Abstract
The solution phase synthesis of a discovery library of 178 tricyclic pyrrole-2-carboxamides was accomplished in nine steps and seven purifications starting with three benzoyl protected amino acid methyl esters. Further diversity was introduced by two glyoxaldehydes and forty-one primary amines. The combination of Pauson-Khand, Stetter and microwave assisted Paal Knorr reactions was applied as a key sequence. The discovery library was designed with the help of QikProp 2.1 and physicochemical data are presented for all pyrroles. Library members were synthesized and purified in parallel and analyzed by LC-MS. Selected compounds were fully characterized.
Introduction
Currently, medicinal chemists rely largely on the notion of “drug-like” space2,3 and chemical intuition as a basis for pre-selecting compounds to be screened as potential drug candidates. This established approach will certainly lead to the development of new drug candidates. However, arguments are being made that “drug-like” space is limiting and opportunities are being missed by not venturing outside this area. Drugs such as nitrous oxide, lithium and insulin, which do not fall into the realm of “drug-like” space support the premise that expanding drug discovery space may be valuable. A potential solution to this drug discovery limitation is the preparation and assaying of “discovery libraries.” A discovery library is defined as compounds that occupy a heretofore poorly populated chemical space. There are many compounds that fall into this category, however, when designing a discovery library there are a few criteria that should be considered: 1) the number of synthetic steps should be minimized while structural and functional diversity is maximized; 2) the amount of each compound synthesized should be adequate so that numerous biological evaluations can be performed; 3) properties like lipophilicity and solubility4 should be taken into consideration since this is an issue for most of the assays; 4) and finally precautions should be taken in the design phase so that very reactive functional groups that covalently bind to the targets are avoided.5 These guidelines will then lead to compounds that will function as tools2,6 for probing biological activity.
The synthesis of compounds that occupy new chemical space depends upon new method development to access these unique chemical structures.7 Recently, protocols have been developed by Brummond and Mitasev demonstrating that structurally unique scaffolds can be generated by subjecting a common intermediate to different transition metal catalyzed conditions.8 The compounds that have been synthesized using this diverging strategy are new and are being evaluated for interesting bioactivity, however, it is the unique reactivity profile of each scaffold and its potential to be differentially functionalized that is deemed the most useful feature of this approach. Thus far, most of our efforts have been devoted to back-end diversifications of these scaffolds.
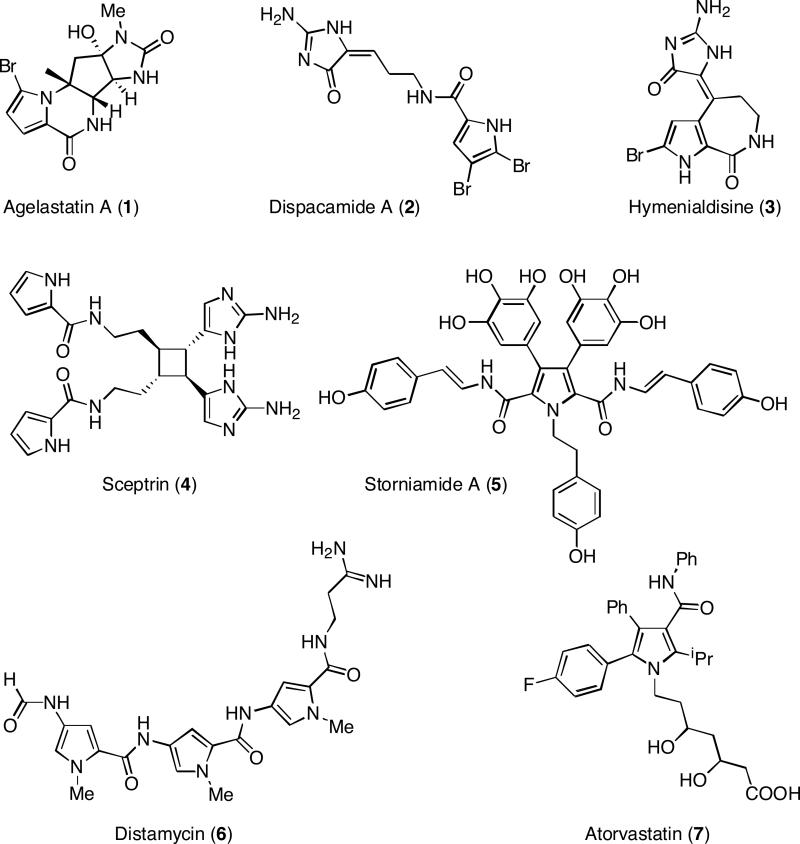
A discovery library of pyrrole-carboxamides9 is of special interest because of the pharmacological activity of this unit. The pyrrole-carboxamide skeleton is a core unit in many marine natural products10 (Figure 1) like agelastatin A (1, antitumor activity),11 dispacamide A (2, anti-histaminic activity),12 hymenialdisine (3, kinase inhibitor),13 sceptrin (4, antiviral activity)14 or storniamide A (5, antibacterial activity)15. One of the most important natural products with a pyrrole-carboxamide unit is distamycin (6), a well-established DNA minor groove binder.16 Synthetic pyrrole-carboxamides exert a wide range of pharmacological activities, e.g. they show antimalarial activity,17 bind to dopamine-D2 like receptors,18 inhibit the enzymes MAO-A and MAO-B,19 inhibit tyrosine kinase,20 show growth hormone secretagogue agonist activity21 or modulate protein kinase activity.22 The calcium salt of atorvastatin (7, Lipitor, Pfizer, 1997, hypolipidemic activity) became a blockbuster drug.
Figure 1.
Examples of bioactive pyrrole-carboxamides.
Results and Discussion
Library Design
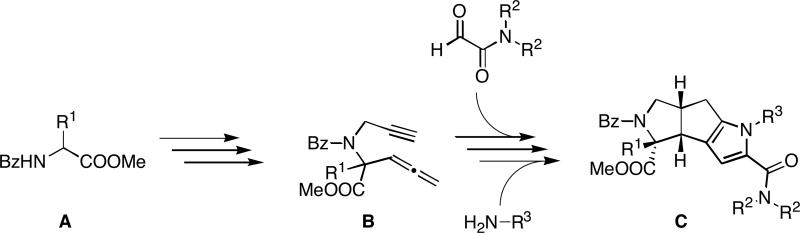
In order to obtain a defined skeleton in a few steps, a complexity generating reaction or reaction sequence is needed. We recently developed such a sequence consisting of a Pauson-Khand/Stetter/Paal-Knorr reaction that allows us to approach the tricyclic pyrrole-carboxamides C in only four steps from the allenynes B which are derived from the protected amino acid esters A (Figure 2).8a,23 Installing appendages on the core unit by using appropriate building blocks leads to a set of compounds with diverse physicochemical properties – the discovery library.
Figure 2.
Forward synthetic analysis of the title compounds C.
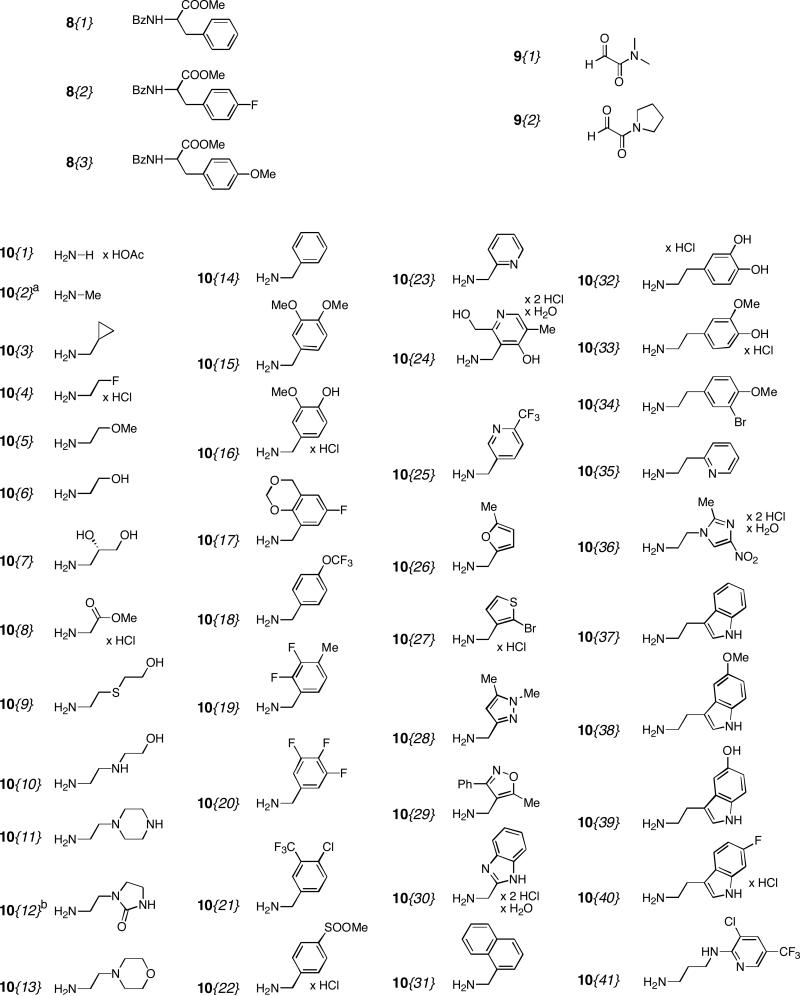
This library has three points of diversity: the amino acid (R1), the glyoxaldehyde (R2) and the primary amine (R3). The first two building blocks were introduced in early stages, therefore it was decided to work with only three amino acids and two glyoxaldehydes. In our earlier work, we reported that R1 has to be an aromatic substituent, otherwise a diastereomer is formed in the Pauson-Khand reaction that does not undergo the Stetter reaction.8a,23 Therefore the benzoyl protected phenylalanine methyl ester derivatives 8{1-3} were chosen (Figure 3). The carboxamide functionality was introduced by the two glyoxaldehydes 9{1-2}.
Figure 3.
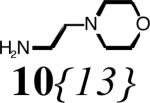
Diversity elements 8{1-3} for R1, 9{1-2} for R2 and 10{1-41} for R3; a 33% in ethanol. b 50% in iso-propanol.
To identify subsets for a library with three points of diversity a reagent-based selection is normally preferred over a product based selection as it deals with less compounds that have to be compared.24 In the library reported here, a design was chosen where the amines 10{1-41} that were incorporated in the last step were the major point of diversity. This approach allowed a product-based design of the library without a large number of calculations as the properties of the products were mainly influenced by the amine that was used in the Paal-Knorr reaction.
