Abstract
Amblyopia is a common visual disorder that results in a spatial acuity deficit in the affected eye. Orthodox treatment is to occlude the unaffected eye for lengthy periods, largely determined by the severity of the visual deficit at diagnosis. Although this treatment is not without its problems (poor compliance, potential to reduce binocular function etc.) it is effective in many children with moderate to severe amblyopia. Diagnosis and initiation of treatment early in life are thought to be critical to the success of this form of therapy. Occlusion is rarely undertaken in older children (over 10 years old) as the visual benefits are considered to be marginal. Therefore, in subjects where occlusion is not effective or those missed by mass screening programmes there is no alternative therapy available later in life. More recently, burgeoning evidence has begun to reveal previously unrecognised levels of residual neural plasticity in the adult brain and scientists have developed new genetic, pharmacological and behavioural interventions to activate these latent mechanisms in order to harness their potential for visual recovery. Prominent amongst these is the concept of perceptual learning - the fact that repeatedly practicing a challenging visual task leads to substantial and enduring improvements in visual performance over time. In the normal visual system the improvements are highly specific to the attributes of the trained stimulus. However, in the amblyopic visual system learned improvements have been shown to generalize to novel tasks. In this paper we ask whether amblyopic deficits can be reduced in adulthood and explore the pattern of transfer of learned improvements. We also show that developing training protocols that target the deficit in stereo acuity allows the recovery of normal stereo function even in adulthood. This information will help guide further development of learning-based interventions in this clinical group.
Keywords: Amblyopia, perceptual learning, plasticity, visual acuity, contrast sensitivity, stereo acuity
Background
Amblyopia is a relatively common developmental disorder (affecting 2-4% of the population) that results in a dramatic loss of spatial acuity in the affected eye and subsequent binocular dysfunction. The condition is caused by disruption of normal visual input during the critical period(s) of visual development - post-natal windows of experience-dependent neural plasticity 1. The neural locus of the amblyopic deficit is widely thought to be primary visual cortex 1-4 although extrastriate areas may also have a supplementary role 5,6. Amblyopia is usually associated with amblyogenic factors such as anisometropia (unequal refractive errors between the eyes), strabismus (misalignment of the visual axes) or a combination of the two. Orthodox treatment for amblyopia involves optical correction of refractive error or surgical realignment of the eyes, followed by a period of ‘refractive adaptation’ 7 and then penalization of the good eye, typically by covering it with a patch, for lengthy periods of time.
Recovery of visual function to normal or near normal levels is possible if the obstacle to normal visual development is removed early in life 8. The age of onset of the amblyogenic factor 9-11, the duration that this is present 12-15, combined with the degree of imbalance between the two eyes 16-19, appears to be strongly associated with the severity of the visual defect. Early detection and treatment is supported by data from screening studies, where better visual outcome (lower prevalence of amblyopia) has been found in those that have undergone intensive screening 20. Therefore, early detection and initiation of treatment is justifiably given a high priority.
Occlusion therapy has remained relatively unchanged since it introduction more than 250 years ago. Unfortunately, this form of therapy can be distressing to the child 21, is unpopular with parents and can adversely effect social and educational development 22. Allergies to the adhesive used on patches can also be problematic 23 and long periods of occlusion can in itself lead to binocular visions problems such as reduced stereopsis. For these reasons, compliance tends to be poor. It is possible to objectively measure the time a patch is worn with an occlusion dose monitor 24 and therefore to assess treatment dose response as well as compliance with prescribed wearing times. Children wear their patch for approximately half the prescribed period 25. The amount of occlusion prescribed varies greatly 26 from 2-6 hours per day for mild to moderate amblyopia 27 to more than 10 hours per day for severe amblyopia 28. A treatment-dose response function has been determined from objective measurements of wearing times 27: an improvement of one line (0.1 logMAR) on a visual acuity chart (Bailey-Lovie chart) requires approximately 120 hours of occlusion.
Despite its drawbacks, a randomized controlled trial demonstrated that occlusion can be an effective form of therapy for many children 29, particularly for those with poor levels of visual acuity at the start of treatment. Orthodox treatment is rarely undertaken in older children or adults. This practice has been supported by clinical trial data showing that patching (or penalisation) is largely ineffective beyond the age of 10 years old 30, supporting the widely held clinical view that the critical period for the development and treatment of amblyopia are one in the same. As neuronal circuits stabilize during development, plasticity was thought to dissipate thus consolidating the neural architecture established through early visual experience. However, a collection of studies have shown that individuals, at an age beyond what which would be considered outside the critical period(s) of visual development can show visual improvements with occlusion 31-40. Common to many of these studies is that treatment usually involved more than simply passive occlusion. For example, Kupfer 32 demonstrated large improvements in the amblyopic eyes of adults with strabismus, but treatment was aggressive (full time occlusion and subjects hospitalised) and supplemented with fixation training. Furthermore, a loss of macular function through progressive pathology in the non-amblyopic eye can lead to concomitant improvements in the visual acuity of the amblyopic eye 41. Taken together, these results point to the existence of residual neural plasticity in the visual system of adults with amblyopia that supports recovery of lost function after visual maturation.
Until relatively recently, adult visual cortex had not been considered capable of retaining any of the experience-dependent neural plasticity so prominent during early visual development. However, it is now abundantly clear that experience can reshape visual brain function throughout the lifespan, and plasticity can be expressed in many different forms – from molecular and synaptic changes 42 to complete reorganization of topographic cortical maps 43. A much studied behavioural manifestation of neural plasticity in normal vision is ‘perceptual learning’, where repeatedly practicing a challenging task can lead to substantial and enduring improvements in visual performance over time. Perceptual learning effects have been widely documented in adulthood, well beyond the critical period(s) of development. In visually normal adult subjects, perceptual learning improves performance on a wide range of visual tasks 44-48, but one of its key characteristics are that improvements in performance are strongly coupled to trained visual attributes such as the orientation 47, spatial frequency 47, retinal position 44,49, size and binocular disparity 50 of a stimulus (but see also 51). In contrast to the task specific learning found in subjects with normal vision, trained improvements in amblyopic visual performance have been shown to generalize to untrained tasks and novel stimuli, including visual acuity 52-55, visual counting 56 and stereoacuity 57. Generalisation of perceptual learning to untrained tasks is key to harnessing this form of plasticity as an effective treatment for amblyopia, whether as a primary intervention or supplementary to traditional methods, such as occlusion therapy. For a detailed treatment of this area, including a thorough discussion of the neural mechanisms thought to mediate perceptual learning, see excellent review articles by Levi and Li 58,59 and Gilbert and colleagues 60.
