Abstract
A thrice weekly schedule dominates hemodialysis practice today. Inherent in such a schedule is a 72-hour inter-week break over the weekend. A growing body of evidence suggests that this break may be associated with increased cardiovascular morbidity and mortality. Five recent studies have linked dialysis session timing to higher cardiovascular event rates and have shed light on possible underlying physiologic mechanisms. We reviewed outcome data linking the “long break” to cardiovascular outcomes and suggest physiologic rationale for this relationship while identifying knowledge gaps that require further study to inform discussions regarding the application and composition of individualized dialysis prescriptions. Further work is needed to determine the relative importance of electrolyte perturbations and hemodynamic shifts in the relationship between the long break and cardiovascular mortality. The evidence suggests that at least in some at-risk patients, an individualized approach to the dialytic schedule and prescription is warranted.
Chronic hemodialysis (HD) treatment schedules today are divided into the Monday-Wednesday-Friday (MWF) and the Tuesday-Thursday-Saturday (TTS) routines. Inherent in these thrice-weekly models are two 48-hour breaks and one 72-hour break between the start of HD sessions – the latter often referred to as the “long weekend break.” This time interval occurs before the HD treatment on Monday for MWF patients and before the Tuesday session for TTS patients. While this structure is advantageous in terms of patient satisfaction and operational efficiency, the long weekend break has been linked to unintended consequences, including an increased risk of cardiovascular mortality, particularly sudden cardiac death (SCD).
Evidence linking the long break and sudden cardiac death
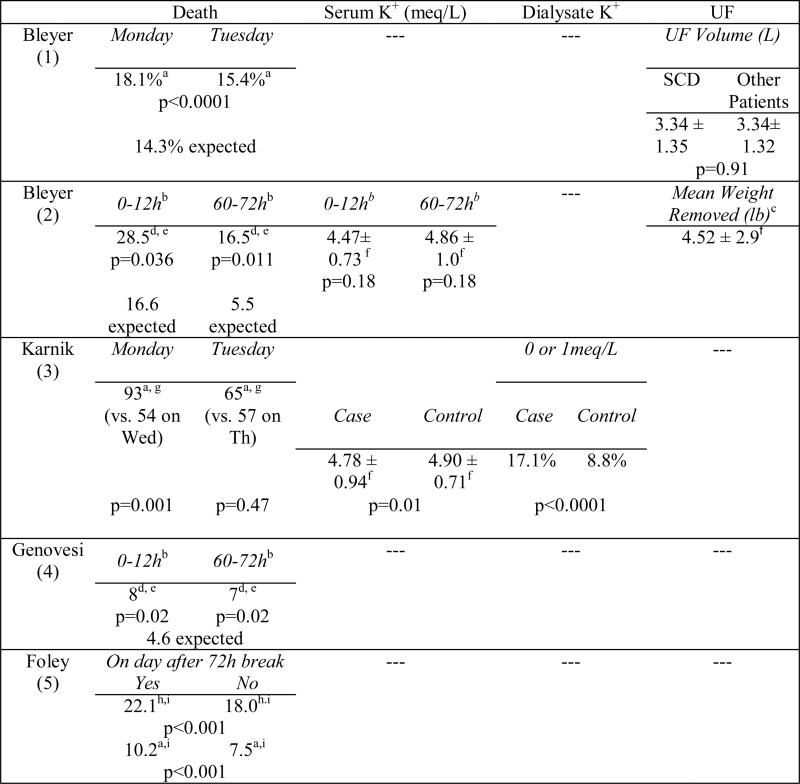
In a retrospective analysis (Table 1), Bleyer and colleagues demonstrated that SCD occur most commonly following the 72-hour break. They reported that 18.3% of SCD occurred on Mondays and 15.4% on Tuesdays, compared to the expected daily rates of 14.3% (p<0.0001).(1) In contrast, deaths in peritoneal dialysis (PD) patients did not demonstrate clustering by day of the week. In a subsequent analysis, Bleyer and colleagues isolated a threefold increased risk of SCD to within the 12 hour period prior to the dialysis session at the conclusion of the long interdialytic interval (p=0.011).(2) These findings support the assertion that the increased rate of SCD on Mondays and Tuesdays reported in their prior study was more related to the time elapsed since the last dialysis session rather than to a true “day-of-week” effect.
Table 1.
Published associations between the long interdialytic break and death.
|
Cardiac death
12h period: starting with hemodialysis session; 60–72h period: 12h period before HD at end of weekend interval
During dialysis treatment preceding sudden death
Sudden death
Values presented as absolute number (N= 228 deaths for Bleyer and N=32 for Genovesi)
Values presented as mean and standard deviation
Values presented as absolute number (N= 400 cardiac deaths)
All-cause death
Values presented as rate per 100 person-years
K+, potassium; UF, ultrafiltration; SCD, sudden cardiac death; Wed, Wednesday; Th, Thursday; HD, hemodialysis
Karnik and colleagues examined patient- and HD-specific factors associated with a higher risk of cardiac arrest and SCD during dialysis treatments in a 2001 case cohort study.(3) In their analysis of 5,744,708 HD treatment sessions, there were 400 documented cardiac arrests, corresponding to an incidence rate of 7 deaths per 100,000 HD sessions; 93 of these arrests occurred on Mondays, 54 on Wednesdays (p=0.001), and 58 on Fridays (p=0.004). Interestingly, a similar pattern was not detected for death patterns in TTS patients: 65 deaths on Tuesdays, 57 on Thursdays, and 68 on Saturdays (p=0.33 and 0.47, respectively). Characteristics of patients experiencing sudden cardiac arrest included dialysis with a lower dialysate potassium (0 or 1 meq/L), older age, diabetes, catheter HD access, recent hospitalization, and intradialytic hypotension (defined as systolic blood pressure drop of 30 mmHg).(3)
Genovesi and colleagues conducted a similar analysis in the European dialysis population in which they evaluated the incidence of SCD and associated risk factors in a cohort of 476 Italian chronic HD patients, examining risk both before and during HD after the long break.(4) The cumulative incidence of SCD was 6.9% (SE 1.2) and was significantly (p=0.02) highest during two 24 hour periods during the week: a) the 24 hours that included the first weekly dialysis session (consistent with Karnik); and b) the last 24 hours of the long break (consistent with Bleyer).
