| rα9hα10 | 3.48 | 2.78–4.34 | 0.97 ± 0.07 |
| hα9rα10 | 245 | 148–405 | 0.50 ± 0.07 |
| hα9hα10 | 494 | 348–703 | 0.56 ± 0.07 |
Table 2.
IC50s for α-CTx RgIA on wildtype rat, wildtype human, and rat mutant α9α10 nAChRs. The human receptor and the only mutant that shifts the IC50 of RgIA towards the human subtype are in bold. r, rat; h, human. Data are average from at least three different oocytes. CI: confidence interval.
| nAChR/mutant | IC50 (nM) | 95% CI (nM) | Hill slope |
|---|---|---|---|
| rα9rα10 | 1.49 | 1.13–1.95 | 1.24 ± 0.19 |
| hα9hα10 | 494 | 348–703 | 0.56 ± 0.07 |
| rα9S6Nrα10 | 2.31 | 1.71–3.12 | 1.16 ± 0.13 |
| rα9S14Nrα10 | 1.83 | 1.30–2.57 | 1.26 ± 0.21 |
| rα9A24Krα10 | 1.24 | 0.87–1.76 | 0.86 ± 0.12 |
| rα9T56Irα10 | 2,560 | 1,230–5,330 | 0.71 ± 0.16 |
| rα9R71Grα10 | 0.95 | 0.55–1.62 | 0.93 ± 0.21 |
| rα9S117Arα10 | 1.73 | 1.39–2.16 | 1.39 ± 0.18 |
| rα9S136Nrα10 | 2.47 | 2.00–3.05 | 0.77 ± 0.05 |
A single amino acid residue in the rat α9 subunit confers high potency for α-CTx RgIA
α-conotoxins are water soluble peptides that bind to residues that lie within the extracellular N-terminal binding domain of nAChRs. The rat and human α9 subunits are highly homologous to each other, yet differ substantially from the rat and human α10 subunits, indicating divergence in evolutionary origin (Elgoyhen and Katz 2011). We first sought to examine whether the difference in species sensitivity for α-CTx RgIA was primarily due to differences in the α9 or α10 subunit. We therefore mixed species of α9 and α10 subunits and heterologously expressed these receptors in Xenopus oocytes. Thus, the rat (r) α9 subunit was co-expressed with the human (h) α10 subunit and the hα9 with the rα10. The potency of α-CTx RgIA was strongly dependent on which species of the α9 subunit was present. α-CTx RgIA blocked rα9hα10 with an IC50 similar to the IC50 obtained on rα9rα10 combination (Table 1). When tested on hα9rα10 combination, the potency of α-CTx RgIA decreased, approaching that observed for the hα9hα10 nAChR (Table 1), indicating that the α9 subunit is the major contributor to α-CTx RgIA potency species difference.
