Abstract
OBJECTIVE
Superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) prevent cellular damage produced by free radicals. Our objective was to evaluate if prenatal alcohol exposure decreased the expression of antioxidant enzymes in the brain, liver or placenta of fetal mice.
STUDY DESIGN
Timed, pregnant C57BL6/J mice were treated on gestational day 8 (E8) with intraperitoneal injection of alcohol (0.03 ml/g) or saline (control). Fetuses were harvested on E18. Fetal brain, liver and placenta were analyzed for mRNA expression of SOD, GPx and CAT by real-time PCR, with 18S RNA used as reference.
RESULTS
SOD, GPx and CAT expression was lower in fetal brains exposed to alcohol with no differences detected in the liver or placenta between the two groups.
CONCLUSION
Maternal alcohol consumption causes a decrease in SOD, GPx and CAT expression in the fetal brain. This may explain the long term neurologic findings in fetal alcohol syndrome.
Keywords: antioxidant enzymes, fetal alcohol syndrome, fetus, mice
Introduction
Alcohol exposure in utero can result in significant fetal effects that are detrimental to offspring throughout their life. In the United States, 0.5 to 3 per 1,000 live births each year suffer from fetal alcohol syndrome (FAS).1 Maternal alcohol consumption is the most commonly identifiable nongenetic cause of mental retardation.2 Children with FAS have a characteristic set of facial features, microcephaly, growth restriction and central nervous system impairment. Children with FAS can have mental and learning delay as well as structural brain defects.3
The brain oxygen consumption is relatively high, and is estimated to be at about 20% of the total oxygen delivered in humans.4 Oxygen consumption in the brain results in generation of free radicals, an effect that is magnified with alcohol exposure. Superoxide dismutase (SOD), Glutathione peroxidase (GPx) and Catalase (CAT) are members of the antioxidant system. They are present in the cerebral cortex, cerebellum and hypothalamus,5 and function to prevent the cellular damage produced by free radicals.
SOD is an enzyme that catalyzes the formation of hydrogen peroxide from superoxide radicals. GPx catalyzes a reaction that converts the reduced monomeric glutathione (GSH) to glutathione disulfide (GSSG). CAT functions to catalyze the decomposition of hydrogen peroxide to water and oxygen. In rodent models of alcohol consumption, the enzymatic activity of SOD,6,7 GPx8 and CAT6 were all reduced in response to alcohol exposure.
The above studies illustrate that alcohol treatment results in decreased enzymatic activity of SOD, GPx and CAT. However, it is not known whether this effect is present at the transcriptional level, and whether it is a generalized effect or localized to the brain. Exploring alcohol's effect on transcription is of importance as a decrease in mRNA transcript may, in part, explain the reduction in enzymatic activity of antioxidant enzymes. Therefore, our aim in this study is to test the hypothesis that alcohol-induced decrease in enzymatic activity is related to reduction in mRNA expression of SOD, GPx and CAT, and that this effect is specific to the fetal brain.
Materials and Methods
The study protocol and all related procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Texas Medical Branch, Galveston, Texas. The mice were maintained in the animal care facility at The University of Texas Medical Branch. They were housed separately in temperature- and humidity-controlled quarters and kept in a 12-hour light/12-hour dark regimen with food and water available at all times.
Study Design
The FAS model by Webster was used. This animal model has been previously validated and exhibits the main features of FAS, including fetal anomalies (maxillary hypoplasia, median cleft lip/palate and mandibular hypoplasia) and an increase in fetal demise.9 Briefly, pregnant C57BL/6J mice were received from Jackson laboratory (Barharbour, ME) on gestational day 7. On gestational day 8, animals were randomly injected intraperitoneally (i.p.) with 25% ethyl alcohol in saline (alcohol) solution or vehicle alone (saline solution) at 0.03 mL/gm of body weight. Because animals receiving alcohol were incapacitated for 6 hours, water and food were withheld from both groups for 6 hours.
