Abstract
OBJECTIVE
Optimizing flow and diminishing power loss in the Fontan circuit can improve hemodynamic efficiency potentially improving long-term outcomes. Computerized modeling has predicted improved energetics with a Y-graft Fontan.
METHODS
From August to December, 2010, six consecutive children had a completion Fontan (n=3) or a Fontan revision (n=3) using a bifurcated polytetrafluoroethylene Y-graft (18×9×9 mm in 2, 20×10×10 mm in 4) connecting the inferior vena cava (IVC) to the right and left pulmonary arteries (PAs) with separate graft limbs. Patents were imaged by magnetic resonance imaging (MRI; n-5) or computerized tomography (n=1). Computational fluid dynamics (CFD) assessed Fontan hemodynamics, power loss, and IVC flow splits to the branch PAs. Clinical parameters were compared with 12 patients immediately preceding this series who had a lateral Fontan procedure.
RESULTS
Despite longer crossclamp and bypass times (not statistically significant), the Y-graft Fontan patients had postoperative courses similar to the conventional Fontan patients. Other than two early readmissions for pleural effusions managed with diuretics, on 6–12 months follow-up (mean 8 months), all six patients have done well. Postoperative flow modeling demonstrated balanced distribution of IVC flow to both PAs with minimal flow disturbance. Improvements in hemodynamics and efficiency were noted when the Y-graft branches were anastomosed distally and aligned tangentially with the branch PAs.
CONCLUSIONS
This preliminary surgical experience demonstrates clinical feasibility of the bifurcated Y-graft Fontan. CFD shows acceptable hemodynamics with low calculated power losses and balanced distribution of IVC flow to the PAs as long as the branch grafts are anastomosed distally.
INTRODUCTION
The surgical management of children with single ventricle physiology using a Fontan procedure has changed considerably over the past three decades since the original operation described by Fontan and Baudet in 1977(1). The introduction of the concept of total cavopulmonary connection (TCPC) described by de Leval and colleagues(2) was based partially on in vitro flow modeling. Subsequent studies with computational fluid dynamics (CFD)(3) have demonstrated designs of the TCPC connection that can cause flow disturbances and energy dissipation. Optimizing flow and diminishing power loss in the Fontan circuit presumably can improve hemodynamic efficiency and potentially improve long-term outcomes. Previously, in vitro and CFD studies have shown reduced energy losses with caval offset(4) and with flaring of the cavopulmonary anastomosis(5) thus prompting modification of the TCPC surgical procedure.
In 2007, using CFD, we proposed an optimized TCPC connection using a bifurcated Y-graft for the superior vena cava (SVC) to pulmonary artery connection and another bifurcated Y-graft for the inferior vena cava (IVC) to pulmonary artery connection(6) (U.S. Patent #7811244). Although this “Optiflo” connection had superior flow characteristics predicted by flow modeling, the optimized design appeared to be cumbersome from a surgical standpoint. Subsequent studies developing surgical designs to balance hepatic blood flow distribution to the right and left pulmonary arteries in children with acquired pulmonary arteriovenous malformations showed favorable results with a bifurcated Y-graft connection from the IVC to the branch pulmonary arteries(7). Comparing the Y-graft Fontan connection with more conventional Fontan connections using CFD, others demonstrated improved flow characteristics, reduced energy losses and balanced hepatic flow distribution to the pulmonary arteries(8;9). Based on these improvements in flow dynamics predicted by computerized modeling, we used a commercially available polytetrafluoroethylene bifurcated Y-graft directing IVC flow with separate graft limbs to the right pulmonary artery (RPA) and to the left pulmonary artery (LPA) in six consecutive children undergoing a Fontan procedure.
PATIENTS AND METHODS
This study was approved by the Human Investigation Committee of the Emory University School of Medicine. Informed consent was obtained from the patient or from one of the parents of each of the children studied.
Patient population
From August to December, 2010, six consecutive children undergoing a Fontan procedure aged 2.1–18.9 years (mean 7.2 years; median 4.6 years) weighing 10.3–50.1 kg (mean 23.6 kg; median 18.1 kg) had a completion Fontan (n=3) or a Fontan revision (n=3) using a commercially available bifurcated polytetrafluoroethylene (PTFE) Y-graft marketed for aorto-iliac reconstruction (Gore-Tex, Flagstaff, AZ) as an off-label use. Patient characteristics are shown in Table 1. Two patients had a prior Norwood palliation, 2 had pulmonary atresia with intact ventricular septum, and 2 had heterotaxy/unbalanced atrioventricular septal defect. Of the three patients undergoing a Fontan revision, both of the heterotaxy patients had developed unilateral pulmonary arteriovenous malformations, the other patient had a Fontan baffle leak unable to be closed with an occluder device in the catheterization laboratory. In these three patients with a Fontan revision, the interval from the original lateral tunnel fenestrated Fontan procedure was 14.0, 2.6 and 9.3 years. All six patients had a prior superior cavopulmonary anastomosis (a Glenn procedure) with bilateral bidirectional anastomoses in Patient 6.