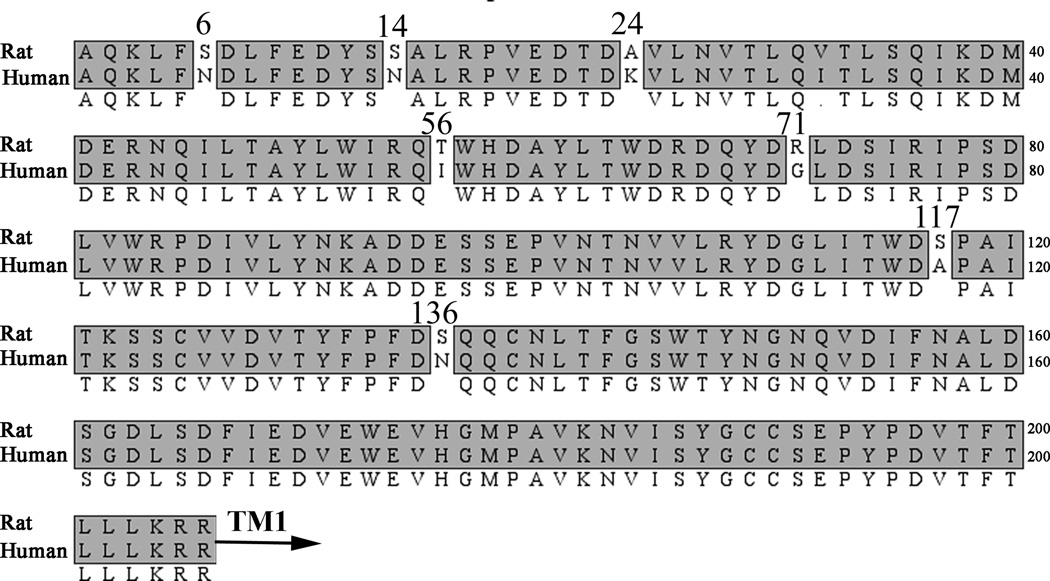
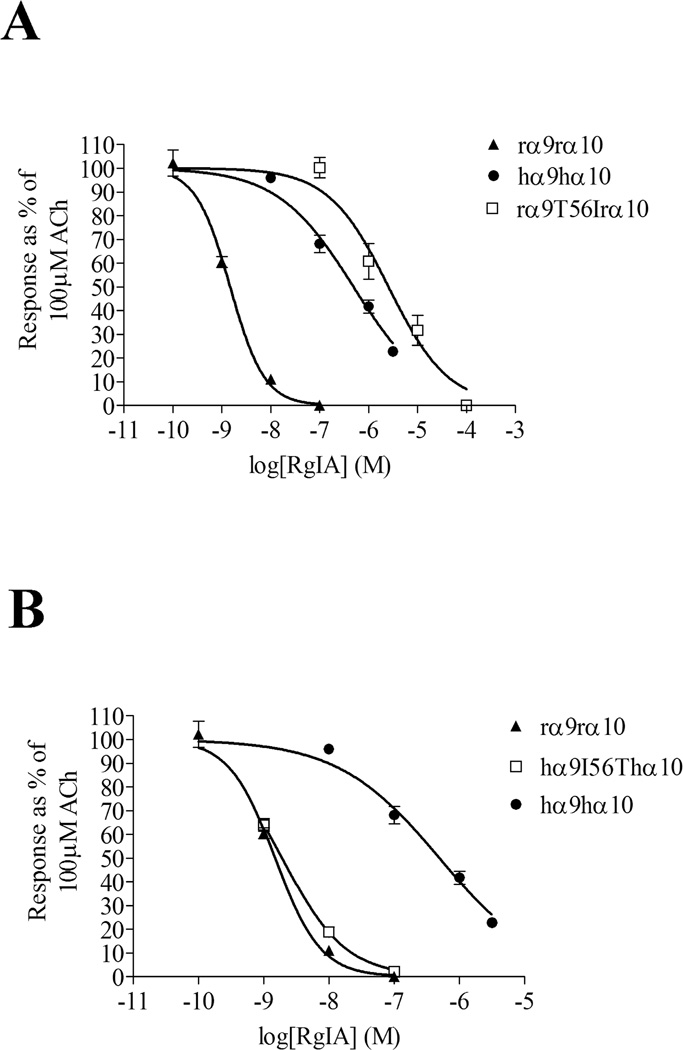
The extracellular N-terminal ligand binding regions of rat and human α9 subunits are highly homologous, differing in only 7 residues from a total of 206 amino acids (Fig. 2). To determine which residue(s) were responsible for conferring the higher potency of α-CTx RgIA for the rat subunit, each amino acid residue that was different between the rat and human subunits was individually switched. Of the 7 residues that were exchanged, only the switch from rat Thr56 to Ile, found in homologous position in the human subunit, affected the potency of α-CTx RgIA (Table 2; Fig. 3A). The IC50 of α-CTx RgIA for rα9T56Irα10 was increased to 2.5 µM (Table 2).
Figure 2.
Amino acid sequence alignment of N-terminal binding regions of rat and human α9 nAChR subunits. The α9 N-terminal binding region is highly homologous between the two species, differing in only 7 amino acids. All the non-homologous residues in the rat α9 subunit were individually mutated to the human counterpart. TM1 indicates the beginning of the first transmembrane region.
Figure 3.
Thr56/Ile is responsible for the rat vs. human difference in potency of block by α-CTx RgIA. (A) Mutation of Thr56 in the rat α9 subunit to Ile, found in homologous position in the human subunit, shifts the potency of α-CTx RgIA towards that of the human receptor. (B) Replacement of Ile56 in human α9 to Thr56, found in rat α9, shifts the potency of RgIA towards that of the rat receptor. Values are mean ± SEM from at least three oocytes and are shown in Table 2.