In the largest study to-date, Foley and colleagues conducted a retrospective analysis of 32,065 participants in the End-Stage Renal Disease Clinical Performance Measures Project, examining all-cause and specific-cause mortality and hospitalization rates on the final day of the long interdialytic interval.(5) Compared to death rates on other days, the following specific-cause related mortality rates were higher on the day after the long break: all-cause (22.1 deaths vs. 18.0 per 100 person-years, p<0.001), cardiovascular (10.2 vs. 7.5; p<0.001), infection (2.5 vs. 2.1, p=0.007), cardiac arrest (1.3 vs. 1.0,p=0.004), and myocardial infarction (6.3 vs. 4.4, p<0.001). The authors were unable to examine the timing of death in relation to the dialytic procedure (i.e. before, during, or after) and did not report serum or dialysate potassium or ultrafiltration volume/rate.(5)
Potential mechanisms linking the long break with cardiovascular mortality
The temporal and circadian nature of sudden death in the general population reflects a similar pattern with increased risk of myocardial infarction on Mondays and in the mornings.(6–8) The GISSI 2 Study investigators demonstrated a 10% increase in myocardial infarction on Mondays, while Willich and colleagues found a 33% increase in myocardial infarction on Mondays among working people.(6,7) Physiologic mechanisms for this pattern may include the temporal plasma catecholamine surge associated with increased heart rate,(9) plus the peak and fall in cortisol levels.(10) Increased arterial pressures and vasoconstriction associated with enhanced activity of the sympathetic nervous system and decreased left ventricular ejection time related to the increased catecholamine levels may also contribute.(11,12)
Dialysis patients are particularly susceptible to circadian-related cardiovascular changes as they often have a high burden of underlying cardiovascular disease, in addition to impaired vascular compensatory mechanisms. Along with structural cardiac changes associated with a high prevalence of diabetes and hypertension, many patients also suffer from autonomic dysfunction, vascular stiffness, and increased levels of circulating inflammatory mediators— additional factors that impair their ability to counter-regulate surges in catecholamines and sympathetic activity.(13,14)
The HD procedure itself activates the sympathetic nervous system,(15) exposing a particularly vulnerable substrate to an additional catecholamine surge – with norepinephrine concentration recognized as an independent predictor of fatal and nonfatal cardiovascular events.(16) In this study by Zoccali and colleagues, the adjusted relative risk for cardiovascular complications in patients with plasma norepinephrine >75th percentile was 1.92 times (95% confidence interval: 1.20–3.07; p=0.006) greater than in patients with normal norepinephrine concentrations.(16) Dialysis patients are thus particularly susceptible to temporal fluctuations in sympathetic nervous system activation and the associated pro-arrhythmic state.
While findings that dialysis patients experience a disproportionate burden of SCD on Mondays is consistent with that of the general population, it does not explain the higher SCD rates observed on Tuesdays among TTS patients. Rather, the overall temporal association of death to the 12-hour period preceding the week's first HD session points to the relevance of the interdialytic period.
The intermittent nature of HD creates a unique physiologic milieu that is not shared by patients undergoing other forms of renal replacement therapy such as PD or renal transplant. The Peak Concentration Hypothesis put forth in 1989 by Kesheviah may help explain this point. The original hypothesis focused on the saw tooth pattern of urea levels over a week of HD and suggested that the “peak” level (at the end of the long weekend break) was the primary determinant of uremic toxicity.(17) Although this hypothesis was not embraced universally with regards to uremic toxicity,(18) the principle, as it applies to the accumulation of fluid and other solutes (particularly potassium), may be relevant to the etiology of SCD in hemodialysis patients.
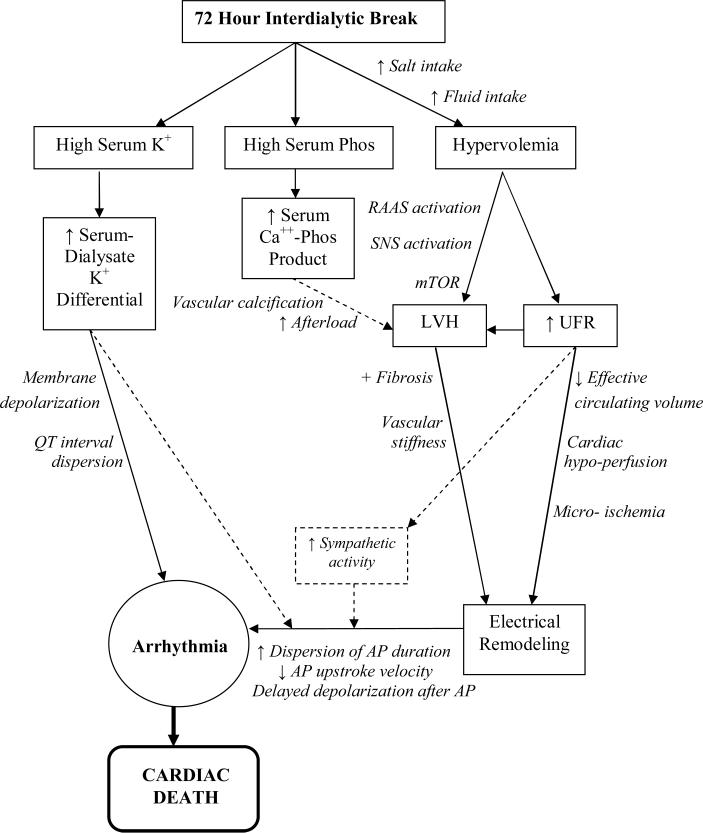
Hemodialysis following the long break introduces a kind of “perfect storm” scenario as patients are subject to many stresses of the procedure when they are most likely to experience extremes in potassium shifts, sustain the largest amount of interdialytic weight gain, and require the most aggressive ultrafiltration. Conceptually, these mortality risk factors may pertain to two separate periods - one right before dialysis after the long break as well as one during the first weekly HD treatment when dialytic conditions are most stressful. The overall mechanistic schema describing how the long interdialytic break and its associated electrolyte and hemodynamic consequences may lead to increased cardiovascular death is shown in Figure 1. Pathways are discussed in greater detail below.
