Abstract
The major heat shock protein Hsp72 is expressed at elevated levels in many human cancers and its expression correlates with tumor progression. Here we investigated the role of Hsp72 in Her2 oncogene-induced neoplastic transformation and tumorigenesis. Expression of Her2 in untransformed MCF10A mammary epithelial cells caused transformation, as judged by foci formation in culture and tumorigenesis in xenografts. However, expression of Her2 in Hsp72-depleted cells failed to induce transformation. The anti-tumorigenic effects of Hsp72 downregulation were associated with cellular senescence due to accumulation of p21 and depletion of survivin. Accordingly, either knockdown of p21 or expression of survivin reversed this senescence process. Further, we developed an animal model of Hsp72-dependent breast cancer associated with expression of Her2. Knockout of Hsp72 almost completely suppressed tumorigenesis in the MMTVneu breast cancer mouse model. In young Hsp72 KO mice, expression of Her2 instead of mammary tissue hyperplasia led to suppression of duct development and blocked alveolar budding. These effects were due to massive cell senescence in mammary tissue, which was associated with upregulation of p21 and downregulation of survivin. Therefore Hsp72 plays an essential role in Her2-induced tumorigenesis by regulating oncogene-induced senescence pathways.
Keywords: Hsp72, HER2, senescence, p21, survivin
Introduction
Two recent groundbreaking reports demonstrated that tumorigenesis in several animal models depends on the heat shock transcription factor Hsf1 (1, 2). For example, hsf1 knockout dramatically delayed overall development of various tumors and increased survival of p53 knock-in mutant (R172H) mice (1). Similarly, Hsf1 deficiency drastically delayed chemical skin carcinogenesis and increased survival (2). Importantly, knockdown of Hsf1 in several cancer cell lines led to significant growth inhibition. The growth dependence of cancer cells on Hsf1 was the basis for a novel concept of “non-oncogene addiction” of cancer cells (3, 4). According to this concept, cancer cells acquire properties that make their survival dependent on proteins which are dispensable for a normal cell, which makes these proteins attractive targets for drug development.
Recently, we developed a cell culture model to investigate effects of Hsf1 on tumor development. In this model, growth of untransformed immortalized human mammary epithelial cells MCF10A was not affected by the shRNA knockdown of Hsf1 (5). On the other hand, expression of the activated Her2 (ErbB2) oncogene in the Hsf1 knockdown MCF10A cells, instead of transformation, triggered growth arrest and senescence. As a result, these cells could not form tumors in xenografts (5). Accordingly, MCF10A cells expressing Her2 oncogene became “addicted” to Hsf1. Hsf1 is a transcription factor that affects expression of more than 5% of human genes, many of which could potentially influence tumor development, e.g. MDR, proinflammatory cytokines or MTA (6). However, the Hsf1 targets that define the “addiction” of tumor cells to Hsf1 remain unclear. We hypothesized that the major effect of Hsf1 on tumor development is via regulation of expression of the inducible heat shock protein Hsp72 (Hspa1). Indeed, we have previously demonstrated that Her2 oncogene induces Hsp72 in Hsf1-dependent fashion, and that knockdown of Hsp72 similarly promotes growth arrest and senescence of MCF10A cells expressing Her2 (5). One of the goals of this study was to investigate how Hsp72 regulates Her2-induced senescence of mammary epithelial cells.
In addition to mediating protein folding and degradation (7), Hsp72 can regulate cell signaling pathways, e.g. JNK, ERK or NF-kB, that play important roles in apoptosis, inflammation, diabetes and other physiological responses (8–12). There are multiple reports in the literature implicating Hsp72 in tumorigenesis (10, 13–15). The majority of these reports are correlative studies, demonstrating that in many tumors and cancer cell lines Hsp72 levels are constitutively elevated (10, 11, 14, 15). Moreover, high levels of Hsp72 correlate with metastasis, resistance to anticancer drugs, and poor prognosis in many human cancers (13). In addition, there are studies demonstrating that Hsp72 plays a critical role in apoptosis survival and proliferation of cancer cell lines in culture. For example, depletion of Hsp72 along with its constitutively expressed housekeeping homolog, Hsc73 (Hspa8), led to apoptosis of certain cancer lines (8). Furthermore, downregulation of Hsp72 alone caused apoptosis or enhanced drug sensitivity (8, 16, 17). The latter observation suggests that Hsp72 may be a potential candidate for targeted drug design. In fact, a novel Hsp72 inhibitor has been shown to have tumor suppressor properties (18, 19), however, the drug currently lacks sufficient specificity, and has other targets as well (18, 19).
Previously we have shown that knockdown of Hsp72 induces senescence in a variety of cancer lines but not in untransformed epithelial cells (5, 20). Interestingly, depending on the presence of either PIK3CA or Ras oncogenes depletion of Hsp72 triggered either p53- or MAPK-dependent senescence signaling pathways (20). Based on these data, we proposed a novel concept that Hsp72 plays a major role in cancer cell evasion of oncogene-induced senescence (OIS). According to this concept, oncogene expression in an epithelial cell activates distinct senescence signaling pathways that are kept in check by elevated levels of Hsp72 allowing cell proliferation, and eventually formation of tumors. Conversely, decreasing Hsp72 levels shifts the balance to favor senescence signaling over mitogenic signaling, triggering cell senescence that prevents tumor development.
None of these works, however, addressed genetically effects of Hsp72 in a relevant animal model. Another goal of this investigation was to establish animal breast cancer models, both xenografts and knockouts, which are dependent on Hsp72. Further, we aimed to dissect the stage in tumor development that requires Hsp72.