Individuals with amblyopia typically present with a wide range of spatial deficits, many of which can be collapsed along two basic visual dimensions that together account for the virtually all of the variation in performance of the amblyopic visual system 61. A large factor analysis study of visual function in over four hundred individuals with amblyopia revealed two orthogonal dimensions of variation: visual acuity and contrast sensitivity 61. Where individuals lie in this deficit space is largely governed by their degree of residual binocularity 61.
In the following sections we review our attempts to exploit this deficit space in order to fully characterize the pattern of learned improvements and generalisation in adult amblyopic subjects62. We also ask whether learned improvements in monocular visual function provide a platform from which abnormal binocular function (stereoacuity loss) can be ameliorated63. Below we give a brief description of methods and results but for a more detailed treatment, the reader should consult the relevant published papers62, 63.
General Methods
35 visually normal and 24 amblyopic subjects participated - Table 1 shows clinical details of the amblyopic subjects. Subjects underwent a full ocular examination and refraction to determine their best optical correction and visual acuity was measured in each eye using the Bailey-Lovie chart. Subjects were classified as amblyopic if they had a visual acuity difference between the two eyes of at least 0.2 logMAR, could not be corrected optically, and had no evident ocular pathology.
All visual stimuli were generated on a PC computer using custom software written in Python 63 and presented on a gamma corrected IIyama Vision Master Pro 514 CRT monitor with a refresh rate of 85 Hz, and resolution of 1024 × 768 pixels. A digital-to-analogue converter (Bits++, Cambridge Research Systems, Cambridge UK) was used to increase the dynamic contrast range.
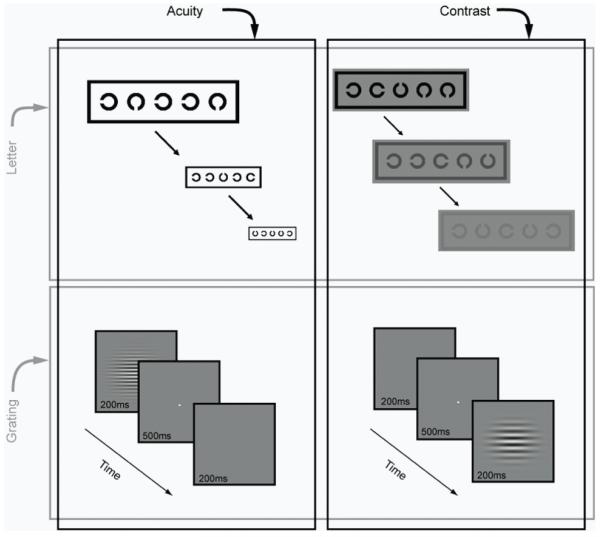
Figure 1 shows the tasks and stimuli used in to characterise performance in the acuity-contrast space. Llandolt C’s were used for the letter-based tasks. The gap width was equal to the stroke width and 1/5th of letter width and height. Five letters were arranged in a row, each randomly oriented in one of 4 cardinal orientations, spaced half a letter-width away from each other and surrounded by a crowding bar to control for contour interactions.
Figure 1.
To measure letter acuity, subjects indicated via a key press the orientation of the gap in each C. On completing a line, the size of all letters was reduced in logarithmic steps and letter acuity was scored in LogMAR units on a letter-by-letter basis. Each letter scored 0.02 logMAR and a letter-by-letter (complete-line) termination criterion of four mistakes was used 64. For letter contrast measures, the stimulus configuration and judgement were the same with the exception that letter size was fixed well above the acuity limit of amblyopic subjects. Michelson contrast was varied in logarithmic steps, with each letter scoring 0.02 log contrast.
We also obtained estimates of acuity and sensitivity using grating stimuli. These consisted of Gabor patches (see Figure 1): a horizontal sinusoidal luminance carrier modulated on a uniform background (~90 cdm−2), windowed by a two-dimensional Gaussian function (SD 0.5°). To measure grating acuity the starting spatial frequency was set to two-thirds of the high spatial frequency cut-off, estimated from Bailey-Lovie letter acuity. In a temporal two-alternative forced choice task, subjects indicated which of two intervals contained the Gabor stimulus. Gabors were presented at 80% Michelson contrast and spatial frequency was varied using an adaptive staircase procedure. Grating acuity thresholds were estimated as the geometric mean of the last 4 reversals of the staircase. For measures of contrast sensitivity, the timing, procedure and staircases were identical, with the following exception. Contrast sensitivity was measured at a range of spatial frequencies (0.5-32 cpd) and quantified as the area under the log contrast sensitivity function. Contrast sensitivity was calculated as the reciprocal of the geometric mean of the contrast threshold for the last 4 reversals. Staircases for different frequencies were randomly interleaved and terminated once all staircases had completed.
Before and after training, we measured subjects’ performance on all four tasks in a random order. During training, subjects were randomly assigned to train on one of the tasks for ten daily sessions. We compared task-specific and generalization performance improvements in both amblyopic and visually normal subjects to untrained test and retest confidence intervals (CIs derived using 10,000 bootstrapped samples from 30 visually normal subjects).
