TRAF3, a cytoplasmic adaptor protein, was recently identified as a candidate tumor suppressor in a range of human B-cell lineage neoplasms. Homozygous deletions and inactivating mutations of the Traf3 gene were detected in non-Hodgkin lymphoma, including splenic marginal zone lymphoma (MZL), B-cell chronic lymphocytic leukemia, mantle cell lymphoma, as well as multiple myeloma and Waldenström's macroglobulinemia.1-4 However, whether TRAF3 deletions or mutations have a causative role in B lymphomagenesis awaits further in vivo study using TRAF3-knockout mice.
We have recently generated a conditional loss-of-function allele of the Traf3 gene that allows specific deletion of TRAF3 in B lymphocytes in mice (B-TRAF3–/– mice).5 These mice exhibit greatly expanded B-cell populations in secondary lymphoid organs, due to remarkably prolonged, BAFF-independent survival of mature B cells, a finding subsequently confirmed by Gardam et al.6 TRAF3 and TRAF2 assemble to form a regulatory complex with cIAP1/2 and NIK to inhibit the NF-κB2-signaling pathway.7,8 These observations define a central role of the TRAF3-NIK-NF-κB2 axis in regulating B-cell survival. Prolonged survival of B cells is a known predisposing factor to the development of autoimmunity and B lymphoma.9,10 Indeed, aging B-TRAF3–/– mice developed high levels of serum autoantibodies to double-stranded DNA as well as immune-complex glomerulonephritis.5 Autoimmunity itself is recognized as a risk factor of B lymphoma in patients with systemic lupus erythematosus or Sjogren's syndrome.11 Hence, B-TRAF3–/– mice may be predisposed to B-cell malignancies. Here we report that B-TRAF3–/– mice spontaneously developed clonal splenic MZL or B1a lymphomas by the age of 18 months.
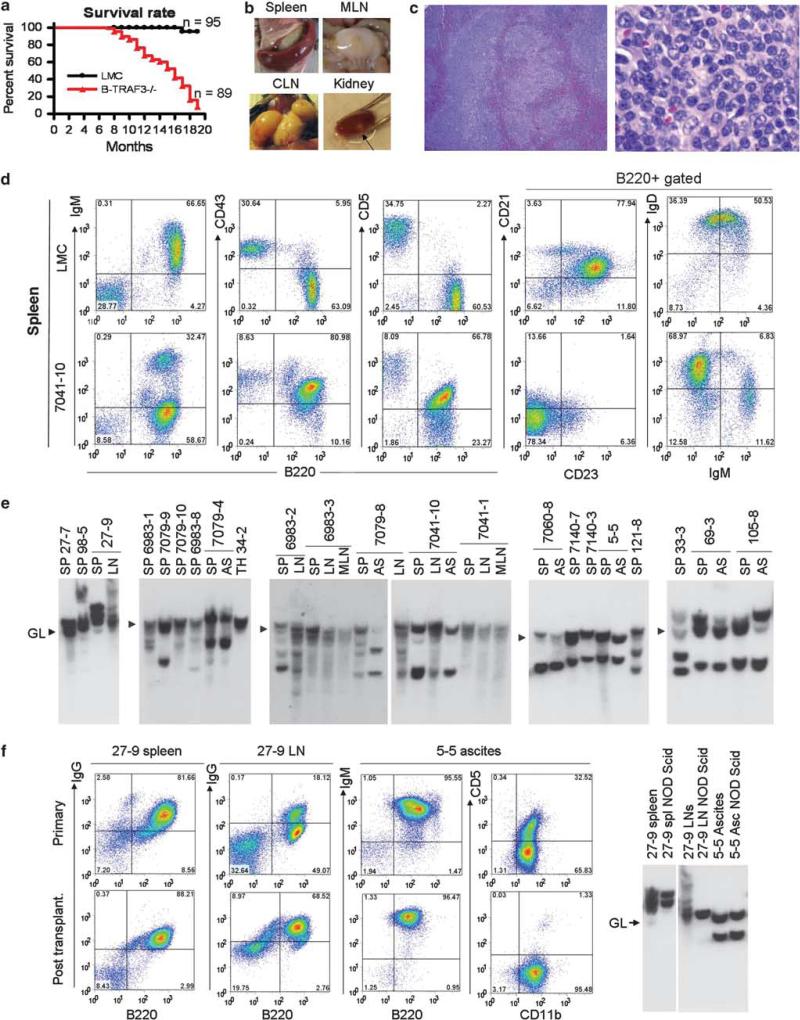
We first noticed that B-TRAF3–/– mice have markedly decreased survival rate at ages >9 months (Figure 1a). We examined tissues of 50 B-TRAF3–/– mice 9-18 months of age, 18 that were necropsied when moribund and 32 that had no external signs of disease at the time of study. All 18 moribund B-TRAF3–/– mice and 20 out of the other 32 (62.5%) had splenic B lymphoma (Supplementary Table 1). Gross observations and microscopic studies also revealed involvement by lymphoma of other tissues including bone marrow, cervical and mesenteric lymph nodes, kidney, lung and liver as well as ascites (Figures 1b and c and Supplementary Figure S1, and Supplementary Materials and Methods). Based on criteria of the Bethesda classification of lymphoid neoplasms,12 the tumors of B-TRAF3–/– mice were splenic MZL, including both high-grade MZL with the cytology of centroblastic diffuse large B-cell lymphomas and low-grade MZL with features of normal MZ B cells.