Materials and methods
Animals
Animal maintenance and experiments were conducted in compliance with IACUC guidelines. MMTVneu+/+ mice (FVB/N, Jackson’s lab) were crossed with WT mice or hsp70−/− mice (C57/129)to generate WT-MMTVneu+/−, hsp70+/−MMTVneu+/−, and hsp70−/−MMTVneu+/− mice. Mice were sacrificed at 3-months of age to study mammary gland hyperplasia or were kept to monitor tumor development.
Cell cultures and reagents
MCF10A cells were cultured in DMEM/F12 medium supplemented with 5% horse serum, 20 ng/mL epidermal growth factor, 0.5 μg/mL hydrocortisone, 10 μg/mL human insulin, and 100 ng/mL cholera toxin. HEK293T cells were from ATCC and were cultivated in Dulbecco’s modified Eagle’s medium supplemented with 10% heat inactivated FBS. SK-BR3 cells were cultured in McCoy’s 5A medium with 10% FBS and MDA-MB453 cells were cultured in Leibovitz’s L-15 medium with 10% FBS.
Retroviral vectors and infection
RNAi-Ready pSIREN-RetroQ vector from BD Biosciences retroviral delivery system was used for knockdown of Hsp72. The sequence of human Hsp72 gene was selected as reported before (19). Her2 and control (pBABE) retroviral vectors were a kind gift of C. Spangenberg (21). This version of Her2 carries the activating V664E mutation (NeuT). Retroviruses were produced as reported before (16, 20). Briefly, HEK293T cells were co-transfected with plasmids expressing retroviral proteins Gag-Pol, VSV-G pseudotype, and enhanced green fluorescent protein (EGFP) or our constructs using lipofectamine 2000 (Invitrogen). 48 hours after transfection, supernatants containing the retroviral particles were collected and frozen at −70 until use. MCF10A cells were infected with diluted supernatant in the presence of 10 μg/ml Polybrene overnight, and were selected with puromycin (0.75 μg/ml) 48 hours after infection. Retroviral vectors expressing EGFP was used as infection efficiency indicator: usually ~90% of cells were fluorescent 2 days after infection.
Immunoblot analysis
Cells were washed twice with PBS and lysed in lysis buffer (40 mM HEPES [pH 7.5], 50 mM KCl, 1% Triton X-100, 1mM Na3VO4, 50 mM glycerophosphate, 50 mM NaF, 5 mM EDTA, 5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, and 5 μg/ml each leupeptin, pepstatin A, aprotinin). Protein concentration of the lysates was measured with the Bio-Rad protein assay reagent, after which they were diluted with lysis buffer to achieve equal protein concentrations. Antibodies used for this study were listed as follow: anti-Hsp72 from Stressgen; anti-p21 and anti-PARP from BD PharMingen; anti-vimentin from LabVision (Thermo Scientific); anti-survivin from Santa Cruz; anti-β-actin from Sigma.
Cell growth and foci formation
Cell growth curve was performed by seeding 5000 cells per well in 12-well plate and cell number was monitored every two days. To study foci formation, cells were seeded at 3×105 per well in 6-well plate and were let grow for 2 days until foci can be observed under microscope. Then cells were washed twice with PBS, fixed with 2% formaldehyde/0.2% glutaraldehyde in PBS for 10 min, and stained with hematoxylin.
Xenografts
For establishing tumor xenografts, cells were trypsinized, mixed at 1:1 ratio with matrigel, and 0.25 million cells of each culture were injected subcutaneously into 6-week-old female NCR nude mice (Taconic). Tumor growth was monitored twice a week using caliper.
β-galactosidase assay
β-galactosidase assay was carried out using X-gal (pH 6.0) as described previously (16, 20). Cells were plated at a low density and fixed with 2% formaldehyde and 0.2% glutaraldehyde. The β-galactosidase activity was determined by incubation with 1 mg/ml of solution of X-gal (5-bromo-4-chloro-3-indolyl β-D-galactopyranoside) in 40 mM sodium citrate, 5 mM K3FeCN6, 5 mM K4FeCN6, 150 mM NaCl, 2 mM MgCl2 diluted in phosphate-buffered saline (pH 6.0). The number of stained cells was counted under microscope from five different fields and the proportion of stained cells was calculated. The results were expressed as mean value ± S.D. on three independent experiments. Mammary gland whole mounts were fixed in formalin overnight at 4°C and were stained with the same β-galactosidase activity assay buffer mentioned above.
Immunohistochemistry
Mammary tissues from 3-month old virgin mice were fixed in formalin overnight and were processed into parafin sections (5 μm in thickness). Immunohistochemical staining was performed using the standard ABC method (Vector Laboratory).