For those amblyopic subjects that demonstrated gross stereo acuity (as measured on the TNO test) at the end of monocular training, we took additional measures of stereo acuity and stereo training using a mirror stereoscope arrangement (for full methodological details see 63). Stereo acuity was measured with stereogram pairs, where random dot images (viewed independently by each eye) created a disparity-defined target (Landolt C) and subjects had to identify the orientation of this target. Each target was presented 5 times and the disparity reduced until the subject made 4 errors at any single disparity level. Subjects trained on the stereo acuity task for 9 sessions (1 session/day; 10mins/session).
Results and Discussion
Normal Variation of Acuity and Sensitivity Measures
Quite often improvement in amblyopic performance is compared to that in the fellow eye 55, which is not a reliable control since performance of one eye can change following training of the other eye 66-69. Moreover, the fellow eye of amblyopic subjects is not considered by many to be completely normal 70,71. To avoid this problematic comparison, we compared performance of amblyopic subjects to visually normal controls and calculated CIs for each task. This sets a minimum baseline and level of variation against which any improvements in visual performance can be compared. One advantage is that CIs can be compared directly other similar measures. For example, the CI for the letter acuity task were in accordance with those found for a Landolt C test 72, the Freiburg Visual Acuity test 73 and other letter acuity tests 74,75. The CI for the Letter Contrast task (0.28 log units) was also is in agreement with the Pelli-Robson contrast sensitivity chart, where it has been suggested that a change of 0.3 log units should be classed as significant 76.
Task Specific Learning in Adult Amblyopia
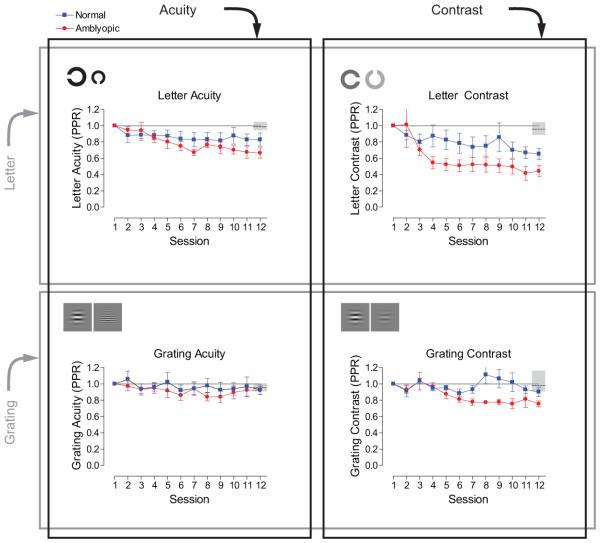
We expressed learned improvements in performance relative to performance before training (post/pre-training ratio for the letter tests and pre/post-training ratio for the grating tests, hereafter referred to as PPR), where numbers less than one constitute learning. Group mean PPR scores were calculated for each of the tasks and are shown in Figure 2. Since the performance on the letter acuity task is expressed in LogMAR units, some scores are negative. This is problematic when calculating ratios, like the PPR. To circumvent this, letter acuity scores were converted to MAR and letter contrast scores converted into raw Michelson contrast units before calculating the PPR.
figure 2.
Normal subjects showed limited improvements in performance over the course of training, but those who trained on the letter contrast task improved by the largest amount (mean PPR 0.65, SEM ±0.07), followed by letter acuity (0.82, ±0.08), grating contrast (0.90, ±0.05), and grating acuity (0.93, ±0.06) over the period of training.
Amblyopic subjects, on the other hand, improved more than normal subjects on all of the tasks apart from grating acuity, where both groups showed little or no change in performance. Mean amblyopic performance significantly exceeded the change in performance of normal subjects who did not train. Amblyopic subjects who trained on letter contrast improved the most (PPR 0.41, ±0.09), followed by letter acuity (0.66, ±0.06) and grating contrast (0.75, ±0.04) over the period of training. Taken together, these data suggest that letter-based contrast sensitivity is much more amenable to learning in both normal and amblyopic subjects than any of the other tasks. Perceptual learning has been shown to improve visual function in amblyopia on a wide range of tasks, though the significance of these effects are hard to judge without appropriate control data and estimates of measurement variability. However, the tasks that show the greatest levels of learned improvements have been contrast-based 59, although all are more similar to our grating contrast task, rather than the letter contrast task.
Even though subjects were randomly assigned to the training groups, we wondered whether the composition of these groups could have contributed to the differential levels of learning on each task. One possibility is that the age or starting acuity of subjects could be confounded with the amount of learning. Amblyopic subjects who trained on the letter contrast task had the lowest mean age of the amblyopic groups and showed the greatest amount of learning on the trained task. However, there was no significant correlation between age and improvements on the trained task for amblyopic subjects (r(27)=0.22, p=0.27). Nor was there a significant relationship between visual acuity and the magnitude of improvement in these subjects (r(27)=−0.16, p=0.41). Therefore, age and starting level of visual acuity are not predictive of the magnitude of learned improvements.
For amblyopic subjects trained on the letter contrast task, greater levels of improvement are found in subjects with poorer start performance compared to those with better start performance (r(8)=0.76, p<0.05). This is in agreement with data from a large group of normal subjects, where poorer initial performance was associated with greater training effects 77. This pattern of results was not, however, replicated in amblyopic subjects that trained on a positional task 78. Whether the amount of learning is governed by task difficulty 79 or set by internal precision (threshold) 80 is currently a matter of debate.
Interestingly, the lack of learning in the grating acuity group was not related to the particular individuals assigned to this treatment group. These subjects, at the end of the study, were offered training on the letter contrast task and most underwent further training. The magnitude of learning found (PPR approx.0.5, 50% improvement) was broadly comparable to the amblyopic group that had trained on the letter contrast task (60% improvement). These data are presented in Figure 3. A previous report has documented improvements in grating acuity with perceptual learning 81. However, this study used only a single subject, who had lost their fellow eye and it is likely that the mechanisms of visual recovery in this case might be very different to our cohort.
figure 3.