Figure 1.
B-TRAF3–/– mice spontaneously developed B lymphomas. (a) Accelerated mortality of B-TRAF3–/– mice. Survival curves of LMC and B-TRAF3–/– mice are generated using the Kaplan-Meier method. P<0.001 as determined by the Mantel-Cox log-rank test. (b) Representative images of spleen, cervical lymph nodes (CLN) and mesenteric lymph nodes (MLN), and kidney of B-TRAF3–/– mice with B lymphomas. Arrow indicates a nodal B lymphoma in the kidney. (c) Representative photomicrographs of the spleen of moribund B-TRAF3–/– mice. Sections of the spleen of B-TRAF3–/– mice with B lymphomas were stained with hematoxylin and eosin. Right panel shows higher magnification of left panel. Normal architecture of the spleen was obliterated, and the red pulp was invaded by lymphoma cells. Lymphoma cells in the spleen had centroblast-like morphology with round or ovoid nuclei, and with prominent central nucleoli or two nucleoli adherent to the nuclear membrane. (d) Representative FACS profiles of the splenocytes of B-TRAF3–/– mice with B lymphomas. Splenocytes were stained with fluorochrome-conjugated B220, IgM, CD43, CD5, CD21, CD23 and IgD Abs, and then analyzed by a FACSCalibur. (e) Representative Southern blot analysis of IgH gene rearrangements of TRAF3–/– B lymphomas. Genomic DNA was prepared from the spleen (SP), ascites (AS), cervical lymph node (LN), mesenteric LN (MLN) or thymus (TH) of B-TRAF3–/– mice. DNA was digested with EcoRI, and hybridized with a JH4 probe. Germline bands (GL) are indicated by arrowheads. All bands of sizes different from the germline band are recombined IgH bands, representing clonal expansion of B-lymphoma cells. (f, g) Representative transplantation data of TRAF3–/– B lymphomas into NOD SCID mice. Spleen or LN cells (25 × 106) from mouse ID 27-9, or ascites (5 × 106 cells) from mouse ID 5-5 were intraperitoneally injected into NOD SCID recipient mice. 8-12 weeks post transplantation, spleens, LNs and ascites were harvested from injected NOD SCID mice, and analyzed by FACS (f) and Southern blots of the IgH gene (g).
We next used flow cytometry to characterize the immunophenotypic features of tumor cells present in spleen, bone marrow and ascites (Figure 1d and Supplementary Figure S2). Flow cytometric data of 21 individual mice are summarized in Supplementary Table 2. In keeping with the histologic diagnoses, the immunophenotypic profiles of the tumors were characteristic of mature B-cell lymphomas. With one exception, all tests for canonical mature B-cell markers–B220, CD19, IgM, IgD and Igκ or Igλ–were positive to varying extents. One exceptional case (27-9) was IgG+IgM–IgD– with 20% CD138+. The immunophenotypic profiles of nine examined lymphomas resembled that of B1a cells whereas two additional cases resembled that of B1b cells. Out of the remaining 10 cases, 5 were CD5 CD11b–CD21–CD23+, resembling normal MZ B cells. Thus, over half (11/21) of TRAF3–/– B lymphomas originated from B1 B cells, and another 23% (5/21) originated from MZ B cells. This suggests that many cases diagnosed histologically as centroblastic diffuse large B-cell lymphomas were actually high-grade splenic MZL.