The importance of Thr56 in the rat α9 subunit in interacting with α-CTx RgIA was further tested by placing the inverse mutation into the human α9 subunit, i.e. replacing human Ile56 with Thr. The IC50 for α-CTx RgIA for hα9I56Thα10 was 1.9 nM (95% CI: 1.6–2.3 nM; Fig. 3B), 250-fold lower than that of wildtype hα9hα10 (Table 2). Thus, a single amino acid change in the human α9 subunit produced a receptor whose affinity for α-CTx RgIA was comparable to that of the rat α9α10 nAChR.
Discussion
In this study, we report the differential sensitivity of rat vs. human α9α10 nAChR to α-CTx RgIA. This toxin is 300-fold more potent on rat vs. human α9α10 nAChR. For non-α9α10 heteromeric nAChRs, the ligand binding site is composed of the interface between the principal (+) face of the α subunit and the complementary (−) face of the β subunit. The α9α10 subtype is unique among mammalian nAChRs in being composed solely of two different α subunits. Based on amino acid homology, the α9 subunit distinctly differs from other α subunits (Elgoyhen et al. 1994) and may represent an early evolutionary branch point for nAChRs (Le Novere and Changeux 1995). α9 subunits will form a homopentameric receptor when expressed in Xenopus oocytes and therefore α9 has been assumed to be similar to other α subunits in providing the principal (+) ligand binding site. In contrast, neither the human nor rat α10 subunit forms a functional receptor when injected alone (Elgoyhen et al. 2001; Sgard et al. 2002). Expression of α9 alone, however, is very low and addition of the α10 subunit not only modifies function but also boosts expression levels by 100-fold or more (Elgoyhen et al. 2001; Sgard et al. 2002). The α10 subunit has therefore been considered as a necessary, possibly “structural” subunit, analogous to the function served by β subunits present in other nAChR subtypes. The three-dimensional solution structure of α-CTx RgIA has been solved by NMR (Clark et al. 2008; Ellison et al. 2008). Using this information, Perez et al. used molecular modeling, docking and molecular dynamics simulations to determine the binding sites of α-CTx RgIA on the α9α10 nAChR (Perez et al. 2009). Their results indicated that α-CTx RgIA binds to the (+) face of the α9 subunit and the (−) face of the α10 subunit. A role for Pro197 and Asp198 in the (+) face of the α9 subunit in interacting with α-CTx RgIA was suggested (Perez et al. 2009). However, both of these residues are conserved between the rat and human subunits and therefore cannot explain the differential sensitivity of α-CTx RgIA for the rat vs. human subtype The results of the present study indicate the converse situation. That is, α-CTx RgIA interacts with the (−) complementary face of the α9 subunit. The critical residue that determined high vs. lower potency of α-CTx RgIA for rat vs. human α9α10 receptors was an α9 residue at position 56. Thr56 in the rat subunit conferred the higher sensitivity and Ile56 in the human subunit conferred the lower sensitivity. Thr/Ile56 is located in the (−) complementary binding face of the α9 subunit.
By aligning the sequences of the rat α9 and AChBP from Aplysia using the solved structure of the apo form of Aplysia californica acetylcholine binding protein (Ac-AChBP) (Hansen et al. 2005) as a template, the location of Thr56 was determined (Figure 4). Thr56 lies in close proximity to Trp60, one of the residues of the (−) complementary binding site of Lymnae stagnalis (Ls) AChBP involved in interacting with agonists and antagonists (Brejc et al. 2001; Bourne et al. 2005). Moreover, Arg57 of Ac-AChBP, located in the homologous position to α9 Thr56, makes contacts with residues in both α-CTx ImI and α-CTx PnIA [A10L;D14K] (Ulens et al. 2006). Ser59 in the α7 subunit, homologous to Thr56 in α9, also confers α-CTx ImI selectivity (Quiram and Sine 1998). Additionally, α9Thr56 in only two residues away from the Thr59 in the β2 nAChR subunit that has been shown to interact with α-CTx MII (Harvey et al. 1997). Lys59 (homologous to Thr59 in the β2 subunit) facilitates the binding of α-CTx BuIA to the β4 subunit (Shiembob et al. 2006). Thus, α-CTx RgIA, like other conotoxins, interacts with the (−) binding face of the nAChR subunit. Unexpectedly, however, this (−) face is presented by the α9 rather than α10 subunit. Our results do not exclude the possibility that α-CTx RgIA also binds to the α9(+)/α10(−) interface. In this case, there may be as many as five binding sites for the toxin on the α9α10 receptor. Indeed, five toxins are bound in an AChBP-α-CTx complex, which serves as a surrogate model of nAChRs and by analogy, five α-CTx may be bound to the homomeric α7 nAChRs (Celie et al. 2005). We are not aware, however, of a report indicating 5 binding sites present for a heteromeric nAChR. It is of interest that although the α10 subunit will not self-assemble to form a functional nAChR, if the N-terminal binding domain of the α10 nAChR subunit is fused to the C-terminal portion of the 5-HT3 receptor, a functional chimera that responds to ACh is formed (Baker et al. 2004). Thus, it appears that the α10 subunit contains the requisite recognition sites, minimally on the (+) face, to functionally bind ACh.