Hence, a virtual library with 300 α-unsubstituted primary amines that are commercially available from four major suppliers was created.25 This set was analyzed according to the following properties:
chemoselective reaction
regioselective reaction
diastereoselective reaction
molecular weight
clogP
functional group diversity
cost
A few exceptions were made when the amines showed interesting properties, e.g. 10{7}. After examination of these properties a very diverse set of 41 primary amines 10{1-41} was obtained. This set contained aliphatic, benzylic, heteroaromatic, and phenethyl amines bearing hydroxyl groups, secondary amines, ureas, esters, nitro groups, ethers, halides, thio ethers, and sulfonyl groups.
Library Synthesis
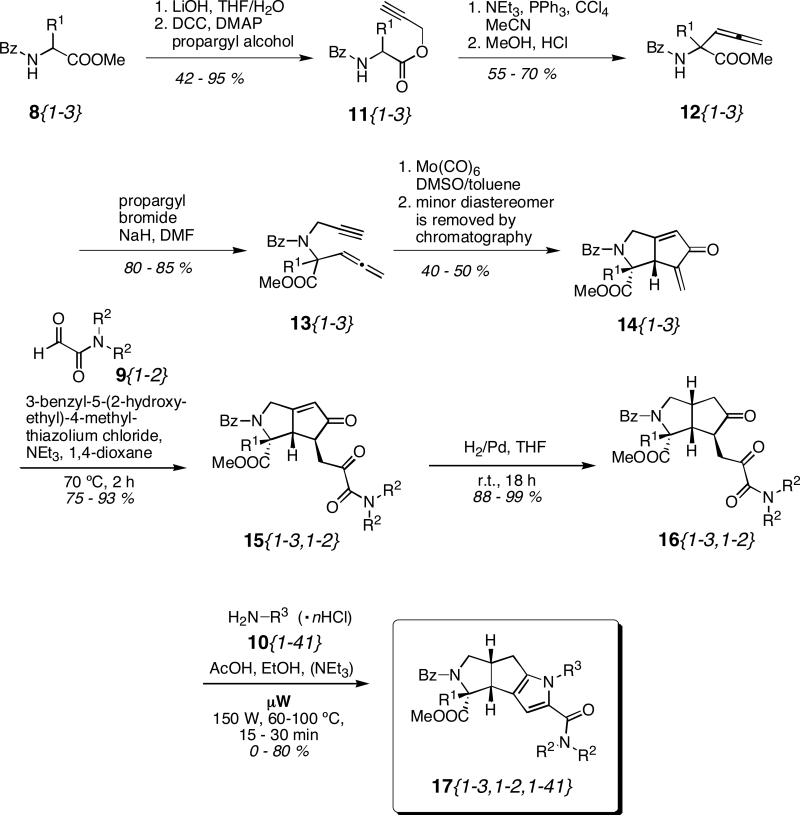
As described previously8a,23 the benzoyl protected amino acid methyl esters 8{1-3} were hydrolyzed and esterified with propargyl alcohol to give the propargyl esters 11{1-3} in yields of 42 to 95% (Scheme 1). Applying the protocol developed by Castelhano and Krantz26 esters 11{1-3} were then converted by a Claisen-rearrangement to allenes 12{1-3} that were obtained in yields of 55 to 70%. Propargylation gave eneynes 13{1-3} in yields of 80 to 85%. In the next step the three key intermediates 14{1-3} were prepared by a Pauson Khand reaction in the presence of Mo(CO)6 (40 to 50% isolated yield of the major diastereomer). 14{1-3} were obtained in a diastereomeric ratio of 3:1, the minor diastereomer was removed by chromatography. The relative stereochemistry of 14{1}, the major diastereomer in the reaction of 13{1}, was determined by single crystal X-ray crystallography. The stereochemistry of 14{2} and 14{3} are based on similarity of the 1H and 13C NMR spectra with those of 14{1}.23
Scheme 1.
Synthesis route to the tricyclic pyrrole-carboxamides 17.
In the following Stetter reaction, the three cyclopentenones 14{1-3} were converted to the six α,β-unsaturated 1,4-diketones 15{1-3} using 3-benzyl-5-(2-hydroxyethyl)-4-methyl-thiazolium chloride as catalyst. In this step the second building block 9{1-2} was incorporated and the six reactions were performed in parallel in a Radley's Carousel Reaction Station. Heating the reaction mixtures to 70 °C for 20 min gave 15{1-3,1-2} in yields ranging from 75 to 93%. Unfortunately, the bicyclic systems 15{1-3,1-2} had to be purified by chromatography twice since traces of impurities poisoned the palladium catalyst in the next step. Compounds 15{1-3,1-2} were obtained as single diastereomers as observed by 1H NMR and LC analysis. The relative stereochemistry was assigned by comparison with a compound previously prepared and characterized by single crystal X-ray crystallography.23 Next, reduction of the double bond gave the 1,4-diketones 16{1-3,1-2} in yields of 88 to 99% as single diastereomers in quantities of 1.0 g (16{1,1-2}) or 2.0 g (16{2,1-2} and 16{3,1-2}), respectively. Compounds 16{1-3,1-2} were used in the following Paal-Knorr reaction without further purification.
While optimizing the protocol for the Paal-Knorr reaction, it was noticed that this reaction is not only 3 to 5 times faster in the microwave, as anticipated, but that it also gave higher yields when performed under microwave irradiation. The higher yields are presumably due to the shorter reaction times and less thermal decomposition.27 In preliminary studies, the reactivity of several amines was compared and it was found that most reactions were complete by TLC analysis within 10 min when irradiated in an automated Emrys Optimizer single mode microwave reactor at 80 °C. To ensure complete reaction, every reaction mixture was irradiated for 15 min at 80 °C. In a typical example, the 1,4-diketone 16 (0.06 mmol) was dissolved in ethanol (600 μL) and acetic acid (60 μL) in an Emrys Process Vial. The corresponding amine (3 equivalents) was added and the microwave tube was sealed and irradiated with an initial power of 150 W at 80 °C for 15 min. When the hydrochloride ammonium salts of the amine were used (Figure 3), triethylamine was added prior to irradiation and the reaction times were found to be longer, therefore the irradiation time was doubled. In the case of very volatile amines like 10{1}, 10{2}, and 10{3} the temperature was reduced to 60 °C and the reaction time was extended to 30 min. Five equivalents of amine had to be used in the case of 10{1} and 10{3}, and 10 equivalents in the case of 10{2}. 10{36} was the least reactive amine and required a temperature of 100 °C and irradiation for 30 min.
Pyrroles 17{1-3,1-2,1-41} were synthesized in batches of 10 or 20. After the reaction was complete, the microwave tubes were opened and all volatile components were removed within 10 min using a stream of argon in a Radley's GreenHouse Blowdown Evaporator, which fits up to 24 microwave tubes. The crude mixtures were loaded with methylene chloride (500 μL) onto 4 g RediSep Disposable Flash Columns and 10 reactions were purified by automated parallel chromatography using an ISCO Optix 10 system with a gradient of hexanes and ethyl acetate. It proved to be advantageous to group 10 reactions with amines of a similar polarity together as the amine had the strongest effect on the polarity of the final product. For the reaction mixtures 17{1-3,1-2,10} and 17{1-3,1-2,11}, which bear a secondary amine, Reverse Phase (C-18) RediSep Columns were used and they were eluted with acetonitrile and water.
Applying this procedure, 210 reactions were carried out, of a 246 possible reactions (array 3 × 2 × 41 = 246). Because of limited amounts of 16{1-3,1-2} that were available not all 246 compounds could be prepared, but in order to obtain a sufficient amount of every library member for a broad biological evaluation all reactions were carried out on the same scale. The tricyclic pyrrole-2-carboxamides 17{1-3,1-2,1-41} were isolated with an average yield of 53% (Table 1) and an average amount of 14.7 mg.
Table 1.
Library matrix for the tricyclic pyrrole-carboxamides 17.
| compound isolated yielda (average purity)b |