We further performed Southern blot analyses of the IgH gene of primary tumor samples from 21 individual B-TRAF3–/– mice to determine their clonality. The results (Figure 1e) revealed the presence of one or several non-germline bands in each of the DNAs examined, indicating monoclonal or oligoclonal expansions of malignant B cells. DNA prepared from two or three tissues of the same mice often exhibited rearranged bands of similar size, indicating metastatic spread of the same clone.
These observations prompted us to seek more direct evidence for malignant transformation by intraperitoneal transfer of tumor cells with known clonal populations to immunodeficient NOD SCID mice. At 2-3 months after transfer, recipient mice were found to have lymphoma infiltrates in multiple tissues. The transplanted tumors had the same immuno phenotypic features as the donor population (Figure 1f). Southern blot analyses revealed that one or two clonal populations from primary TRAF3–/– B lymphomas were propagated in NOD SCID recipient mice (Figure 1g). Thus, the spontaneous B lymphomas that developed in B-TRAF3–/– mice are clonal, malignant and transplantable.
To gain additional information about the origin, partitioning and history of malignant B cells, we cloned and sequenced the IgH V(D)J regions of lymphomas from 11 B-TRAF3–/– mice (Table 1). The IgH V region sequences of 10 of the 11 examined mice did not exhibit significant somatic hypermutation, suggesting that they were not derived from germinal center-passaged cells but rather from naive pre-germinal center B cells, MZ B cells or B1 cells. Interestingly, all of the major VH sequences identified in TRAF3–/– B lymphomas belonged to one of four VH families, VH1 (J558), VH3 (VH36-60), VH5 (VH7183) and VH12. These gene families are frequently used by MZ, B1, or autoreactive B cells. Thus, IgH V(D)J sequences, together with the histopathological and immunophenotypic data, allow us to classify B lymphomas developed in most B-TRAF3–/– mice as splenic MZL or B1 lymphomas.
Table 1.
Characteristics of IgH gene utilization by lymphomas of B-TRAF3–/– mice
| Mouse | Tissue | Bands on Southerna | Heavy-chain variable region |
DHe | JH | Frequency | SHM | |||
|---|---|---|---|---|---|---|---|---|---|---|
| DNAb | AAb | GLc | VHd | |||||||
| 6983-2 | Spleen | 1 dominant | 98.6 | 99.1 | VH36-60.a2.90 | VH36-60 | DSP2.2 | JH2 | 11/20 | No |
| 1 minor | 98.9 | 97.2 | J558.37.127 | J558 | DSP2.8 | JH2 | 5/20 | No | ||
| 99.7 | 99.1 | VHD6.96 (or VH7183.a4.6) | VHD6 | DST4.3 | JH2 | 4/20 | No | |||
| 7041-10 | Spleen | 1 dominant | 98.6 | 99.1 | VH36-60.a2.90 | VH36-60 | DSP2.5 | JH2 | 18/20 | No |
| 99.3 | 99.2 | VH7183.a19.31 | VH7183 | DSP2.11 | JH3 | 2/20 | No | |||
| 7060-8 | Spleen | 1 dominant | 99.0 | 98.2 | VH7183.a25.43 (or VH283) | VH7183 | DST4.3 | JH4 | 8/19 | No |
| 98.6 | 99.1 | VH36-60.a2.90 | VH36-60 | DSP2.9 | JH2 | 11/19 | No | |||
| Ascites | 1 dominant (same) | 99.0 | 98.2 | VH7183.a25.43 (or VH283) | VH7183 | DST4.3 | JH4 | 11/18 | No | |