On gestational day 18, pregnant mice were euthanized with carbon dioxide and fetuses were collected. Fetal demise/absorption was recorded. Each litter was weighed as a group to determine the average fetal weight between the groups. Fetal blood was pooled from all pups within each litter from 5 alcohol and 5 saline treated mothers that were randomly selected, centrifuged to obtain serum and stored at -80°C. Fetal brain, liver and placenta were harvested, immediately flash frozen in liquid nitrogen and stored at -80°C.
Tissue samples from each fetus were analyzed individually. For brain tissue, 4 to 7 brains from 4 alcohol treated litters and 4 to 6 brains from 5 saline treated litters were randomly chosen. For liver and placental tissue, 4 individual samples were randomly selected from 3 litters in the alcohol and saline group. Tissues from demised fetuses were excluded.
To measure the degree of systemic oxidative RNA damage, we quantified 8-hydroxyguanosine (8-OHG) levels from pooled fetal serum using OxiSelect™ RNA damage ELISA kit (Cell Biolabs, Inc. San Diego, CA) according to the protocol provided by the company.
For RNA extraction, samples were homogenized using Bullet Blender from Next Advance (Averill Park, NY). Reverse transcription into cDNA was performed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosytems, Foster City, CA) according to manufacturer's instructions. The cDNA was amplified by Real-time PCR with 7500 Fast Real-Time PCR System (Applied Biosystems) using mouse specific, performed TaqMan probe sets (all purchased from Applied Biosystems) for SOD (catalog number Mm01344232_g1), GPx (catalog number Mm00656767_g1), and CAT (catalog number Mm00437992_m1) according to the manufacturer's protocol. Target gene expression was expressed in relation to 18S mRNA levels in each specific sample (catalog number Hs99999901_s1; Applied Biosystems). All PCR reactions for each sample were performed simultaneously on the same plate.
Statistical analysis was performed using Shapiro-Wilk test for normality testing and then Mann-Whitney rank sum test accordingly. The unit of analysis was the litter. When more than one pup per litter was evaluated, the average for that litter was used in the analysis. Two sided p value < 0.05 was considered significant.
Results
There were 65 fetuses from 9 litters in the saline group and 73 fetuses from 9 litters in the alcohol group. Seventeen (23%) pups in the alcohol exposed group were either demised or absorbed compared with none (0%) in the control group.
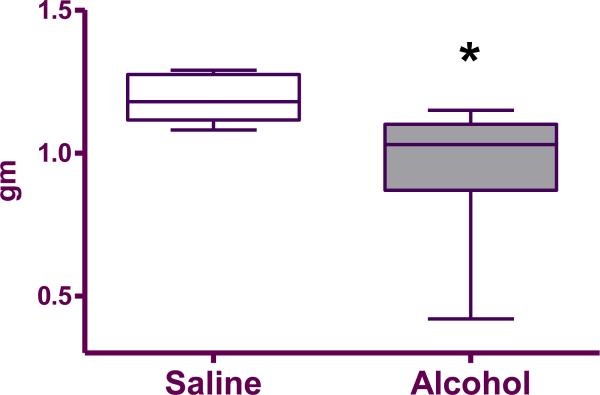
Fetuses exposed to alcohol in utero were on average smaller than the saline treated controls (0.96±0.08 grams vs. 1.19±0.03 grams, respectively; p=0.02; Figure 1).
Figure 1.
Box plot of the average weight of live fetuses per liter in saline (n=9) and alcohol-exposed (n=9) groups. The box extends from the 25th percentile to the 75 percentile with a line at the median (the 50th percentile). Whiskers show the highest and the lowest values. *denotes statistically significant difference (p=0.01) between the groups.
8-OHG Quantitation
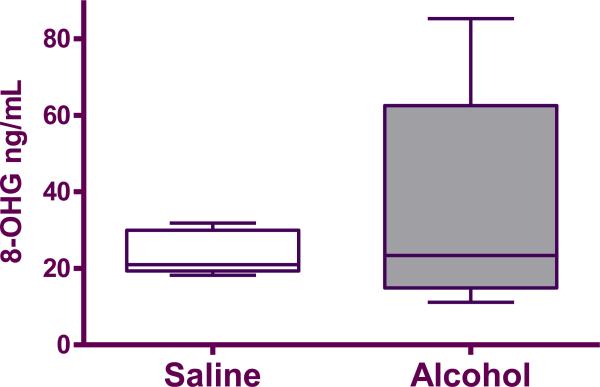
There was no significant difference in 8-OHG levels in the pooled fetal serum between alcohol and control group (35.65±13.26 ng/mL vs. 23.9±2.59 ng/mL; p=0.84; Figure 2).
