Abstract
Purpose
Determine prognostic factors and build a model to predict one-year overall survival (1Y-OS) and six-month progression free survival (6M-PFS) in advanced non-small cell lung cancer (NSCLC) patients treated with first-line paclitaxel and carboplatin (PC) with or without bevacizumab.
Materials and Methods
We analyzed 26 pretreatment clinical variables in 850 NSCLC patients treated in the randomized study ECOG 4599. Univariate and multivariate analyses were performed to identify prognostic factors. Cox regression with 50% randomly sampled data was used to build nomograms with a prognostic score assigned to each factor. The model was validated with the remaining 50% of data.
Results
Eleven poor factors for OS (hazard ratio) were: skin metastasis (4.49), body mass index <18.5 (2.09), increased serum LDH (1.74), adrenal metastasis (1.52), performance status >0 (1.45), low serum albumin (1.45), male (1.39), bone metastasis (1.39), large cell/not other wise specified histology (1.29), mediastinal nodal metastasis (1.23) and treatment without bevacizumab (1.18). Seven poor factors for PFS were: skin metastasis (3.13), treatment without bevacizumab (1.52), bone metastasis (1.41), liver metastasis (1.40), low serum albumin (1.39), performance status >0 (1.21) and mediastinal nodal metastasis (1.14). Based on these factors, we built and validated two nomograms predicting 1Y-OS and 6M-PFS.
Conclusion
Using our proposed models, the probability of survival with first-line paclitaxel and carboplatin with or without bevacizumab in non-squamous NSCLC patients can be estimated. These prognostic models provide a tool for research design and clinical decision making, such as patient stratification and therapy selection.
Keywords: Prognostic Models, Nomograms, Non-Small Cell Lung Cancer, Chemotherapy, Bevacizumab
INTRODUCTION
In 2005, we reported a clinical model to predict survival in chemo-naïve patients with advanced non-small cell lung cancer (NSCLC) treated with third-generation platinum-based chemotherapy doublets.1 Six clinical prognostic factors were determined and a nomogram was built based on data of the two randomized phase III studies ECOG 5592 and ECOG 1594.2,3 Since then, ECOG investigators have published the pivotal randomized study ECOG 4599 which demonstrated the benefit of adding the VEGF targeted monoclonal antibody bevacizumab to the standard regimen of paclitaxel and carboplatin (PC) in non-squamous NSCLC.4 Patients receiving the triplet regimen of paclitaxel, carboplatin and bevacizumab (PCB) lived significantly longer than those treated PC alone with a hazard ratio (HR) of 0.79 (p=0.003). Based on these results, bevacizumab was approved for use in combination with PC as first-line therapy for non-squamous NSCLC.
In spite of proven survival benefits, bevacizumab is associated with certain specific, sometimes fatal adverse events, including hemorrhages, organ perforation, hypertension, and proteinuria. In addition, significant neutropenia and thrombocytopenia are seen more often in those treated with the triplet PCB. Therefore, it is critical to identify patients who may benefit the most, and conversely those who may benefit the least, with bevacizumab. Unfortunately, despite extensive investigation on several potential biomarkers for anti-angiogenic therapy, to date no biomarkers for bevacizumab have been validated for clinical use. Instead, the decision to use bevacizumab in NSCLC is based upon clinical features such as histology and history of hemoptysis.
Therefore, we retrospectively analyzed ECOG 4599 data to identify clinical and laboratory characteristics which would predict survival of NSCLC patients receiving first-line treatment with PC with or without bevacizumab. We used this data to build a nomogram to estimate the probability of survival in this patient population.
MATERIALS AND METHODS
Patients
We retrospectively analyzed data of 850 eligible patients with advanced NSCLC who were randomized to receive first-line therapy of PCB or PC in the study ECOG 4599. The inclusion/exclusion criteria and treatment regimens was previously described in the original report by Sandler et al.4 In short, patients were randomly assigned to receive paclitaxel 200 mg/m2 and carboplatin at AUC of 6, with or without bevacizumab 15 mg/kg every 3 weeks for up to 6 cycles. Patients with at least stable disease in the PCB arm continued bevacizumab until disease progression or unacceptable toxicity.
Statistical Analysis Methods
The primary objectives of this analysis are to determine prognostic factors to predict survival of NSCLC patients treated with first-line PC with or without bevacizumab, and to build nomograms to predict one-year survival (1Y-OS) and six-month progression free survival (6M-PFS). Based on our prior clinical model1 as well as other analyses on prognosis in lung cancer,5–8 we selected 26 pretreatment clinical and laboratory variables recorded in the database for this study, including (Table 1): treatment with or without bevacizumab, patient demographics, disease stage, performance status, histology subtypes, metastatic sites/organs, number of metastatic sites, prior radiation therapy, body mass index or BMI (underweight < 18.5, normal weight 18.5–24.9, overweight 25–29.9, and obesity ≥ 30); body surface area BSA (</≥ 2 m2), and baseline laboratory tests including serum albumin level (</≥ lower limit of normal LLN), serum lactate dehydrogenase LDH (≤/> upper limit of normal ULN), and proteinuria by urinary analysis (negative, trace or 1+/higher).
Table 1.
Patient Characteristics.
| Patient Characteristics | Patients (Total n= 850) | ||
|---|---|---|---|
| n | % | ||
| Bevacizumab Treatment | Yes | 417 | 49 |
| No | 433 | 51 | |
| Age | <70 | 648 | 76 |
| ≥ 70 | 202 | 24 | |
| Gender | Female | 387 | 46 |
| Male | 463 | 54 | |
| Race | White | 730 | 86 |
| Other | 120 | 14 | |
| Stage | IIIB | 105 | 12 |
| IV/Recurrent | 745 | 88 | |
| Performance Status | 0 | 343 | 40 |
| 1 | 507 | 60 | |
| Histology* | AC | 586 | 69 |
| ACLP | 23 | 3 | |
| LCC | 46 | 5 | |
| NOS | 160 | 19 | |
| Other | 32 | 4 | |
| Hilar Nodes | Present | 335 | 39 |
| Mediastinal Nodes | Present | 439 | 52 |
| Supraclavicular/Scalene Nodes | Present | 72 | 8 |
| Pleural Effusion | Present | 322 | 38 |
| Malignant Effusion | Present | 169 | 20 |
| Pleural Metastasis | Present | 223 | 26 |
| Ipsilateral Lung Metastasis | Present | 381 | 45 |
| Contralateral Lung Metastasis | Present | 280 | 33 |
| Liver Metastasis | Present | 163 | 19 |
| Bone/Bone Marrow Metastasis | Present | 267 | 31 |
| Adrenal Metastasis | Present | 125 | 15 |
| Skin Metastasis | Present | 12 | 1.4 |
| Distant Metastasis > 3 sites** | Present | 165 | 19 |
| Prior Radiation Therapy | Yes | 70 | 8 |
| No | 780 | 92 | |
| Body Mass Index (BMI) | < 18.5 | 30 | 4 |
| 18.5–24.9 | 349 | 41 | |
| 25–29.9 | 304 | 36 | |
| ≥ 30 | 167 | 20 | |
| Body Surface Area (BSA) | ≥ 2 | 269 | 32 |
| < 2 | 581 | 68 | |
| Low Serum Albumin (< LLN) | Present | 240 | 28 |
| High Serum LDH (> ULN) | Present | 273 | 32 |
| Proteinuria | Negative | 673 | 80 |
| Trace | 136 | 16 | |
| 1+ or Higher | 34 | 4 | |
AC, adenocarcinoma; ACLP, adenocarcinoma with lepidic pattern (bronchio-alveolar carcinoma); LCC, large cell carcinoma; NOS, not otherwise specified.