| 98.6 | 99.1 | VH36-60.a2.90 | VH36-60 | DSP2.9 | JH2 | 6/18 | No | |||
| 7140-7 | Spleen | 1 dominant | 97.9 | 96.6 | J558.22 | J558 | DSP2.2 | JH3 | 9/17 | No |
| 99.7 | 99.1 | VH98-3G (VH7183.a21.35) | VH98-3G | DSP2.6 | JH3 | 5/17 | No | |||
| 99.3 | 98.3 | V11 (VHS107.a3.106) | V11 | DSP2.2 | JH2 | 3/17 | No | |||
| 7140-3 | Spleen | 1 dominant | 97.9 | 97.5 | J558.17 | J558 | DSP2.2 | JH4 | 7/18 | No |
| 99.7 | 100 | VH7183.a47.76 | VH7183 | DFL16.2 | JH3 | 4/18 | No | |||
| Ascites | Not determined | 99.0 | 99.1 | VH12.a3.101 | VH12 | DSP2.8 | JH1 | 12/19 | No | |
| 98.6 | 98.3 | J558.17 | J558 | DSP2.2 | JH4 | 3/19 | No | |||
| 7079-8 | Ascites | 2 equimolar | 99.7 | 99.1 | VH7183.a2.3 (7183.2.3) | VH7183 | DSP2.11 | JH3 | 18/21 | No |
| 95.6 | 94.2 | V98-3G | VH98-3G | DSP2.9 | JH4 | 2/21 | No | |||
| 5-5 | Spleen | 2 equimolar | 99.7 | 99.1 | VH98-3G (VH7183.a21.35) | VH98-3G | DSP2.9 | JH1 | 15/20 | No |
| 99.3 | 98.3 | VS107.a3.106 | VS107 | DFL16.1 | JH4 | 3/20 | No | |||
| 7079-4 | Ascites | 2 equimolar | 98.0 | 98.3 | VH7183.a19.31 | VH7183 | DQ52 | JH2 | 4/22 | No |
| 99.7 | 99.1 | V98-3G (VH7183.a21.35) | VH98-3G | DSP2.x | JH2 | 4/22 | No | |||
| 100 | 100 | VH7183.a47.76 | VH7183 | DSP2.7 | JH2 | 3/22 | No | |||
| 99.7 | 99.1 | VH7183.a30.50 | VH7183 | DST4.2 | JH3 | 2/22 | No | |||
| 33-3 | Spleen | 2 equimolar dominant | 97.6 | 96.7 | VH7183.a19.31 | VH7183 | DFL16.1 | JH4 | 8/23 | No |
| 2 equimolar minor | 99.0 | 99.1 | VOx-1 | Vox | DSP2.7 | JH4 | 4/23 | No | ||
| 100 | 100 | VH7183.a47.76 | VH7183 | DQ52 | JH4 | 3/23 | No | |||
| 99.7 | 99.2 | VH7183.a7.10 | VH7183 | DSP2.2 | JH2 | 2/23 | No | |||
| 115-6 | Spleen | Not determined | 93.0 | 87.5 | J558.39.129 | J558 | DSP2.9 | JH2 | 21/21 | Yes |
| Ascites | Not determined | 90.9 | 88.9 | J558.39.129 | J558 | DSP2.9 | JH2 | 8/19 | Yes | |
| 99.7 | 99.1 | VHD6.96 | VHD6 | DSP2.2 | JH1 | 5/19 | No | |||
| 99.2 | 98.2 | VHF102 | 2,36,48 | DST4.3 | JH4 | 3/19 | No | |||
| 105-8 | Spleen | 2 dominant | 98.6 | 98.3 | J558.17 | J558 | DSP2.8 | JH4 | 8/22 | No |
| 100 | 100 | VH7183.a47.76 | VH7183 | DSP2.2 | JH4 | 6/22 | No | |||
| Ascites | 2 dominant | 98.6 | 100 | J558.17 | J558 | DFL16.2 | JH4 | 10/21 | No | |
| 99.3 | 98.2 | J558.39.129 | J558 | DSP2.9 | JH2 | 5/21 | No | |||
| 100 | 100 | VH7183.a47.76 | VH7183 | DFL16.2 | JH4 | 3/21 | No | |||
Abbreviations: AA, amino acid; GL, germline sequences; SHM, somatic hypermutation.
The IgH VDJ regions of primary TRAF3–/– B lymphomas were cloned by reverse transcription and PCR using primers VH consensus (5′-GTGCAGCTGGTGGAG TCTGG-3′) and C-μ4(5′-CCTGGATGACTTCAGTGTTGTTCTG-3′). The high-fidelity polymerase, Pfu UltraII, was used in the amplification reaction. PCR products were subsequently subcloned into pBlueScript vector. Mini-prep DNAs of about 20 clones of each B-lymphoma sample were sequenced using the primer C-μSq (5′-CCA-CCA-GAT-TCT-TAT-CAG-ACA-GGG-3′).
