Abstract
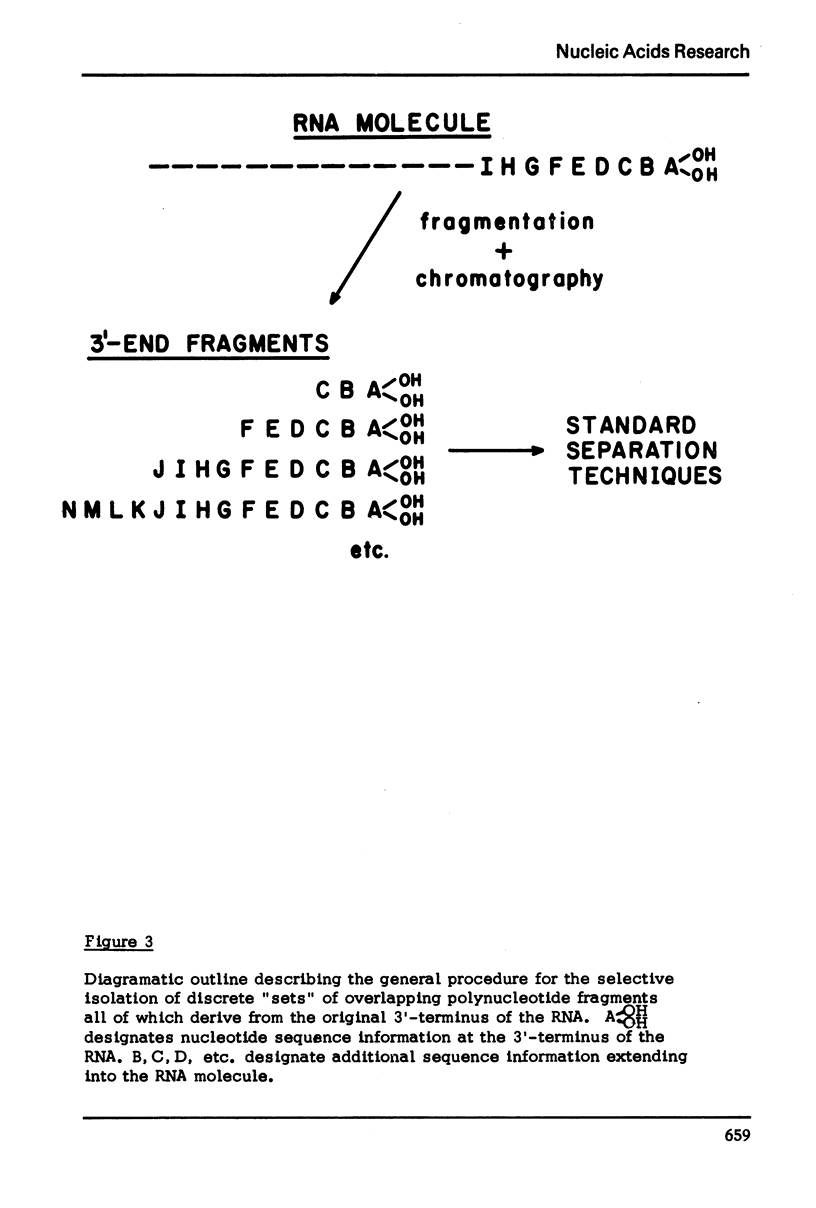
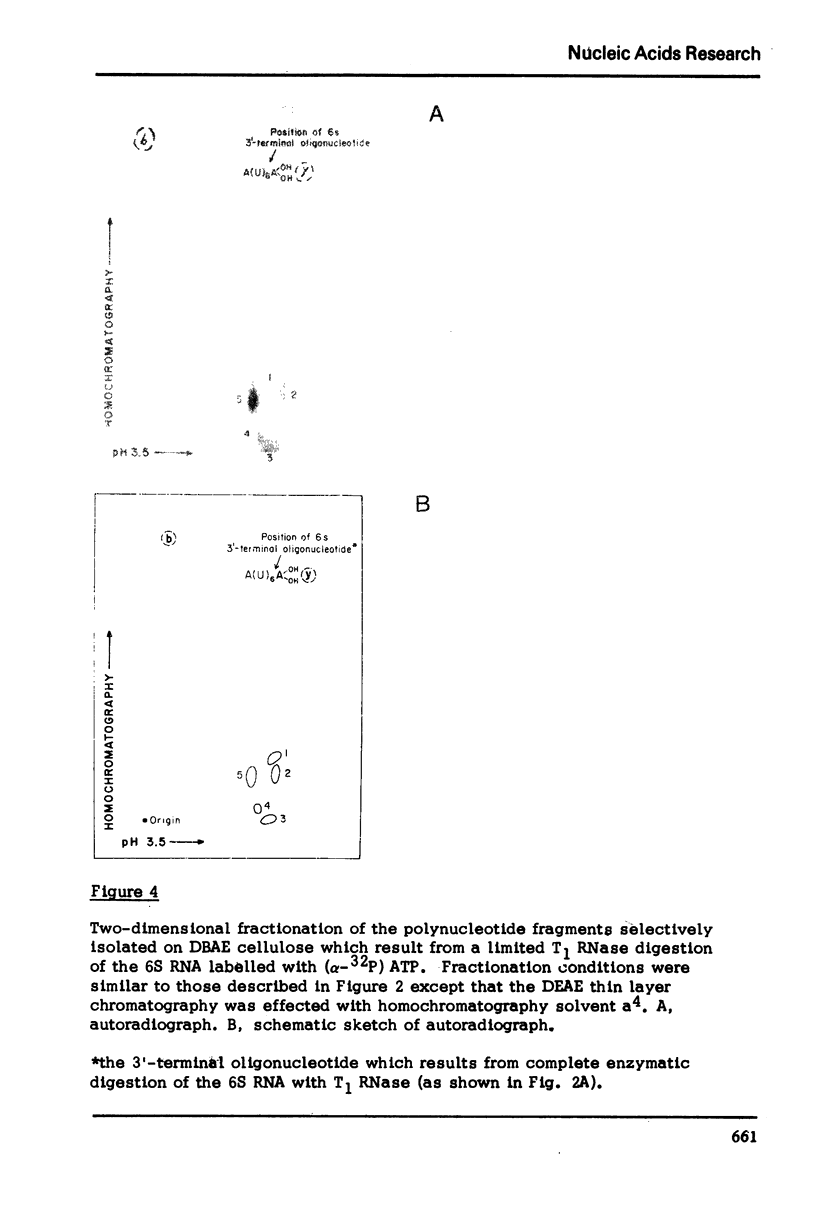
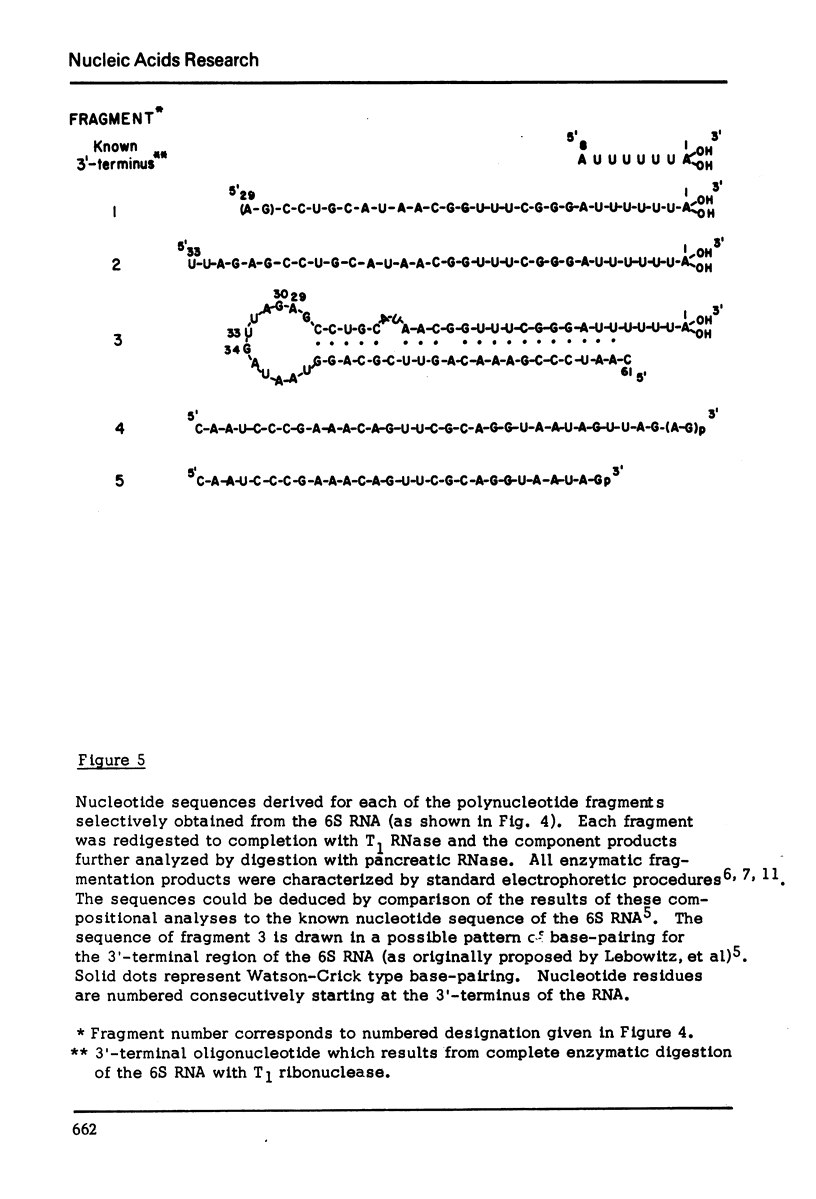
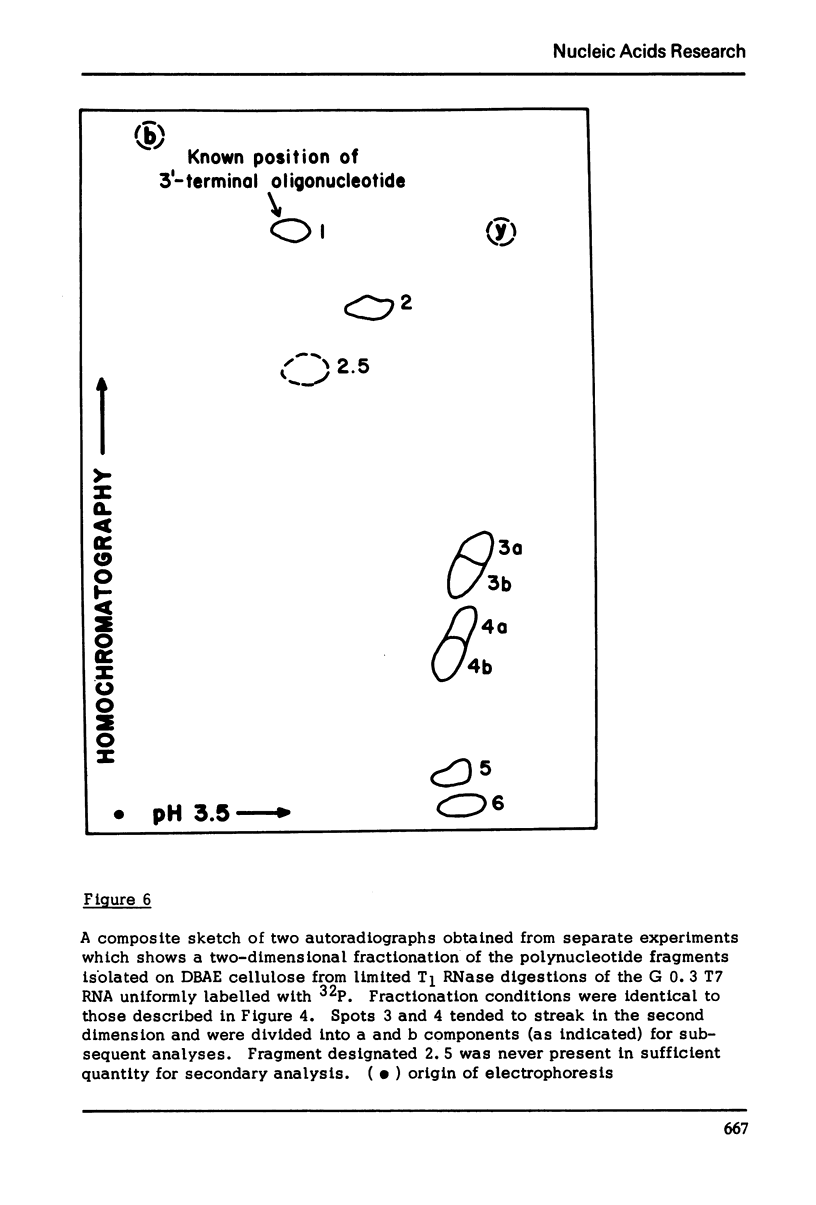
The method which was developed for the selective isolation of 3′-terminal polynucleotides from large RNA molecules on columns of cellulose derivatives containing covalently bound dihydroxyboryl groups has been modified and adapted for use on radioactively labelled RNAs. The 3′-terminal polynucleotide fragments which result from specific ribonuclease digestion of isotopically detectable quantities of RNA can be