Figure 1.
Mechanistic pathway linking the interdialytic “long break” to cardiovascular mortality.
K+, potassium; Phos, phosphorus; Ca++, calcium; RAAS, renin angiotension aldosterone system; SNS, sympathetic nervous system; mTOR, mammalian target of rapamycin; AP, action potential
Potassium
The long interdialytic break coincides with the weekend, a time period often associated with greater dietary indiscretion, thus placing patients at risk for two adverse phenomena: a higher absolute pre-dialysis potassium level and a wider serum-todialysate potassium gradient. Bleyer found that patients dying in the 60–72h period after their last dialysis session had higher average serum potassium levels than surviving patients (4.86 ± 1.0 vs. 4.47 ± 0.73; p=0.18).(2) Genovesi made a similar observation and also reported a 2.7 fold increased risk of SCD (95% CI 1.3–5.8) in patients with a pre-dialysis serum potassium >6mmol/L.(4)
In addition to the serum potassium level, the width of the potassium gradient may also be important. Karnik observed that dialysis performed with 0 mEq/L potassium dialysate was associated with an increased risk of death.(3) More recently, Pun and colleagues employed a case-control study design to identify modifiable risk factors associated with cardiac arrest during HD.(19) While timing of events were not their primary focus, their findings paralleled those of Karnik in that patients dialyzed on a low potassium bath (<2 meq/L) were at higher risk for SCD (p<0.0001).(19)
Potassium fluxes have been associated with increased cardiac ectopy, a prolonged QT interval, and an increased QT interval dispersion during the interdialytic period.(20–24) The use of a low potassium bath against an elevated serum potassium sets up the greatest potassium gradient at the start of an HD session. This high gradient is present not only in the serum but also at the cellular level, leading to an atypical intracellular to extracellular potassium ratio.(25) Such imbalance may lead to intracellular depletion of potassium and associated membrane hyperpolarization, thus increasing the risk of arrhythmia.(26–28) QT interval change and QT interval dispersion may represent two of the electrochemical drivers for these arrhythmias, and both have been linked to a steep potassium gradient.(21,27)
Left Ventricular Hypertrophy
In addition to their relationship to electrolyte shifts inherent to dialysis, ventricular arrhythmias have also been linked to the electrical remodeling that occurs as a result of left ventricular hypertrophy (LVH). Dialysis patients have a high prevalence of LVH, a condition identified as an independent risk factor for SCD.(29,30) Cardiac myofibers experience the greatest stretch at times of greatest volume overload. Such a time of enhanced stress naturally occurs after the long weekend break associated with the greatest cumulative fluid intake and accumulated interdialytic weight gain. LVH leads to many changes in the electrical conduction system of the heart including an increase in dispersion of the action potential duration,(31–33) a decrease in action potential upstroke velocity,(34) and delayed depolarization following the action potential.(35,36) The abnormal electrical conduction system combines with triggers such as electrolyte shifts, electrolyte imbalances, sympathetic activation, and underlying fibrosis, small vessel disease, and arterial stiffness to induce arrhythmias.
Ultrafiltration Rates
Increased interdialytic weight gain during the long weekend break also necessitates more aggressive ultrafiltration rates during the subsequent HD session. Rapid ultrafiltration has been linked to increased rates of mortality,(37–40) particularly cardiovascular mortality,(39) and can induce frank hypotension as well as subclinical cardiac stunning and micro-ischemia.(41–44) Dialysis patients are particularly susceptible to insults from intravascular volume depletion due to their impaired counter-regulatory responses and high burden of LVH, arterial stiffness, and microvascular disease.(45,46) Over time, clinical and subclinical ischemic insults contribute to adverse cardiac remodeling by worsening underlying LVH and fibrosis, ultimately predisposing patients to conduction arrhythmias - implicated as a common cause of SCD.(47)
Indeed, Pun's data showed that patients experiencing SCD during dialysis had greater ultrafiltration volumes (p=0.0002).(19) Similarly, Karnik and colleagues reported that intradialytic hypotension (systolic blood pressure drop ≥30 mm Hg) preceded sudden death in 16% of the cases,(3) providing clinical evidence that fluid shifts and associated hemodynamic instability may play a role in the underlying pathophysiology of dialysis-associated SCD.
Limitations of the data and residual gaps in knowledge
While SCD following the long weekend break has been linked to electrolyte shifts and hemodynamic changes, the underlying physiology and the relative importance of these two phenomena is not clear. For example, Karnik, Bleyer, Genovesi, and Pun's works all found that a low potassium dialysate concentration is a risk factor for death, (1–4,19) but Pun's analysis failed to show a significant association between pre-dialysis serum potassium and SCD.(19) We can conclude that a low potassium bath is harmful, but we cannot as yet implicate the steepness of the serum-dialysate potassium gradient as the firm operative mechanism. There are also conflicting data regarding the causative role of fluid shifts and volume removal in the long break-cardiovascular mortality risk relationship. Pun's analysis found that greater ultrafiltration volumes were associated with SCD,(19) but Bleyer did not find a significant correlation between interdialytic weight gain (a surrogate for required ultrafiltration volume) and death.(2) Additional mechanistic studies are needed to identify the discrete roles of ion shifts (e.g. potassium, calcium) and hemodynamics in cardiac deaths following the long break. A few other areas of interest are discussed below.
Calcium
Calcium has been shown to exert an effect on cardiac myocytes and vascular smooth muscle function, thus influencing both myocardial contractility and vascular reactivity.(48–51) Dialysate calcium influences intradialytic blood pressure stability presumably via effects on serum calcium levels.(52,53) Pun observed that patients experiencing SCD were more likely to be exposed to a low calcium dialysate (<2.5meq/L) (11.8% vs. 6.2%, p<0.0001).(19) While calcium transfer between the serum and dialysate is driven by the serum-dialysate gradient, it is also influenced by convective losses from ultrafiltration.(54,55) The change in serum calcium during hemodialysis shows considerable variability among patients. It remains to be elucidated whether some patients, particularly those with sudden drops in ionized calcium concentration, may be susceptible to adverse cardiac effects that are amplified by high ultrafiltration rates and the accentuated potassium and hydrogen ion shifts often required after the long weekend break. Additionally, the more frequent borderline and overt hypocalcemia associated with the increased use of calcimimetics may be of particular interest,(56) particularly during the first weekly HD treatment.