Clonal IgH rearrangements identified by Southern blot hybridization (Figure 1e).
Percent homology between tumor DNA and amino acid sequences and germline sequences.
Closest matched GL gene.
VH family.
DH segment.
We previously showed constitutive activation of NF-κB2 and decreased nuclear translocation of PKCδ, but normal CD40-induced NF-κB1 activation in premalignant TRAF3–/– B cells.5 Here, we extended our analyses of these pathways to lymphomas of B-TRAF3–/– mice. We found that the elevated nuclear levels of the NF-κB2 subunits, p52 and RelB, and the decreased nuclear levels of PKCδ were also features of primary B lymphomas (Figure 2a). Interestingly, TRAF3–/– B lymphomas also had moderately increased nuclear levels of the NF-κB1. Hence, the signaling landscape of TRAF3–/– B lymphomas is characterized by constitutive activation of both the NF-κB2 and NF-κB1 (albeit to a lesser extent) pathways as well as reduced nuclear translocation of PKCδ.
Figure 2.
Oridonin, an inhibitor of NF-κB, exhibited potent tumoricidal activity on primary TRAF3–/– B-lymphoma cells. (a) Signaling pathways in TRAF3–/– B lymphoma cells. Cytosolic and nuclear extracts were prepared from splenic B cells purified from LMC mice (LMC) or tumor-free, young B-TRAF3–/– mice (8-16-week old, Young), or B lymphomas of different individual B-TRAF3–/– mice (Tumor). Proteins were immunoblotted for NF-κB2 (p100–p52), RelB, NF-κB1 (p50), RelA, c-Rel and PKCδ. Immunoblots of actin and HDAC1 were used as loading control for cytosolic and nuclear proteins, respectively. (b, c) Tumoricidal activity of oridonin. Primary splenic B-lymphoma cells were purified from several individual B-TRAF3–/– mice with spontaneous lymphomas. Cells were treated with BMS-345541 and oridonin for 24 h. Viability and proliferation of cells were subsequently determined by MTT assay. Panel (b) shows the activity of each drug examined with a wide range of doses (1:10 serial dilutions). Panel (c) shows refined dose-dependent effects of oridonin (examined at 1:2 serial dilutions). The graphs depict the results of two independent experiments with duplicate samples in each experiment (mean ± s.d.). Similar results were also obtained with primary B-lymphoma cells purified from ascites, cervical and mesenteric LNs of several individual B-TRAF3–/– mice with spontaneous tumors. (d) Oridonin inhibited both NF-κB2 and NF-κB1 pathways. Primary TRAF3–/– B-lymphoma cells purified from B-TRAF3–/– mice were treated without (CTL) or with 0.5 μm BMS-345541 (BMS) or 2 μg/ml oridonin. Cytosolic and nuclear extracts were prepared at indicated time points (3 or 6 h) after treatment, and then immunoblotted for NF-κB2 (p100–p52), RelB, NF-κB1 (p50), RelA, c-Rel and PKCδ. Immunoblots of actin and HDAC1 were used as loading control for cytosolic and nuclear proteins, respectively. Data are representative of three independent experiments.
To test whether aberrant NF-κB activation pathways could serve as therapeutic targets for B-cell neoplasms associated with TRAF3 inactivation, we evaluated the effects on cell viability of drugs that modulate NF-κB activation using primary B-lymphoma cells purified from B-TRAF3–/– mice. We used an inhibitor of IKK2, BMS-345541,13 and an inhibitor of NF-κB, oridonin.14 The effects of these agents were compared with the activities of drugs used clinically to treat B lymphomas or leukemias–vincristine, all trans-retinoic acid, doxorubicin and cyclophosphamide. We found that oridonin exhibited potent dose-dependent tumoricidal activity on primary lymphoma cells, whereas BMS-345541 and the four clinical drugs were inactive (Figures 2b and c and Supplementary Figure S3).