selectively obtained in both high yield and purity by the modified procedure and can be subsequently analyzed by standard electrophoretic and chromatographic techniques. In addition, when the extent of enzymatic fragmentation of the RNA is controlled, the procedure permits the selective isolation of discrete “sets” of fragments of variable chain length, all of which derive from the 3′-terminus of the RNA molecule. These overlapping polynucleotides can be used directly to obtain extensive sequence information regarding the primary structure in the 3′-region of the RNA.
Full text
PDF








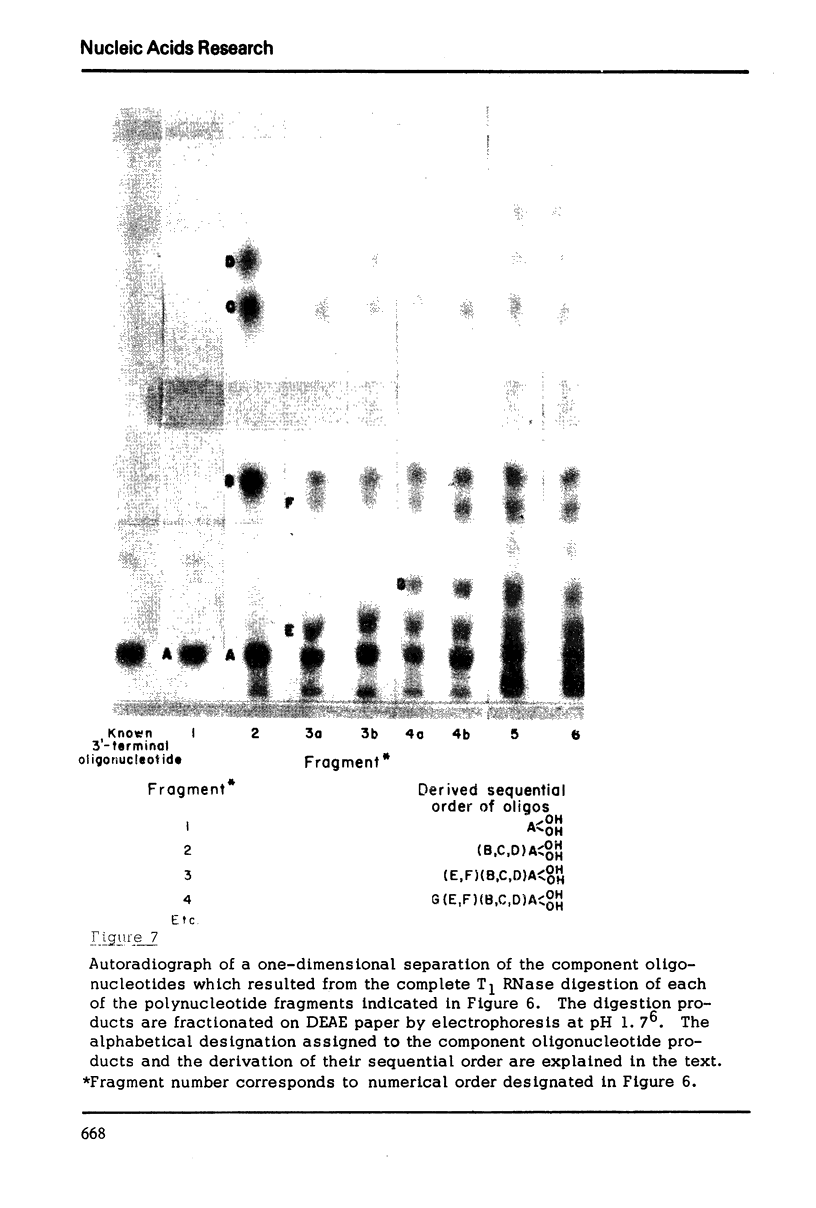



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- DuBuy B., Weissman S. M. Nucleotide sequence of Pseudomonas fluorescens 5 S ribonucleic acid. J Biol Chem. 1971 Feb 10;246(3):747–761. [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. Oligonucleotides produced by digestion of KB cell ribosomal 5 S ribonucleic acid with specific nucleases. J Biol Chem. 1968 Nov 10;243(21):5709–5723. [PubMed] [Google Scholar]
- Gilham P. T., Rosenberg M. The isolation of 3'-terminal polynucleotides from RNA molecules. Biochim Biophys Acta. 1971 Aug 26;246(2):337–340. doi: 10.1016/0005-2787(71)90143-2. [DOI] [PubMed] [Google Scholar]
- Lebowitz P., Weissman S. M., Radding C. M. Nucleotide sequence of a ribonucleic acid transcribed in vitro from lambda phage deoxyribonucleic acid. J Biol Chem. 1971 Aug 25;246(16):5120–5139. [PubMed] [Google Scholar]
- Rosenberg M., Wiebers J. L., Gilham P. T. Studies on the interactions of nucleotides, polynucleotides, and nucleic acids with dihydroxyboryl-substituted celluloses. Biochemistry. 1972 Sep 12;11(19):3623–3628. doi: 10.1021/bi00769a020. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Brunovskis I., Hyman R. W. The process of infection with coliphage T7. VII. Characterization and mapping of the major in vivo transcription products of the early region. J Mol Biol. 1973 Mar 5;74(3):291–300. doi: 10.1016/0022-2836(73)90374-4. [DOI] [PubMed] [Google Scholar]
- Weith H. L., Wiebers J. L., Gilham P. T. Synthesis of cellulose derivatives containing the dihydroxyboryl group and a study of their capacity to form specific complexes with sugars and nucleic acid components. Biochemistry. 1970 Oct 27;9(22):4396–4401. doi: 10.1021/bi00824a021. [DOI] [PubMed] [Google Scholar]