Table 1.
Patient Characteristics
| Patient | A7ge (y) | Wt (kg) | Prior Norwood |
Glenn Age (y) |
Interval from Original Fontan (y) |
Primary Diagnosis | Indication for Fontan |
|---|---|---|---|---|---|---|---|
| 1 | 18.9 | 50.1 | 1.38 | 14.0 | Left atrial isomerism, common AV valve, heterotaxy, interrupted inferior vena cava with azygos continuation | Unilateral pulmonary | |
| 2 | 5.4 | 17.8 | 0.34 | Pulmonary atresia with intact ventricular septum | Primary Fontan | ||
| 3 | 2.1 | 12.3 | Yes | 0.39 | Unbalanced CAVSD, hypoplastic LV/aorta | Primary Fontan | |
| 4 | 3.7 | 18.4 | 0.49 | 2.6 | Pulmonary atresia with intact ventricular septum | Fontan baffle leak | |
| 5 | 2.3 | 10.3 | Yes | 0.35 | HLHS (AA/MA) | Primary Fontan | |
| 6 | 11.0 | 32.5 | 0.61 | 9.3 | Right atrial isomerism, hypoplastic RV, heterotaxy, bilateral superior venae cavae | Unilateral pulmonary |
Legend: AA/MA = aortic atresia/mitral atresia; AV = atrioventricular; AVM = arteriovenous malformations; CAVSD = complete atrioventricular septal defect; HLHS = hypoplastic left heart syndrome; LV = left ventricle; RV = right ventricle
To establish a two to one non-matched comparison group with the Y-graft Fontan patients, the records of the most recent consecutive twelve patients undergoing a more conventional Fontan procedure immediately preceding the initiation of the Y-graft series were examined to compare preoperative, operative and postoperative variables. The patients in the comparison group were all operated upon by the same surgeon as the Y-graft Fontan patients (K.R.K.). No attempt was made to match the clinical characteristics of the six Y-graft Fontan patients with the twelve “conventional” fenestrated lateral tunnel Fontan patients; the choice for the comparison group was based solely on the most recent patients having a Fontan operation with two “conventional” Fontan patients for every one Y-graft patient. The purpose of using a nearly contemporaneous comparison group was simply to determine if any of the common early measures of clinical progress differed significantly in the Y-graft patients. All twelve comparison patients had a Fontan completion operation; none had a Fontan revision. A lateral tunnel Fontan technique with a single 2.8 mm fenestration was used in all. The “standard” Fontan comparison group had their operations performed between August, 2009, and August, 2010.
Operative procedure
All operations were performed through a reoperative sternotomy using cardiopulmonary bypass with aortic and direct caval cannulation, moderate hypothermia (30–32°C), and cold cardioplegic arrest. No attemp t was made to avoid cardiopulmonary bypass using temporary venous bypass as described by Okano and colleagues(10). After cardioplegic arrest, the right atrium was opened through a short lateral incision. For the three patients undergoing a Fontan revision (Patients 1, 4, and 6), the previous intra-atrial lateral tunnel conduit was excised and the atriopulmonary connection repaired. Using a bifurcated polytetrafluoroethylene (PTFE) Y-graft (18×9×9 mm in 2, 20×10×10 mm in 4), a single fenestration was created in the 18 mm or 20 mm portion of the graft 1 cm from its bifurcation. In Patient 1, because of diminished ventricular function preoperatively, a 4.0 mm fenestration was employed; for the remaining five patients, a single 2.8 mm fenestration was used. In order to reliably assure the patency of the fenestration, an intra/extracardiac conduit Fontan as described by Jonas was used(11). The graft was sewn inside the atrium around the orifice of the IVC. The 18 mm or 20 mm portion of the graft was brought out through the atriotomy incision which was closed around the graft with polypropylene suture. The two limbs of the bifurcated graft were anastomosed to the undersurface of the right and left branch pulmonary arteries striving to flare the anastomosis(5). We attempted to anastomose the right limb of the graft to the RPA lateral to the insertion of the superior vena cava to the pulmonary artery where the Glenn anastomosis had been performed to create an offset(4). The left limb of the graft was routed behind the ascending aorta and was anastomosed either to the proximal LPA or the medial portion of the proximal RPA. Postoperative management was identical to that for our “standard” Fontan patient. Only chronic low-dose daily aspirin was used routinely for thrombus prophylaxis postoperatively.