Results
Hsp72 depletion precipitates senescence in MCF10A cells expressing Her2
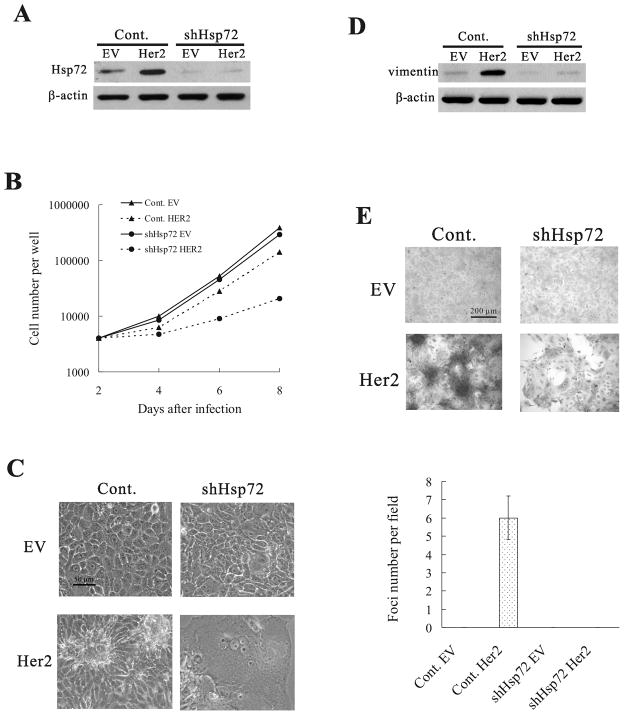
Previously, we described that Hsf1 knockdown dramatically changes the response of immortalized mammary epithelial cells MCF10A to Her2 oncogene. In these cells, expression of Her2 instead of transformation precipitated senescence (5). We hypothesized that this effect was associated with the failure of Hsf1-mediated Hsp72 induction. This hypothesis was consistent with our observation that Her2 expression in the Hsp72 knockdown MCF10A cells also triggers senescence (5). To further explore this possibility, we investigated whether knockdown of Hsp72 changes the responses of MCF10A cells to expression of Her2 similarly to Hsf1. Accordingly, late passage MCF10A cells were infected with control retrovirus or retrovirus expressing the shRNA against Hsp72 (shHsp72) (as described previously (5)) and selected with puromycin for 48h, which resulted in more than 80% downregulation of Hsp72 (Fig. 1A). This treatments did not affect cell viability, and only slightly diminished growth rate (Fig. 1B), indicating that Hsp72 is dispensable for growth of normal cells. Then cells were infected with retrovirus expressing the constitutively active Her2 mutant V664E (neuT). On day 2 post-infection, cells were plated and the cell number was counted every 2 days. Knockdown of Hsp72 slightly decreased the growth of control MCF10A cells (Fig. 1B). Expression of Her2 also caused a minor reduction of growth rate in control cells (probably because of a slight p21 upregulation, see bellow, Fig. 2A). Importantly, when we expressed Her2 in Hsp72-depleted cells, a dramatic growth inhibition was observed (Fig. 1B). This slowdown of cell proliferation did not result from apoptosis, since little PARP cleavage was found under these conditions (Fig. S1). On the other hand, there was a pronounced change in cell morphology indicative of senescence, including enlarged, flattened morphology, extensive vacuolization (Fig. 1C) and appearance of the senescence-associated β-galactosidase (β-gal) activity(5).
Figure 1.
Her2 overexpression in Hsp72-depleted MCF10A cells precipitates senescence. (A) Hsp72 depletion by shHsp72 retrovirus. MCF10A cells were infected with control (Cont.) or shHsp72 retroviruses and following three days selection, cells were infected with empty virus (EV) or Her2-expressing virus (Her2). After four more days, Hsp72 levels were measured by immunoblotting. (B) Cell growth curves of control (Cont.) and shHsp72 MCF10A cells with (Her2) or without (empty vector, EV) Her2 overexpression. (C) Morphology of cells described in (A) on day 4 after Her2 overexpression. (D) Effect of Hsp72 depletion on Her2-induced vimentin expression. Samples from (A) were re-blotted for vimentin. (E) Effect of Hsp72 depletion on Her2-induced foci formation. Cells described in (A) were stained with hematoxylin to visualize foci. Dark masses in the bottom left panel represent foci. Average foci numbers in five fields were quantified (lower panel).
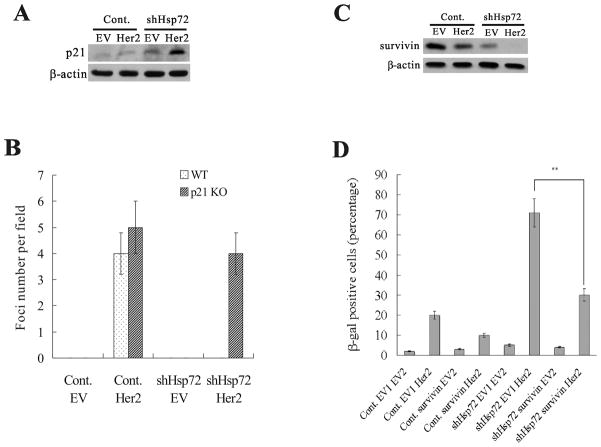
Figure 2.
p21 and survivin are involved in Her2-induced senescence in Hsp72-deficient cells. (A) p21 levels in control and shHsp72 MCF10A cells with (Her2) or without (EV) Her2 overexpression. (B) Average foci numbers in five fields were quantified from cultures described in (A). (C) Survivin levels in control or shHsp72 MCF10A cells with (Her2) or without (EV) Her2 overexpression. (D) Control and shHsp72 MCF10A cells were infected with retrovirus expressing survivin (surv), and two days later these cells were infected with Her2-expressing (Her2) retrovirus. EV1 – control empty vector for survivin. EV2 – control empty vector for Her2. Populations were stained for β-gal and quantification of β-gal staining is shown.
Since Hsp72 was critical for proliferation of Her2-expressing MCF10A cells, we further explored if Hsp72 knockdown has effects on cellular transformation. Expression of Her2 in control MCF10A cells led to increased expression of the epithelial-mesenchymal transition marker vimentin, and promoted formation of foci in control MCF10A cells (Fig. 1D), demonstrating that cells underwent neoplastic transformation. In contrast, expression of Her2 in cells with knockdown of Hsp72 led to minimal upregulation of vimentin, and failed to promote foci formation (Fig. 1E), indicating that Hsp72 is critical for Her2-induced transformation in cell culture. Importantly, knockdown of Hsp72 did not reduce Her2 signaling, as judged by activation of the ERK and Akt pathways. In fact, the ERK signaling pathway was even over-activated by Her2 in Hsp72-depleted cells (Fig. S2), similar to what we observed with Ras oncogene expression (20). Taken together with the observation that expression of Her2 in control cells led to increased Hsp72 levels (Fig. 1A and ref. 5), this provides further support for the “chaperone addiction” model relating increased Hsp levels to oncogenic transformation.