Phosphorus
In addition to cardiovascular mortality, the long break has been linked to changes in calcium-phosphorus balance and protein metabolism. Sigrist and colleagues showed that mean serum phosphate was 0.43 mg/dL higher after the 3 day break compared to the 2 day break in a cohort of patients with well-controlled phosphate levels (<5 mg/dL).(57) There was no difference in serum albumin and hemoglobin after the long break, suggesting that hemodilution did not play a significant role. This incremental increase in phosphorus during the last 24-hours of the long break is likely greater in patients with more liberal phosphorus control as demonstrated by Ring and colleagues, with mean increase of 0.7±0.1 mg/dL (0.239±0.022 mmol/L) in the 24 hours preceding dialysis.(58) The impact of hyperphosphatemia is the subject of intense interest. A weekly spike in serum phosphate on the last day of the long break may well be a contributing factor towards the panoply of adverse consequences linked to hyperphosphatemia(59–61). While acute changes in serum phosphate are not likely to have acute consequences, this weekly spike in serum phosphate may, nevertheless be of clinical importance. It may, for example, exacerbate large artery tunica media calcification and alter vascular distensibility potentially contributing to LVH.
Protein Metabolism
In contrast to the numerous ill effects that may be ascribed to the long break, protein metabolism may benefit from it. In one study, the protein catabolic rate was decreased from 0.96 to 0.82 g/kg/d when the interdialytic period was extended.(62) This work suggests that longer interdialytic intervals allow the body to resume a more anabolic state. Nevertheless, bicarbonate levels are lower after the long break,(63) indicating continued buffering and protein turnover during the additional non-dialysis day. These findings have not been linked to the observed risk for SCD though the transient deterioration of acid-base status may have as yet undocumented ill effects.
>Cardiovascular Hospitalization
To what extent cardiovascular hospitalization contributes to SCD has not been studied. However, Foley found higher hospitalization rates for myocardial infarction, congestive heart failure, stroke, dysrhythmia, and any cardiovascular event on the day following the long break (p<0.001 for all).(5) Similarly, at the 2010 national meeting of the American Society of Nephrology (Abstract #THPO518, JASN 20:230A, 2010), we reported hospitalization data from 70,374 patients treated in Fresenius Medical Care North America (FMCNA) facilities over a 3-month period (January to March, 2009) and found that 36% disproportionately occurred on Mondays (MWF group) and 37% occurred on Tuesdays (TTS group). Furthermore, 44% of hospitalizations from fluid-overload conditions (including congestive heart failure and acute pulmonary edema) occurred on either Monday or Tuesday, with the proportion increasing to half of these events with the addition of those occurring during the long weekend break.
Patient Preferences
An updated formal assessment of patients' informed opinions and willingness to adopt alternative treatment schedules is needed.(64)
Implications for practice
Alterations aimed at reducing the impact of electrolyte and fluid shifts may be beneficial to reducing cardiovascular death and hospitalizations associated with the long interdialytic break. Apart from obviating the long break, potential practice modifications should include attenuating accumulation of solute and fluid and/or individualizing dialysis prescriptions for the first treatment thereafter.
There exists considerable interest in more frequent HD schedules that eliminate the long break. The Frequent Hemodialysis Network Trial on short daily HD demonstrated favorable changes in the composite outcomes of death, left ventricular mass, and physical functioning among patients dialyzed six times per week compared to three times per week.(65) Patients in the six times a week HD group had substantially lower IDWG with average UF volumes of 2.12 ± 0.74L per treatment compared to 3.06 ± 0.99L in the conventional group (p<0.001); this likely reduced cardiac strain from interdialytic volume accumulation. There was improved phosphorus control with daily HD (p<0.002). The study did not follow serum potassium and calcium levels.
In an earlier evaluation, Mastrangelo and colleagues reported on their 20 year experience with “Lecce dialysis”, a dialysis protocol characterized by shorter interdialytic periods and HD sessions and fewer dietary restrictions.(66) From 1978 to 1993, they assigned 224 patients to dialytic protocols based on body size: patients with body surface area >1.55m2 underwent HD four sessions per week and patients with body surface area <1.55m2 underwent HD every other day; no patient experienced >48 hours between HD sessions. While the study design did not include a control group, results showed improved mortality, nutritional parameters, and hemoglobin parameters when compared to European rates and pre-Lecce schedule measurements. Of interest, only 69 patients (30.8%) died during a 17 year period and, of these, only 6 patients (8.6%) died of SCD.(66)
More recently, the Australian model (N=145) focused on home dialysis, including alternate night HD.(67,68) Excellent fluid, blood pressure, and phosphorus control were reported. Similar results were noted in Hong Kong (N=14).(69) Unfortunately, more prospective trials showing the benefit of every-other-day or more frequent HD are required before changes to the Medicare reimbursement policy can occur given the cost associated with additional therapy (particularly for in center treatment). Cost effectiveness analyses evaluating the cost tradeoff of additional therapy and decreased disease complications are also needed.
However, Medicare policy does allow for additional treatment sessions if medically justified. Justifications include large interdialytic weight gain and associated compromising fluid burden, intolerance of ultrafiltration, and intradialytic hypotension.(70) Physicians should consider documenting the medical necessity of an additional treatment for appropriate patients and providing such treatment to optimize the cardiovascular outcomes of these high risk patients.