Postoperative imaging
Postoperatively, routine transthoracic echocardiography was inadequate for providing satisfactory images of the distal anastomosis of the individual limbs of the Y-graft to the branch pulmonary arteries. As is our institutional standard of care for early postoperative Fontan patients in whom the branch pulmonary arteries cannot be adequately visualized, the Fontan baffle was imaged by magnetic resonance imaging (MRI; n=5) or chest computerized tomography (CT; n=1). Approximately 40–50 axial slices were collected through the thoracic region of the patient. Three-dimensional geometries were constructed. Using computational fluid dynamics (CFD), phase contrast MRI derived flows (or echocardiography/catheterization derived flows in the patient imaged with a CT scan) were used to assess Fontan flows from which power losses and IVC flow splits to the branch pulmonary arteries were calculated using previously described methods(3;12;13).
Statistics
Continuous variables were compared by unpaired t-test and are presented as mean ± standard deviation. Nominal variables were compared by chi-square analysis with Fisher’s exact test. Statistical tests were considered significant if the P value was <0.05.
RESULTS
Operative and postoperative data are shown in Table 2. Mean aortic cross clamp and cardiopulmonary bypass times were 72±33 and 122±56 minutes respectively. Mean ventilation was 20.0±4.2 hours; mean hospitalization was 9.0±3.3 days. Systemic oxygen saturation rose from 82.0±7.1% preoperatively to 90.8±7.7% postoperatively. The only patient who did not have an immediate postoperative increase in oxygen saturations was an 18.9 year-old who had severe pulmonary arteriovenous malformations (Patent 1) and was the only patient to have a 4.0 mm fenestration rather than a 2.8 mm fenestration; she had higher saturations of 85% on examination eight months postoperatively. Other than two early readmissions for pleural effusions managed with increased diuretic therapy, on 6–12 months follow-up (mean 8 months), all six patients have done well clinically.
Table 2.
Operative Results
| Patient | Conduit Size (mm) |
AXC (min) |
CPB (min) | Other Procedures | Inotropic Support Duration (d) |
Ventilation (hrs) | ICU Stay (d) |
Hosp |
|---|---|---|---|---|---|---|---|---|
| 1 | 18×9×9 | 128 | 215 | CAVV repair | 2 | 22.3 | 5 | |
| 2 | 20×10×10 | 50 | 80 | Atrial septectomy | 1 | 18.0 | 1 | |
| 3 | 18×9×9 | 52 | 88 | None | 1 | 17.0 | 1 | |
| 4 | 20×10×10 | 56 | 100 | Remove ASD device | 1 | 14.5 | 1 | |
| 5 | 20×10×10 | 49 | 80 | None | 1 | 22.4 | 2 | |
| 6 | 20×10×10 | 97 | 168 | None | 1 | 25.8 | 3 | |
| Average | 72.0 | 121.8 | 20.0 | 2.2 | 9.0 |
Legend: ASD = atrial septal defect occluder; AXC = aortic cross clamp time; CAVV = common atrioventricular valve; CPB = cardiopulmonary bypass time; ICU = intensive care unit
Comparison of the six Y-graft Fontan patients with the twelve immediately preceding conventional Fontan patients is shown in Table 3. The Y-graft Fontan patients were significantly older and heavier than the lateral tunnel Fontan patients. This was because three (50%) of the Y-graft patients had revision of a previous Fontan procedure whereas all twelve of the comparison group patients had primary Fontan completion. The Y-graft Fontan patients had longer mean aortic crossclamp times (72 vs. 49 minutes) and longer mean cardiopulmonary bypass times (122 vs. 87 minutes) compared with the lateral Fontan patients, but these differences did not achieve statistical significance (Table 3). These longer times were due to the more extensive dissection and additional anastomosis required for the Y-graft Fontan procedure. Despite the more complex operation, postoperative duration of inotropic support, ventilation time, ICU stay and hospital stay in the Y-graft patients were comparable to the values obtained for the lateral tunnel Fontan comparison group (Table 3). Preoperative and early postoperative central venous pressure measurements as well as duration of chest tube drainage and selected laboratory values were not statistically different between the Y-graft patients and the “conventional” Fontan patients (Table 3).