Figure 4.
Top panel: Model of the α9α10 nAChR showing two adjacent subunits. The location of the critical residues conferring the species difference, Thr/Ile56, for binding of α-CTx RgIA to the α9 subunit is shown. This residue is located on the (−) complementary binding face of the α9 subunit. The adjacent subunit could either be an α9 or an α10 subunit. Bottom panel: Sequence alignment of rat subunits aligned with Aplysia californica acetylcholine binding protein (Ac-AChBP). Arrows indicate residues shown to interact with α-conotoxins. Numbering indicates that of the particular subunit or AChBP (see Discussion). Note that α9 Thr56 aligns with residues in the (−) face of the the nAChR subunits and AChBP.
The current studies were performed on mammalian nAChRs heterologously expressed in Xenopus oocytes. It would also be desirable to perform similar experiments using nAChRs expressed in a mammalian cell line. However, reliable expression of α9α10 nAChRs in such cell lines has been problematic (Baker et al. 2004). We note that oocyte-expressed rat α9α10 nAChRs have similar biophysical and pharmacological properties compared to native rat α9α10 nAChRs found in cochlear hair cells (Elgoyhen et al. 2001; Gomez-Casati et al. 2005). Sensitivity to antagonists, including α-CTx RgIA, has also been comparable between native and oocyte-expressed α9α10 nAChRs (McIntosh et al. 2005; Ellison et al. 2006).
The α9 nAChR is increasingly recognized for its potential role in physiological and pathological processes. Other α9α10 antagonists are being investigated as possible human therapeutics (Satkunanathan et al. 2005; Adams et al. 2011; Lewis et al. 2012). One α9α10 antagonist, α-conotoxin Vc1.1 (ACV1), was advanced through human clinical trials for chronic pain by Metabolic Pharmaceuticals (Melbourne, VIC, Australia). Vc1.1 development was stopped after completion of a phase 2A trial due to concerns about lower affinity at human versus rat α9α10 nAChRs. Small molecule antagonists of α9α10 nAChRs are also being investigated as novel analgesics (Holtman et al. 2011; Zheng et al. 2011). The α9 subunit has been shown to play a key role in regulating initial events of keratinocyte migration and adhesion (Nguyen et al. 2000; Nguyen et al. 2004; Chernyavsky et al. 2007). Manipulation of α9 nAChR signaling may, therefore, be applicable to treatment of non-healing wounds. Nicotine is regularly consumed in the form of tobacco by a substantial portion of the world’s population. Nicotine is known to stimulate growth of various cancers. Recently the α9 receptor was shown to be ubiquitously expressed in breast cancer. Although non-malignant breast tissue also had α9 subunits, breast cancer tissue from patients had dramatically increased levels of α9 expression (Lee et al. 2011). Furthermore, stimulation of the α9 nAChR leads to breast cancer growth (Lee et al. 2011) and block of α9 receptors inhibits growth (Tu et al. 2011). Also, different forms of the α9 nAChR affect transformation and proliferation of bronchial cells (Chikova and Grando 2011). Increased risk of lung cancer is associated with single nucleotide polymorphism at position 442 of the gene coding for the α9 subunit (Chikova and Grando 2011; Chikova et al. 2012). Thus, the development of novel agents, with activity at α9 nAChRs, may provide novel therapeutic avenues. The molecular information of the present study may help inform the development of conotoxin analogs of α-CTx RgIA and other conotoxins targeting the α9α10 nAChR (Halai et al. 2009) and possibly the development of small molecule α9α10 antagonists with analgesic activity (Vincler et al. 2006; Vincler and McIntosh 2007; Olivera et al. 2008; Azam and McIntosh 2009).