Notwithstanding reimbursement, there are logistical challenges to converting to every-other-day outpatient dialysis. Facilities would need to operate seven days per week, and this would be challenging within the current context of a nursing shortage and difficulties in recruitment and training of patient care technicians. In addition, more frequent equipment maintenance will be required due to greater use, and scheduling maintenance work (e.g. disinfecting pipes or regenerating the water systems often performed in the evenings or on Sundays) would be challenging, particularly for facilities with 3rd shifts or nocturnal programs. Finally, in a bi-weekly calendar, there is potential for confusion among patients, caregivers, and transportation agents with regards to which cycle they are on: M-W-F-Su vs. a T-Th-S schedule, especially for new admissions and following hospital discharges.
Home HD and its associated flexible treatment schedule is a highly recommended option that should be explored for appropriate patients, along with a modality shift to PD in eligible patients. In-center nocturnal HD albeit performed thrice weekly, is another alternative treatment regimen that decreases the long break from 72 hours to <65 hours and allows for lower ultrafiltration rates and greater solute removal, including phosphorus.(71) In a limited FMCNA cohort of 746 patients treated with in-center nocturnal dialysis, two-year mortality rate was low at 19% and there was no pattern of increased mortality on either Mondays or Tuesdays (unpublished data). However, patients opting for this therapy differed (e.g. younger, larger body size, etc.) from the general HD population.
Since patients may be resistant to the use of more intensive therapies or alternative treatment schedules,(64) physicians should have a heightened sense of awareness with regards to selectively addressing patients at higher risk for solute and fluid accumulation during the long break. For example, targeting increased colonic excretion of potassium during the long break may blunt potassium peaks in hyperkalemic patients. Potential therapies include fludrocortisone administration (demonstrated to lower the interdialytic potassium increase by 0.7mEq/L)(72) and the use of non cathartic doses of bisacodyl(73). Glycyrrhetinic acid food supplementation has also been shown to decrease hyperkalemia,(74) but toxicity studies are needed prior to clinical implementation given its association with hypertension.(75,76) Converting from sevelamer hydrochloride to lanthanum carbonate may lower mean potassium levels (4.99 ± 0.75 to 4.70 ± 0.65 mEq/L; p<0.001).(77) Patients dialyzing with a lower sodium dialysate (138 vs. 143 mmol/L) show a decrease in the post-dialysis potassium rebound(78), a potential strategy for the week's final session (Friday or Saturday) to minimize potassium rebound over the long break. Of course, implementing a low dialysate sodium for all treatments is also justified as a general intervention to decrease interdialytic weight gain.(79–81)
Finally, given the predominance of SCD during the first HD session after the extended break, modifications to the first weekly HD session may be of benefit. Extending HD treatment time may alleviate high ultrafiltration requirements while ameliorating the need for very low potassium dialysate in eligible patients. Individualized prescription of potassium dialysate baths, tailored to more frequently monitored serum levels when appropriate, may also be helpful. In practice, some physicians have abandoned 0 and 1 meq/L potassium baths in the outpatient setting, while others have time-limited the use of 1 meq/L potassium baths with instructions to draw pre-HD serum potassium before the following treatment. Looking to the future, potassium profiling (i.e. adjusting dialysate potassium bath to maintain a constant plasma-dialysate potassium concentration gradient) has been shown to decrease premature ventricular contractions.(82)
Conclusions
A growing body of evidence suggests that the 72-hour weekend dialysis interval is associated with increased all-cause and cardiovascular risk among HD patients. Possible physiologic mechanisms for this relationship include electrolyte and ion concentration changes and unfavorable hemodynamic shifts that ultimately predispose patients to fatal cardiac arrhythmias. Further studies are needed to better delineate the relative importance of these proposed mechanisms and to identify practice pattern alterations that may improve patient cardiovascular outcomes. Such changes may require a new approach to dialysate make-up and concentration and/or increased frequency or duration of dialysis. Physicians should consider prescribing a 4th HD or hemofiltration treatment during the week in eligible patients. In patients with increased risk for complications during the long interdialytic period, options for PD or home HD should definitely be explored.
References
- 1.Bleyer AJ, Russell GB, Satko SG. Sudden and cardiac death rates in hemodialysis patients. Kidney International. 1999;55:1553–1559. doi: 10.1046/j.1523-1755.1999.00391.x. [DOI] [PubMed] [Google Scholar]
- 2.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney International. 2006;69:2268–2273. doi: 10.1038/sj.ki.5000446. [DOI] [PubMed] [Google Scholar]
- 3.Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazaraus JM, Chertow GM. Cardiac arrest and sudden death in dialysis units. Kidney Int. 2001;60:350–357. doi: 10.1046/j.1523-1755.2001.00806.x. [DOI] [PubMed] [Google Scholar]