If asked to define the quality of vision using a single number, most clinicians would opt for an estimate of letter acuity. Due to the familiarity and sensitivity of this measure, it is also used as a key indicator of treatment success in amblyopia 82,83. Amblyopic subjects that trained on a letter acuity task showed a 34% change in letter score, which equates to an improvement of 0.2 logMAR. The maximum improvement for any amblyopic subject was more than 0.3 logMAR. An improvement of this magnitude would require around 380 hrs of patching in a child 27. Here we were able to generate these changes in a fraction of the time (<10 hrs).
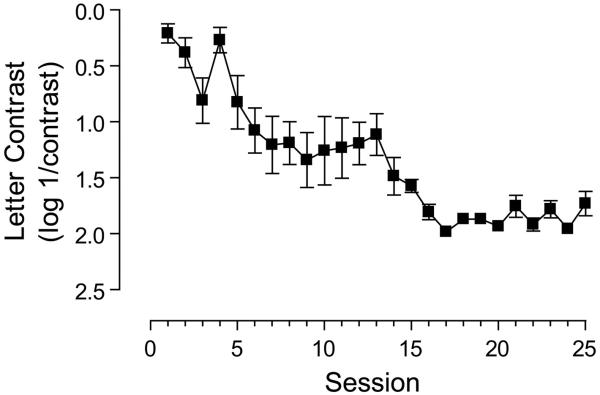
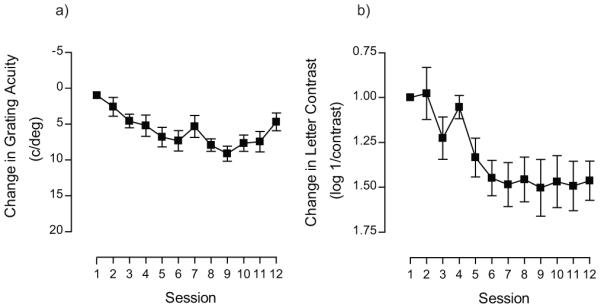
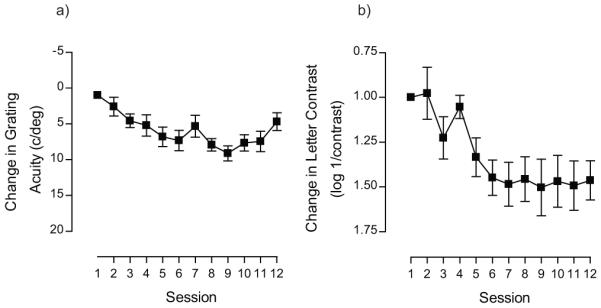
It is possible that extended periods of training may deliver additional improvements. We explored this possibility in one subject that trained on the letter contrast task. Rather than terminate training after 12 days, this subject trained for a total of 25 sessions. Performance improved over the initial stage of training (up to around day 9) and then reached an asymptote, but extending this training period revealed a second stage to the learning process (see Figure 4). This suggests that for some observers more extended periods of training may be required to achieve optimum visual performance, consistent with a previous report showing that the amount of training should be tailored to the initial start performance on a positional task 84. Amblyopic subjects with poor starting thresholds require longer periods of training: exponential time constants for learned improvements have been estimated as ~19hrs, ~6hrs and 3hrs for deep amblyopia, moderate amblyopia and the normal visual system respectively 84.
figure 4.
Generalization of Learning in Adult Amblyopia
We now consider how these learned improvements in monocular performance transfer to untrained tasks. Figure 5 shows the transfer of learning for both normal and amblyopic subjects to all untrained tasks. Each panel shows the average trained improvement on each task (bars in lower contrast) and how these transferred to the three other tasks (bars in higher contrast). Subjects with normal vision showed modest amounts of transfer to other tasks. Amblyopic subjects who trained on letter contrast, not only improved significantly on the task itself, but also improved on all other tasks (exceeding the retest CIs for all the untrained tasks).
figure 5.
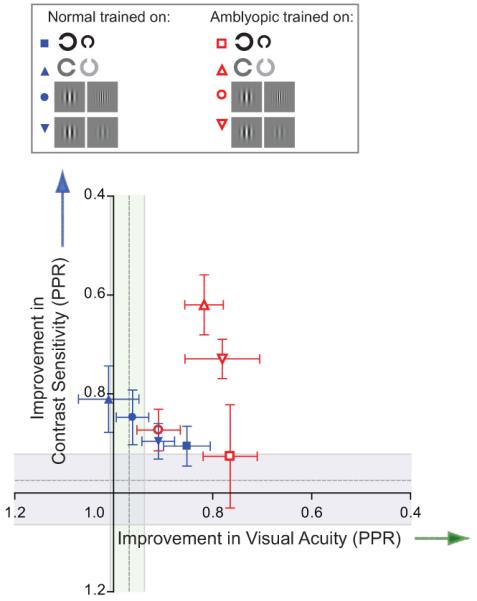
These results show that it is possible for learned improvements to transfer to different types of stimuli along the same visual dimension. When collapsed across dimensions and represented in acuity-contrast space (see Figure 6), the most notable features of the amblyopic data are that training on contrast-based task confers significant visual benefits along both dimensions, whereas acuity training produces benefits that are tightly coupled to the trained dimension.
figure 6.
It has been established that the spatial frequency bandwidth of learning is broader in amblyopia 52,85. That is, learning generalizes broadly across spatial scales. However, it is less clear whether a similar pattern of generalization holds for orientation. The extensive generalization we observe for the letter contrast task suggests that the use of broadband (in orientation and spatial frequency) stimuli facilitates learning within and between deficit scales. In keeping with this, studies that have trained contrast sensitivity using narrowband stimuli (gratings), but present these at multiple orientations, have shown considerable transfer to visual acuity 54. More recently, it has been shown that playing action video games produces improvements in monocular visual acuity and stereo acuity in amblyopic subjects 86. This may be due to the broadband nature of the visual images or the fact that focussed attention is required during game play.
From a clinical perspective, any therapeutic intervention needs to optimise the magnitude, timescale and generalization of learned visual improvements in amblyopia. Mapping the pattern of learning onto the known deficit space for amblyopia enables us to identify which task best met these conditions for a fixed training period. Letter-based contrast training confers the largest magnitude of within dimension learning and across dimension generalization over very short time scales. This makes contrast-based letter tasks ideal candidates for further development of learning-based interventions in this clinical group.