|

|

|
|||
|---|---|---|---|---|---|---|

|

|

|

|

|

|
|
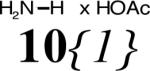
|
17{1,1,1} 63 (100) |
17{1,2,1} 66 (100) |
17{2,1,1} 58 (87) |
d |
17{3,1,1} 78 (100) |
17{3,2,1} 43 (100) |

|
17{1,1,2} 29 (87) |
17{1,2,2} 40 (94) |
17{2,1,2} 30 (87) |
17{2,2,2} 36 (88) |
17{3,1,2} 29 (97)c |
d |

|
- |
17{1,2,3} 47 (98) |
17{2,1,3} 57 (100)c |
17{2,2,3} 80 (92)c |
- | - |

|
d | - |
17{2,1,4} 70 (92)c |
17{2,2,4} 48 (89) |
17{3,1,4} 86 (100)c |
17{3,2,4} 39 (96) |

|
- |
17{1,2,5} 70 (100) |
17{2,1,5} 61 (100)c |
17{2,2,5} 76 (92) |
17{3,1,5} 69 (87) |
17{3,2,5} 61 (99) |

|
17{1,1,6} 58 (100) |
17{1,2,6} 56 (100) |
17{2,1,6} 72 (100) |
17{2,2,6} 67 (95) |
17{3,1,6} 50 (100) |
17{3,2,6} 62 (94) |

|
17{1,1,7} 46 (93) |
17{1,2,7} 46 (100) |
17{2,1,7} 46 (100) |
17{2,2,7} 64 (100) |
17{3,1,7} 62 (100) |
17{3,2,7} 48 (100) |

|
17{1,1,8} 31 (94)c |
17{1,2,8} 56 (100) |
17{2,1,8} 72 (98)c |
17{2,2,8} 73 (89) |
17{3,1,8} 76 (99)c |
17{3,2,8} 34 (95)c |

|
17{1,1,9} 38 (100)c |
17{1,2,9} 55 (91) |
17{2,1,9} 49 (89) |
17{2,2,9} 62 (92) |
17{3,1,9} 57 (88) |
17{3,2,9} 47 (100) |

|
d | d | - | d | - | d |

|
d | d | - | d | - | d |

|
17{1,1,12} 54 (87)c |
- |
17{2,2,12} 40 (86) |
17{3,1,12} 58 (93)c |
17{3,2,12} 63 (86) |
|
|
- | d |
17{2,1,13} 50 (96) |
- | d |
17{3,2,13} 35 (100) |

|
17{1,1,14} 53 (98)c |
17{1,2,14} 77 (94) |
17{2,1,14} 56 (81) |
17{2,2,14} 74 (96)c |
17{3,1,14} 57 (98)c |
17{3,2,14} 57 (93) |

|
17{1,1,15} 39 (97)c |
17{1,2,15} 50 (96) |
17{2,1,15} 67 (95)c |
- |
17{3,1,15} 51 (98)c |
17{3,2,15} 55 (100) |

|
17{1,1,16} 31 (89) |
17{1,2,16} 22 (98) |
17{2,1,16} 64 (87) |
- |
17{3,1,16} 64 (91)c |
17{3,2,16} 48 (100) |

|
17{1,2,17} 66 (100)c |
17{2,1,17} 80 (98)c |
17{2,2,17} 33 (98)c |
d |
17{3,2,17} 56 (92)c |
|

|
17{1,2,18} 57 (96)c |
17{2,1,18} 70 (86) |
17{2,2,18} 64 (95)c |
17{3,1,18} 67 (98)c |
17{3,2,18} 31 (89)c |
|

|
17{1,1,19} 68 (100) |
- |
17{2,1,19} 72 (98)c |
17{2,2,19} 66 (97)c |
17{3,1,19} 54 (98)c |
17{3,2,19} 73 (91) |

|
17{1,1,20} 47 (95)c |
- |
17{2,1,20} 94 (96)c |
- |
17{3,1,20} 63 (93)c |
17{3,2,20} 66 (93)c |

|
17{1,1,21} 45 (99) |
d |
17{2,1,21} 67 (96)c |
17{2,2,21} 73 (88)c |
17{3,1,21} 81 (96)c |
17{3,2,21} 64 (95) |

|
17{1,1,22} 53 (100)c |
17{1,2,22} 63 (98) |
17{2,1,22} 64 (81) |
17{2,2,22} 61 (100)c |
17{3,1,22} 60 (94)c |
17{3,2,22} 66 (88) |

|
17{1,1,23} 54 (100) |
17{1,2,23} 71 (99) |
17{2,1,23} 59 (97) |
17{2,2,23} 68 (95) |
17{3,1,23} 30 (88) |
17{3,2,23} 46 (99) |

|
17{1,1,24} 51 (100) |
17{1,2,24} 54 (100) |
17{2,1,24} 66 (100) |
17{2,2,24} 67 (100) |
17{3,1,24} 55 (100) |
17{3,2,24} 48 (100) |

|
17{1,1,25} 28 (97) |
17{1,2,25} 64 (97) |
17{2,1,25} 69 (91) |
17{2,2,25} 62 (87) |
17{3,1,25} 30 (92)c |
17{3,2,25} 26 (97)c |

|
17{1,1,26} 22 (95)c |
17{1,2,26} 62 (96) |
17{2,1,26} 71 (88) |
17{2,2,26} 75 (89) |
17{3,1,26} 71 (92) |
17{3,2,26} 28 (100)c |

|
17{1,1,27} 73 (91)c |
17{1,2,27} 54 (89) |
17{2,1,27} 65 (98)c |
d |
17{3,1,27} 43 (97)c |
17{3,2,27} 75 (91)c |

|
17{1,1,28} 51 (94) |
- |
17{2,1,28} 63 (98) |
17{2,2,28} 62 (98) |
17{3,1,28} 41 (100) |
17{3,2,28} 59 (100) |

|
17{1,1,29} 56 (89)c |
- | d | - | d |
17{3,2,29} 31 (89)c |

|
17{1,1,30} 39 (87) |
17{1,2,30} 42 (98) |
17{2,1,30} 57 (93) |
- |
17{3,1,30} 38 (89) |
17{3,2,30} 62 (100) |

|
- |
17{1,2,31} 61 (94)c |
17{2,1,31} 47 (97)c |
17{2,2,31} 63 (90)c |
17{3,1,31} 68 (90)c |
17{3,2,31} 26 (96)c |

|
d | - |
17{2,1,32} 23 (89) |
17{2,2,32} 47 (96) |
d |
17{3,2,32} 48 (89) |

|
17{1,1,33} 54 (94)c |
- |
17{2,1,33} 17 (91)c |
- |
17{3,1,33} 35 (86) |
17{3,2,33} 46 (93)c |

|
- | - |
17{2,1,34} 54 (91) |
17{2,2,34} 61 (95) |
17{3,1,34} 53 (88) |
17{3,2,34} 64 (97)c |

|
- |
17{1,2,35} 49 (95) |
17{2,1,35} 49 (93) |
17{2,2,35} 52 (90) |
17{3,1,35} 35 (94) |
17{3,2,35} 68 (98) |

|
17{1,1,36} 20 (95)c |
17{1,2,36} 37 (92) |
17{2,1,36} 21 (93)c |
17{2,2,36} 56 (96) |
- |
17{3,2,36} 41 (98) |