Acknowledgements
This work was supported by National Institute of Health grants GM48677 and MH53631.
Abbreviations
- nAChR
Nicotinic acetylcholine receptors
- CTx
Conotoxin
- CI
confidence interval
Footnotes
Authors have no conflict of interest. Layla Azam and J. Michael McIntosh both contributed to conceptual design of the experiments. Layla Azam contributed to acquisition and analysis of data. Layla Azam and J. Michael McIntosh contributed to interpretation of data, article drafting and revising and both approved the version for publication.
References
- Adams DJ, Callaghan B, Berecki G. ANALGESIC CONOTOXINS: BLOCK AND G PROTEIN-COUPLED RECEPTOR MODULATION OF N-TYPE (Ca(V) 2.2) CALCIUM CHANNELS. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armishaw CJ. Synthetic alpha-Conotoxin Mutants as Probes for Studying Nicotinic Acetylcholine Receptors and in the Development of Novel Drug Leads. Toxins (Basel) 2010;2:1471–1499. doi: 10.3390/toxins2061471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, McIntosh JM. Characterization of nicotinic acetylcholine receptors that modulate nicotine-evoked [3H]norepinephrine release from mouse hippocampal synaptosomes. Mol Pharmacol. 2006;70:967–976. doi: 10.1124/mol.106.024513. [DOI] [PubMed] [Google Scholar]
- Azam L, McIntosh JM. Alpha-conotoxins as pharmacological probes of nicotinic acetylcholine receptors. Acta Pharmacol Sin. 2009;30:771–783. doi: 10.1038/aps.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Dowell C, Watkins M, Stitzel JA, Olivera BM, McIntosh JM. Alpha-conotoxin BuIA, a novel peptide from Conus bullatus, distinguishes among neuronal nicotinic acetylcholine receptors. J Biol Chem. 2005;280:80–87. doi: 10.1074/jbc.M406281200. [DOI] [PubMed] [Google Scholar]
- Baker ER, Zwart R, Sher E, Millar NS. Pharmacological properties of alpha 9 alpha 10 nicotinic acetylcholine receptors revealed by heterologous expression of subunit chimeras. Mol Pharmacol. 2004;65:453–460. doi: 10.1124/mol.65.2.453. [DOI] [PubMed] [Google Scholar]
- Bourne Y, Talley TT, Hansen SB, Taylor P, Marchot P. Crystal structure of a Cbtx-AChBP complex reveals essential interactions between snake alpha-neurotoxins and nicotinic receptors. Embo J. 2005;24:1512–1522. doi: 10.1038/sj.emboj.7600620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- Celie PH, Kasheverov IE, Mordvintsev DY, Hogg RC, van Nierop P, van Elk R, van Rossum-Fikkert SE, Zhmak MN, Bertrand D, Tsetlin V, Sixma TK, Smit AB. Crystal structure of nicotinic acetylcholine receptor homolog AChBP in complex with an alpha-conotoxin PnIA variant. Nat Struct Mol Biol. 2005;12:582–588. doi: 10.1038/nsmb951. [DOI] [PubMed] [Google Scholar]
- Chernyavsky AI, Arredondo J, Vetter DE, Grando SA. Central role of alpha9 acetylcholine receptor in coordinating keratinocyte adhesion and motility at the initiation of epithelialization. Exp Cell Res. 2007;313:3542–3555. doi: 10.1016/j.yexcr.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikova A, Grando SA. Naturally occurring variants of human Alpha9 nicotinic receptor differentially affect bronchial cell proliferation and transformation. PLoS One. 2011;6:e27978. doi: 10.1371/journal.pone.0027978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikova A, Bernard HU, Shchepotin IB, Grando SA. New associations of the genetic polymorphisms in nicotinic receptor genes with the risk of lung cancer. Life Sci. 2012 doi: 10.1016/j.lfs.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RJ, Daly NL, Halai R, Nevin ST, Adams DJ, Craik DJ. The three-dimensional structure of the analgesic alpha-conotoxin, RgIA. FEBS Lett. 2008;582:597–602. doi: 10.1016/j.febslet.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Dowell C, Olivera BM, Garrett JE, Staheli ST, Watkins M, Kuryatov A, Yoshikami D, Lindstrom JM, McIntosh JM. Alpha-conotoxin PIA is selective for alpha6 subunit-containing nicotinic acetylcholine receptors. J Neurosci. 