Ipsilateral or contralateral lung, pleura, liver, adrenal, brain, bone, BM, skin.
A univariable Cox regression analysis was used to assess the association between each variable and outcome. Multivariable stepwise Cox models were then fitted for final variable selection of prognostic factors. Using 50% randomly sampled data (test set), we built the two nomograms predicting 1Y-OS and 6M-PFS. Prognostic scores used in each nomogram are derived from the absolute values of the estimated log hazard ratios (from the multivariate Cox model built on the test set) multiplied by ×100. The prediction models were then validated with the remaining 50% of data (validation set).
RESULTS
As reported in the original ECOG 4599 paper by Sandler et al, PCB significantly prolonged survival in comparison to chemotherapy alone, with median OS of 12.3 months vs. 10.3 months, 1Y-OS of 51% vs. 44%, with a death HR of 0.79 (p=0.003), and median PFS of 6.2 months vs. 4.5 months, with a HR for disease progression of 0.66 (p<0.001).4 A summary of baseline clinical and laboratory variables of 850 patients included in this analysis is presented in Table 1. Patients were randomized equally to PC and PCB arms. One out of four patients were 70 years or older. More than two-thirds had adenocarcinoma and almost half were women. A small number of patients had adenocarcinoma with lepidic pattern (formerly bronchioalveolar carcinoma) (3%) or large cell carcinoma (5%), while 19% had not otherwise specified (NOS) NSCLC. Of all metastatic organs, the most common site was mediastinal nodes (52%). Other common metastatic sites included ipsilateral (45%) or contralateral (33%) lung, pleura (20% reported as malignant effusion and 26% pleural metastasis), and bone (31%). The least reported metastatic organ was skin (1.4%). More than half of patients were overweight (36%) or obese (20%), while only 4% were considered underweight with BMI <18.5. Serum albumin was low in 28% of patients, serum LDH was high in 32%, and proteinuria (trace or higher) was seen in 20%.
Prognostic Factors and Nomogram Predicting One-Year Overall Survival
To determine independent prognostic factors predicting OS, univariate and multivariate Cox models were fitted. Out of 26 variables analyzed in univariate analysis, 15 are associated with poor survival. The Cox multivariate regression model further identified 11 independent poor prognostic factors (Table 2).
Table 2.
Independent Poor Prognostic Factors of Overall Survival
| Patient Characteristics | Hazard Ratio | p-value | Prog Score |
|---|---|---|---|
| Skin Metastasis | 4.49 | <0.0001 | 130 |
| Low BMI < 18.5 | 2.09 | 0.0003 | 81 |
| High Serum LDH (> ULN) | 1.74 | <0.0001 | 48 |
| Adrenal Metastasis | 1.52 | 0.0002 | 52 |
| PS 1 (vs. PS 0) | 1.45 | <0.0001 | 35 |
| Low Serum Albumin (< LLN) | 1.45 | <0.0001 | 32 |
| Gender - Male | 1.39 | 0.0002 | 37 |
| Bone Metastasis | 1.39 | 0.0002 | 29 |
| Histology - LCC/NOS (vs. AC/ACLP) | 1.29 | 0.006 | 30 |
| Mediastinal Nodes | 1.23 | 0.02 | 42 |
| No Bevacizumab (PC Alone) | 1.18 | 0.05 | 25 |
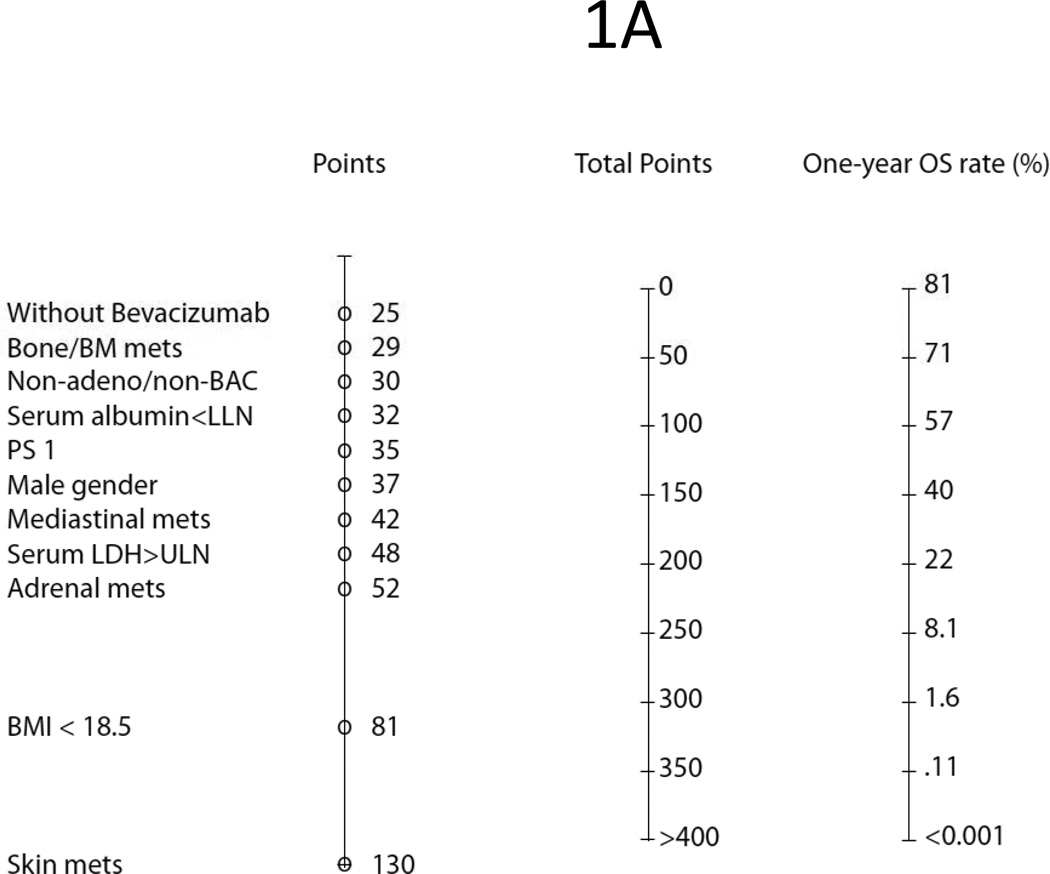
Based on those 11 independent prognostic markers, we built a nomogram to predict 1Y-OS using 50% randomly sampled data (Figure 1A). In this model, each poor prognostic factor is “assigned” a prognostic score (P/S) correlating with 1Y-OS prognosis. A higher score implies a poorer survival outcome. The factor with the poorest prognosis is skin metastasis (HR = 4.49, P/S = 130), followed by a low BMI <18.5 (HR = 2.09, P/S = 81). The remaining nine poor independent prognostic factors are: increased serum LDH (HR = 1.74, P/S = 48), adrenal metastasis (HR = 1.52, P/S = 52), performance status ECOG 1 (HR 1.45, P/S = 35), low serum albumin (HR = 1.45, P/S = 32), male gender (HR = 1.39, P/S = 37), bone metastasis (HR 1.39, P/S = 29), large cell or NOS histology (HR = 1.29, P/S = 30), mediastinal nodal involvement (HR = 1.23, P/S = 42), and treatment without bevacizumab (HR = 1.18, P/S = 25).