Figure 2.
8-OHG in pooled fetal serum from saline (n=5) and alcohol-exposed (n=5) groups. The box extends from the 25th percentile to the 75 percentile with a line at the median (the 50th percentile). Whiskers show the highest and the lowest values.
mRNA expression of SOD, GPx and CAT
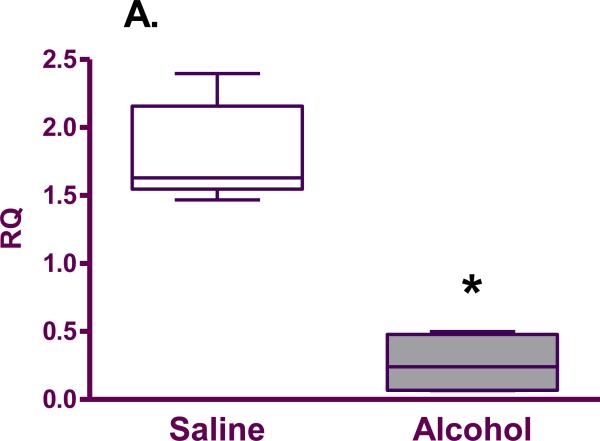
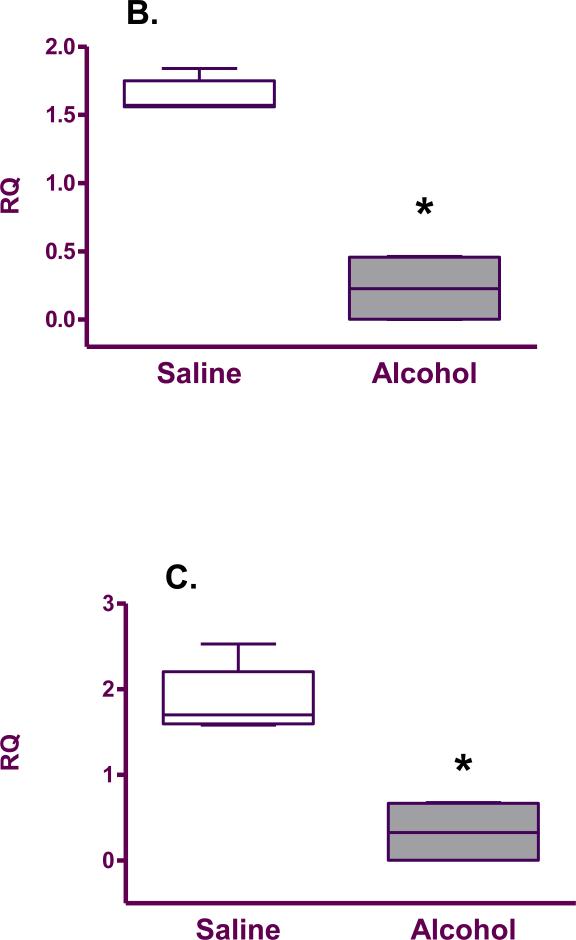
mRNA expression of SOD (0.26±0.11 vs. 1.81±0.16; p=0.02), GPx (0.23±0.13 vs. 1.64±0.05; p=0.02) and CAT (0.33±0.19 vs. 1.86±0.18; p=0.02) was found to be significantly decreased in the brains of pups born to mice exposed to alcohol compared with the saline group (Figure 3).
Figure 3.
Antioxidant enzyme mRNA expression (relative quantity; RQ) in the fetal brain from saline (n=5) and alcohol-exposed (n=4) groups: A. SOD; B. GPx; C. CAT. The n is the number of litters. The box extends from the 25th percentile to the 75 percentile with a line at the median (the 50th percentile). Whiskers show the highest and the lowest values.*denotes statistically significant difference (p=0.02) between the groups.