Table 3.
Comparison of Y-graft Fontan patients and lateral tunnel Fontan patients
| Y-graft Fontan (n=6) |
Lateral Tunnel Fontan (n=12) |
P-Value | |
|---|---|---|---|
| Age at Fontan (yrs) | 7.2 ± 6.6 | 2.5 ± 0.6 | .022 |
| Weight at Fontan (kg) | 23.6 ± 15.1 | 12.1 ± 1.6 | .016 |
| Prior Norwood (n; %) | 2 (33%) | 4 (33%) | >.99 |
| Aortic crossclamp time (min) | 72.0 ± 33.0 | 48.8 ± 14.6 | .051 |
| Cardiopulmonary bypass time (min) | 121.9 ± 56.5 | 87.2 ± 25.7 | .087 |
| Preoperative CVP in OR (mmHg) | 10.0 ± 2.2 | 9.3 ± 2.8 | .622 |
| Postoperative CVP in OR (mmHg) | 14.0 ± 2.0 | 14.6 ± 2.3 | .607 |
| CVP postoperative day 1 (mmHg) | 14.2 ± 1.9 | 14.7 ± 3.4 | .748 |
| Postoperative inotropic support duration (d) | 1.7 ± 0.4 | 2.0 ± 1.5 | .199 |
| Postoperative ventilation (hours) | 20.0 ± 4.2 | 26.7 ± 13.3 | .253 |
| Intensive Care Unit stay (d) | 2.2 ± 1.6 | 2.4 ± 0.8 | .659 |
| Duration of chest tube drainage (d) | 6.2 ± 2.8 | 6.5 ± 1.7 | .754 |
| Hospital stay (d) | 9.0 ± 3.3 | 7.1 ± 1.7 | .124 |
| Highest postoperative lactic acid (mg/dL) | 41.3 ± 21.1 | 30.0 ± 13.1 | .116 |
| Highest postoperative creatinine (mg/dL) | 0.7 ± 0.10 | 0.6 ± .04 | .372 |
| Highest postoperative AST (U/L) | 88.8 ± 21.0 | 89.3 ± 35.2 | .975 |
| Highest postoperative ALT (U/L) | 24.2 ± 5.8 | 22.4 ± 6.9 | .600 |
Legend: ALT = alanine aminotransferase; AST = aspartate aminotransferase; CVP = central venous pressure; OR = Operating Room
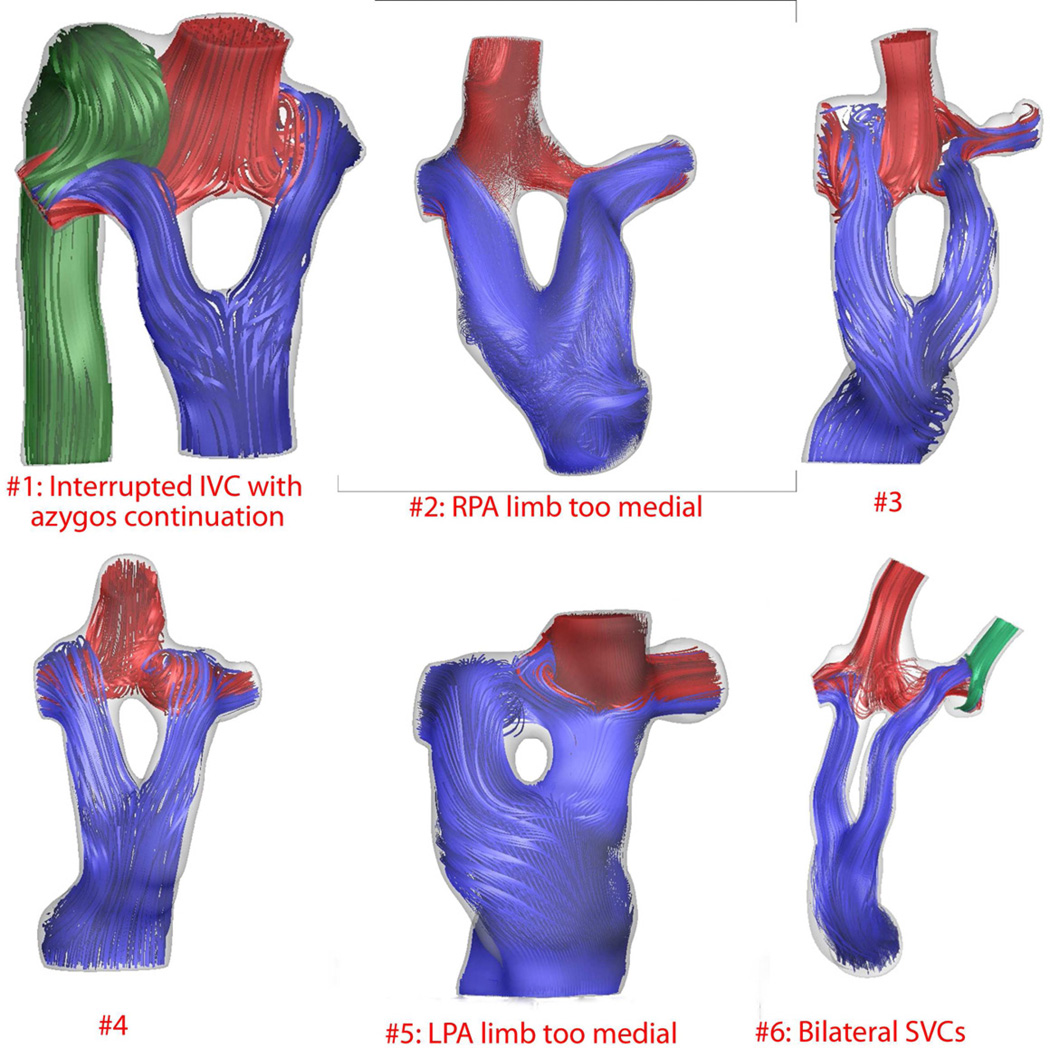
Postoperative flow modeling generally demonstrated balanced distribution of IVC flow to both pulmonary arteries with minimal flow disturbances (Figure 1 and Table 4). Slight improvements in hemodynamics and efficiency were noted when the Y-graft branches were anastomosed distally and aligned tangentially with the branch pulmonary arteries (Table 4). In Patient 2, failure to sufficiently offset the anastomosis of the right limb of the graft to the right pulmonary artery (Figure 1) resulted in an increased calculated power loss of 2.47 mW and 70% of the IVC flow going to the LPA. (Table 4). Similarly, in Patient 5 in whom the LPA limb of the graft was brought too medially with minimal offset from the Glenn anastomosis (Figure 1), only 34% of the IVC flow was calculated to flow to the LPA (Table 4). The calculated resistances across the TCPC (Table 4) averaged 0.34 Wood units and the average calculated power loss was 1.23 mW.