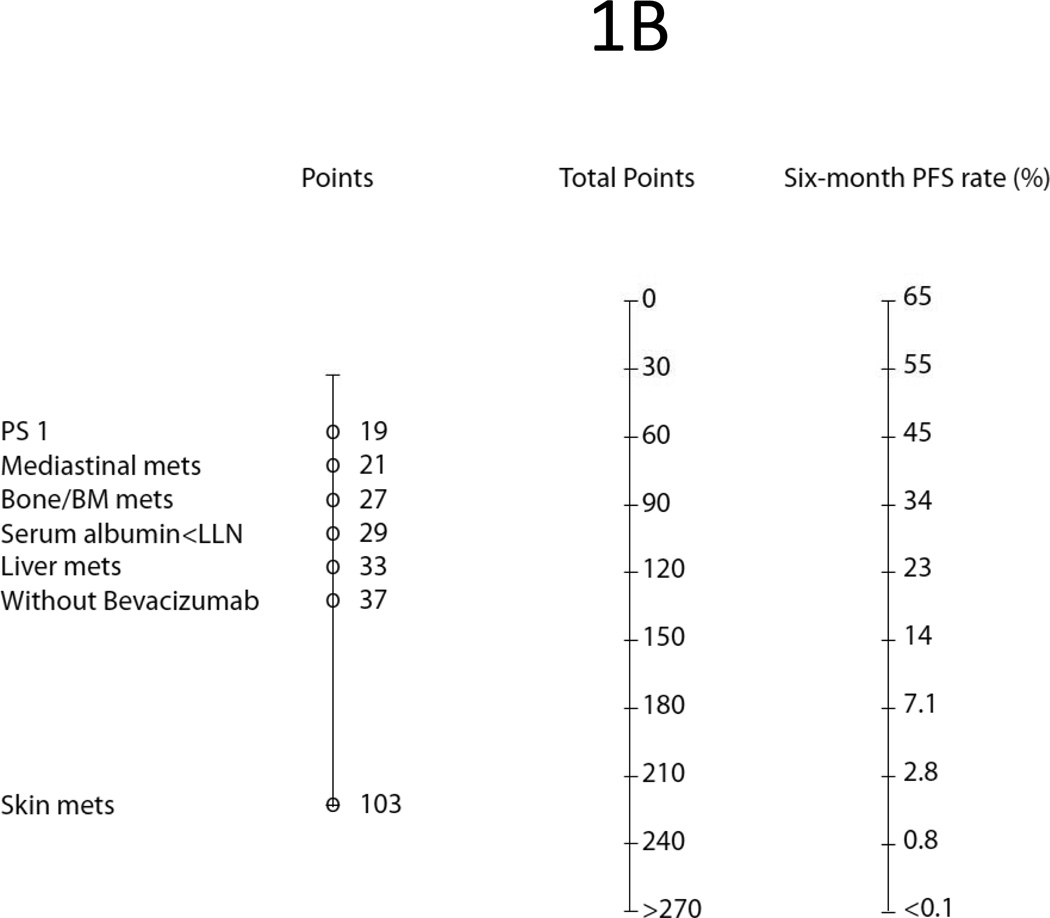
Figure 1.
Prognostic Nomograms Predicting: (A) One-Year Survival; (B) Six-Month Progression Free Survival.
Prognostic Factors and Nomogram Predicting Six-Month Progression Free Survival
Using a similar method, we fit clinical data of ECOG 4599 into Cox models to identify independent poor prognostic factors (Table 3) and build a nomogram (Fig. 1B) to predict 6M-PFS. From 26 clinical variables, we identified 15 poor factors in univariate analysis. In multivariate analysis, only seven stood out as independent poor factors for 6M-PFS, including: skin metastasis (HR = 3.13, P/S = 103), treatment without bevacizumab (HR = 1.52, P/S = 37), bone metastasis (HR = 1.41, P/S = 27), liver metastasis (HR = 1.40, P/S = 33), low serum albumin (HR = 1.39, P/S = 29), performance status ECOG 1 (HR = 1.21, P/S = 19), and mediastinal nodal involvement (HR = 1.14, P/S = 21).
Table 3.