SOD, GPx and CAT mRNA expression in the fetal liver was not significantly different in the alcohol versus saline group (SOD: 1.91±0.30 vs. 1.48±0.28, p=0.4; GPx: 3.33±0.98 vs. 2.07±0.41, p=0.4; CAT: 1.92±0.36 vs. 1.46±0.34; p=0.4). Similarly, there were no significant difference in the placenta between the alcohol and saline groups (SOD: 1.26±0.27 vs. 1.08±0.07, p=0.7; GPx: 1.00±0.36 vs. 0.88±0.05, p=0.7; and CAT: 0.64±0.24 vs. 0.46±0.06; p=1.00).
Comment
In this study, we found that exposure to alcohol in utero decreased the expression of the antioxidant enzymes (SOD, GPx, and CAT) in the fetal brain, with no difference in fetal liver or placenta. This indicates that impairment in oxidant/redox system in fetal alcohol syndrome is specific to the fetal brain. We also found that levels of 8-OHG in the serum of fetuses exposed to alcohol were not increased. 8-hydroxyguanosine is formed when free radicals cause RNA oxidative damage on the nucleoside guanosine and serves as a biomarker for oxidative stress. As RNA is repaired, 8-OHG is passed out of cells into the fetal serum. The lack of difference in 8-OHG levels between the two groups further supports our finding that oxidative stress in fetal alcohol syndrome is specific to the fetal brain. The reduction in antioxidant enzyme expression may explain some of the cytotoxic effects seen in FAS. To our knowledge, this is the first study on the effects of fetal alcohol exposure on the expression of antioxidant enzymes in the developing fetal brain, using an animal model of FAS. This information is critical in understanding the pathways leading to the development of FAS and helps to focus research on development of potential therapy or prevention measures.
The rate of fetal demise seen in our study is consistent with previously published work by Spong et al.10 Fetuses exposed to alcohol were significantly smaller than controls, which is consistent with growth restriction seen in FAS. These findings validate our mouse model for FAS research.
It is known that alcohol exposure results in decreased enzymatic activity of SOD, GPx and CAT in the brains of rodents exposed to alcohol.6-8 Our study shows that this effect is transcriptional. This finding opens another point for intervention in the pathway leading to FAS. Consumption of alcohol in humans is associated with increased oxidative stress metabolites as well as decreased antioxidant enzyme activity in the brain.11,12 In the developing fetal brain, a decrease in expression of antioxidant enzymes may help explain some of the mental and learning impairments seen in FAS secondary to the brain's ability to counter increased oxidative stress attributed to maternal alcohol consumption. We did not find any difference in expression of antioxidant enzymes in the liver or placenta. These results indicate that at E18 the decreased transcription of antioxidant enzymes in this model of FAS is specific to the developing brain. This specificity is more evidence that supports a causative and direct role for antioxidant-oxidant imbalance in the pathogenesis of FAS. We speculate two potential explanations for this finding. First, DNA damage by alcohol-induced oxidative stress at E8 is more pronounced in the brain and results in a reduction of DNA suitable for transcription and this leads to decreased mRNA expression. Second, alcohol may not damage DNA directly but can influence cell signaling mechanisms that affect the rate of transcription. For example, Glycogen Synthase Kinase-3β (GSK-3β) is cell signaling enzyme that effects mRNA transcription by influencing expression of nuclear transcription factors and is known to be reduced in response to alcohol exposure.13
Our study has few limitations. Maternal alcohol consumption in this model of FAS is most consistent with a binge drinking episode rather than a model of chronic alcohol consumption. This well-established model represents a severe form of FAS associated with high rates of fetal anomalies or demise. We only evaluated the fetuses at a single time point during development (E18), 10 days after exposure to alcohol. It is therefore not possible to determine whether changes in the antioxidant system in the liver and placenta occurred at an earlier or later time point. However, the alterations in antioxidants in the brain 10 days following exposure indicate that these changes are not just acute, but may persist long term. In addition, we did not pool samples of brain tissues within the control or alcohol group demonstrating that each result obtained was specific to the brain being studied. Our experiment focused on mRNA expression and not protein expression. A decrease in transcription may lead to a decrease in protein translation of these enzymes or have no effect. In addition, we did not evaluate enzymatic activity of SOD, GPx and CAT in relation to mRNA expression in the mouse model of FAS. However, these were previously evaluated by others, and their findings are consistent with the downregulation of mRNA expression in our study.