Longevity of Perceptual Learning Effects
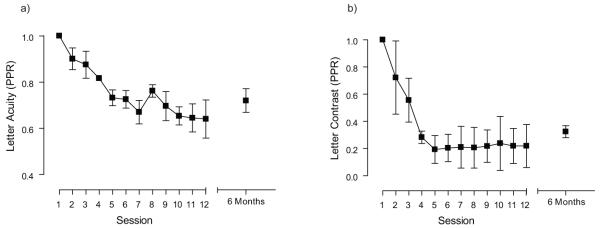
A subset of the subjects that trained on letter acuity and letter contrast were retested 6 months after the cessation of training. Mean training data for these subjects are shown in Figure 7 and are expressed in PPR units. The letter acuity test does show some slippage of improvement, but this is not evident in the data for letter contrast. The change in performance from day 12 to 6 months later did not differ by an amount greater than the confidence limit for the trained test for all observers retested. These results are consistent with previous work suggesting that perceptual learning is long lasting. For example, Polat et al., have re-assessed subjects from 3 months up to 1 year after training and found only minimal slippage of the gains made during training 54. Zhou et al., have used an even longer follow-up period (18 months) and report almost complete retention of improvements in acuity 55. Therefore, unlike occlusion, where acuity often regresses back towards pre-therapy levels 87, the effects of perceptual learning appear to endure. Moreover, when learned improvements show some slippage they can be reinstated very rapidly 78.
figure 7.
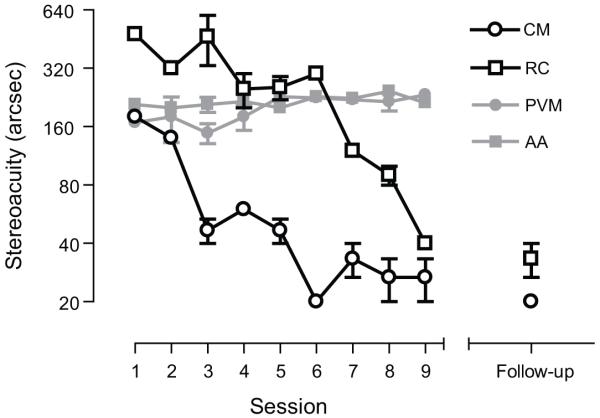
Recovery of Stereo Acuity in Adult Amblyopia
Recently, we have also shown that monocular training puts in place the necessary neural precursors required to fully recover stereo acuity in adult amblyopic subjects 63. After monocular training, 2 subjects demonstrated gross stereopsis on a standard clinical test (TNO). These subjects then underwent training on a disparity-defined task specifically designed to ameliorate their stereo deficit. The data in Figure 8 shows that both adult amblyopic subjects improved their stereo acuity to normal levels over 9 training sessions (open symbols). As a control, we used monocular dioptric blur to degrade stereo acuity to around 200 seconds of arc in two visually normal subjects (grey symbols). In contrast to the amblyopic subjects the controls with blur-limited acuity showed little or no improvement over the same time course, ruling out simple procedural explanations for the visual improvements of adult amblyopic subjects. In each case, the improvements in stereo acuity are gained independently of visual acuity, which remained stable over the course of stereo training and were retained completely 7 months after training had finished. These cases further support the view that the critical period for visual development and the window for treating amblyopia, in this case deficient stereopsis, can be decoupled.
figure 8.
Conclusions
A large body of work now suggests that in many adults with amblyopia it is possible to restore several aspects of visual function using perceptual learning. Although, it should be noted that this approach has not yet been subjected to the scrutiny of a large-scale randomized controlled trial. The key ingredients for designing a learning-based therapy for this group are listed below. Some of these are now well established and supported by data from several independent labs. Others, such as the role of crowding in the task, or introducing a binocular aspect to training 88, are not yet fully understood but are likely to be important.
Use a contrast-based discrimination task
Use broadband stimuli with energy at multiple orientations and spatial frequencies
Repeated exposure to near-threshold stimuli (individualized for observer)
Provide feedback on visual performance
Attentional engagement of subject (make task interesting and challenging)
Daily training sessions
Duration of training coupled to start performance on task
Stringent stopping rule for termination of training
At present, it is unknown whether the inclusion of ‘crowding’ elements in the stimulus configuration is important.
The relationship between these improvements and other non-occlusive forms of therapy, such as refractive adaptation, remain open to question. Clearly these processes appear to operate over very different timescales. One possibility is that refractive adaptation in amblyopic children represents a diluted and unsupervised binocular form of perceptual learning. Simply performing everyday tasks, whilst learning to interpret higher quality visual images, could engage the same cortical mechanisms that drive perceptual improvements in adults. It would be interesting to know whether it is possible to accelerate refractive adaptation effects by supplementing it with active perceptual training.
Acknowledgements
The research leading to these results has received funding from the European Community’s Seventh Framework Programme [FP2007-2013] under grant agreement no 223326. Andrew Astle was funded by the College of Optometrists and Ben Webb by a Wellcome Trust Research Career Development Fellowship.
Footnotes
Declaration: The authors have no financial or personal conflicts of interest.