- 4.Genovesi S, Valsecchi MG, Rossi E, Pogliani D, Acquistapace I. Sudden death and associated factors in a historical cohort of chronic hemodialysis patients. Nephrol Dial Transplant. 2009;24:2529–2536. doi: 10.1093/ndt/gfp104. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. NEJM. 2011;365(12):1099–1107. doi: 10.1056/NEJMoa1103313. [DOI] [PubMed] [Google Scholar]
- 6.Ruscone TG, Piccaluga E, Guzzetti S, Contini M, Montano N, Nicolis E, on behalf of GISSI 2 Study Investigators Morning and Monday: Critical periods for the onset of acute myocardial infarction. The GISSI 2 Study experience. Eur Heart J. 1994;15:882–887. doi: 10.1093/oxfordjournals.eurheartj.a060605. [DOI] [PubMed] [Google Scholar]
- 7.Willich SN, Lowel H, Lewish M, Hormann A, Arntz HR, Keil U. Weekly variation of acute myocardial infarctions: Increased Monday risk in the working population. Circulation. 1994;90:87–94. doi: 10.1161/01.cir.90.1.87. [DOI] [PubMed] [Google Scholar]
- 8.Muller JE, Stone PH, Rutherford TG, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, SObel BE, Willerson JT, Braunwald E. Circadian variation in the frequency of onset of acute myocardial infarction. New Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 9.Turton MB, Deegan T. Circadian variation of plasma catecholamine, cortisol, and immunoreactive insulin concentration in supine subjects. Clin Chim Acta. 1974;55:389–397. doi: 10.1016/0009-8981(74)90014-x. [DOI] [PubMed] [Google Scholar]
- 10.Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour patterns of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 11.Miller-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood pressure. Lancet. 1978;1:797–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 12.Wertheimer L, Hassen AZ, Delman AJ. The 24-hour (circadian) rhythm of the cardiovascular system. Clin Res, (abstract) 1972;20:404. [Google Scholar]
- 13.Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 14.Pecoits-Filho R, Heimburger O, Barany P, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41:1212–1218. doi: 10.1016/s0272-6386(03)00353-6. [DOI] [PubMed] [Google Scholar]
- 15.Daugirdas JT. Pathophysiology of dialysis hypotension: an update. Am J Kidney Dis. 2001;38:S11–S17. doi: 10.1053/ajkd.2001.28090. [DOI] [PubMed] [Google Scholar]
- 16.Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanellii B, Malatino LS. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- 17.Kesheviah PR, Nolph KD, Van Stone JC. The peak concentration hypothesis: a urea kinetic approach to comparing the adequacy of continuous ambulatory peritoneal dialysis and hemodialysis. Perit Dial Int. 1989;9(4):257–260. [PubMed] [Google Scholar]
- 18.Sherman RA. The peak concentration hypothesis–a justification for inadequate therapy? Sem Dial. 1994;7:318–320. [Google Scholar]
- 19.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79:218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 20.Morrison G, Michelson EL, Brown S, Morganroth J. Mechanism and prevention of cardiac arrhythmias in chronic hemodialysis patients. Kidney Int. 1980;17:811–819. doi: 10.1038/ki.1980.93. [DOI] [PubMed] [Google Scholar]
- 21.Lorincz I, Maytus J, Zilahi Z, Kun C, Karanyi Z, Kakuk G. QT dispersion in patients with end-stage renal failure and during hemodialysis. J Am Soc Nephrol. 1999;10:1297–1302. doi: 10.1681/ASN.V1061297. [DOI] [PubMed] [Google Scholar]
- 22.Cupisti A, Galetta F, Caprioli R, Morelli E, Tintori GC, Franzoni F, Lippi A, Meola M, Rindi P, Barsotti G. Potassium removal increases the QTc interval dispersion during hemodialysis. Nephron. 1999;82:122–126. doi: 10.1159/000045387. [DOI] [PubMed] [Google Scholar]
- 23.Morris S, Galiatsou E, Stewart GA, Rodger RS, Jardine AG. QT dispersion before and after hemodialysis. J Am Soc Nephrol. 1999;10:160–163. doi: 10.1681/ASN.V101160. [DOI] [PubMed] [Google Scholar]
- 24.Buemi M, Aloisi E, Coppolino G, Loddo S, Crasci E, Aloise C, Barilla A, Cosentini V, Nostro L, Caccamo C, Floccari F, Romeo A, Frisina N, Teti D. The effect of two different protocols of potassium haemodiafiltration on QT dispersion. Nephrol Dial Transplant. 2005;20:1148–54. doi: 10.1093/ndt/gfh770. [DOI] [PubMed] [Google Scholar]
- 25.Rombola G, Colussi G, De Ferrari ME, Frontini A, Minetti L. Cardiac arrhythmias and electrolyte changes during haemodialysis. Nephrol Dial Transplant. 1992;7:318–322. doi: 10.1093/oxfordjournals.ndt.a092135. [DOI] [PubMed] [Google Scholar]
- 26.Butkus DE, Alfrey AC, Miller NL. Tissue potassium in chronic dialysis patients. Nephron. 1974;13:314–324. doi: 10.1159/000180407. [DOI] [PubMed] [Google Scholar]
- 27.Johny KV, Lawrence JR, O'Halloran MW, Wellby ML, Worthley BW. Studies on total body, serum and erythrocyte potassium in patients on maintenance haemodialysis. The value of erythrocyte potassium as a measure of body potassium. Nephron. 1970;7:230–240. doi: 10.1159/000179825. [DOI] [PubMed] [Google Scholar]
- 28.Cohen JD, Neaton JD, Preineas RJ, Daniels KA, the Multiple Risk Factor Intervention Trial Research Group Diuretics, serum potassium and ventricular arrhythmia in the multiple risk factor intervention trial. JAMA. 1982;60:548–554. doi: 10.1016/0002-9149(87)90303-1. [DOI] [PubMed] [Google Scholar]
- 29.Paoletti E, Specchia C, Di Maio G. The worsening of left ventricular hypertrophy is the strongest predictor of sudden cardiac death in haemodialysis patients: a 10 year survey. Nephrol Dial Transplant. 2004;19:1829–34. doi: 10.1093/ndt/gfh288. [DOI] [PubMed] [Google Scholar]
- 30.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with an increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–9. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 31.Cameron JS, Myerburg RJ, Wong SS. Electrophysiologic consequences of chronic experimentally induced left ventricular pressure overload. J Am Coll Cardiol. 1983;2:481–7. doi: 10.1016/s0735-1097(83)80275-7. [DOI] [PubMed] [Google Scholar]