|
d | - | d |
17{2,2,37} 38 (95)c |
d |
17{3,2,37} 62 (92) |

|
d | - |
17{2,1,38} 19 (88)c |
17{2,2,38} 54 (96) |
d |
17{3,2,38} 57 (91) |

|
17{1,1,39} 34 (87) |
- | - | - |
17{3,1,39} 27 (89)c |
17{3,2,39} 45 (92) |

|
d | - | d |
17{2,2,40} 22 (92) |
d |
17{3,2,40} 29 (97) |

|
d | - |
17{2,1,47} 60 (94) |
17{2,2,41} 56 (86) |
d |
17{3,2,41} 59 (86) |
Isolated yield for the last step after parallel chromatography (ISCO Optix 10 System).
Purity was determined by HPLC-MS on an Alltech Varian column, 1 mL/min, 35% H2O/MeOH (95/5), 15% MeOH, 50% MeCN.
Compound was repurified on a serial HPLC system (Gilson) with a Varian C-18 column (250 × 21.4 mm), 15 mL/min, 15 min runtime, 70 - 95% MeOH/MeCN (1/3), 30 - 5% H2O.
Reaction failed because no molecular mass was found or the purity after repurification was lower than 85% at 210, 220 or 240 nm.
Purity Analysis
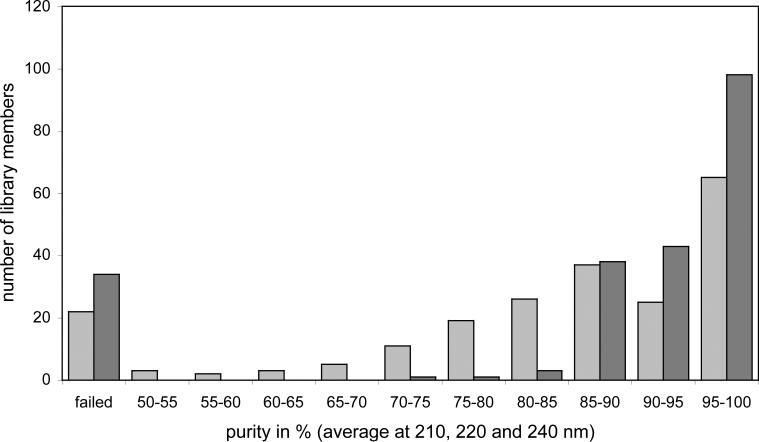
To pass the purity criteria for our compound collection, library members must be at least 85% pure at 210, 220 and 240 nm and the structure is confirmed by MS or 1H NMR spectroscopy. All 210 tricyclic pyrroles were analyzed by reverse-phase HPLC with UV and MS detection using a Thermo Finnigan Surveyor LC and LCQ Advantage MS system before purification by HPLC (Table 1 and Supporting Information).28 One hundred and twelve library members met these requirements. Twenty-four compounds were analyzed by 1H NMR since the molecular mass was not observed. Seventy-four library members showed the correct molecular mass, but the purity was lower than 85% for at least one of the three wavelengths, so these were purified on an automated HPLC system (Gilson). After purification they were reanalyzed by LC-MS and sixty-six compounds passed the purity criteria (Figure 4). Altogether 178 library members were added to our compound collection, with an average purity of 94% at 210, 220, and 240 nm. Thirty-two reactions did not lead to the desired pyrroles or gave purities below 85% even after purification by HPLC (Table 1). It has to be assumed that the differences in yields and purities are due to the fact that the applied protocol could only be optimized for a few representative amines but not for every single reaction. 2-(2-Aminoethylamino)ethanol (10{10}) and 1-(2-aminoethyl)piperazine (10{11}) were the only amines that gave completely failed reactions. Both bear an additional secondary amine functionality and it was assumed that this led to unselective reactions.
Figure 4.
Purity of the 178 library members before purification by HPLC (light grey) and after purification by HPLC (dark grey); if the purity was lower than 50% or no molecular mass was detected by LC-MS analysis the reaction was classified as “failed”.
All library members 17{1-3,1-2,1-41} were analyzed by LC-MS, 34 library members (20%) were analyzed by 1H NMR, and 10 library members (5%) were fully characterized by 1H, 13C, 19F NMR, IR, MS, HRMS and mp. These compounds are all currently stored as DMSO solution (20 mg/mL) at – 78 °C.
Stability studies29 are part of the physicochemical profiling of a compound library and were performed on selected library members before synthesizing the final library. To test the stability of the tricyclic pyrrole-2-carboxamides in DMSO, 17{1,2,14} was dissolved in DMSO-d6 and stored at room temperature in a sealed NMR-tube. 4-Phthalic acid ethyl ester was used as internal standard and 1H NMR monitoring indicated no decomposition over a period of 60 days. During this time the NMR tube was sealed with a rubber septum and kept on the laboratory bench top without specific care being taken to exclude air or light. In a second experiment, 17{3,2,16}, dissolved in dry DMSO (20 mg/mL) and sealed with a silica septum, was submitted to 30 freezing (-78 °C) and thawing (r.t.) cycles (one cycle/day) and the purity was checked by HPLC-MS with biphenyl as internal standard, again no decomposition was observed.
Computational Analysis
3-D structures of all library members 17{1-3,1-2,1-41} were built and minimized using the MM2 force field in MacroModel 8.6. The physicochemical profiling of the 178 library members was analyzed computationally using QikProp 2.1.30 (Table 2). Most of the molecular descriptors like molecular weight, molecular volume, the hydrophilic component of the solvent accessible surface area (FISA), number of rotatable bonds, number of hydrogen bond donors and acceptors, number of primary metabolites and Caco and MDCK permeability were within the range of 95% of current drugs. In contrast, several pyrroles 17{1-3,1-2,1-41} had logP and logS values outside this range, they are more lipophilic than most drugs. Another concern was the serum protein binding (log Khsa). The pyrroles were predicted to bind very well to human serum albumin and this could reduce the biological activity.
Table 2.
Computational Analysis of Molecular Descriptors for the Pyrroles 17{1-3,1-2,1-41} using QikProp 2.1.30
| Parameter | Range for 95% of all Drugs | Average ± Standard Deviation for 179 Pyrroles | Number of Compounds out of Range |
|---|---|---|---|
| molecular weight (g/mol) | 130 to 725 | 620 ± 57 | 5 |
| molecular volume (Å3)a | 500 to 2000 | 1800 ± 100 | 3 |
| FISA (Å2)b | 7 to 330 | 64 ± 33 | 0 |
| number of rotatable bondsc | 0 to 15 | 8.3 ± 1.5 | 0 |
| number of hydrogen bond donorsd | 0 to 6 | 0.5 ± 0.7 | 0 |
| number of hydrogen bond acceptorse | 2 to 20 | 9.6 ± 1.3 | 0 |
| logP (octanol/water) | -2 to 6 | 6.6 ± 1.3 | 119 |
| logS (aqueous solubility) | -6 to 0.5 | -7.4 ± 1.5 | 149 |