To understand the mechanisms of oridonin, we determined the levels of NF-κB2 and NF-κB1 subunits in cytosolic and nuclear extracts of primary B lymphomas. The levels of activated NF-κB2 and NF-κB1 in the nucleus were markedly reduced in B lymphoma cells treated with oridonin (Figure 2d). Furthermore, nuclear translocation of PKCδ, or activation of ERK, p38, JNK, and AKT was unaffected by oridonin (Figure 2d and data not shown). Thus, the potent tumoricidal effects of oridonin can be ascribed to its activity in inhibiting the activation of both NF-κB2 and NF-κB1, suggesting that oridonin or closely related agents should be considered as new candidates for the treatment of B-cell neoplasms characterized by genetic/epigenetic inactivation of TRAF3.
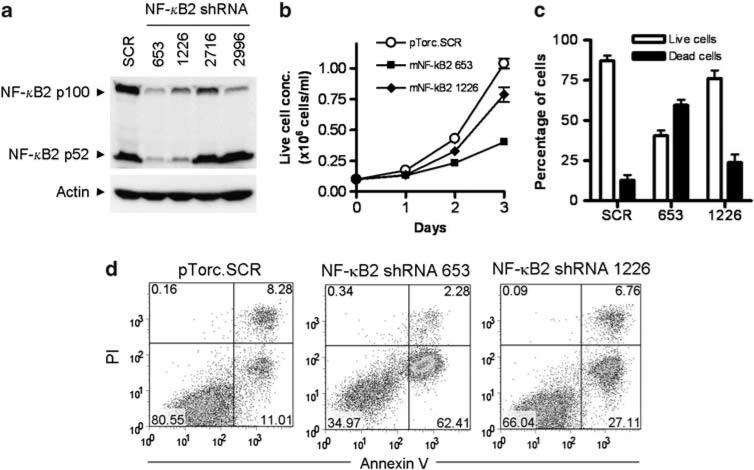
We next sought to specifically decrease the NF-κB2 level using lentiviral shRNA vectors to evaluate the role of constitutive NF-κB2 activation in TRAF3–/– B lymphomagenesis. We screened 4 NF-κB2 shRNA lentiviral vectors. The NF-κB2 shRNA 653 and 1226 knocked down both p100 and p52 NF-κB2 proteins to ~95% and ~75% reduction, respectively. We subsequently used these shRNA lentiviruses to transduce the TRAF3–/– B-lymphoma cell line 27-9.5.3 generated in this study (Supplementary Materials and Methods). Interestingly, both NF-κB2 shRNA 653 and 1226 inhibited the proliferation and induced apoptosis in 27-9.5.3 cells. Importantly, the potency of the two shRNAs in knocking down NF-κB2 protein levels correlated with their ability in inhibiting the proliferation and inducing apoptosis in TRAF3–/– B-lymphoma cells (Figure 3). Although confirmation using additional TRAF3–/– B-lymphoma lines is necessary, our data suggest that constitutive NF-κB2 activation is one major oncogenic pathway in TRAF3–/– B cells.
Figure 3.
NF-κB2 shRNAs induced apoptosis in TRAF3–/– B-lymphoma cells. Mouse B-lymphoma cells (M12.4.1 cell line in (a), and TRAF3–/– B lymphoma cell line 27-9.5.3 in (b, c, d)) were transduced with lentiviruses expressing mouse NF-κB2 shRNAs or a scrambled shRNA (SCR). (a) Total cellular protein levels of p100 and p52 NF-κB2 determined by immunoblot analysis. Proteins were prepared from B-lymphoma cells transduced with a scrambled shRNA, or NF-κB2 shRNA 653, 1226, 2716 or 2996. Immunoblot of actin was used as loading control. (b) Growth curves of live cells and (c) percentage of live versus dead cells determined by trypan blue-stained cell counting. The graphs depict the results of three independent experiments (mean ± s.d.). (d) Representative FACS profiles of transduced cells analyzed by annexin V and propidium iodide (PI) staining. Apoptotic cells were identified as annexin V+ PI–, dead cells were annexin V+ PI+ and live cells were annexin V– PI–. Data are representative of three independent experiments.