Hsp72 controls Her2-induced senescence by regulating p21 and survivin
To clarify the Her2-induced senescence mechanisms controlled by Hsp72, we assessed the levels of p21Cip, which is one of the major regulators of senescence in cancer cells (22, 23). Furthermore, Hsf1 exerts its action on Her2-induced senescence in part through p21 (5). As expected, p21 was mildly up-regulated by Her2 expression in MCF10A cells. Similarly, Hsp72-depleted cells showed a minor increase in p21 levels (Fig. 2A). However, there was a strong induction of p21 upon expression of Her2 in shHsp72 cells (Fig. 2A). To investigate the importance of p21 in the onset of Her2-induced senescence in cells that lack Hsp72, we used wild type (WT) and p21 knockout (KO) MCF10A cells (a kind gift of Dr. Park, ref. 24). In WT MCF10A cells, Hsp72-knockdown caused Her2 expression to induce senescence whereas in p21 KO cells there was little senescence and the cells continue forming foci (Fig. 2B and S3A). Therefore, p21 plays an important role in Her2-induced senescence and suppression of transformation in Hsp72-depleted cells.
Another component of the Her2-induced senescence regulated by Hsf1 was survivin (5), which affected senescence pathway independent of p21. Therefore, we addressed whether Hsp72 can also regulate the effect of Her2 on survivin. Survivin plays a critical role in cell proliferation (24), and its expression closely correlates with tumor grade and progression (24, 25). In MCF10A cells, expression of Her2 alone had little effect on survivin (Fig. 2C). Hsp72 knockdown in MCF10A cells caused about 60% downregulation of survivin, which however did not significantly affect cell growth, as shown on Fig. 1B. However, expression of Her2 in shHsp72 cells led to more than 90% depletion of survivin. To test for the importance of survivin in Her2-induced senescence, we infected control and shHsp72 MCF10A cells with lentivirus expressing survivin, and following brief selection infected cells with Her2-expressing virus. Survivin expression rescued the senescence phenotype in Hsp72-knockdown cells upon Her2 expression, as judged by cell morphology and β-gal staining (Fig. 2D and S3B), indicating that suppression of survivin observed in Hsp72 knockdown cells significantly contributes to growth inhibition and onset of senescence under these conditions.
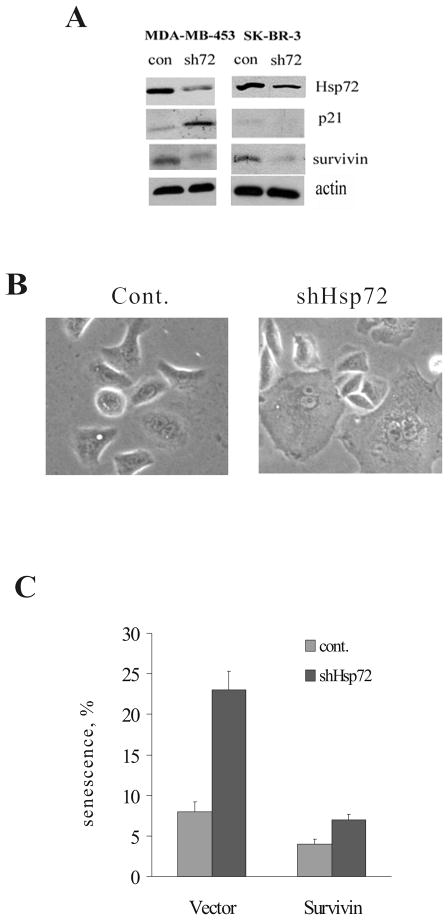
To understand the relevance of the Hsp72 effects to human cancer, we studied the consequence of Hsp72 knockdown on proliferation of human cell line derived from Her2-positive breast cancer MDA-MB453. In line with data with MCF10A cells, in the MDA-MB453 cells (having WT p53) we observed growth inhibition and induction of p21 upon depletion of Hsp72 (Fig. 3A). Furthermore, there was significant downregulation of survivin in this cell line (Fig. 3A). Of note, growth inhibition under these conditions was not associated with development of the acidic β-gal activity (data not shown), suggesting that a component of the senescence mechanism responsible for β-gal upregulation is inactivated in this cancer line.
Figure 3.
Effects of Hsp72 knockdown on p21 and survivin levels in Her2-positive human breast cancer cells. Of note, MDA-MB453 line has WT p53 while SK-BR-3 line has mutant p53. (A) Hsp72 was depleted from MDA-MB453 and SK-BR-3 cell lines and four days later samples were harvested for evaluation of Hsp72, p21 and survivin by immunoblotting. (B) Effect of Hsp72 knockdown on development of cellular senescence in SK-BR-3 cells. Morphology of Hsp72-depleted cells is shown. (C) Expression of recombinant survivin reverses the effect of Hsp72 on β-gal staining in SK-BR-3 cells. Survivin was expressed using retroviral expression system prior to depletion of Hsp72.
Her2-positive human cancers often lack p53. To understand the relationship between Hsp72-regulated senescence programs, we investigated Her2-positive cancer line SK-BR3, which carries mutant p53. In contrast to MDA-MB453 cells that carry WT p53, Hsp72 depletion did not induce p21 in SK-BR3 cells (Fig. 3A). Nevertheless, depletion of Hsp72 in SK-BR3 led to growth inhibition, which was associated with development of senescent morphology and accumulation of acidic β-gal activity (Fig. 3B and C), pointing to a p53-p21-independent mechanism of senescence. Importantly, knockdown of Hsp72 in SK-BR3 cells led to a strong decrease in survivin levels (Fig. 3A), indicating that effects of Hsp72 on downregulation of survivin were p53-independent.