| log Khsa (serum protein binding) | -1.5 to 1.2 | 1.2 ± 0.5 | 94 |
| Caco permeability (nm/sec) | <25 poor, >500 great | 3200 ± 2100 | 0 |
| MDCK permeability (nm/sec) | <25 poor, >500 great | 3400 ± 3300 | 0 |
| number of primary metabolites | 1 to 8 | 5.0 ± 1.3 | 6 |
total solvent accessible volume, using a probe with a 1.4 Å radius.
hydrophilic component of the solvent accessible surface area (SASA), using a probe with a 1.4 Å radius (SASA on N, O and attached H).
non-trivial (not CX3), non-hindered (not alkene, amide, small ring).
estimated number of hydrogen bonds that would be donated by the solute from water molecules in an aqueous solution.
estimated number of hydrogen bonds that would be accepted by the solute from water molecules in an aqueous solution.
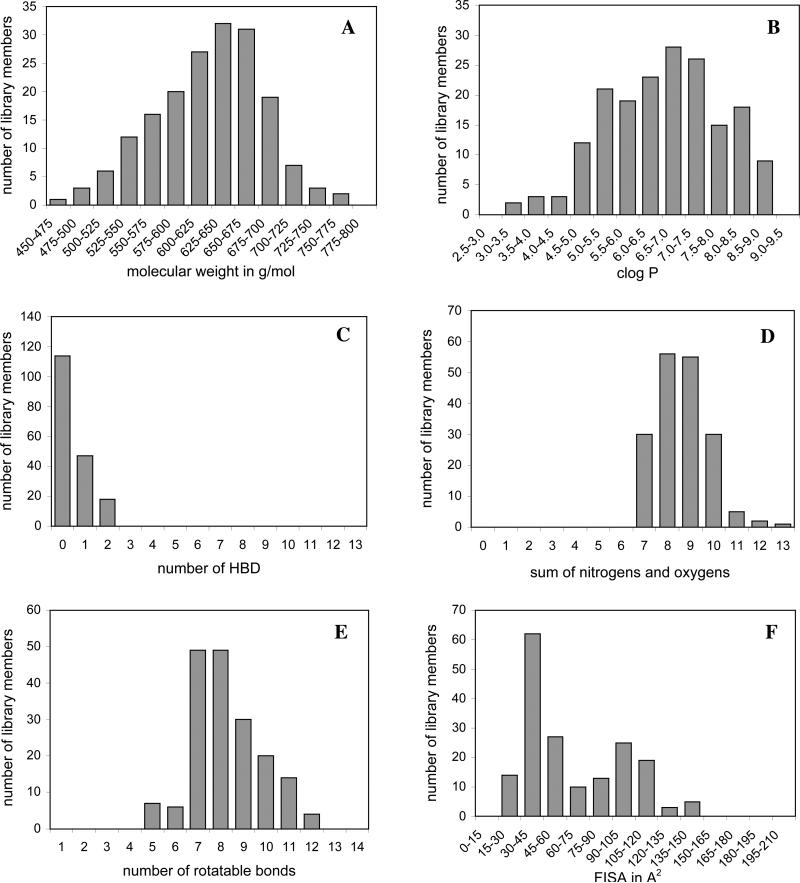
An important factor for diversity in a discovery library and especially for a library of tool-like compounds is the distribution of those physicochemical parameters. In Figure 5 the distribution for the classical “Rule of Five” parameters, the number of rotatable bonds and FISA is shown. Molecular weight and logP show a very broad distribution what is essential for a diverse library. The average molecular weight is 620 g/mol, with a range from 472 to 763 g/mol. The logP distribution is even broader with a minimum of 3.3 and a maximum of 8.9, the average being 6.6. The average number of hydrogen bond acceptors is 9.6 (range from 8 to 13.25) and the average number of hydrogen bond donors was found to be 0.5 (range from 0 to 2). The number of rotatable bonds ranges from 5 to 12, the average being 8.3. The mean of the FISA is 64.3 2 Å2, ranging from 15.4 to 153.1 Å2.
Figure 5.
Distribution for the Rule of Five parameters molecular weight (A), log of the octanol/water partition coefficient (logP) (B), number of hydrogen donor bonds (HBD) (C), sum of nitrogen and oxygen atoms (D) and for the number of rotatable bonds (E) and the hydrophilic component of the solvent accessible surface area (FISA) (F); calculated for the 178 pyrroles 17{1-3,1-2,1-41} using QikProp 2.1.30
Conclusions
We have developed a method for a mid-size solution phase library of tricyclic pyrrole-2-carboxamides. The application of parallel synthesis methods and automated purification techniques allowed us to prepare this library in only 2 months. One hundred and seventy-eight library members were obtained with an average purity of 94% and an average amount of 14 mg. They were added to our compound collection and are tested currently against a variety of different targets. Preliminary biological data show promising potential, and detailed studies are on-going.
Supplementary Material
Acknowledgment
We gratefully acknowledge the financial support provided by NIGMS (P50-GM067082) and thank Dr. Sukhdev Manku from the research group of Prof. Dennis P. Curran and Dr. Donald Probst from the research group of Prof. Kay M. Brummond (both University of Pittsburgh) for providing us with compounds 14{1} and 14{2}, respectively.
Experimental Section
General
All air and moisture sensitive reactions were performed under an argon atmosphere. THF was dried by distillation from Na/benzophenone and 1,4-dioxane was used as obtained (quality “extra dry” from Acros). Other solvents or reagents were used without further purification. NMR spectra were recorded in CDCl3 (298 K) at either 300.1 MHz (1H), 75.5 MHz (13C) or 282.3 MHz (19F) using a Bruker Avance 300 with XWIN-NMR software. Chemical Shifts (δ) are reported in parts per million (ppm). Tetramethylsilane (1H), chlorotrifluoromethane (19F) or chloroform-d (13C) were used as internal standards. Data are reported as follows: chemical shift, multiplicity (s = singlet, d = dublet, t = triplet, q = quartet, quin = quintet, m = multiplet, bs = broad singlet, app. = apparent), integration and coupling constants. IR spectra were obtained on a Nicolet AVATAR 360 FTIR E.S.P. Spectrometer. Mass spectra were obtained on a Waters QTof API US. Melting points were obtained using a heating rate of 2 °C/min on a MelTemp melting point apparatus with digital temperature reading and are reported uncorrected. All microwave assisted reactions were performed in an Emrys Optimizer microwave reactor (Biotage) using Emrys Process Vials 0.5-2 mL. The synthesis of the Pauson Khand products 14{1-3,1-2} was described previously.23
General procedure for the Stetter Reaction
The conversions of the cyclopentenones 14 to the 1,4-diketones 15 were performed in parallel in a Radley's Carousel Reaction Station on a 1 g scale under an argon atmosphere. To a solution of cyclopentenone 14{1}, 14{2} or 14{3} (1.00 g, 1 equivalent) in dry 1,4-dioxane (15 mL) at room temperature was added glyoxaldehyde 9{1} or 9{2} (5 equivalents) and triethylamine (3 equivalents), followed by 3-benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chloride (0.3 equivalents). The reaction mixtures were heated to 70 °C for 20 min, then cooled to room temperature, poured into water (50 mL) and extracted with diethyl ether (3 × 50 mL). The organic layers were combined, washed with brine (2 × 50 mL), dried over magnesium sulfate and concentrated in vacuo. The residues were purified by parallel chromatography using an ISCO Optix 10 chromatography system (40 g RediSep cartridges, 40 mL/min, gradient hexanes/ethyl acetate 60/40 to 20/80). Two chromatographies were necessary in order to remove impurities that otherwise poisoned the palladium catalyst in the following step. The products 15{1-3,1-2} were obtained as colorless crystals in 75 - 93% yield.