Independent Poor Prognostic Factors of Progression Free Survival
| Patient Characteristics | Hazard Ratio | p-value | Prog Score |
|---|---|---|---|
| Skin Metastasis | 3.13 | 0.0001 | 103 |
| No Bevacizumab (PC Alone) | 1.52 | <0.0001 | 37 |
| Bone Metastasis | 1.41 | <0.0001 | 27 |
| Liver Metastasis | 1.40 | 0.0002 | 33 |
| Low Serum Albumin (< LLN) | 1.39 | <0.0001 | 29 |
| PS 1 (vs. PS 0) | 1.21 | 0.01 | 19 |
| Mediastinal Nodes | 1.14 | 0.07 | 21 |
Validation of the Nomograms
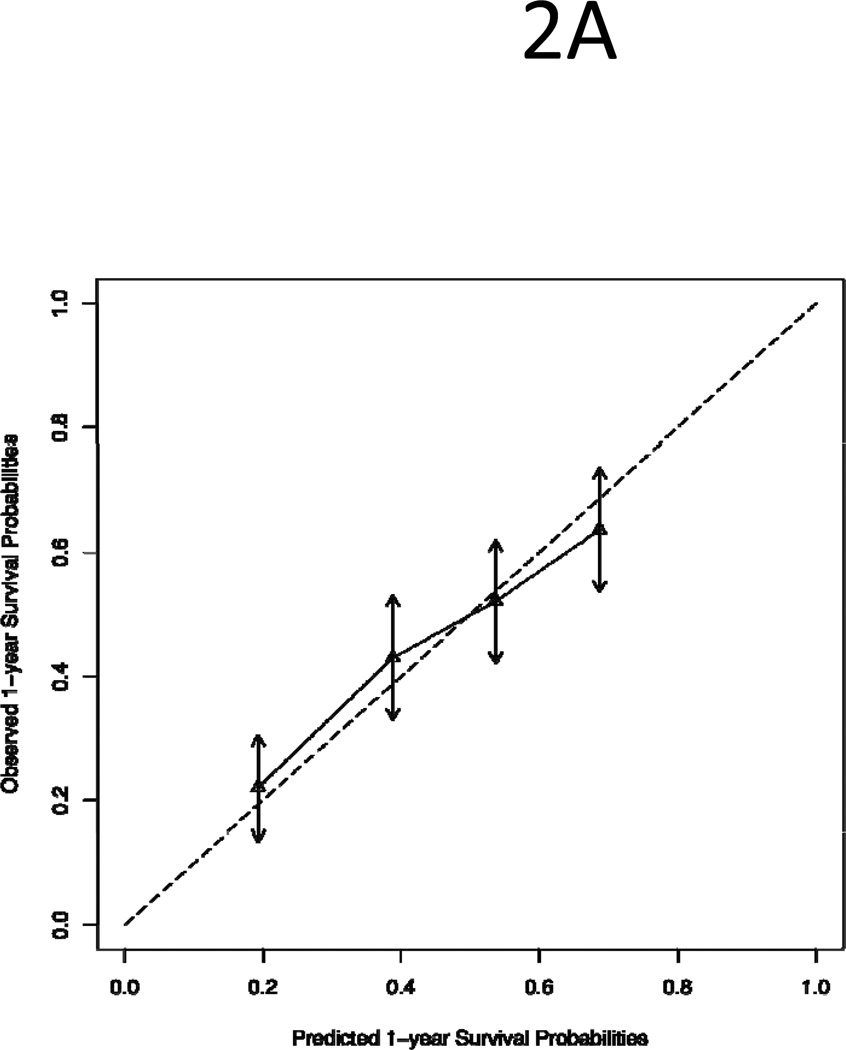
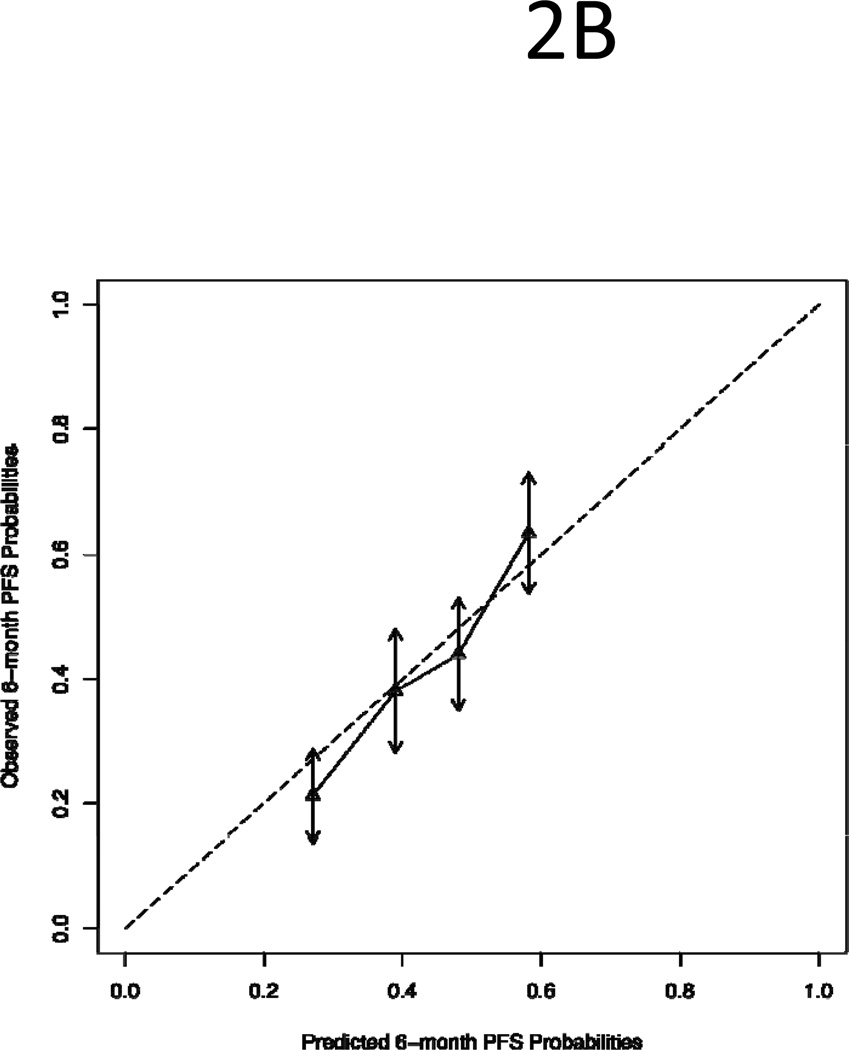
The 1Y-OS and 6-M PFS nomograms were constructed from the test set of 50% randomly sampled data and validated by the validation set of the remaining 50% data. To validate the models, patients in the validation set were divided into quartile groups based on their prognostic scores: low risk, low-intermediate risk, high-intermediate risk and high risk. As shown in Figure 2, the predicted survival (x-axis) for each quartile group was compared with the observed/actual survival (y-axis). The dotted line in the figure illustrates the ideal scenario in which the predicted survival perfectly matches the observed survival. We found that the predicted survival was within the 95% confidence intervals of the observed survival (arrows) and both 1Y-OS and 6M-PFS lines follow the “ideal line”. The finding demonstrates a good agreement between predicted and observed survivals.
Figure 2.
Comparison of Predicted Survival with Observed Survival of Quartile Groups of Patients in the Validation Set: (A) One-Year Survival; (B) Six-Month Progression Free Survival. The dotted line is the “ideal” line if there is a perfect match between predicted and observed survival. The vertical arrows represent 95% confidence intervals of observed survival.