Figure 1.
Computational fluid dynamics (CFD) derived three-dimensional flow streaming in frontal orientation for all six patients reconstructed using magnetic resonance imaging (n=5) or computerized tomography (n=1). In general, there are minimal flow disturbances and balanced distribution of hepatic flow to the branch pulmonary arteries. Patients 2 and 5 show inadequate offset of one of the graft limbs resulting in decreased hepatic flow distribution to the ipsilateral lung. Patient 3 demonstrates the deleterious effect of an uncorrected left pulmonary artery stenosis which resulted in diminished flow to the left lung and increased power loss and calculated resistance (Table 4).
Table 4.
Postoperative calculations
| Patient | Flow (L/min) |
Power Loss (mW) |
IVC Flow Split (per cent to LPA) |
Resistance (Wood Units) |
||
|---|---|---|---|---|---|---|
| IVC | LPA | RPA | ||||
| 1 | 0.50 | 0.43 | 1.95 | 0.79 | 35%* | 0.21 |
| 2 | 0.74 | 1.10 | 1.20 | 2.47 | 70%** | 0.33 |
| 3 | 0.29 | 0.16 | 0.32 | 2.46 | 28% | 0.88 |
| 4 | 0.65 | 0.32 | 0.63 | 0.42 | 45% | 0.14 |
| 5 | 0.37 | 0.64 | 0.23 | 0.25 | 34%** | 0.16 |
| 6 | 0.44 | 0.84 | 0.39 | 0.99 | 30%*** | 0.33 |
Interrupted IVC with azygos continuation
Postoperative MRI showed RPA or LPA limb of graft directly opposite Glenn
Bilateral SVCs
Legend: IVC = inferior vena cava; LPA = left pulmonary artery; MRI = magnetic resonance imaging; RPA = right pulmonary artery; SVC = superior vena cava
DISCUSSION
The clinical use of a Y-graft Fontan procedure previously has been reported infrequently, and always for anatomic reasons rather than for predicted hemodynamic efficiencies. Goksel and colleagues from Istanbul, Turkey, used a 20×10×10 mm PTFE graft in a six year-old with an unbalanced complete atrioventricular septal defect who had separate entry of the IVC and hepatic veins into the inferior portion of the right atrium(14). The 10 mm limbs of the bifurcated graft were sewn inferiorly, one to the IVC and the other to the hepatic veins. Only one connection to the pulmonary artery was made with the 20 mm portion of the Y-graft as an extracardiac Fontan procedure. A variation of this technique using two inferior limbs for separate IVC and hepatic veins connecting to a single conduit to the pulmonary arteries was also mentioned in a paper from Children’s Hospital, Boston, dealing with heterotaxy syndrome(15).