rac-(1R,6S,6aS)-2-Benzoyl-6-(2,3-dioxo-3-pyrrolidin-1-ylpropyl)-1-(4-fluorobenzyl)-5-oxo-1,2,3,5,6,6a-hexahydrocyclopenta[c]pyrrole-1-carboxylic acid methyl ester (15{2,2})
mp 92-94 °C; IR 2953, 2883, 1716, 1638 cm-1; 1H NMR (CDCl3) δ 7.53-7.43 (m, 5 H), 7.19 (dd, 2 H, J = 8.5, 5.5 Hz), 7.01 (app. t, 2 H, J = 8.6 Hz), 5.93 (s, 1 H), 4.28 (d, 1 H, J = 15.9 Hz), 4.14 (d, 1 H, J = 14.1 Hz), 4.09 (d, 1 H, J = 15.3 Hz), 3.81 (s, 3 H), 3.63 (app. t, 2 H, J = 6.4 Hz), 3.57 (app. t, 2 H, J = 6.4 Hz), 3.50 (dd, 1 H, J = 18.9, 5.0 Hz), 3.40 (m, 1 H), 3.31 (dd, 1 H, J = 18.8, 5.3 Hz), 3.21 (d, 1 H, J = 14.5 Hz), 2.45 (app. q, 1 H, J = 4.7 Hz), 2.02-1.88 (m, 4 H); 13C (CDCl3) δ 207.1, 198.2, 173.8, 171.4, 171.1, 162.2, 162.1 (d, JCF = 244.7 Hz), 135.6, 132.6 (d, JCF = 7.8 Hz), 131.3 (d, JCF = 3.3 Hz), 131.1, 128.7, 127.1, 123.5, 115.3 (d, JCF = 21.0 Hz), 71.6, 53.3, 52.5, 51.7, 47.2, 46.3, 46.0, 37.7, 36.4, 26.2, 23.6; 19F NMR (CDCl3) δ –115.7 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 555 (M+ + Na, 100), 533 (M+ + H, 17), 473 (17); HRMS (ESI) m/z calculated for C30H29N2O6FNa 555.1907, found 555.1918.
15{1,1} and 15{1,2} were previously described,2315{2,1}, 15{3,1} and 15{3,2} are described in the Supporting Information.
General procedure for the Hydrogenation
Each of the diketones 15{1-3,1-2} (1 equivalent) was dissolved in THF (10 mL) and Pd/C (10 wt%, 0.15 equivalents) was added at room temperature. The flask was immersed into a warm water bath (40 °C) and the atmosphere was replaced three times with hydrogen. After vigorous stirring for 16 h at room temperature the suspension was filtered over 4 cm Celite 545 and washed with ethyl acetate (100 mL) and dichloromethane (100 mL). Evaporation of the solvents gave the products 16{1-3,1-2} as colorless crystals in 88 to 99% yield. The 1,4-diketones 16{1-3,1-2} were used in the Paal Knorr reaction without further purification.
rac-(1R,3aS,6S,6aR)-2-Benzoyl-6-(2,3-dioxo-3-pyrrolidin-1-ylpropyl)-1-(4-fluorobenzyl)-5-oxo-octahydrocyclopenta[c]pyrrole-1-carboxylic acid methyl ester (16{2,2})
mp 196-198 °C; IR 2979, 2959, 1738, 1635 cm-1; 1H NMR (CDCl3) δ 7.48-7.43 (m, 5 H), 7.23 (dd, 2 H, J = 8.7, 5.5 Hz), 7.04 (app. t., 2 H, J = 8.7 Hz), 4.15 (d, 1 H, J = 14.0 Hz), 3.86 (s, 3 H), 3.65 (app. t, 2 H, J = 6.6 Hz), 3.53 (app. t, 2 H, J = 6.7 Hz), 3.33 (dd, 1 H, J = 10.9, 8.3 Hz), 3.27 (d, 1 H, J = 14.4 Hz), 3.24 (dd, 1 H, J = 10.8, 9.2 Hz), 3.12-3.08 (m, 2 H), 2.90-2.85 (m, 2 H), 2.26 (dd, 1 H, J = 19.3, 8.5 Hz), 2.08 (dd, 1 H, J = 19.2, 3.3 Hz), 2.01-1.85 (m, 5 H); 13C (CDCl3) δ 216.2, 198.0, 171.2, 169.5, 161.9 (d, JCF = 244.4 Hz), 161.8, 136.6, 132.3 (d, JCF = 3.5 Hz), 131.9 (d, JCF = 7.5 Hz), 129.9, 128.4, 126.1, 115.3 (d, JCF = 21.0 Hz), 72.6, 55.3, 52.6, 52.0, 47.1, 46.3, 45.4, 39.1, 38.6, 38.4, 36.1, 26.2, 23.4; 19F NMR (CDCl3) δ –115.8 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 557 (M+ + Na, 100), 535 (M+ + H, 10), 503 (13), 475 (18); HRMS (ESI) m/z calculated for C30H31N2O6FNa 557.2064, found 557.2089.
16{1,1} and 16{1,2} were previously described,2316{2,1}, 16{3,1} and 16{3,2} are described in the Supporting Information.
General procedure for the Paal Knorr reaction
The Paal Knorr reaction was performed in batches of 10 reactions. All reaction mixtures were prepared at the same time and then the microwave tubes were queued up for irradiation in an automated single mode microwave reactor.
To a solution of 16{1-3,1-2} (30 mg, 1 equivalent) in ethanol (600 μL) in an Emrys Process Vial (0.5-2 mL) was added glacial acetic acid (60 μL) and amine 10{1-41} (3 equivalents). In the case of hydrochloride ammonium salts triethylamine (1.5 equivalents for monohydrochloride salts, 3.0 equivalents for dihydrochloride salts) was added. The microwave tube was sealed without using an inert gas atmosphere and irradiated in the microwave at 80 °C for 15 min (in the case of hydrochloride ammonium salts for 30 min). An initial power of 150 W was applied. The reactions with 10{1}, 10{2} and 10{3} were performed at 60 °C for 30 min. Five equivalents of amine had to be used in the case of 10{1} and 10{3} and 10 equivalents in the case of 10{2}. 10{36} required a temperature of 100 °C and irradiation for 30 min. The tube was opened and completion of the reaction was confirmed by TLC analysis, then all volatile components were removed using a Radley's GreenHouse Blowdown Evaporator.31 Up to 2 batches of 10 reactions each were dried within 10 min using a stream of argon. The crude reaction mixtures were loaded in dichloromethane (500 μL) onto SiO2 cartridges and purified using an ISCO Optix 10 chromatography system (4g RediSep cartridge, 18 mL/min). Ten compounds were purified in parallel using customized hexanes/ethyl acetate gradients based on their Rf by TLC. All volatile components were removed in vacuo and the compounds were dried in a Christ alpha RVC evaporator for 12 h at 40 °C (<0.1 mbar) prior to analysis.
All compounds were analyzed by LC-MS (APCI positive and negative mode, Alltech Prevail C-18, 100 × 4.6 mm, 1 mL/min, 50% MeCN, 35% H2O/MeOH 95/5, 15% MeOH), 20% of the library members were analyzed by 1H NMR and 5% were completely characterized (mp, 1H NMR, 13C NMR, 19F NMR, IR, MS, HRMS). All compounds that were not more than 85% pure at 210, 220 and 240 nm were further purified by serial automated HPLC (Varian Dynamix C-18, 250 × 21.4 mm, 15 mL/min, 70-95% MeCN/MeOH 3/1, 30-5% H2O).
rac-(3bR,4R,6aS)-5-Benzoyl-1-cyclopropylmethyl-4-(4-fluorobenzyl)-2-(pyrrolidine-1-carbonyl)-3b,4,5,6,6a,7-hexahydro-1H-1,5-diazacyclopenta[a]pentalene-4-carboxylic acid methyl ester (17{2,2,3})