2003;23:8445–8452. doi: 10.1523/JNEUROSCI.23-24-08445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre S, Lewis RJ. Toxin insights into nicotinic acetylcholine receptors. Biochem Pharmacol. 2006;72:661–670. doi: 10.1016/j.bcp.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Katz E. The efferent medial olivocochlear-hair cell synapse. J Physiol Paris. 2011 doi: 10.1016/j.jphysparis.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79:705–715. doi: 10.1016/0092-8674(94)90555-x. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison M, Haberlandt C, Gomez-Casati ME, Watkins M, Elgoyhen AB, McIntosh JM, Olivera BM. Alpha-RgIA: a novel conotoxin that specifically and potently blocks the alpha9alpha10 nAChR. Biochemistry. 2006;45:1511–1517. doi: 10.1021/bi0520129. [DOI] [PubMed] [Google Scholar]
- Ellison M, Feng ZP, Park AJ, Zhang X, Olivera BM, McIntosh JM, Norton RS. Alpha-RgIA, a novel conotoxin that blocks the alpha9alpha10 nAChR: structure and identification of key receptor-binding residues. J Mol Biol. 2008;377:1216–1227. doi: 10.1016/j.jmb.2008.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Casati ME, Fuchs PA, Elgoyhen AB, Katz E. Biophysical and pharmacological characterization of nicotinic cholinergic receptors in rat cochlear inner hair cells. J Physiol. 2005;566:103–118. doi: 10.1113/jphysiol.2005.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74:1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Haberberger RV, Bernardini N, Kress M, Hartmann P, Lips KS, Kummer W. Nicotinic acetylcholine receptor subtypes in nociceptive dorsal root ganglion neurons of the adult rat. Auton Neurosci. 2004;113:32–42. doi: 10.1016/j.autneu.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Halai R, Clark RJ, Nevin ST, Jensen JE, Adams DJ, Craik DJ. Scanning mutagenesis of alpha-conotoxin Vc1.1 reveals residues crucial for activity at the alpha9alpha10 nicotinic acetylcholine receptor. J Biol Chem. 2009;284:20275–20284. doi: 10.1074/jbc.M109.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TS, Teichert RW, Olivera BM, Bulaj G. Conus venoms - a rich source of peptide-based therapeutics. Curr Pharm Des. 2008;14:2462–2479. doi: 10.2174/138161208785777469. [DOI] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. Embo J. 2005;24:3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, McIntosh JM, Cartier GE, Maddox FN, Luetje CW. Determinants of specificity for alpha-conotoxin MII on alpha3beta2 neuronal nicotinic receptors. Mol Pharmacol. 1997;51:336–342. doi: 10.1124/mol.51.2.336. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Dwoskin LP, Dowell C, Wala EP, Zhang Z, Crooks PA, McIntosh JM. The novel small molecule alpha9alpha10 nicotinic acetylcholine receptor antagonist ZZ-204G is analgesic. Eur J Pharmacol. 2011;670:500–508. doi: 10.1016/j.ejphar.2011.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes RW. alpha-Conotoxins as selective probes for nicotinic acetylcholine receptor subclasses. Curr Opin Pharmacol. 2005;5:280–292. doi: 10.1016/j.coph.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Kasheverov IE, Utkin YN, Tsetlin VI. Naturally occurring and synthetic peptides acting on nicotinic acetylcholine receptors. Curr Pharm Des. 2009;15:2430–2452. doi: 10.2174/138161209788682316. [DOI] [PubMed] [Google Scholar]