Case Examples on How to Use the Prognostic Nomograms
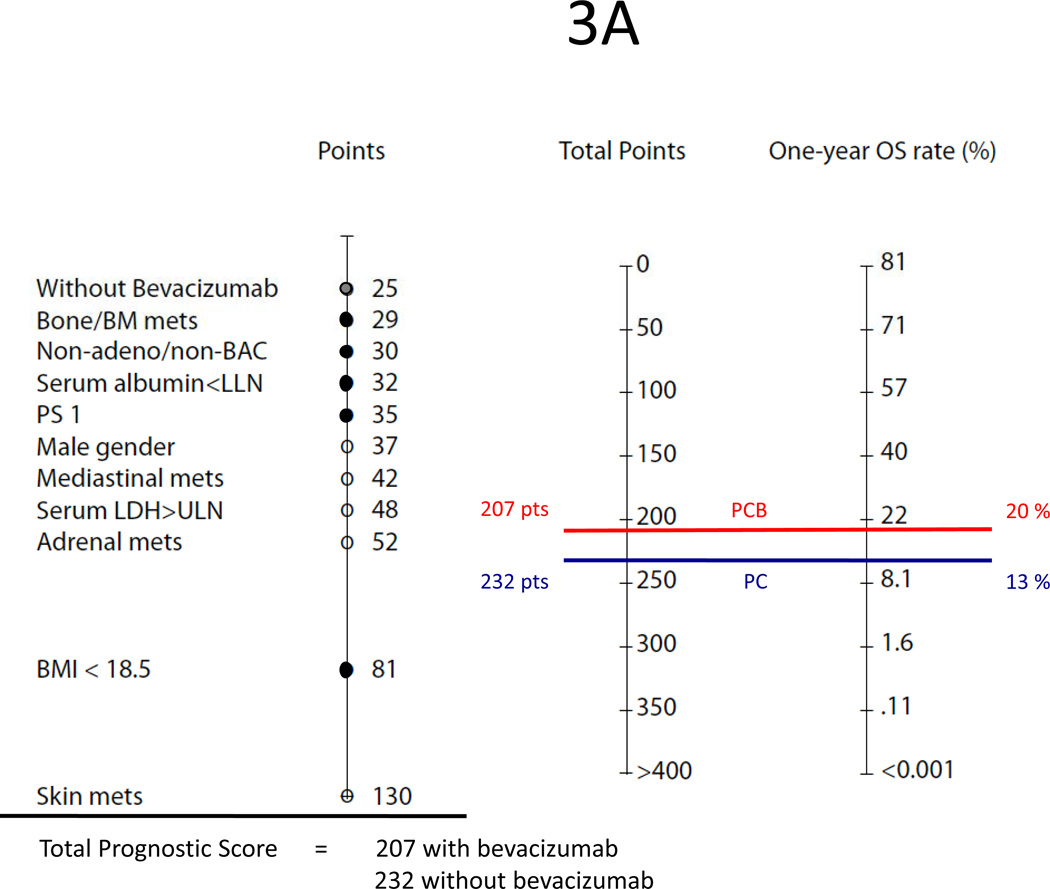
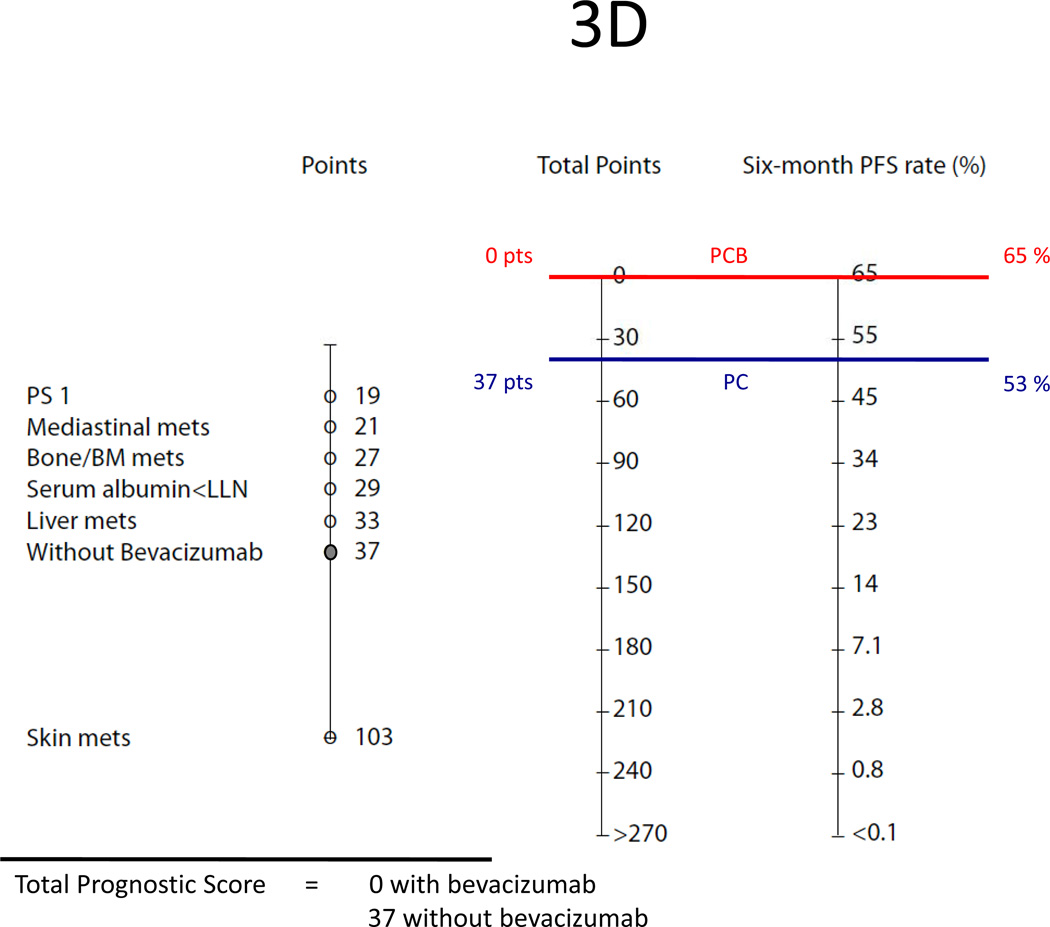
Using our nomograms, one could predict the probability of a NSCLC patient surviving one year or longer and achieving a six-month or longer disease-free progression interval with first-line paclitaxel and carboplatin with or without bevacizumab. For example, patient 1, a woman with large cell lung cancer, ECOG performance status 1, BMI <18.5, bone metastasis, serum albumin below the lower limit of normal, and receiving PC as first-line treatment, has a total prognostic score of 232 for overall survival and 112 for progression free survival (Figures 3A–B). On the nomograms, those scores correspond to an estimate 1Y-OS chance of 13% and 6M-PFS chance of 26%. However, if this patient were treated with the triplet therapy of PCB, her total prognostic score would be 207 for overall survival and 75 for PFS. Thus, her predicted 1Y-OS and 6M-PFS would be 20% and 40%, respectively.