mp 102-104 °C; IR 2949, 2874, 1737, 1640, 1605, 1509, 1446, 1400 cm-1; 1H NMR (CDCl3) δ 7.48-7.38 (m, 5 H), 7.31 (dd, 2 H, J = 8.5, 5.6 Hz), 7.04 (app. t, 2 H, J = 8.6 Hz), 6.18 (s, 1 H), 4.20 (d, 1 H, J = 13.8 Hz), 4.06 (dd, 1 H, J =14.0, 7.0 Hz), 3.86-3.77 (m, 2 H), 3.72-3.55 (m, 4 H), 3.62 (s, 3 H), 3.38 (d, 1 H, J = 13.4 Hz), 3.37 (dd, 1 H, J = 10.3, 8.1 Hz), 3.21 (dd, 1 H, J = 10.4, 8.6 Hz), 2.59 (dd, 1 H, J = 15.1, 7.0 Hz), 2.34 (app. quin, 1 H, J = 7.5 Hz), 2.26 (d, 1 H, J =15.3 Hz), 2.00-1.88 (m, 4 H), 1.13-1.00 (m, 1 H), 0.48-0.37 (m, 2 H), 0.24-0.16 (m, 2 H); 13C (CDCl3) δ 172.2, 169.9, 162.2, 162.0 (d, JCF = 243.8 Hz), 140.2, 137.4, 132.7 (d, JCF = 3.2 Hz), 132.3 (d, JCF = 7.7 Hz), 129.7, 129.5, 128.4, 126.2, 122.1, 115.0 (d, JCF = 20.9 Hz), 108.4, 72.9, 56.3, 52.1 (2 resonances overlap), 50.9, 50 (br), 46 (br), 45.5, 38.5, 28.9, 27 (br), 24 (br), 12.6, 3.8, 3.5; 19F NMR (CDCl3) δ –116.5 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 592 (M+ + Na, 100), 570 (M+ + H, 25), 510 (32); HRMS (ESI) m/z calculated for C34H36N3O4FNa 592.2588, found 592.2589.
rac-(3bR,4R,6aS)-5-Benzoyl-4-(4-fluorobenzyl)-1-(2-methoxyethyl)-2-(pyrrolidine-1-carbonyl)-3b,4,5,6,6a,7-hexahydro-1H-1,5-diazacyclopenta[a]pentalene-4-carboxylic acid methyl ester (17{2,2,5})
mp 95-97 °C; IR 2949, 2873, 1734, 1640, 1604, 1509, 1446, 1399 cm-1; 1H NMR (CDCl3) δ 7.43-7.38 (m, 5 H), 7.31 (dd, 2 H, J = 8.4, 5.5 Hz), 7.04 (app. t, 2 H, J = 8.6 Hz), 6.22 (s, 1 H), 4.33 (ddd, 1 H, J = 13.9, 5.6, 4.2 Hz), 4.20 (d, 1 H, J = 13.8 Hz), 4.08 (ddd, 1 H, J = 14.0, 6.0, 4.4 Hz), 3.83 (d, 1 H, J = 7.4 Hz), 3.68-3.55 (m, 9 H), 3.40-3.34 (m, 2 H), 3.24-3.20 (m, 4 H), 2.60 (dd, 1 H, J = 15.3, 7.0 Hz), 2.38-2.29 (m, 1 H), 2.27 (d, 1 H, J = 15.9 Hz), 1.98-1.89 (m, 4 H); 13C (CDCl3) δ 172.0, 169.8, 161.8, 161.8 (d, JCF = 243.9 Hz), 141.6, 137.3, 132.6 (d, JCF = 3.1 Hz), 132.2 (d, JCF = 7.7 Hz), 129.5, 129.1, 128.3, 126.0, 121.8, 114.9 (d, JCF = 20.9 Hz), 108.8, 72.9, 72.8, 58.6, 56.2, 52.1, 51.9, 49.0 (br), 46.9, 46 (br), 45.2, 38.4, 28.6, 27 (br), 24 (br); 19F NMR (CDCl3) δ –116.5 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 596 (M+ + Na, 100), 574 (M+ + H, 72), 514 (30); HRMS (ESI) m/z calculated for C33H36N3O5FNa 595.2537, found 596.2544.
rac-(3bR,4R,6aS)-5-Benzoyl-4-(4-fluorobenzyl)-1-(2-hydroxyethyl)-2-(pyrrolidine-1-carbonyl)-3b,4,5,6,6a,7-hexahydro-1H-1,5-diazacyclopenta[a]pentalene-4-carboxylic acid methyl ester (17{2,2,6})
mp 111-113 °C; IR 2950, 2873, 1735, 1636, 1602, 1509, 1446, 1400 cm-1; 1H NMR (CDCl3) δ 7.45-7.40 (m, 5 H), 7.31 (dd, 2 H, J = 8.6, 5.5 Hz), 7.04 (app. t, 2 H, J = 8.7 Hz), 6.25 (s, 1 H), 4.22-4.08 (m, 3 H), 3.84-3.76 (m, 3 H), 3.73-3.53 (m, 4 H), 3.63 (s, 3 H), 3.37 (d, 1 H, J = 13.7 Hz), 3.36 (dd, 1 H, J = 10.6, 8.3 Hz), 3.25 (dd, 1 H, J = 10.6, 8.2 Hz), 2.58 (dd, 1 H, J = 15.2, 7.1 Hz), 2.41 (app. quind, 1 H, J = 7.6, 2.2 Hz), 2.26 (dd, 1 H, J = 15.3, 1.9 Hz), 2.00-1.90 (m, 4H); 13C (CDCl3) δ 172.1, 169.8, 162.0, 162.0 (d, JCF = 243.9 Hz), 141.2, 137.3, 132.6 (d, JCF = 3.2 Hz), 132.3 (d, JCF = 7.7 Hz), 129.7, 129.7, 128.4, 126.2, 123.0, 115.1 (d, JCF = 20.9 Hz), 109.1, 72.9, 62.5, 56.2, 52.1, 52.0, 49.8 (br), 48.8, 46.7 (br), 45.6, 38.4, 28.5, 26.5 (br), 24.0 (br); 19F NMR (CDCl3) δ –116.3 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 582 (M+ + Na, 70), 560 (M+ + H, 100), 528 (20), 500 (42); HRMS (ESI) m/z calculated for C32H35N3O5F 560.2561, found 560.2545.
rac-(3bR,4R,6aS)-5-Benzoyl-4-(4-fluorobenzyl)-1-methoxycarbonylmethyl-2-(pyrrolidine-1-carbonyl)-3b,4,5,6,6a,7-hexahydro-1H-1,5-diazacyclopenta[a]pentalene-4-carboxylic acid methyl ester (17{2,2,8})
mp 114-116 °C; IR 2952, 2874, 1739, 1640, 1604, 1509, 1447, 1402 cm-1; 1H NMR (CDCl3) δ 7.43-7.38 (m, 5 H), 7.30 (dd, 2 H, J = 8.7, 5.5 Hz), 7.04 (dd, 2 H, J = 8.7 Hz), 6.30 (s, 1 H), 4.98 (d, 1 H, J = 17.3 Hz), 4.67 (d, 1 H, J = 17.3 Hz), 4.20 (d, 1 H, J = 13.8 Hz), 3.87 (d, 1 H, J = 7.6 Hz), 3.74-3.54 (m, 4 H), 3.72 (s, 3 H), 3.64 (s, 3 H), 3.39 (dd, 1 H, J = 10.5, 8.3 Hz), 3.38 (d, 1 H, J = 13.8 Hz), 3.23 (dd, 1 H, J = 10.6, 8.5 Hz), 2.55 (ddd, 1 H, J = 15.2, 6.6, 0.8 Hz), 2.39 (app. quind, 1 H, J = 7.4, 2.0 Hz), 2.20 (dd, 1 H, J = 15.3, 1.1 Hz), 1.98-1.88 (m, 4 H) ; 13C (CDCl3) δ 172.1, 170.0, 169.4, 162.0 (d, JCF = 243.9 Hz), 161.1, 141.4, 137.5, 132.7 (d, JCF = 3.1 Hz), 132.3 (d, JCF = 7.7 Hz), 129.7, 129.6, 128.4, 126.2, 122.7, 115.0 (d, JCF = 20.8 Hz), 109.4, 72.9, 56.3, 52.2, 52.2, 52.1, 49 (br), 48.4, 47 (br), 45.4, 38.6, 28.3, 27 (br), 24 (br); 19F NMR (CDCl3) δ –116.4 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 610 (M+ + Na, 100), 588 (M+ + H, 35), 528 (12); HRMS (ESI) m/z calculated for C33H34N3O6FNa 610.2329, found 610.2344.
rac-(3bR,4R,6aS)-5-Benzoyl-1-benzyl-4-(4-fluorobenzyl)-2-(pyrrolidine-1-carbonyl)-3b,4,5,6,6a,7-hexahydro-1H-1,5-diazacyclopenta[a]pentalene-4-carboxylic acid methyl ester (17{2,2,14})
mp 101-103 °C; IR 2949, 2873, 1737, 1640, 1604, 1509, 1446, 1398 cm-1; 1H NMR (CDCl3) δ 7.43-7.38 (m, 5 H), 7.30 (dd, 2 H, J = 8.7, 5.5 Hz), 7.26-7.19 (m, 3 H), 7.02 (app. t, 2 H, J = 8.7 Hz), 6.99-6.96 (m, 2 H), 6.21 (s, 1 H), 5.43 (d, 1 H, J = 15.4 Hz), 5.14 (d, 1 H, J = 15.4 Hz), 4.20 (d, 1 H, J = 13.8 Hz), 3.83 (d, 1 H, J = 7.2 Hz), 3.61 (s, 3 H), 3.58-3.46 (m, 4 H), 3.38 (d, 1 H, J = 13.9 Hz), 3.35 (dd, 1 H, J = 10.6, 8.1 Hz), 3.17 (dd, 1 H, J = 10.4, 8.6 Hz), 2.46-2.28 (m, 2 H), 2.13 (d, 1 H, J = 14.9 Hz), 1.93-1.77 (m, 4 H); 13C (CDCl3) δ 172.1, 170.0, 162.1, 162.0 (d, JCF = 244.0 Hz), 140.8, 138.6, 137.5, 132.8 (d, JCF = 2.9 Hz), 132.3 (d, JCF = 7.7 Hz), 130.1, 129.7, 128.4, 128.4, 127.2, 126.9, 126.2, 122.6, 115.0 (d, JCF = 21.0 Hz), 108.5, 73.0, 56.4, 52.1, 52.0, 49.9, 49 (br), 46 (br), 45.6, 38.6, 28.7, 26 (br), 24 (br); 19F NMR (CDCl3) δ –116.5 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 628 (M+ + Na, 100), 606 (M+ + H, 84), 546 (30); HRMS (ESI) m/z calculated for C37H37N3O4F 606.2768, found 606.2788.