Studies that identified TRAF3 deletions and mutations in B-cell chronic lymphocytic leukemia, MZL, mantle cell lymphoma, Waldenström's macroglobulinemia and multiple myeloma suggest potential tumor-suppressive function of TRAF3.1-4 Paradoxically, transgenic mice overexpressing TRAF3 in B cells display autoimmune disease, systemic inflammation and are predisposed to cancers.15 One possibility raised by Zapata et al.15 is that TRAF3 may promote plasma cell differentiation. Alternatively, the unexpected phenotype of TRAF3-transgenic mice may potentially relate to the site of transgene insertion in the genome that may affect the expression or function of additional important gene(s), considering that only a single transgenic founder line was examined in detail.15 Here, the spontaneous, highly penetrant development of B lymphomas in B-TRAF3–/– mice provides conclusive evidence that Traf3 is a tumor-suppressor gene in B cells.
Collectively, data presented in the present study indicate that B-TRAF3–/– mice closely model human splenic MZL and B-cell chronic lymphocytic leukemia. Using B lymphoma cells derived from B-TRAF3–/– mice as model systems, we demonstrated that oridonin and NF-κB2 shRNAs have therapeutic potential. Our findings suggest that restoration of TRAF3 protein or its downstream signaling pathways represents important therapeutic avenues of B lymphomas. In this context, B-TRAF3–/– mice provide a useful tool for developing and testing therapeutic drugs for the treatment of human B-cell neoplasms involving TRAF3 inactivation or relevant genetic/epigenetic alterations.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Dr Janet Hartley for expert advice on mouse B-lymphoma dissection, and Dr Hongsheng Wang for expert advice on IgH VDJ cloning as well as for the critical review of the manuscript. We also thank Jessica Kim, Anano Zangaladze and Will Meng for providing technical assistance to this study. This study was supported by a seed grant from the New Jersey Commission on Cancer Research (10-1066-CCR-EO, P Xie), a Busch Biomedical Grant (P Xie), and the Arthur Herrmann Endowed Cancer Research Fund (P Xie), and in part by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases (H Morse III). The FACS analyses described in this paper were supported by the Flow Cytometry Core Facility of The Cancer Institute of New Jersey (P30CA072720).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
REFERENCES
- 1.Nagel I, Bug S, Tonnies H, Ammerpohl O, Richter J, Vater I, et al. Biallelic inactivation of TRAF3 in a subset of B-cell lymphomas with interstitial del(14)(q24.1q32.33). Leukemia. 2009;23:2153–2155. doi: 10.1038/leu.2009.149. [DOI] [PubMed] [Google Scholar]
- 2.Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braggio E, Keats JJ, Leleu X, Van Wier S, Jimenez-Zepeda VH, Valdez R, et al. Identification of copy number abnormalities and inactivating mutations in two negative regulators of nuclear factor-kappaB signaling pathways in Walden-strom's macroglobulinemia. Cancer Res. 2009;69:3579–3588. doi: 10.1158/0008-5472.CAN-08-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- 10.Mackay F, Tangye SG. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr Opin Pharmacol. 2004;4:347–354. doi: 10.1016/j.coph.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Caligaris-Cappio F. Autoimmune disorders and lymphoma. Ann Oncol. 2008;19(Suppl 4):iv31–iv34. doi: 10.1093/annonc/mdn190. [DOI] [PubMed] [Google Scholar]
- 12.Morse III HC, Anver MR, Fredrickson TN, Haines DC, Harris AW, Harris NL, et al. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood. 2002;100:246–258. doi: 10.1182/blood.v100.1.246. [DOI] [PubMed] [Google Scholar]
- 13.Burke JR, Pattoli MA, Gregor KR, Brassil PJ, MacMaster JF, McIntyre KW, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278:1450–1456. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- 14.Ikezoe T, Yang Y, Bandobashi K, Saito T, Takemoto S, Machida H, et al. Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits the proliferation of cells from lymphoid malignancies in association with blockade of the NF-kappa B signal pathways. Mol Cancer Ther. 2005;4:578–586. doi: 10.1158/1535-7163.MCT-04-0277. [DOI] [PubMed] [Google Scholar]
- 15.Zapata JM, Llobet D, Krajewska M, Lefebvre S, Kress CL, Reed JC. Lymphocyte-specific TRAF3-transgenic mice have enhanced humoral responses and develop plasmacytosis, autoimmunity, inflammation, and cancer. Blood. 2009;113:4595–4603. doi: 10.1182/blood-2008-07-165456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.