References
- 1.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 2.Barnes GR, Hess RF, Dumoulin SO, Achtman RL, Pike GB. The cortical deficit in humans with strabismic amblyopia. J Physiol (Lond) 2001;533:281–297. doi: 10.1111/j.1469-7793.2001.0281b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965;28:1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- 4.Kiorpes L, McKee SP. Neural mechanisms underlying amblyopia. Curr Opin Neurobiol. 1999;9:480–486. doi: 10.1016/s0959-4388(99)80072-5. [DOI] [PubMed] [Google Scholar]
- 5.Barrett BT, Bradley A, McGraw PV. Understanding the neural basis of amblyopia. Neuroscientist. 2004;10:106–117. doi: 10.1177/1073858403262153. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Dumoulin SO, Mansouri B, Hess RF. Cortical deficits in human amblyopia: their regional distribution and their relationship to the contrast detection deficit. Invest Ophthalmol Vis Sci. 2007;48:1575–1591. doi: 10.1167/iovs.06-1021. [DOI] [PubMed] [Google Scholar]
- 7.Moseley MJ, Neufeld M, McCarry B, et al. Remediation of refractive amblyopia by optical correction alone. Ophthal Phys Opt. 2002;22:296–299. doi: 10.1046/j.1475-1313.2002.00034.x. [DOI] [PubMed] [Google Scholar]
- 8.Vaegen, Taylor D. Critical period for deprivation amblyopia in children. Trans Ophthalmol Soc UK. 1979;99:432–439. [PubMed] [Google Scholar]
- 9.Awaya S, Sugawara M, Miyake S. Observations in patients with occlusion amblyopia: results of treatment. Trans Ophthalmol Soc UK. 1979;99:447–454. [PubMed] [Google Scholar]
- 10.Harwerth RS, Smith EL, Crawford ML, von Noorden GK. Behavioral studies of the sensitive periods of development of visual functions in monkeys. Behav Brain Res. 1990;41(3):179–198. doi: 10.1016/0166-4328(90)90107-p. [DOI] [PubMed] [Google Scholar]
- 11.von Noorden GK. New clinical aspects of stimulus deprivation amblyopia. Am J Ophthalmol. 1981;92(3):416–421. doi: 10.1016/0002-9394(81)90534-1. [DOI] [PubMed] [Google Scholar]
- 12.Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1996;37(8):1532–1538. [PubMed] [Google Scholar]
- 13.Birch EE, Swanson WH, Stager DR, et al. Outcome after very early treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1993;34(13):3687–3699. [PubMed] [Google Scholar]
- 14.Kugelberg U. Visual acuity following treatment of bilateral congenital cataracts. Doc Ophthalmol. 1992;82(3):211–215. doi: 10.1007/BF00160767. [DOI] [PubMed] [Google Scholar]
- 15.Parks MM. Visual results in aphakic children. Am J Ophthalmol. 1982;94(4):441–449. doi: 10.1016/0002-9394(82)90237-9. [DOI] [PubMed] [Google Scholar]
- 16.Harwerth RS, Crawford MLJ, Smith EL, et al. Behavioral studies of stimulus deprivation amblyopia in monkeys. Vision Res. 1981;21(6):779–789. doi: 10.1016/0042-6989(81)90175-9. [DOI] [PubMed] [Google Scholar]
- 17.Kivlin JD, Flynn JT. Therapy of anisometropic amblyopia. J Ped Ophthalmol Strab. 1981;18(5):47–56. doi: 10.3928/0191-3913-19810901-12. [DOI] [PubMed] [Google Scholar]
- 18.Smith EL, Hung L-F, Harwerth RS. The degree of image degradation and the depth of amblyopia. Invest Ophthalmol Vis Sci. 2000;41(12):3775–3781. [PubMed] [Google Scholar]
- 19.Smith EL, Harwerth RS, Crawford ML. Spatial contrast sensitivity deficits in monkeys produced by optically induced anisometropia. Invest Ophthalmol Vis Sci. 1985;26(3):330–342. [PubMed] [Google Scholar]
- 20.Williams C, Northstone K, Harrad RA, et al. Amblyopia treatment outcomes after screening before or at age 3 years: follow up from randomised trial. BMJ. 2002;324(7353):1–5. doi: 10.1136/bmj.324.7353.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hrisos S, Clarke MP, Wright CM. The emotional impact of amblyopia treatment in preschool children: Randomized controlled trial. Ophthalmol. 2004;111(8):1550–1556. doi: 10.1016/j.ophtha.2003.12.059. [DOI] [PubMed] [Google Scholar]
- 22.Koklanis K, Abel LA, Aroni R. Psychosocial impact of amblyopia and its treatment: a multidisciplinary study. Clin Exp Ophthalmol. 2006;34(8):743–750. doi: 10.1111/j.1442-9071.2006.01317.x. [DOI] [PubMed] [Google Scholar]
- 23.Foley-Nolan A, McCann A, O’Keefe M. Atropine penalisation versus occlusion as the primary treatment for amblyopia. Brit J Ophthalmol. 1997;81(1):54–57. doi: 10.1136/bjo.81.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fielder AR, Irwin M, Auld R, et al. Compliance in amblyopia therapy: objective monitoring of occlusion. Brit J Ophthalmol. 1995;79:585–589. doi: 10.1136/bjo.79.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awan M, Proudlock FA, Gottlob I. A randomized controlled trial of unilateral strabismic and mixed amblyopia using occlusion dose monitors to record compliance. Invest Ophthalmol Vis Sci. 2005;46(4):1435–1439. doi: 10.1167/iovs.04-0971. [DOI] [PubMed] [Google Scholar]
- 26.Loudon SE, Polling J-R, Simonsz B, et al. Objective survey of the prescription of occlusion therapy for amblyopia. Graefe’s Arch Clin Exp Ophthalmol. 2004;2004;242(9):736–740. doi: 10.1007/s00417-004-0896-9. [DOI] [PubMed] [Google Scholar]
- 27.Stewart CE, Moseley MJ, Stephens DA, et al. Treatment dose-response in amblyopia therapy: the monitored occlusion treatment of amblyopia study (MOTAS) Invest Ophthalmol Vis Sci. 2004;45(9):3048–3054. doi: 10.1167/iovs.04-0250. [DOI] [PubMed] [Google Scholar]
- 28.PEDIG A comparison of atropine and patching treatments for moderate amblyopia by patient age, cause of amblyopia, depth of amblyopia, and other factors. Ophthalmol. 2003;110(8):1632–1637. doi: 10.1016/S0161-6420(03)00500-1. [DOI] [PubMed] [Google Scholar]