Figure 3.
Examples of the Nomograms:
(A) Predicted one-year survival of patient 1 who is a woman with large cell lung cancer (PS 30), ECOG performance status 1 (PS 35), BMI <18.5 (PS 81), bone metastasis (PS 29) and low serum albumin (PS 32). Treated with PC alone (PS 25), her total prognostic score is 232 points (30+35+81+29+32+25). If bevacizumab is added to the doublet, her total prognostic score is 207 points (30+35+81+29+32);
(B) Predicted six-month PFS of patient 1. Treated with PC alone, her prognostic score is 112 points (19+27+29+37). If bevacizumab is added to the doublet, her prognostic score is 75 points (19+27+29);
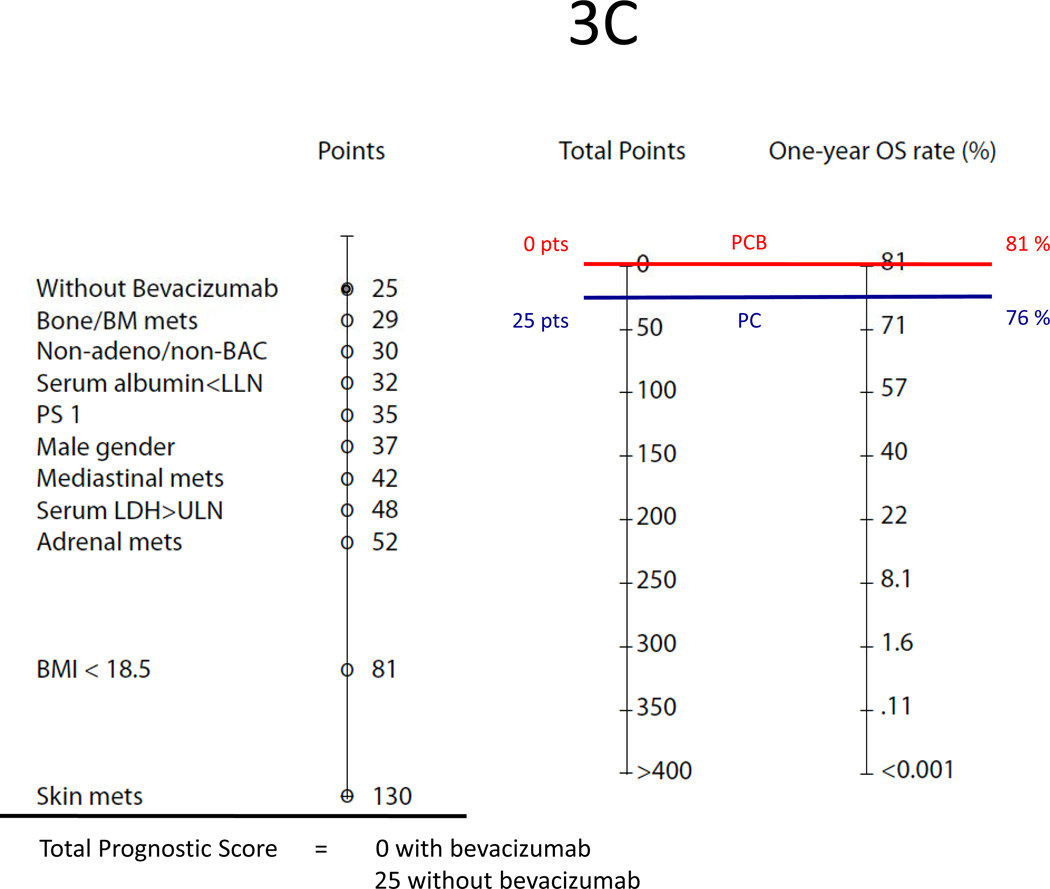
(C) Predicted one-year survival of patient 2 who is a woman with an adenocarcinoma of the lung with malignant effusion, otherwise having none of poor baseline factors. Treated with PC alone, her total prognostic score is 25 points. If bevacizumab is added to the doublet, her total prognostic score is 0;
(D) Predicted six-month PFS of patient 2. Treated with PC alone, her prognostic score is 37 points. If bevacizumab is added to the doublet, her prognostic score is 0.
In another example, patient 2 is a woman with a lung adenocarcinoma with a malignant effusion who has none of the poor baseline factors (Figure 3C–D). Should she be treated with PCB, her total prognostic score would be 0 for both overall survival and PFS, and as such, her estimated 1Y-OS and 6M-PFS chance would be 81% and 65%. However, if she were treated with PC alone, her prognostic score would be 25 for overall survival and 37 for PFS, corresponding to a 1Y-OS and 6M-PFS chance of 76% and 53%, respectively.
In both patients in the above examples, adding bevacizumab to paclitaxel and carboplatin prolongs both 1Y-OS and 6M-PFS. However, the relative gain with bevacizumab appears more profound in the first patient who has a much worse prognosis than the second patient who has none of baseline poor prognostic factors. Indeed, the addition of bevacizumab in patient 1 results in a 50% improvement in 1Y-OS (from 13% to 20%), whereas in patient 2, bevacizumab only leads to a 7% improvement in survival (from 76% to 81%).
DISCUSSION
During the last few decades, progresses in biology, diagnostics and therapy have resulted in a slow but steady improvement in the treatment of advanced NSCLC. Overall median survival of patients with metastatic disease improved from 5–6 months in 1980s to 12 months at present with the incorporation of targeted drugs such as bevacizumab into conventional chemotherapy. However, survival among patients varies significantly, based upon tumor biology and clinical factors, such as poor performance status and significant weight loss. Other factors including gender, metastasis, or laboratory abnormality such as leukocytosis and increased LDH were suggested in some but not all studies. Table 4 shows the findings of five large retrospective analyses based on selected randomized studies from cooperative groups conducted within the last three decades.1,5–7
Table 4.