To test the role of survivin in p53-independent senescence upon depletion of Hsp72, we over-expressed survivin in SK-BR3 cells using lentiviral vector, and then knocked down Hsp72, as described above. As judged by suppression of β-gal activity, expression of survivin strongly suppressed senescence in Hsp72-depleted SK-BR3 cells (Fig. 3B), indicating that survivin depletion is a critical factor in the p53-independent senescence upon Hsp72 knockdown.
Thus, Hsp72 exerts its effects on Her2-induced transformation via regulation of p21 and survivin pathways. Taken together, these data indicate that depletion of Hsp72, a downstream target of Hsf1, has similar to Hsf1 knockdown effects on Her2-positive cells, suggesting that Hsp72 is the major contributor to the effects of Hsf1 on Her2-driven transformation. Furthermore, these experiments directly prove the hypothesis that Hsp72 functions to suppress the intrinsic senescence programs activated by Her2 oncogene.
Hsp72-mediated regulation of tumor development in xenograft model
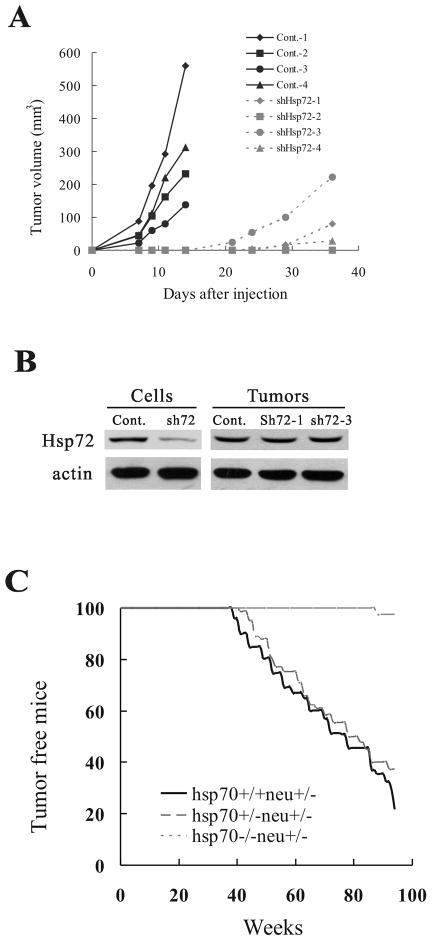
As mentioned in the introduction, so far no animal cancer model has been established to address the Hsp72-dependence of tumor development. Accordingly, we studied the effect of Hsp72 depletion on tumor formation using xenografts in nude mice. Control and Hsp72 knockdown MCF10A cells expressing Her2 were mixed with matrigel and injected subcutaneously in flanks of female nude mice. Her2 appeared to cause very aggressive tumorigenesis, where control Her2-expressing MCF10A cells formed detectable tumors at xenograft injection sites with 100% probability. Tumors appeared by day 3 post-injection and had a very high growth rate (Fig. 4A). In contrast, Her2-expressing Hsp72 knockdown cells did not form tumors for at least three weeks. In a fraction of animals, tumors became detectable in three-four weeks post-injection (Fig. 4A). However, these tumors appear to be selected from cells that escaped Hsp72 knockdown (e.g. have lost the Hsp72 shRNA), since Hsp72 levels in these tumors were comparable to that of control cells (Fig. 4B). Therefore, it appears that only cells that escaped the shRNA control could form tumors, indicating the critical role of Hsp72 for tumorigenesis in the xenograft model.
Figure 4.
Lack of Hsp72 strongly inhibits tumor development. (A) Hsp72 depletion suppresses tumor emergence in xenografts. Growth curves of individual tumors in xenografts of Her2-expressing control (Cont.) and shHsp72 MCF10A cells. (B) Levels of Hsp72 were restored in xenograft tumors derived from shHsp72 cells. (C) Effect of Hsp72 on mammary tumor incidence in Her2-expressing mice.
Hsp72-mediated regulation of tumor development in transgenic model
Since experiments with the cell culture model indicate that Hsp72 controls the oncogene-induced senescence, a simple prediction was that Hsp72 should regulate early stages of tumorigenesis associated with expression of the oncogenic Her2. Accordingly, depletion of Hsp72 might block early hyperplasia. Xenograft model does not allow dissecting where in the tumorigenic process Hsp72 exerts its activity. Therefore, to address this question, we used the transgenic animal model. Accordingly we established a mouse model in which Hsp72 knockout is combined with expression of Her2. For these experiments we used mice with both orthologs of human Hsp72, Hsp70a (Hspa1a) and Hsp70b (Hspa1b), deleted (26) and, hereafter termed hsp70−/−. These animals were bred with mice expressing Her2/neu (neu - a rodent homolog of Her2) under the control of MMTV promoter (MMTVneu) (27). Accordingly, we generated WT-MMTVneu+/−, hsp70+/−MMTVneu+/−, and hsp70−/−MMTVneu+/− mice, and followed tumor development in these animals. Because of the mixed background, in the experiments with transgenic animals we used littermates WT as controls for the knockouts. Immunostaining of sections of mammary glands obtained from three months old mice indicated that there was a strong induction of Her2/neu transgene in all animal lines compared to control animals, and there was no significant difference in the Her2 expression among the lines (Fig. S4). There was similar tumor incidence between heterozygous hsp70+/−MMTVneu+/− and WT-MMTVneu+/− mice (median tumor appearance in this strain was 66 weeks), indicating that one copy of the hsp70 genes is sufficient to support mammary tumorigenesis induced by Her2. In contrast, the absence of Hsp72 in the homozygous knockout animals almost completely blocked mammary tumor development (Fig. 4C). Indeed, within more than 100 weeks no tumors appeared in ten out of eleven animals, and in one hsp70−/−MMTVneu+/− mouse a tumor appeared by 88 weeks of age, which is far later than tumor appearance in control animals. This animal model confirms that Hsp72 is critical for Her2-induced tumor development.