- 29.Clarke MP, Wright CM, Hrisos S, et al. Randomised controlled trial of treatment of unilateral visual impairment detected at preschool vision screening. BMJ. 2003;327(7426):1251. doi: 10.1136/bmj.327.7426.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C, Hunter DG. Amblyopia: diagnostic and therapeutic options. Am J Ophthalmol. 2006;141(1):175–184. doi: 10.1016/j.ajo.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 31.Birnbaum MH, Koslowe K, Sanet R. Success in ambylopia therapy as a function of age: a literature survey. Am J Optom Physiol Opt. 1977;54(5):269–275. [PubMed] [Google Scholar]
- 32.Kupfer C. Treatment of amblyopia exanopsia in adults; a preliminary report of seven cases. Am J Ophthalmol. 1957;43(6):918–922. doi: 10.1016/0002-9394(57)91795-6. [DOI] [PubMed] [Google Scholar]
- 33.Oliver M, Neumann R, Chaimovitch Y, et al. Compliance and results of treatment for amblyopia in children more than 8 years old. Am J Ophthalmol. 1986;102(3):340–345. doi: 10.1016/0002-9394(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 34.Park KH, Hwang JM, Ahn JK. Efficacy of amblyopia therapy initiated after 9 years of age. Eye. 2004;18(6):571–574. doi: 10.1038/sj.eye.6700671. [DOI] [PubMed] [Google Scholar]
- 35.Scheiman MM, Hertle RW, Beck RW, et al. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol. 2005;2005;123(4):437–447. doi: 10.1001/archopht.123.4.437. [DOI] [PubMed] [Google Scholar]
- 36.Selenow A, Ciuffreda KJ. Vision function recovery during orthoptic therapy in an exotropic amblyope with high unilateral myopia. Am J Optom Physiol Opt. 1983;60(8):659–666. doi: 10.1097/00006324-198308000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Selenow A, Ciuffreda KJ. Vision function recovery during orthoptic therapy in an adult esotropic amblyope. J Am Optom Assoc. 1986;57(2):132–140. [PubMed] [Google Scholar]
- 38.Simmers AJ, Gray LS, McGraw PV, et al. Functional visual loss in amblyopia and the effect of occlusion therapy. Invest Ophthalmol Vis Sci. 1999;40(12):2859–2871. [PubMed] [Google Scholar]
- 39.Steven CH, Kenneth JC. Different rates and amounts of vision function recovery during orthoptic therapy in an older strabismic amblyope. Ophthal Physiol Opt. 1986;6(2):213–220. [PubMed] [Google Scholar]
- 40.Wick B, Wingard M, Cotter S, et al. Anisometropic amblyopia: is the patient ever too old to treat? Optom Vision Sci. 1992;69(11):866–878. doi: 10.1097/00006324-199211000-00006. [DOI] [PubMed] [Google Scholar]
- 41.El Mallah MK, Chakravarthy U, Hart PM. Amblyopia: is visual loss permanent? Brit J Ophthalmol. 2000;84(9):952–956. doi: 10.1136/bjo.84.9.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sengpiel F. The critical period. Curr Biol. 2007;17(17):R742–R743. doi: 10.1016/j.cub.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 43.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Ann Rev Neurosci. 1998;1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 44.Fahle M, Morgan M. No transfer of perceptual learning between similar stimuli in the same retinal position. Curr Biol. 1996;6:292–297. doi: 10.1016/s0960-9822(02)00479-7. [DOI] [PubMed] [Google Scholar]
- 45.Poggio T, Fahle M, Edelman S. Fast perceptual learning in visual hyperacuity. Science. 1992;256:1018–1021. doi: 10.1126/science.1589770. [DOI] [PubMed] [Google Scholar]
- 46.Saarinen J, Levi DM. Perceptual learning in vernier acuity: What is learned? Vision Res. 1995;35:519–527. doi: 10.1016/0042-6989(94)00141-8. [DOI] [PubMed] [Google Scholar]
- 47.Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287:43–44. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- 48.Fine I, Jacobs RA. Comparing perceptual learning tasks: A review. J Vis. 2002;2:190–203. doi: 10.1167/2.2.5. [DOI] [PubMed] [Google Scholar]
- 49.Shiu LP, Pashler H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Percept Psychophys. 1992;52:582–588. doi: 10.3758/bf03206720. [DOI] [PubMed] [Google Scholar]
- 50.O’Toole AJ, Kersten DJ. Learning to see random-dot stereograms. Perception. 1992;21:227–243. doi: 10.1068/p210227. [DOI] [PubMed] [Google Scholar]
- 51.Xiao L-Q, Zhang J-Y, Wang R, et al. Complete transfer of perceptual learning across retinal locations enabled by double training. Curr Biol. 2008;2008;18(24):1922–1926. doi: 10.1016/j.cub.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang C, Zhou Y, Lu ZL. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc Natl Acad Sci USA. 2008;105:4068–4073. doi: 10.1073/pnas.0800824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levi DM. Perceptual learning in adults with amblyopia: A reevaluation of critical periods in human vision. Dev Psychobiol. 2005;46:222–232. doi: 10.1002/dev.20050. [DOI] [PubMed] [Google Scholar]
- 54.Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci USA. 2004;101:6692–6697. doi: 10.1073/pnas.0401200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y, Huang C, Xu P, et al. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Res. 2006;46:739–750. doi: 10.1016/j.visres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 56.Li RW, Levi DM. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. J Vis. 2004;4:476–487. doi: 10.1167/4.6.7. [DOI] [PubMed] [Google Scholar]
- 57.Li RW, Provost A, Levi DM. Extended perceptual learning results in substantial recovery of positional acuity and visual acuity in juvenile amblyopia. Invest Ophthalmol Vis Sci. 2007;48:5046–5051. doi: 10.1167/iovs.07-0324. [DOI] [PubMed] [Google Scholar]