Okano from Kyoto, Japan, described the use of a 22×11×11 mm Dacron Y-graft in a 17 year-old with previous bilateral bidirectional Glenn anastomoses who developed severely distorted hypoplastic central pulmonary arteries between the two superior cavopulmonary anastomoses(10). In that case, the two 11 mm limbs of the Y-graft were anastomosed separately to the RPA and the LPA with the LPA limb being brought in a non-anatomic position anterior to the ascending aorta. Unlike the procedures in the current series, the operations described by Goksel and by Okano were performed without the aid of cardiopulmonary bypass and without the creation of a fenestration.
Previous studies from our group predicted significant energy dissipation from head-on collision of the blood flow from the IVC with blood flow from the SVC when the IVC portion of the TCPC connection is anastomosed in a T-fashion to the pulmonary artery directly opposite the connection of the SVC(4). The predicted power loss was reduced 50% with a caval offset compared with no offset(4). Flaring of the anastomosis combined with caval offset resulted in a 68% reduction in power loss comparing designs with no flaring or offset(5). With these concepts in mind, Soerensen proposed an idealized “Optiflo” configuration using a bifurcated Y-graft for the SVC to pulmonary artery connections and another bifurcated Y-graft for the IVC to pulmonary artery connections(6) (U.S. Patent #7811244). Compared with a one-diameter caval offset design, the Optiflo TCPC design was shown by CFD to reduce power loss from 26% to 42% at a wide range of flows due to the avoidance of inflow collisions. It also provided balanced hepatic blood flow distribution to the branch pulmonary arteries. In an accompanying invited commentary to that paper, Hazekamp correctly questioned the clinical feasibility of this design in a small child(16).
Using computer-aided design and computational fluid dynamics, Marsden and colleagues demonstrated that the Y-graft Fontan results in higher energy efficiencies and more equal distribution of IVC flow to both lungs when compared with more traditional TCPC techniques(8). In the same CFD study, they compared a commercially available 18×9×9 mm bifurcated graft with a simulated 18×12×12 mm graft and found the latter to be superior. As shown with the Optiflo model(6), under exercise conditions the efficiencies of the Y-graft design are even more pronounced compared with more standard extracardiac TCPC geometries(8;9).
In patients with acquired pulmonary arteriovenous malformations, we used computer-based surgical modeling to predict distribution of hepatic blood flow to the RPA and LPA(7;17). In some patients, the Y-graft Fontan was predicted to provide the most reliable solution for equalizing hepatic blood flow distribution to both lungs. Thus, based on predicted improved energetics using CFD models with the Optiflo and Y-graft designs described above (especially under simulated exercise conditions) as well as reliably balanced hepatic blood flow distribution to both lungs, we decided to apply the Y-graft Fontan connection clinically which is the basis of this report.
Clinically, all six patients in this series did well with successful operations and reasonable postoperative length of ventilation, ICU stays and hospital stays (Table 2). Compared with an almost contemporaneous non-matched group of twelve patients having a more standard fenestrated lateral tunnel Fontan procedure, despite longer crossclamp and bypass times, postoperative duration of inotropic support, mechanical ventilation, ICU stay, duration of chest tube drainage, selected laboratory results and hospital stay were quite similar (Table 3). The early imaging results demonstrated potential pitfalls to this operation. In two patients (Patients 2 and 5), there was minimal offset of one of the limbs of the bifurcated graft resulting in moderate maldistribution of the hepatic flow to the lungs (Figure 1 and Table 4). We have previously reported that caval offset significantly influences hepatic blood flow distribution in extracardiac and lateral tunnel TCPC connections(12). In Patient 2, there was also an increased calculated power loss which did not occur in Patient 5, possibly explained by the use of a relatively large 20×10×10 mm graft in a small 10.3 kg child. Despite these issues with inadequate offset, in all six patients, the less-perfused lung always received more than a quarter of the IVC flow (Table 2).