- Klimis H, Adams DJ, Callaghan B, Nevin S, Alewood PF, Vaughan CW, Mozar CA, Christie MJ. A novel mechanism of inhibition of high-voltage activated calcium channels by alpha-conotoxins contributes to relief of nerve injury-induced neuropathic pain. Pain. 2011;152:259–266. doi: 10.1016/j.pain.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Kumar P, Meizel S. Nicotinic acetylcholine receptor subunits and associated proteins in human sperm. J Biol Chem. 2005;280:25928–25935. doi: 10.1074/jbc.M502435200. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Berger H, Jager C, Hartschuh W, Naher H, Gratchev A, Goerdt S, Deichmann M. Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J Invest Dermatol. 2004;123:937–949. doi: 10.1111/j.0022-202X.2004.23425.x. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Changeux JP. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J Mol Evol. 1995;40:155–172. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- Lee CH, Huang CS, Chen CS, Tu SH, Wang YJ, Chang YJ, Tam KW, Wei PL, Cheng TC, Chu JS, Chen LC, Wu CH, Ho YS. Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J Natl Cancer Inst. 2010;102:1322–1335. doi: 10.1093/jnci/djq300. [DOI] [PubMed] [Google Scholar]
- Lee CH, Chang YC, Chen CS, Tu SH, Wang YJ, Chen LC, Chang YJ, Wei PL, Chang HW, Chang CH, Huang CS, Wu CH, Ho YS. Crosstalk between nicotine and estrogen-induced estrogen receptor activation induces alpha9-nicotinic acetylcholine receptor expression in human breast cancer cells. Breast Cancer Res Treat. 2011;129:331–345. doi: 10.1007/s10549-010-1209-0. [DOI] [PubMed] [Google Scholar]
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: new twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Dutertre S, Vetter I, Christie MJ. Conus venom Peptide pharmacology. Pharmacol Rev. 2012;64:259–298. doi: 10.1124/pr.111.005322. [DOI] [PubMed] [Google Scholar]
- Lips KS, Pfeil U, Kummer W. Coexpression of alpha 9 and alpha 10 nicotinic acetylcholine receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;115:1–5. doi: 10.1016/s0306-4522(02)00274-9. [DOI] [PubMed] [Google Scholar]
- Livett BG, Sandall DW, Keays D, Down J, Gayler KR, Satkunanathan N, Khalil Z. Therapeutic applications of conotoxins that target the neuronal nicotinic acetylcholine receptor. Toxicon. 2006;48:810–829. doi: 10.1016/j.toxicon.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Lustig LR, Peng H, Hiel H, Yamamoto T, Fuchs PA. Molecular cloning and mapping of the human nicotinic acetylcholine receptor alpha10 (CHRNA10) Genomics. 2001;73:272–283. doi: 10.1006/geno.2000.6503. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Absalom N, Chebib M, Elgoyhen AB, Vincler M. Alpha9 nicotinic acetylcholine receptors and the treatment of pain. Biochem Pharmacol. 2009;78:693–702. doi: 10.1016/j.bcp.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh JM, Plazas PV, Watkins M, Gomez-Casati ME, Olivera BM, Elgoyhen AB. A novel alpha-conotoxin, PeIA, cloned from Conus pergrandis, discriminates between rat alpha9alpha10 and alpha7 nicotinic cholinergic receptors. J Biol Chem. 2005;280:30107–30112. doi: 10.1074/jbc.M504102200. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Millard EL, Daly NL, Craik DJ. Structure-activity relationships of alpha-conotoxins targeting neuronal nicotinic acetylcholine receptors. Eur J Biochem. 2004;271:2320–2326. doi: 10.1111/j.1432-1033.2004.04148.x. [DOI] [PubMed] [Google Scholar]
- Muttenthaler M, Akondi KB, Alewood PF. Structure-activity studies on alpha-conotoxins. Curr Pharm Des. 2011;17:4226–4241. doi: 10.2174/138161211798999384. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Ndoye A, Grando SA. Novel human alpha9 acetylcholine receptor regulating keratinocyte adhesion is targeted by Pemphigus vulgaris autoimmunity. Am J Pathol. 2000;157:1377–1391. doi: 10.1016/s0002-9440(10)64651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Chernyavsky AI, Arredondo J, Bercovich D, Orr-Urtreger A, Vetter DE, Wess J, Beaudet AL, Kitajima Y, Grando SA. Synergistic control of keratinocyte adhesion through muscarinic and nicotinic acetylcholine receptor subtypes. Exp Cell Res. 2004;294:534–549. doi: 10.1016/j.yexcr.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Quik M, Vincler M, McIntosh JM. Subtype-selective conopeptides targeted to nicotinic receptors: Concerted discovery and biomedical applications. Channels (Austin) 2008;2:143–152. doi: 10.4161/chan.2.2.6276. [DOI] [PubMed] [Google Scholar]