- 32.Nordin C, Siri F, Aronson RS. Electrophysiologic characteristics of single myocytes isolated from hypertrophied guinea-pig hearts. J Mol Cell Cardiol. 1989;21:729–39. doi: 10.1016/0022-2828(89)90614-7. [DOI] [PubMed] [Google Scholar]
- 33.Gillis AM, Mathison HJ, Kulisz E, Lester WM. Dispersion of ventricular repolarization and ventricular fibrillation in left ventricular hypertrophy: influence of selective potassium channel blockers. J Pharmacol Exp Ther. 2000;292:381–6. [PubMed] [Google Scholar]
- 34.Li Q, Keung EC. Effects of myocardial hypertrophy on transient outward current. Am J Physiol. 1994;266(5 Pt 2):H1738–45. doi: 10.1152/ajpheart.1994.266.5.H1738. [DOI] [PubMed] [Google Scholar]
- 35.Ben-David J, Zipes DP, Ayers GM, Pride HP. Canine left ventricular hypertrophy predisposes to ventricular tachycardia induction by phase 2 early afterdepolarizations after administration of BAY K 8644. J Am Coll Cardiol. 1992;20:1576–84. doi: 10.1016/0735-1097(92)90453-t. [DOI] [PubMed] [Google Scholar]
- 36.Charpentier F, Baudet S, Le Marec H. Triggered activity as a possible mechanism for arrhythmias in ventricular hypertrophy. Pacing Clin Electrophysiol. 1991;14:1735–41. doi: 10.1111/j.1540-8159.1991.tb02756.x. [DOI] [PubMed] [Google Scholar]
- 37.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69:1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 38.Movilli E, Gaggia P, Zubani R, Camerini C, Vizzardi V, Parrinello G, Savoldi S, Fischer MS, Londrino F, Cancarini G. Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant. 2007;22:3547–3552. doi: 10.1093/ndt/gfm466. [DOI] [PubMed] [Google Scholar]
- 39.Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79:250–257. doi: 10.1038/ki.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flythe JE, Brunelli SM. The risks of high ultrafiltration rate in chronic hemodialysis: implications for patient care. Semin Dial. 2011;24(3):259–265. doi: 10.1111/j.1525-139X.2011.00854.x. [DOI] [PubMed] [Google Scholar]
- 41.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selby NM, Burton JO, Chesterton LJ, McIntyre CW. Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol. 2006;1:1216–1225. doi: 10.2215/CJN.02010606. [DOI] [PubMed] [Google Scholar]
- 44.Selby NM, Lambie SH, Camici PG, Baker CS, McIntyre CW. Occurrence of regional left ventricular dysfunction in patients undergoing standard and biofeedback dialysis. Am J Kidney Dis. 2006;47:830–841. doi: 10.1053/j.ajkd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 45.London GM, Guerin AP, Marchais SJ, Pannier B, Safar ME, Day M, Metivier F. Cardiac and arterial interactions in end-stage renal disease. Kidney Int. 1996;50:600–608. doi: 10.1038/ki.1996.355. [DOI] [PubMed] [Google Scholar]
- 46.London GM, Drueke TB. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997;51:1678–1695. doi: 10.1038/ki.1997.233. [DOI] [PubMed] [Google Scholar]
- 47.U S Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD. 2010. [Google Scholar]
- 48.Maynard JC, Cruz C, Kleerekoper M, Levin NW. Blood pressure response to changes in serum ionized calcium during hemodialysis. Ann Intern Med. 1986;104:358–361. doi: 10.7326/0003-4819-104-3-358. [DOI] [PubMed] [Google Scholar]
- 49.Fellner Sk, Lang RM, Neumann A, Spencer KT, Bushinsky DA, Borow KM. Physiological mechanism for calcium-induced changes in systemic arterial pressure in stable dialysis patients. Hypertenstion. 1989;13:213–218. doi: 10.1161/01.hyp.13.3.213. [DOI] [PubMed] [Google Scholar]
- 50.Leunissen KML, van den Berg BW, van Hoof JP. Ionized calcium plays a pivotal role in controlling blood pressure during hemodialysis. Blood Purif. 1989;7:233–239. doi: 10.1159/000169600. [DOI] [PubMed] [Google Scholar]
- 51.Henrich WL, Hunt JM, Nixon JV. Increased ionized calcium and left ventricular contractility during hemodialysis. N Engl J Med. 1984;310:19–23. doi: 10.1056/NEJM198401053100105. [DOI] [PubMed] [Google Scholar]
- 52.Van Kuijk WHM, Mulder AW, Peels CH, Harff GH, Leunissen KML. Influence of changes in ionized calcium on cardiovascular reactivity during hemodialysis. Clin Nephrol. 1997;47:190–196. [PubMed] [Google Scholar]
- 53.Van der Sande FM, Cheriex EC, van Kuijk WHM, Leunissen KML. Effect of dialysate calcium concentrations on intradialytic blood pressure course in cardiac-compromised patients. Am J Kidney Dis. 1998;32:125–131. doi: 10.1053/ajkd.1998.v32.pm9669433. [DOI] [PubMed] [Google Scholar]
- 54.Malberti F, Ravani P. The choice of the dialysate calcium concentration in the management of patients on hemodialysis and hemodiafiltration. Nephrol Dial Transplant. 2003;(Suppl 7):vii37–40. doi: 10.1093/ndt/gfg1077. [DOI] [PubMed] [Google Scholar]
- 55.Goldsmith RS, Furszfer J, Johnson WJ, Beeler GW, Taylor WF. Calcium flux during dialysis. Nephron. 1978;20:132–140. doi: 10.1159/000181211. [DOI] [PubMed] [Google Scholar]
- 56.Messa P, Alfieri C, Brezzi B. Cinacalcet: pharmacological and clinical aspects. Expert Opin Drug Metab Toxicol. 2008;4:1551–60. doi: 10.1517/17425250802587017. [DOI] [PubMed] [Google Scholar]
- 57.Sigrist MK, Devlin L, Taal MW, Fluck RJ, McIntyre CW. Length of interdialytic interval influences serum calcium and phosphorus concentrations. Nephrol Dial Transplant. 2005;20:1643–1646. doi: 10.1093/ndt/gfh874. [DOI] [PubMed] [Google Scholar]
- 58.Ring T, Sanden AK, Hansen HHT, Halkier HP, Nielsen C, Fog L. Ultradian variation in serum phosphate concentration in patients on haemodialysis. Nephrol Dial Transplant. 1995;10:59–63. [PubMed] [Google Scholar]