Survival Prognostic Factors and Prediction Models for Non-Small Cell Lung Cancer from Five Retrospective Analyses
| ECOG Finkelstein, 19865 |
SWOG Albain, 19916 |
ELCWP Paesmans, 19957 |
ECOG Hoang, 20051 |
ECOG Hoang, 2011 |
|
|---|---|---|---|---|---|
| Database | 2 phase III (EST 2575 & 1581) |
5 phase III 9 phase II |
3 phase III 4 phase II |
2 phase III (ECOG 1592 & 1594) |
1 phase III (ECOG 4599) |
| Patient number | 893 | 2531 | 1052 | 1436 | 850 |
| Disease stage | Metastatic | Locally advanced or metastatic |
Unresectable I-IV | Wet IIIB, IV, or recurrent |
Wet IIIB, IV, or recurrent |
| Histology | Any NSCLC histology | Any NSCLC histology | Any NSCLC histology | Any NSCLC histology | Non-squamous NSCLC |
| Line of treatment | First-line | First-line or previously treated |
First-line or previously treated |
First-line | First-line |
| Chemotherapy regimens |
Various older platin and non-platin based regimens |
Various older platin and non-platin based regimens; mono versus combined |
Various platin-based, second-generation regimens; mono versus combined |
Platin-based, third- generation doublets |
Paclitaxel, carboplatin with or without bevacizumab |
| Survival Median, months 1-year, % 2-year, % |
< 6 19 4 |
5.1 16 - |
7 - 7.4 |
8.2 33 11 |
10.3/12.3* 44/51* 15/23* |
| No. of factors analyzed |
36 | 15 (not all patients) | 23 | 27 | 26 |
| Independent negative survival prognostic factors identified from multivariate analyses |
1. Lower PS (1–2) 2. Bone metastasis 3. Male gender 4. Weight loss 5. Subcutaneous metastasis 6. Large cell carcinoma 7. Shoulder/arm pain 8. Liver metastasis |
All patients: 1. Lower PS (≥2) 2. Non-platin drugs 3. Male gender 4. Age < 70 PS 0–1 patients: 1. Hb < 11 2. Abnormal LDH 3. Abnormal calcium 4. ≥2 metastatic lesions 5. Non-platin drugs |
1. Stage IV (vs. earlier stages) 2. Lower PS (≤70) 3. Increased WBC 4. Skin metastasis 5. Increased calcium 6. Abnormal ANC 7. Age > 60 8. Male gender |
1. Skin metastasis 2. Lower PS (1–2) 3. Loss of appetite 4. Liver metastasis 5. ≥4 metastatic sites 6. No prior lung surgery |
1. Skin metastasis 2. Low BMI <18.5 3. Increased LDH 4. Adrenal metastasis 5. Lower PS (1) 6. Low serum albumin 7. Male gender 8. Bone metastasis 9. LCC/NOS histology 10. Mediastinal nodal metastasis 11. No bevacizumab (PC alone) |
| Survival prediction model or prognostic subgroups |
Discriminant function requiring calculation to predict if an individual will survive > 1 year or not |
Three prognostic subgroups based on recursive partitioning and amalgamation |
Four prognostic subgroups with different 1- and 2-year survival, based on recursive partitioning and amalgamation |
Nomogram with a scoring system to predict 1-and 2-year survival probability of individual patients |
Nomogram with a scoring system to predict probability of 1-year survival and 6-month PFS of individual patients |
without bevacizumab/with bevacizumab
Abbreviations: ECOG, Eastern Cooperative oncology Group; SWOG, Southwest Oncology Group; ELCWP, European Lung Cancer Working Party; PS, performance status; Hb, hemoglobin; LDH, lactic dehydrogenase; WBC, white blood count; ANC, absolute neutrophil count; BMI, body mass index; LCC, large cell carcinoma; NOS, not otherwise specified.
In our prior analysis which is based on ECOG 5592 and ECOG 1594, we found six poor prognostic factors predicting survival of NSCLC patients treated with platinum-based doublets involving paclitaxel, docetaxel, or gemcitabine, including: skin metastasis, lower performance status 1 or 2, loss of appetite, liver metastasis, ≥ four metastatic sites, and no prior surgery.1 There are several differences between our prior and current analysis. This analysis is based on the trial ECOG 4599 which accrued bevacizumab eligible population only (non-squamous histology, no significant hemoptysis). Furthermore, we included both clinical and laboratory variables. More importantly, the treatment regimen involved paclitaxel and carboplatin with or without bevacizumab.
In this analysis, 11 poor survival factors were identified. Similar to our previous report, we found that skin metastasis carries the worst survival prognosis with a highest HR (4.49) and prognostic score (130) in the 1Y-OS model. Skin metastasis also predicted the worst 6M-PFS. Although skin metastasis from primary lung cancer is an uncommon event, reported in only 4–12% of patients in prior ECOG studies involving all NSCLC histology subtypes, and in 1.4% in ECOG 4599, its presence likely suggests an aggressive biological behavior of the disease causing widespread metastasis. Other investigators also found the negative impact of skin or subcutaneous spread on survival in NSCLC.5,7
Significant weight loss of more than 5–10% total body weight within 6-month period and loss of appetite have been considered among the most important negative prognostic factors in NSCLC. However, not all patients are able to quantify their weight loss. Therefore, in this study, we decided to evaluate BMI at baseline, prior to the start of chemotherapy, which can be calculated easily based on patient’s weight and height, as a potential prognostic factor. The results demonstrated that underweight patients with BMI <18.5 had a poorer survival compared to those with normal or overweight (BMI ≥18.5), corresponding to the second highest HR (2.09). Interestingly, the relation between excess body weight and cancer mortality has been documented in many cancer types, although the underlying mechanism is not clear yet. In a report involving more than 900,000 American adults accrued in the prospective Cancer Prevention Study II, high BMI was in general associated with increased cancer related death rates in both obese men and women cohorts with BMI ≥35.0.8 That observation was seen across several solid and hematologic cancers, with the only exception being lung cancer. Indeed, contrary to other cancers, BMI was inversely correlated to lung cancer mortality. The relative risk of lung death in cohorts with BMI ≥35.0 in that study was approximately 0.67. Low serum albumin level is also a poor prognostic overall survival in our model, with a HR of 1.45. It is possible that low BMI, low serum albumin, and weight loss/loss of appetite all are biologic indicators of an aggressive cancer leading to a quick decline in nutritional and overall general health status.
Accumulating data have suggested that there is a gender difference between women and men with lung cancer.9 Some studies have suggested that women are more susceptible to carcinogenic effects of cigarette smoking.10,11 On the other hand, female patients with NSCLC are more likely to be never-smokers,12 have adenocarcinoma,9 harbor mutations in the EGF receptor,13 and live longer than their male counterparts with chemotherapy9. In this analysis, although there was no difference in PFS, men had a lower chance of surviving at one year, with a HR of 1.39. The difference in outcome suggests gender is an important factor in designing clinical trials, and that gender should be a stratification factor in randomized trials.