In all but Patient 3, the calculated resistances across the TCPC (Table 4) were lower than the average TCPC resistance of 0.39±0.26 Wood units previously reported by our group for more standard extracardiac and lateral tunnel Fontan geometries(13). Average calculated power losses of 1.23 mW (Table 4) were also lower than published values predicted for more standard TCPC connections(3). As shown in Table 4, Patient 3 had a higher calculated power loss and TCPC resistance compared with the other Y-graft patients. Most likely, these mildly unfavorable energetics were more related to an uncorrected stenosis of the LPA (Figure 1) rather than a problem with the Y-graft Fontan connection. We have previously shown that distortion of the branch pulmonary arteries far more strongly negatively affects energy loss than the type of TCPC connection(18). Probably for the same anatomic reason, Patient 3 had only 28% of IVC flow directed to the LPA (Table 4).
Since this series presents a preliminary clinical application of the Y-graft Fontan procedure, several questions remain unanswered. We chose to perform all the operations with cardiopulmonary bypass (CPB) and cardioplegic arrest. Undoubtedly, this procedure can be performed safely without CPB with or without venous shunting as suggested previously(10;14). Also, because of ease and reliability of creating a fenestration, we chose to employ elements of the intra/extracardiac Fontan procedure(11) rather than constructing a completely extracardiac Fontan connection. Certainly the Y-graft Fontan can be performed completely as an extracardiac connection.
Finally, for convenience, even though it represented off-label use, we chose commercially available 18×9×9 mm or 20×10×10 mm PTFE bifurcated Y-grafts rather than constructing what theoretically appears to be a more energetically favorable 18×12×12 mm Y-graft as suggested by Marsden and colleagues(8). In their paper, the calculated efficiency at rest of the 18×8×9 graft was 88.5% compared with 90.3% for a 18×12×12 graft. However, the difference in energy efficiency was much more pronounced under simulated exercise conditions. The calculated combined cross-sectional area of the two limbs of a bifurcated graft are one-half that of the main graft if the diameter of each limb is half that of the main graft (A = πr2). For example, for the 18×9×9 mm graft, the combined cross-sectional area of the two 9 mm limbs is 127.2 mm2 compared with a cross-sectional area of 254.4 mm2 for the 18 mm portion. Similarly, for the 20×10×10 mm graft, the combined cross-sectional area of the two 10 mm limbs is 157 mm2 compared with a cross-sectional area of 314 mm2 for the 20 mm portion. To achieve the same cross-sectional area as the main graft, the diameter of each limb would have to be half the diameter of the main graft multiplied by . Thus to maintain equal combined cross-sectional areas, the branches for an 18 mm graft would each have a diameter of 12.7 mm (18×12.7×12.7 mm); for the 20 mm graft, each limb would need a diameter of 14.1 mm (20×14.1×14.1 mm). To construct such a bifurcated graft that preserves the total cross-sectional area of the main portion into the separate limbs would require either collaboration with industry or the fashioning of individual “home-made” grafts. Only time and further study will demonstrate if this theoretical geometric advantage is clinically important.
In summary, we have shown the clinical feasibility of a Y-graft Fontan connection with excellent clinical results on very short follow-up. Early imaging and CFD studies demonstrate acceptable hemodynamics and hepatic blood flow distribution. The degree of offset and flaring of the upper limbs of the Y-graft to the pulmonary arteries appear to be important factors for optimizing favorable hemodynamics. The critical unanswered question from this study is whether or not the computationally predicted improved energetics (especially during exercise), flow characteristics and balanced hepatic flow distribution will translate into improved long-term clinical outcomes.