- 59.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112(17):2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 60.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 61.Di Benedetto A, Marcelli D, D'Andrea A, Cice G, D'Isa S, Cappabianca F, Pacchiano G, D'Amato R, Oggero AR, Bonanno D, Pergamo O, Calabrò R. Risk factors and underlying cardiovascular diseases in incident ESRD. J Nephrol. 2005;18:592–598. [PubMed] [Google Scholar]
- 62.Lim VS, Flanigan MJ. The effect of interdialytic interval on protein metabolism: evidence suggesting dialysis-induced catabolism. Am J Kidney Dis. 1989;14(2):96–100. doi: 10.1016/s0272-6386(89)80183-0. [DOI] [PubMed] [Google Scholar]
- 63.Graham KA, Hoenich NA, Goodship TH. Pre and interdialytic acid-base balance in hemodialysis patients. Int J Artif Organs. 2001;24:192–196. [PubMed] [Google Scholar]
- 64.Ramkumar N, Beddhu S, Eggers P, Pappas LM, Cheung AK. Patient preferences for in-center intense hemodialysis. Hemodial Int. 2005;9:281–295. doi: 10.1111/j.1492-7535.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 65.FHN Trial Group In-center hemodialysis six times per week versus three times per week. New Engl J Med. 2010;363(24):2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mastrangelo F, Alfonso L, Napoli M, DeBlasi V, Russo F, Patruno P. Dialysis with increased frequency of sessions (Lecce dialysis) Nephrol Dial Transplant. 1998;13(Suppl 6):139–147. doi: 10.1093/ndt/13.suppl_6.139. [DOI] [PubMed] [Google Scholar]
- 67.Kerr PG, Agar JWM, Hawley CM. Alternate night nocturnal hemodialysis: the Australian experience. Sem Dial. doi: 10.1111/j.1525-139X.2011.00997.x. In press. [DOI] [PubMed] [Google Scholar]
- 68.Van Eps CL, Jeffries JK, Anderson JA, Bergin PT, Johnson DW, Campbell SB, Carpenter SM, Isbel NM, Mudge DW, Hawley CM. Mineral metabolism, bone histomorphometry and vascular calcification in alternate night nocturnal haemodialysis. Nephrology (Carlton) 2007;12:224–233. doi: 10.1111/j.1440-1797.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 69.Tang HL, Wong JH, Poon CK, Tang CM, Chu KH, Lee W, Fung SK, Chau KF, Tong KL. One year experience of nocturnal home haemodialysis with an alternate night schedule in Hong Kong. Nephrology (Carlton) 2011;16:57–62. doi: 10.1111/j.1440-1797.2010.01371.x. [DOI] [PubMed] [Google Scholar]
- 70.Medicare Benefit Policy Manual, Chapter 11 - End Stage Renal Disease. 01/28/2011. Retrieved from www.cms.gov/manuals/Downloads/bp102c11.pdf.
- 71.Lacson E, Wang W, Lester K, Ofsthun N, Lazarus JM, Hakim RM. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5:220–226. doi: 10.2215/CJN.06070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singhal PC, Desroches L, Mattana J, Abramovici M, Wagner JD, Maesaka JK. Mineralocorticoid therapy lowers serum potassium in patients with end stage renal disease. Am J Nephrol. 1993;13:138–141. doi: 10.1159/000168604. [DOI] [PubMed] [Google Scholar]
- 73.Mathialahan T, Sandle GI. Dietary potassium and laxatives as regulators of colonic potassium secretion in end-stage renal disease. Nephrol Dial Transplant. 2003;18:341–347. doi: 10.1093/ndt/18.2.341. [DOI] [PubMed] [Google Scholar]
- 74.Farese S, Kruse A, Pasch A, Dick B, Frey BM, Uehlinger DE, Frey FJ. Glycyrrhetinic acid food supplementation lowers serum potassium concentration in chronic hemodialysis patients. Kidney Int. 2009;76:877–884. doi: 10.1038/ki.2009.269. [DOI] [PubMed] [Google Scholar]
- 75.Stewart PM, Wallace AM, Vanentino R, Burt D, Shackleton CH, Edwards CR. Mineralocorticoid activity of liquorice: 11-beta-hydroxysteroid dehydrogenase deficiency comes of age. Lancet. 1987;10:821–824. doi: 10.1016/s0140-6736(87)91014-2. [DOI] [PubMed] [Google Scholar]
- 76.Farese RV, Biglieri EG, Shackleton CH, Irony I, Gomez-Gontes R. Licorice-induced hypermineralocorticoidism. N Engl J Med. 1991;325:1223–1227. doi: 10.1056/NEJM199110243251706. [DOI] [PubMed] [Google Scholar]
- 77.Filiopoulos V, Koutis I, Trompouki S, Hadjiyannakos D, Lazarou D, Vlassopoulos D. Lanthanum carbonate versus sevelamer hydrochloride: improvement of metabolic acidosis and hyperkalemia in hemodialysis patients. Therapeutic Apheresis and Dialysis. 2011;15:20–27. doi: 10.1111/j.1744-9987.2010.00868.x. [DOI] [PubMed] [Google Scholar]
- 78.De Nicola L, Bellizzi V, Minutolo R, Cioffi M, Giannattasio P, Terracciano V, Iodice C, Uccello F, Memoli B, Iorio BR, Conte G. Effect of Dialysate Sodium Concentration on Interdialytic Increase of Potassium. J Am Soc Nephrol. 2000;11:2337–2343. doi: 10.1681/ASN.V11122337. [DOI] [PubMed] [Google Scholar]
- 79.Keen ML, Gotch FA. The association of the sodium “setpoint” to interdialytic weight gain and blood pressure in hemodialysis patients. Int J Artif Organs. 2007;30:971–979. doi: 10.1177/039139880703001105. [DOI] [PubMed] [Google Scholar]
- 80.Mendoza JM, Sun S, Chertow GM, Moran J, Doss S, Schiller B. Dialysate sodium and sodium gradient in maintenance hemodialysis: a neglected sodium restriction approach? Nephrol Dial Transplant. 2011;26:617–626. doi: 10.1093/ndt/gfq807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mendoza JM, Bayes LY, Sun S, Doss S, Schiller B. Effect of lowering dialysate sodium concentration on interdialytic weight gain and blood pressure in patients undergoing thrice-weekly in-center nocturnal hemodialysis: A quality improvement study. Am J Kidney Dis. 2011 doi: 10.1053/j.ajkd.2011.06.030. E-pub ahead of print, Aug 27: PMID: 21875769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Redaelli B, Locatelli F, Limido D, Andrulli S, Signorini MG, Sforzini S, Bonoldi L, Vincenti A, Cerutri, Orlandini G. Effect of a new model of hemodialysis potassium removal on the control of ventricular arrhythmias. Kidney Int. 1996;50:609–617. doi: 10.1038/ki.1996.356. [DOI] [PubMed] [Google Scholar]