Our findings improve our understanding of the pathogenesis of FAS and have the potential to lead to new preventive approaches at the translational level. This model may be used to further investigate the underlying molecular pathways that may help explain the reduction of mRNA expression of SOD, GPx and CAT in the developing fetal brain.
Acknowledgments
This manuscript was accepted for an oral presentation on February 11, 2011 at the SMFM 2011 Annual Meeting, San Francisco, CA.
Footnotes
DISCLOSURE: None of the authors have a conflict of interest
References
- 1.Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention and Treatment. National Academy Press; Washington, DC: 1996. [Google Scholar]
- 2.Windham GC, Vonbehren J, Fenster L, Schaefer C, Swan SH. Moderate maternal alcohol consumption and risk of spontaneous abortion. Epidemiology. 1997;8:509–14. doi: 10.1097/00001648-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;19:1088–93. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- 4.Bailey DM, Bärtsch P, Knauth M, Baumgartner RW. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: from the molecular to the morphological. Cell Mol Life Sci. 2009;66:3583–94. doi: 10.1007/s00018-009-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augustyniak A, Michalak K, Skrzydlewska E. The action of oxidative stress induced by ethanol on the central nervous system (CNS). Postepy Hig Med Dosw. 2005;59:464–71. [PubMed] [Google Scholar]
- 6.Pushpakiran G, Mahalakshmi K, Anuradha CV. Taurine restores ethanol-induced depletion of antioxidants and attenuates oxidative stress in rat tissues. Amino Acids. 2004;27:91–6. doi: 10.1007/s00726-004-0066-8. [DOI] [PubMed] [Google Scholar]
- 7.Ledig M, M'Paria JR, Mandel P. Superoxide dismutase activity in rat brain during acute and chronic alcohol intoxication. Neurochem Res. 1981;6:385–90. doi: 10.1007/BF00963853. [DOI] [PubMed] [Google Scholar]
- 8.Kumral A, Tugyan K, Gonenc S, Genc K, Genc S, Sonmez U, et al. Protective effects of erythropoietin against ethanol-induced apoptotic neurodegenaration and oxidative stress in the developing C57BL/6 mouse brain. Dev Brain Res. 2005;160:146–56. doi: 10.1016/j.devbrainres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Webster WS, Walsh DA, Lipson AH, McEwen SE. Teratogenesis after acute alcohol exposure in inbred and outbred mice. Neurobehav Toxicol. 1980;2:227–34. [Google Scholar]
- 10.Spong CY, Abebe DT, Gozes I, Brenneman DE, Hill JM. Prevention of fetal demise and growth restriction in a mouse model of fetal alcohol syndrome. J Pharmacol Exp Ther. 2001;297:774–9. [PubMed] [Google Scholar]
- 11.Biller A, Bartsch AJ, Homola G, Solymosi L, Bendszus M. The effect of ethanol on human brain metabolites longitudinally characterized by proton MR spectroscopy. J Cereb Blood Flow Metab. 2009;29:891–902. doi: 10.1038/jcbfm.2009.12. [DOI] [PubMed] [Google Scholar]
- 12.Götz ME, Janetzky B, Pohli S, Gottschalk A, Gsell W, Tatschner T, et al. Chronic alcohol consumption and cerebral indices of oxidative stress: is there a link? Alcohol Clin Exp Res. 2001;25:717–25. [PubMed] [Google Scholar]
- 13.Acquaah-Mensah GK, Kehrer JP, Leslie SW. In utero ethanol suppresses cerebellar activator protein-1 and nuclear factor-kappa B transcriptional activation in a rat fetal alcohol syndrome model. J Pharmacol Exp Ther. 2002;301:277–83. doi: 10.1124/jpet.301.1.277. [DOI] [PubMed] [Google Scholar]