In our analysis, patients with large cell carcinoma or NOS histology had a poorer survival compared to those with adenocarcinoma or adenocarcinoma with lepidic pattern suptype, with a HR of 1.29. It was unclear if the histology of NOS cases was undefined due to poor histological differentiation or lacking of tissue samples from fine needle aspiration. Patients with poorly differentiated, high-grade cancer may have more aggressive course and poorer prognosis.
Our study demonstrated that increase in LDH was significantly associated with shorter one-year survival with a HR of 1.74. LDH is an enzyme which catalyzes the forward and backward conversion of pyruvate to lactate, an important step in the process of cellular glycolysis and energy production. Cancer cells primarily metabolize glucose into lactate through the anaerobic glycolysis (Warburg effect), while most normal cells rely on the more efficient oxidative phosphorylation in mitochondria to produce ATP. The isozyme M-LDH (LDH-5) is increased in several human tumors compared to normal tissues,14 and elevated serum LDH is associated with chemotherapy and radiation resistance,15 as well as poor prognosis in a variety of cancers including NSCLC.6,15,16 The overexpression of LDH-5 in NSCLC tumor samples was linked to the expression of several angiogenic markers (VEGF, bFGF, bFGFR), the hypoxia-inducible factor HIF 1∝, and survival in patients with resected disease.15,17 Findings from our study as well as others suggest that serum LDH and albumin are two simple blood tests which can be used to evaluate prognosis in NSCLC patients. However, it should be noted that LDH is not specific, as it can also be elevated in patients with underlying medical diseases such as cardiac, lung and liver disorders. Prospective studies are needed to validate the prognostic value of tumor LDH-5 expression for clinical use.
Finally, the addition of bevacizumab to chemotherapy is a positive prognostic factor for improved PFS and one-year survival rate. In regard to PFS, bevacizumab is the second most important factor, second only to skin metastasis. Compared to triplet therapy PCB, treatment with paclitaxel and carboplatin alone correlated with a HR of 1.52 for PFS. However, the benefit of bevacizumab on one-year survival appeared relatively modest in comparison to other factors. Indeed, among the 11 poor factors predicting one–year survival, bevacizumab impacts the least with a HR of 1.18 in patients receiving chemotherapy alone. Although survival advantages of bevacizumab in NSCLC have been proven, the drug is associated with certain morbid and fatal adverse effects such as bleeding, GI perforation or fistula. Because of the modest gain by adding bevacizumab to chemotherapy in patients without poor prognostic factors (such as patient 2 in the above example), patients with good factors and borderline risks to bevacizumab (for example, hemoptysis <1/2 teaspoon, labile hypertension) may elect a non-bevacizumab containing regimen, either with standard chemotherapy or enrolling into clinical trials.
Multiple potential biomarkers for bevacizumab and other antiangiogenic agents such as VEGF, basic fibroblast growth factor (b-FGF), intercellular adhesion molecule (ICAM), E-selectin, thrombospondin-2, microvessel density have been investigated in clinical trials in lung cancer as well as other cancers.18–21 However, to date, no biomarkers for anti-angiogenic therapy have yet been validated for clinical use. Thus, these nomograms may be helpful in identifying NSCLC patients most likely to benefit from the addition of bevacizumab to chemotherapy. By doing so, one may maximize the therapeutic gain, while minimizing potential morbidity and mortality and reduce the cost to the health care system.
In conclusion, our analysis identified several clinical factors predicting survival in non-squamous NSCLC patients treated with first-line paclitaxel and carboplatin with or without bevacizumab. Our nomograms offer a tool to estimate the possibility of survival. Those prognostic factors and models may help investigators in designing clinical trials and assist clinicians in selecting the most appropriate therapy for their patients.
Acknowledgments
This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA21076, CA49957, CA16116 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hoang T, Xu R, Schiller JH, et al. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with thirdgeneration chemotherapy regimens based on eastern cooperative oncology group data. J Clin Oncol. 2005;23:175–183. doi: 10.1200/JCO.2005.04.177. [DOI] [PubMed] [Google Scholar]
- 2.Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623–631. doi: 10.1200/JCO.2000.18.3.623. [DOI] [PubMed] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of Four Chemotherapy Regimens for Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein DM, Ettinger DS, Ruckdeschel JC. Long-term survivors in metastatic non-small-cell lung cancer: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1986;4:702–709. doi: 10.1200/JCO.1986.4.5.702. [DOI] [PubMed] [Google Scholar]
- 6.Albain KS, Crowley JJ, LeBlanc M, et al. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618–1626. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 7.Paesmans M, Sculier JP, Libert P, et al. Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. J Clin Oncol. 1995;13:1221–1230. doi: 10.1200/JCO.1995.13.5.1221. [DOI] [PubMed] [Google Scholar]
- 8.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 9.Patel JD, Bach PB, Kris MG. Lung cancer in US women: a contemporary epidemic. JAMA. 2004;291:1763–1768. doi: 10.1001/jama.291.14.1763. [DOI] [PubMed] [Google Scholar]
- 10.Kure EH, Ryberg D, Hewer A, et al. p53 mutations in lung tumours: relationship to gender and lung DNA adduct levels. Carcinogenesis. 1996;17:2201–2205. doi: 10.1093/carcin/17.10.2201. [DOI] [PubMed] [Google Scholar]
- 11.Toyooka S, Tsuda T, Gazdar AF. The TP53 gene, tobacco exposure, and lung cancer. Hum Mutat. 2003;21:229–239. doi: 10.1002/humu.10177. [DOI] [PubMed] [Google Scholar]
- 12.Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25:472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 14.Goldman RD, Kaplan NO, Hall TC. Lactic Dehydrogenase in Human Neoplastic Tissues. Cancer Res. 1964;24:389–399. [PubMed] [Google Scholar]
- 15.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argiris A, Murren JR. Staging and clinical prognostic factors for smallcell lung cancer. Cancer J. 2001;7:437–447. [PubMed] [Google Scholar]
- 17.Danner BC, Didilis VN, Wiemeyer S, et al. Long-term survival is linked to serum LDH and partly to tumour LDH-5 in NSCLC. Anticancer Res. 2010;30:1347–1351. [PubMed] [Google Scholar]
- 18.Dowlati A, Gray R, Sandler AB, et al. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab--an Eastern Cooperative Oncology Group Study. Clin Cancer Res. 2008;14:1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- 19.Jubb AM, Hurwitz HI, Bai W, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217–227. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 20.Dowlati A, Robertson K, Cooney M, et al. A phase I pharmacokinetic and translational study of the novel vascular targeting agent combretastatin a-4 phosphate on a single-dose intravenous schedule in patients with advanced cancer. Cancer Res. 2002;62:3408–3416. [PubMed] [Google Scholar]
- 21.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]