- Peng H, Ferris RL, Matthews T, Hiel H, Lopez-Albaitero A, Lustig LR. Characterization of the human nicotinic acetylcholine receptor subunit alpha (alpha) 9 (CHRNA9) and alpha (alpha) 10 (CHRNA10) in lymphocytes. Life Sci. 2004;76:263–280. doi: 10.1016/j.lfs.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Perez EG, Cassels BK, Zapata-Torres G. Molecular modeling of the alpha9alpha10 nicotinic acetylcholine receptor subtype. Bioorg Med Chem Lett. 2009;19:251–254. doi: 10.1016/j.bmcl.2008.10.094. [DOI] [PubMed] [Google Scholar]
- Quiram PA, Sine SM. Identification of residues in the neuronal alpha7 acetylcholine receptor that confer selectivity for conotoxin ImI. J Biol Chem. 1998;273:11001–11006. doi: 10.1074/jbc.273.18.11001. [DOI] [PubMed] [Google Scholar]
- Satkunanathan N, Livett B, Gayler K, Sandall D, Down J, Khalil Z. Alpha-conotoxin Vc1.1 alleviates neuropathic pain and accelerates functional recovery of injured neurones. Brain Res. 2005;1059:149–158. doi: 10.1016/j.brainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F. A novel human nicotinic receptor subunit, alpha10, that confers functionality to the alpha9-subunit. Mol Pharmacol. 2002;61:150–159. doi: 10.1124/mol.61.1.150. [DOI] [PubMed] [Google Scholar]
- Shiembob DL, Roberts RL, Luetje CW, McIntosh JM. Determinants of alpha-conotoxin BuIA selectivity on the nicotinic acetylcholine receptor beta subunit. Biochemistry. 2006;45:11200–11207. doi: 10.1021/bi0611715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu SH, Ku CY, Ho CT, Chen CS, Huang CS, Lee CH, Chen LC, Pan MH, Chang HW, Chang CH, Chang YJ, Wei PL, Wu CH, Ho YS. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits nicotine- and estrogen-induced alpha9-nicotinic acetylcholine receptor upregulation in human breast cancer cells. Mol Nutr Food Res. 2011;55:455–466. doi: 10.1002/mnfr.201000254. [DOI] [PubMed] [Google Scholar]
- Ulens C, Hogg RC, Celie PH, Bertrand D, Tsetlin V, Smit AB, Sixma TK. Structural determinants of selective alpha-conotoxin binding to a nicotinic acetylcholine receptor homolog AChBP. Proc Natl Acad Sci U S A. 2006;103:3615–3620. doi: 10.1073/pnas.0507889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman J, Heinemann SF, Elgoyhen AB. Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron. 1999;23:93–103. doi: 10.1016/s0896-6273(00)80756-4. [DOI] [PubMed] [Google Scholar]
- Vincler M, McIntosh JM. Targeting the alpha9alpha10 nicotinic acetylcholine receptor to treat severe pain. Expert Opin Ther Targets. 2007;11:891–897. doi: 10.1517/14728222.11.7.891. [DOI] [PubMed] [Google Scholar]
- Vincler M, Wittenauer S, Parker R, Ellison M, Olivera BM, McIntosh JM. Molecular mechanism for analgesia involving specific antagonism of alpha9alpha10 nicotinic acetylcholine receptors. Proc Natl Acad Sci U S A. 2006;103:17880–17884. doi: 10.1073/pnas.0608715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Lee CH, Ho YS. Nicotinic acetylcholine receptor-based blockade: applications of molecular targets for cancer therapy. Clin Cancer Res. 2011;17:3533–3541. doi: 10.1158/1078-0432.CCR-10-2434. [DOI] [PubMed] [Google Scholar]
- Wu J, Lukas RJ. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem Pharmacol. 2011;82:800–807. doi: 10.1016/j.bcp.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Zhang Z, Dowell C, Wala E, Dwoskin LP, Holtman JR, McIntosh JM, Crooks PA. Discovery of non-peptide, small molecule antagonists of alpha9alpha10 nicotinic acetylcholine receptors as novel analgesics for the treatment of neuropathic and tonic inflammatory pain. Bioorg Med Chem Lett. 2011;21:2476–2479. doi: 10.1016/j.bmcl.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]