Acknowledgments
This study was supported by the National Heart Lung and Blood Institute Grant HL67622 and a grant from Children's Healthcare of Atlanta to Dr. Kanter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 37th Annual Meeting of the Western Thoracic Surgical Association, Colorado Springs, CO
REFERENCES
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96:682–695. [PubMed] [Google Scholar]
- 3.Whitehead KK, Pekkan K, Kitajima HD, Paridon SM, Yoganathan AP, Fogel MA. Nonlinear power loss during exercise in single-ventricle patients after the Fontan: insights from computational fluid dynamics. Circulation. 2007;116:I165–I171. doi: 10.1161/CIRCULATIONAHA.106.680827. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S, Goudy S, Walker P, Panchal S, Ensley A, Kanter K, et al. In vitro flow experiments for determination of optimal geometry of total cavopulmonary connection for surgical repair of children with functional single ventricle. J Am Coll Cardiol. 1996;27:1264–1269. doi: 10.1016/0735-1097(95)00598-6. [DOI] [PubMed] [Google Scholar]
- 5.Ensley AE, Lynch P, Chatzimavroudis GP, Lucas C, Sharma S, Yoganathan AP. Toward designing the optimal total cavopulmonary connection: an in vitro study. Ann Thorac Surg. 1999;68:1384–1390. doi: 10.1016/s0003-4975(99)00560-3. [DOI] [PubMed] [Google Scholar]
- 6.Soerensen DD, Pekkan K, de Zelicourt D, Sharma S, Kanter K, Fogel M, et al. Introduction of a new optimized total cavopulmonary connection. Ann Thorac Surg. 2007;83:2182–2190. doi: 10.1016/j.athoracsur.2006.12.079. [DOI] [PubMed] [Google Scholar]
- 7.de Zelicourt DA, Haggerty CM, Sundareswaran KS, Whited BS, Rossignac JR, Kanter KR, et al. Individualized computer-based surgical planning to address pulmonary arteriovenous malformations in patients with a single ventricle with an interrupted inferior vena cava and azygous continuation. J Thorac Cardiovasc Surg. 2011;141:1170–1177. doi: 10.1016/j.jtcvs.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsden AL, Bernstein AJ, Reddy VM, Shadden SC, Spilker RL, Chan FP, et al. Evaluation of a novel Y-shaped extracardiac Fontan baffle using computational fluid dynamics. J Thorac Cardiovasc Surg. 2009;137:394–403. doi: 10.1016/j.jtcvs.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 9.Yang W, Feinstein JA, Marsden AL. Constrained optimization of an idealized Y-shaped baffle for the Fontan surgery at rest and exercise. Comp Meth Appl Mech Eng. 2010;199:2135–2149. [Google Scholar]
- 10.Okano T, Yamagishi M, Shuntoh K, Yamada Y, Hayashida K, Shinkawa T, et al. Extracardiac total cavopulmonary connection using a Y-shaped graft. Ann Thorac Surg. 2002;74:2195–2197. doi: 10.1016/s0003-4975(02)03978-4. [DOI] [PubMed] [Google Scholar]
- 11.Jonas RA. The intra/extracardiac conduit fenestrated fontan. Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann. 2011;14:11–18. doi: 10.1053/j.pcsu.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Dasi LP, Whitehead K, Pekkan K, de ZD, Sundareswaran K, Kanter K, et al. Pulmonary hepatic flow distribution in total cavopulmonary connections: extracardiac versus intracardiac. J Thorac Cardiovasc Surg. 2011;141:207–214. doi: 10.1016/j.jtcvs.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundareswaran KS, Pekkan K, Dasi LP, Whitehead K, Sharma S, Kanter KR, et al. The total cavopulmonary connection resistance: a significant impact on single ventricle hemodynamics at rest and exercise. Am J Physiol Heart Circ Physiol. 2008;295:H2427–H2435. doi: 10.1152/ajpheart.00628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goksel OS, Tireli E, Sungur Z, Harmandar B, Nisli K, Dayioglu E. Use of a bifurcated ePTFE graft for off-pump extracardiac Fontan completion. Thorac Cardiovasc Surg. 2007;55:324–325. doi: 10.1055/s-2006-924703. [DOI] [PubMed] [Google Scholar]
- 15.Stamm C, Friehs I, Duebener LF, Zurakowski D, Mayer JE, Jr, Jonas RA, et al. Improving results of the modified Fontan operation in patients with heterotaxy syndrome. Ann Thorac Surg. 2002;74:1967–1977. doi: 10.1016/s0003-4975(02)04124-3. [DOI] [PubMed] [Google Scholar]
- 16.Hazekamp M. Invited commentary: Introduction of a new optimized total cavopulmonary connection. Ann Thorac Surg. 2007;83:2190. doi: 10.1016/j.athoracsur.2006.12.079. [DOI] [PubMed] [Google Scholar]
- 17.Sundareswaran KS, de Zelicourt D, Sharma S, Kanter KR, Spray TL, Rossignac J, et al. Correction of pulmonary arteriovenous malformation using image-based surgical planning. J Am Coll Cardiol Img. 2009;2:1024–1030. doi: 10.1016/j.jcmg.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasi LP, Krishnankuttyrema R, Kitajima HD, Pekkan K, Sundareswaran KS, Fogel M, et al. Fontan hemodynamics: importance of pulmonary artery diameter. J Thorac Cardiovasc Surg. 2009;137:560–564. doi: 10.1016/j.jtcvs.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]