Hsp72 knockout blocks hyperplasia and triggers senescence in mammary tissue in mice
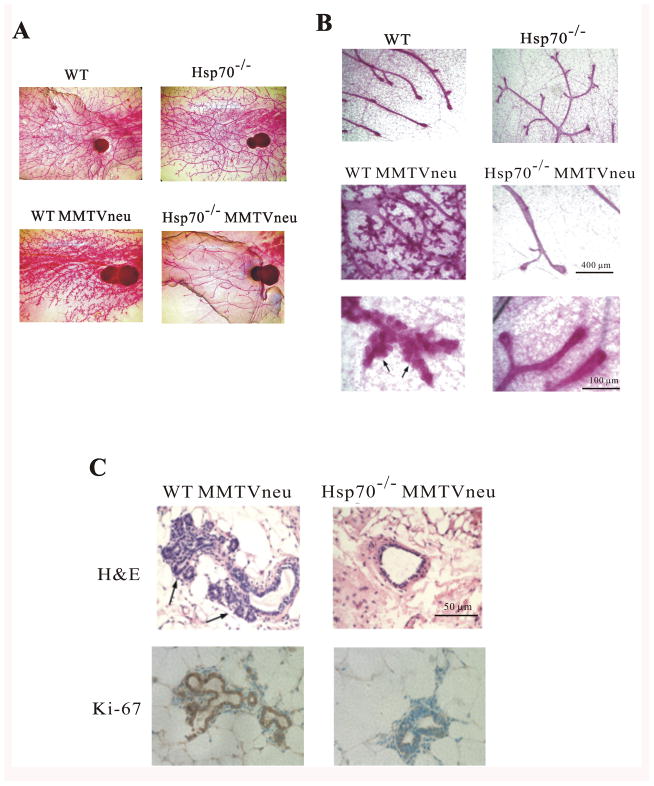
To investigate the role of Hsp72 in Her2-induced hyperplasia, whole mounts of mammary glands were taken from 3-month old virgin mice to evaluate duct branching. By this age Her2 transgene was fully expressed (Fig. S4). Hsp70 KO itself did not affect duct or alveoli morphology (Fig. 4C). Expression of Her2 in WT-MMTVneu+/− mammary gland strongly enhanced the density of ducts and led to more extensive alveoli branching, as reported previously (27). Importantly, expression of Her2 in the hsp70−/− background (hsp70−/− MMTVneu+/−) led to dramatic reduction in duct density, as compared to control or hsp70−/− animals, and completely prevented alveoli branching (Fig. 5A and B). Histological examination by hematoxylin-eosin staining also detected extensive alveoli branching and hyperplasia in WT-MMTVneu+/−, but not in hsp70−/−MMTVneu+/− mice (Fig. 5C). Immunostaining of tissue sections with Ki-67 antibody confirmed extensive cell proliferation in WT-MMTVneu+/− animals, and lack of such proliferation hsp70−/−MMTVneu+/− mice (Fig. 5C). Therefore, Hsp70 KO prevented Her2-induced tissue hyperplasia. In other words, effects of Hsp70 were detected at very early stages, almost ten months before pulpable tumors appeared.
Figure 5.
Hsp72 knockout robustly suppresses Her2-induced mammary duct hyperplasia in vivo. (A) Ducts and alveoli in mammary glands of three months-old WT and hsp70−/− (Hsp72 KO) mice with and without MMTVneu (Her2). Tissue samples were fixed with Carnoy’s fixative and stained with carmine. (B) Higher magnification of dust hyperplasia in (A). (C) Tissue sections described in (A) were stained with hematoxylin or immunostained with Ki-67 antibody.
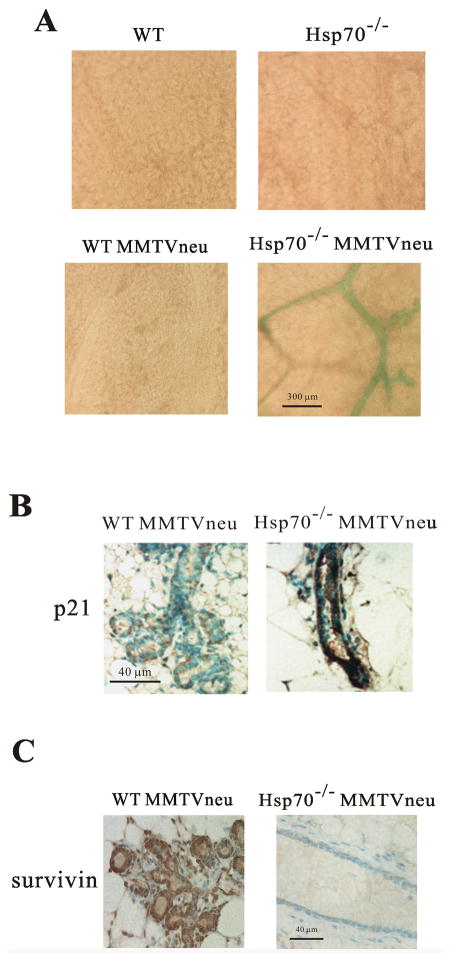
To address whether Hsp72 KO suppresses Her2-dependent tumorigenesis in vivo via precipitating senescence, we performed β-gal staining of mammary glands from 3-month old mice. There was almost no β-gal staining in control, WT MMTVneu+/− and hsp70−/− animals. In contrast, extensiveβ-gal staining of ducts (Fig. 6A) was seen when expression of Her2 was combined with Hsp70 knockout (hsp70−/−MMTVneu+/−). Therefore in an hsp70−/− background, Her2/neu oncogene, instead of epithelial transformation, triggers senescence and suppresses alveoli branching. In other words, Hsp72 appears to become critical for hyperplasia upon expression of Her2 because it allows epithelial cells to escape growth arrest and senescence controls.