- 58.Levi DM, Li RW. Improving the performance of the amblyopic visual system. Phil Trans Roy Soc B. 2009;364(1515):399–407. doi: 10.1098/rstb.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levi DM, Li RW. Perceptual learning as a potential treatment for amblyopia: A mini-review. Vision Res. 2009;49:2535–2549. doi: 10.1016/j.visres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;2001;31(5):681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- 61.McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- 62.Astle AT, Webb BS, McGraw PV. The pattern of learned visual improvements in adult amblyopia. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.11-7584. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Astle A, McGraw PV, Webb BS. Perceptual learning improves stereoacuity in adults with amblyopia. BMJ Case Reports. 2011 doi: 10.1136/bcr.07.2010.3143. doi:10.1136/bcr.07.2010.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peirce JW. PsychoPy-Psychophysics software in Python. J Neurosci Methods. 2007;162:8–13. doi: 10.1016/j.jneumeth.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carkeet A. Modeling logMAR visual acuity scores: effects of termination rules and alternative forced-choice options. Optom Vis Sci. 2001;78:529–538. doi: 10.1097/00006324-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 66.Ball K, Sekuler R. Direction-specific improvement in motion discrimination. Vision Res. 1987;27:953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- 67.Schoups A, Orban G. Interocular transfer in perceptual learning of a pop-out discrimination task. Proc Natl Acad Sci U S A. 1996;93:7358–7362. doi: 10.1073/pnas.93.14.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fahle M. Human pattern recognition: parallel processing and perceptual learning. Perception. 1994;23:411–427. doi: 10.1068/p230411. [DOI] [PubMed] [Google Scholar]
- 69.Lu ZL, Chu W, Dosher BA, Lee S. Perceptual learning of Gabor orientation identification in visual periphery: Complete inter-ocular transfer of learning mechanisms. Vision Res. 2005;45:2500–2510. doi: 10.1016/j.visres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Res. 2003;43:729–738. doi: 10.1016/s0042-6989(02)00684-3. [DOI] [PubMed] [Google Scholar]
- 71.Giaschi DE, Regan D, Kraft SP, Hong XH. Defective processing of motion-defined form in the fellow eye of patients with unilateral amblyopia. Invest Ophthalmol Vis Sci. 1992;33:2483–2489. [PubMed] [Google Scholar]
- 72.Raasch TW, Bailey IL, Bullimore MA. Repeatability of visual acuity measurement. Optom Vis Sci. 1998;75:342–348. doi: 10.1097/00006324-199805000-00024. [DOI] [PubMed] [Google Scholar]
- 73.Wesemann W. Visual acuity measured via the Freiburg visual acuity test (FVT), Bailey Lovie chart and Landolt Ring chart. Klin Monatsbl Augenheilkd. 2002;219:660–667. doi: 10.1055/s-2002-35168. [DOI] [PubMed] [Google Scholar]
- 74.Arditi A, Cagenello R. On the statistical reliability of letter-chart visual acuity measurements. Invest Ophthalmol Vis Sci. 1993;34:120–129. [PubMed] [Google Scholar]
- 75.Bailey IL, Bullimore MA, Raasch TW, Taylor HR. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci. 1991;32:422–432. [PubMed] [Google Scholar]
- 76.Elliott DB, Sanderson K, Conkey A. The Reliability of the Pelli-Robson Contrast Sensitivity Chart. Ophthalmic Physiol Opt. 1990;10:21–24. [PubMed] [Google Scholar]
- 77.Fahle M, Henke-Fahle S. Interobserver variance in perceptual performance and learning. Invest Ophthalmol Vis Sci. 1996;37(5):869–877. [PubMed] [Google Scholar]
- 78.Levi DM, Polat U, Hu YS. Improvement in Vernier acuity in adults with amblyopia. Practice makes better. Invest Ophthalmol Vis Sci. 1997;38(8):1493–1510. [PubMed] [Google Scholar]
- 79.Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387(6631):401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- 80.Lu ZL, Dosher BA. Characterizing human perceptual inefficiencies with equivalent internal noise. J Opt Soc Am. 1999;1999;16(3):764–778. doi: 10.1364/josaa.16.000764. [DOI] [PubMed] [Google Scholar]
- 81.Fronius M, Cirina L, Cordey A, et al. Visual improvement during psychophysical training in an adult amblyopic eye following visual loss in the contralateral eye. Graefe’s Arch Clin Exp Ophthalmol. 2005;243(3):278–280. doi: 10.1007/s00417-004-1014-8. [DOI] [PubMed] [Google Scholar]
- 82.Hiscox F, Strong N, Thompson JR, Minshull C, Woodruff G. Occlusion for amblyopia: a comprehensive survey of outcome. Eye. 1992;6(Pt 3):300–304. doi: 10.1038/eye.1992.59. [DOI] [PubMed] [Google Scholar]
- 83.Stewart CE, Moseley MJ, Fielder AR. Defining and measuring treatment outcome in unilateral amblyopia. Br J Ophthalmol. 2003;87:1229–1231. doi: 10.1136/bjo.87.10.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li RW, Klein SA, Levi DM. Prolonged perceptual learning of positional acuity in adult amblyopia: perceptual template retuning dynamics. J Neurosci. 2008;28(52):14223–14229. doi: 10.1523/JNEUROSCI.4271-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Astle AT, Webb BS, McGraw PV. Spatial frequency discrimination learning in normal and developmentally impaired human vision. Vision Res. 2010;50:2445–2454. doi: 10.1016/j.visres.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li RW, Ngo C, Levi DM. Video game play induces plasticity in the visual system of adults with amblyopia. PLoS Biology. doi: 10.1371/journal.pbio.1001135. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoyt CS. Why is the adult amblyopic eye unstable? Brit J Ophthalmol. 2004;88(9):1105–1106. doi: 10.1136/bjo.2004.053207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Waddingham PE, Butler TKH, Cobb SV, et al. Preliminary results from the use of the novel Interactive Binocular Treatment (I-BiT[trade]) system, in the treatment of strabismic and anisometropic amblyopia. Eye. 2005;20(3):375–378. doi: 10.1038/sj.eye.6701883. [DOI] [PubMed] [Google Scholar]