Figure 6.
Hsp72 knockout allows Her2-induced senescence in mammary ducts. (A) Her2 causes development of acidic β-gal activity in mammary tissue of Hsp72 KO animals. Whole mount mammary tissue samples were fixed and stained for β-gal. (B) Mammary tissue sections from WT-MMTVneu and hsp70−/−MMTVneu mice immunostained for p21. (C) Mammary gland sections from WT-MMTVneu and hsp70−/−MMTVneu mice immunostained for survivin.
To evaluate whether Hsp72 regulates Her2 effects on p21 and survivin in mammary tissue in vivo, we assessed levels of these proteins in mice by immunohistochemistry. Indeed, we observed strong accumulation of p21 in mammary glands from hsp70−/−MMTVneu+/− mouse as compared to tissue from WT-MMTVneu+/− animals (Fig. 6B). Furthermore, we observed a strong decrease of survivin levels in hsp70−/−MMTVneu+/− mouse mammary glands compared to mammary tissue from WT-MMTVneu+/− animals (Fig. 6C). Therefore, expression of Her2 in cells lacking Hsp72 leads to upregulation of p21 and downregulation of survivin both in vitro and in vivo.
Discussion
This work, which is built upon our previous publication on the role of Hsf1 in Her2-induced tumorigenesis, had two goals: first to investigate whether induction Hsp72 is the major contributor to the effects of Hsf1, and second to develop animal models that allow investigating the role of Hsp72 in tumor development. The involvement of Hsp72 in Hsf1-mediated effects on tumorigenesis was hinted by previous observations that (a) Hsp72 is induced by Her2 in Hsf1-dependent manner, and (b) similar to Hsf1 knockdown, expression of Her2 in Hsp72 knockdown precipitates senescence of MCF10A cells instead of promoting transformation (5). Here we confirmed these observations, and demonstrated that other effects of Hsp72 knockdown on Her2-mediated transformation also recapitulate effects of Hsf1 depletion. In fact, we observed that in the shHsp72 cells Her2 expression blocks proliferation and suppresses EMT, as judged by suppression of foci formation and reduced expression of vimentin (Fig. 1D and E).
Importantly, as with Hsf1 depletion, expression of Her2 in Hsp72 knockdown cells upregulated p21 and downregulated survivin. Both of these effects were found to be involved in Her2-mediated senescence. Indeed, we observed partial suppression of senescence by either knockout of p21 or expression of recombinant survivin (Fig. 2B and D). It appears that p21 and survivin represent parallel and independent pathways of senescence controlled by Hsp72. This is evident from the experiments with human Her2-positive cancer cell lines. Indeed, in MDA-MB-453 cells that carry normal p53 depletion of Hsp72 both upregulated p21 and downregulated survivin. These effects were associated with growth inhibition (although β-gal activity was not upregulated, probably because of an additional defect in this pathway). In contrast, in Her2-positive SK-BR-3 cell line, which carry mutant p53, depletion of Hsp72 did not cause upregulation of p21, but still triggered downregulation of survivin, indicating that these pathways are independent, and that survivin regulation is not associated with functional p53. Furthermore, expression of survivin in this cell line was sufficient to almost completely reverse Hsp72 depletion-mediated senescence (Fig. 3C). On the other hand, expression of survivin only partially reversed Hsp72 depletion-mediated senescence in MCF10A cells which have functional p21 pathway, suggesting a significant contribution of p21 in this system (Fig. 2).
Altogether these experiments with several cell culture models indicated that effects of Hsp72 on Her2-induced transformation are very similar to effects of Hsf1. This finding strongly suggest that Hsp72 in the major factor which mediates effects of Hsf1 on tumorigenesis. This is an important advance since as mentioned in Introduction almost 5% of human genes are regulated by Hsf1 either directly or indirectly, and identifying the downstream target critical for cancer development is important. On the other hand, while claiming that Hsp72 downregulation is sufficient for effects of Hsf1 knockdown, we realize that it may not be essential, since Hsp27 and possibly other downstream targets could play a role in this regulation as well. In fact, Her2 similarly precipitates senescence in Hsp27-depleted cells. Furthermore, overexpression of Hsp72 is unable to suppress effects of Hsf1 depletion (unpublished data). Nevertheless, strong similarity of effects of Hsp72 knockdown to effects of Hsf1 knockdown indicates that Hsp72 is the important contributor to these effects.
Another goal of this study was to develop animal models to investigate effects of Hsp72 on tumor development. In fact, so far all studies that experimentally addressed the critical role of Hsp72 in tumorigenesis were either done with cell culture or were correlative using biopsies. An exception was recent publication with a novel compound that inhibits Hsp72, which was tested with tumor xenografts (18). However, these data should be taken cautiously because of the obvious question of specificity of the compound. Here we first demonstrated that shHsp72 MCF10A cells that express Her2 cannot form tumor xenografts in contrast to control Her2-expressing MCF10A cells (Fig. 4A). Although tumors appeared from shHsp72 cells with almost three weeks delay, these tumors only support the notion that Hsp72 is essential for Her2-induced tumor formation, since in all of them the shRNA control of Hsp72 expression was lost.
Further we addressed at what stage of tumor development Hsp72 exerts its activity. Cell culture experiments suggested that Hsp72 should act early in tumor development when the process of the oncogene-induced senescence may take place. Accordingly, we expected that Hsp72 knockout could suppress Her2-induced hyperplasia of mammary tissue. This kind of analyses is not possible with the xenograft models, and we developed the transgenic model. With this model we observed an extremely strong effect of Hsp72 KO. In fact, there was almost complete prevention of Her2-induced mammary tumors in Hsp72 KO animals. As predicted, this effect was associated with suppression of hyperplasia and cell proliferation, as judged by histology and Ki-67 immunostaining (Fig. 5). Very importantly, we observed strong β-gal staining indicative of extensive senescence in mammary ducts from Her2-expressing Hsp72 KO animals (Fig. 6A). Senescence was so strong that it led not only to suppression of hyperplasia, but also to suppression of normal alveolar branching. These effects were specifically associated with Her2 oncogene, since neither senescence nor suppression of alveolar branching was seen in Hsp72 KO animals that do not express Her2 (Fig. 5A and B). It should be noted that these effects were seen in young mice, at least several months before tumors appeared. Another important note is that in the transgenic model we observed both upregulation of p21 and downregulation of survivin in Her2-expressing Hsp72 KO animals specifically (Fig. 6B and C), which recapitulates effects seen in cell culture.
Recently there was a publication that Hsp72 KO does not prevent, but rather promotes carcinogen-induced colon cancer (28). Cancer caused by this carcinogen requires chronic inflammatory response. The authors argued that Hsp72 KO promotes inflammation and thus facilitates cancer development. This may be the main difference with Her2-induced cancer, which does not require chronic inflammation. The other difference could be in tissue type, since in this work colon cancer was studied, while we study breast cancer.
We conclude that Hsp72 could potentially be an anti-cancer drug target similar to Hsf1. Furthermore, animal models developed in this work demonstrating full dependence of Her2-induced tumor development on Hsp72 could be used for drug testing.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Health CA081244. We thank Dr. B. Park and Dr. C. Spangenberg for their kind supply of MCF10A cells and HER2-overexpressing retroviral vector.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Min JN, Huang L, Zimonjic DB, Moskophidis D, Mivechi NF. Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene. 2007;26(35):5086–97. doi: 10.1038/sj.onc.1210317. [DOI] [PubMed] [Google Scholar]
- 2.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130(6):1005–18. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136(5):823–37. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell. 2007;130(6):986–8. doi: 10.1016/j.cell.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Meng L, Gabai VL, Sherman MY. Heat-shock transcription factor HSF1 has a critical role in human epidermal growth factor receptor-2-induced cellular transformation and tumorigenesis. Oncogene. 29(37):5204–13. doi: 10.1038/onc.2010.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 11(8):545–55. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–77. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 8.Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell. 2008;14(3):250–62. doi: 10.1016/j.ccr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Tang D, Kang R, Xiao W, Wang H, Calderwood SK, Xiao X. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J Immunol. 2007;179(2):1236–44. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31(3):164–72. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Jaattela M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999;248(1):30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- 12.Gabai VL, Meriin AB, Mosser DD, et al. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem. 1997;272(29):18033–7. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- 13.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10(2):86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23(16):2907–18. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- 15.Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92(19):1564–72. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- 16.Yaglom JA, Gabai VL, Sherman MY. High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res. 2007;67(5):2373–81. doi: 10.1158/0008-5472.CAN-06-3796. [DOI] [PubMed] [Google Scholar]
- 17.Gabai VL, Budagova KR, Sherman MY. Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene. 2005;24(20):3328–38. doi: 10.1038/sj.onc.1208495. [DOI] [PubMed] [Google Scholar]
- 18.Leu JI, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Mol Cell. 2009;36(1):15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaarur N, Gabai VL, Porco JA, Jr, Calderwood S, Sherman MY. Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res. 2006;66(3):1783–91. doi: 10.1158/0008-5472.CAN-05-3692. [DOI] [PubMed] [Google Scholar]
- 20.Gabai VL, Yaglom JA, Waldman T, Sherman MY. Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Mol Cell Biol. 2009;29(2):559–69. doi: 10.1128/MCB.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trost TM, Lausch EU, Fees SA, et al. Premature senescence is a primary fail-safe mechanism of ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res. 2005;65(3):840–9. [PubMed] [Google Scholar]
- 22.Mooi WJ, Peeper DS. Oncogene-induced cell senescence--halting on the road to cancer. N Engl J Med. 2006;355(10):1037–46. doi: 10.1056/NEJMra062285. [DOI] [PubMed] [Google Scholar]
- 23.Sherr CJ. Principles of tumor suppression. Cell. 2004;116(2):235–46. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- 24.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22(53):8581–9. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 25.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8(1):61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 26.Hunt CR, Dix DJ, Sharma GG, et al. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol. 2004;24(2):899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller WJ, Arteaga CL, Muthuswamy SK, et al. Synergistic interaction of the Neu proto-oncogene product and transforming growth factor alpha in the mammary epithelium of transgenic mice. Mol Cell Biol. 1996;16(10):5726–36. doi: 10.1128/mcb.16.10.5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao Y, Hart J, Lichtenstein L, Joseph LJ, et al. Inducible heat shock protein 70 prevents multifocal flat dysplastic lesions and invasive tumors in an inflammatory model of colon cancer. Carcinogenesis. 2009;30:175–82. doi: 10.1093/carcin/bgn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.