rac-(3bR,4R,6aS)-5-Benzoyl-4-(4-fluorobenzyl)-1-(4-methanesulfonylbenzyl)-2-(pyrrolidine-1-carbonyl)-3b,4,5,6,6a,7-hexahydro-1H-1,5-diazacyclopenta[a]pentalene-4-carboxylic acid methyl ester (17{2,2,22})
mp 133-135 °C; IR 2951, 2874, 1737, 1636, 1602, 1509, 1446, 1405, 1149 cm-1; 1H NMR (CDCl3) δ 7.83 (d, 2 H, J = 8.5 Hz), 7.43-7.36 (m, 5 H), 7.30 (dd, 2 H, J = 8.7, 5.5 Hz), 7.14 (d, 2 H, J = 8.5 Hz), 7.03 (app. t, 2 H, J = 8.7 Hz), 6.32 (s, 1 H), 5.53 (d, 1 H, J = 16.2 Hz), 5.29 (d, 1 H, J = 16.2 Hz), 4.20 (d, 1 H, J = 13.8 Hz), 3.86 (d, 1 H, J = 7.4 Hz), 3.69-3.50 (m, 4 H), 3.66 (s, 3 H), 3.39 (d, 1 H, J = 17.4 Hz), 3.38 (dd, 1 H, J = 10.6, 7.7 Hz), 3.15 (dd, 1 H, J = 10.9, 8.0 Hz), 3.01 (s, 3 H), 2.45-2.35 (m, 2 H), 2.16-2.08 (m, 1 H), 1.95-1.87 (m, 4 H); 13C (CDCl3) δ 172.0, 170.0, 162.0 (d, JCF = 243.8 Hz), 161.4, 145.1, 141.1, 139.2, 137.2, 132.5 (d, JCF = 3.0 Hz), 132.3 (d, JCF = 7.7 Hz), 129.8, 129.8, 128.5, 127.6, 127.2, 126.2, 123.2, 115.1 (d, JCF = 20.8 Hz), 109.3, 72.9, 56.3, 52.1, 52.0, 49.7, 49 (br), 46 (br), 45.6, 44.4, 38.5, 28.4, 27 (br), 24 (br); 19F NMR (CDCl3) δ –116.3 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 706 (M+ + Na, 100), 684 (M+ + H, 23), 413 (23); HRMS (ESI) m/z calculated for C38H38N3O6FSNa 706.2363, found 706.2391.
rac-(4R,6aR,3bR)-5-Benzoyl-4-(4-fluorobenzyl)-1-pyridin-2-ylmethyl-2-(pyrrolidine-1-carbonyl)-3b,4,5,6,6a,7-hexahydro-1H-1,5-diazacyclopenta[a]pentalene-4-carboxylic acid methyl ester (17{2,2,23})
mp 105-107 °C; IR 2950, 2872, 1374, 1640, 1604, 1509, 1446, 1399 cm-1; 1H NMR (CDCl3) δ 8.46 (ddd, 1 H, J = 4.9, 1.7, 0.9 Hz), 7.57 (td, 1 H, J = 7.7, 1.8 Hz), 7.43-7.38 (m, 5 H), 7.29 (dd, 2 H, J = 8.7, 5.4 Hz), 7.11 (ddd, 1 H, J = 7.5, 4.9, 1.1 Hz), 7.01 (app. t, 2 H, J = 8.7 Hz), 6.96 (dt, 1 H, J = 7.8, 1.2 Hz), 6.27 (s, 1 H), 5.56 (d, 1 H, J = 15.8 Hz), 5.25 (d, 1 H, J = 15.8 Hz), 4.20 (d, 1 H, J = 13.8 Hz), 3.85 (d, 1 H, J = 7.3 Hz), 3.69-3.51 (m, 4 H), 3.63 (s, 3 H), 3.38 (d, 1 H, J = 14.0 Hz), 3.35 (dd, 1 H, J = 10.6, 8.2 Hz), 3.17 (d, 1 H, J = 10.5, 8.6 Hz), 2.44 (dd, 1 H, J = 15.4, 7.1 Hz), 2.31 (app. quind, 1 H, J = 8.2, 1.2 Hz), 2.16 (d, 1 H, J = 15.6 Hz), 1.95-1.85 (m, 4 H); 13C (CDCl3) δ 172.1, 169.9, 161.9 (d, JCF = 243.9 Hz), 161.8, 158.1, 148.9, 141.3, 137.3, 136.7, 132.6 (d, JCF = 3.1 Hz), 132.2 (d, JCF = 7.7 Hz), 129.9, 129.7, 128.4, 126.2, 122.7, 122.1, 121.3, 115.0 (d, JCF = 20.9 Hz), 108.8, 72.9, 56.3, 52.1, 52.1, 52.0, 49.7 (br), 46.2 (br), 45.5, 38.5, 28.5, 26.5 (br), 24.1 (br); 19F NMR (CDCl3) δ –116.4 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 629 (M+ + Na, 100), 607 (M+ + H, 52), 536 (10); HRMS (ESI) m/z calculated for C36H35O4N4FNa 629.2540, found 629.2555.
rac-(4R,6aR,3bR)-5-Benzoyl-4-(4-fluorobenzyl)-1-(4-hydroxy-2-hydroxymethyl-5-methyl-pyridin-3-ylmethyl)-2-(pyrrolidine-1-carbonyl)-3b,4,5,6,6a,7-hexahydro-1H-1,5-diazacyclopenta[a]pentalene-4-carboxylic acid methyl ester (17{2,2,24})
mp 177-179 °C; IR 2952, 2875, 1737, 1634, 1602, 1576, 1552, 1508, 1475, 1447, 1399 cm-1; 1H NMR (CDCl3) δ 11.27 (bs, 1 H), 7.68 (s, 1 H), 7.42-7.33 (m, 5 H), 7.24 (dd, 2 H, J = 8.6, 5.4 Hz), 7.00 (app. t, 2 H, J = 8.7 Hz), 6.30 (s, 1 H), 5.39 (d, 1 H, J = 15.8 Hz), 5.27 (d, 1 H, J = 15.7 Hz), 4.59 (s, 2 H), 4.11 (d, 1 H, J = 13.9 Hz), 3.82-3.70 (m, 3 H), 3.70-3.60 (m, 2 H), 3.56 (s, 3 H), 3.30 (d, 1 H, J = 14.1 Hz), 3.25 (dd, 1 H, J = 10.7, 8.3 Hz), 3.08 (dd, 1 H, J = 10.7, 7.4 Hz), 2.39 (app. quind, 1 H, J = 7.7, 3.2 Hz), 2.38 (s, 3 H), 2.16 (dd, 1 H, J = 15.6, 7.6 Hz), 2.05-1.95 (m, 4 H), 1.93 (dd, 1 H, J = 15.9, 1.8 Hz); 13C (CDCl3) δ 171.9, 170.0, 162.0 (d, JCF = 244.1 Hz), 162.9, 151.5, 149.8, 143.0, 138.5, 137.0, 132.4, 132,4 (d, JCF = 3.4 Hz), 132.2 (d, JCF = 7.7 Hz), 129.9, 129.6, 128.4, 127.5, 126.3, 124.3, 115.1 (d, JCF = 21.3 Hz), 109.5 (br), 72.8, 61.1, 56.1, 51.9, 51.2, 49.9, 46.6, 46.0, 42.6, 38.3, 29.3, 26.4, 24.1, 19.2; 19F NMR (CDCl3) δ –116.3 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 689 (M+ + Na, 30), 667 (M+ + H, 100); HRMS (ESI) m/z calculated for C38H40N4O6F 667.2932, found 667.2940.
rac-(4R,6aR,3bR)-5-Benzoyl-4-(4-fluorobenzyl)-2-(pyrrolidine-1-carbonyl)-1-(6-trifluoromethyl-pyridin-3-ylmethyl)-3b,4,5,6,6a,7-hexahydro-1H-1,5-diazacyclopenta[a]pentalene-4-carboxylic acid methyl ester (17{2,2,25})
mp 110-112 °C; IR 2951, 2875, 1737, 1636, 1604, 1509, 1447, 1402, 1336 cm-1; 1H NMR (CDCl3) δ 8.32 (s, 1 H), 7.59 (s, 2 H), 7.43-7.38 (m, 5 H), 7.30 (dd, 2 H, J = 8.1, 5.6 Hz), 7.04 (app. t, 2 H, J = 8.5 Hz), 6.32 (s, 1 H), 5.55 (d, 1 H, J = 16.0 Hz), 5.29 (d, 1 H, J = 15.9 Hz), 4.20 (d, 1 H, J = 13.8 Hz), 3.85 (d, 1 H, J = 7.1 Hz), 3.64 (s, 3 H), 3.70-3.49 (m, 4 H), 3.42-3.38 (m, 1 H), 3.38 (d, 1 H, J = 13.9 Hz), 3.17 (dd, 1 H, J = 10.4, 8.3 Hz), 2.49-2.37 (m, 2 H), 2.17 (d, 1 H, J = 13.7 Hz), 1.95-1.85 (m, 4 H); 13C (CDCl3) δ 172.0, 170.0, 162.1 (d, JCF = 244.0 Hz), 161.4, 148.4, 147.2 (q, JCF = 34.3 Hz), 141.0, 137.5, 137.3, 136.0, 132.6 (d, JCF = 2.9 Hz), 132.3 (d, JCF = 7.5 Hz), 129.8, 129.8, 128.5, 126.3, 123.6, 121.5 (q, JCF = 272.3 Hz), 119.7 (q, JCF = 2.2 Hz), 115.1 (d, JCF = 20.6 Hz), 109.7, 72.9, 56.3, 52.1, 52.0, 49 (br), 47.6, 47 (br), 45.7, 38.6, 28.6, 26 (br), 24 (br); 19F NMR (CDCl3) δ –68.3 (s, 3 F), –116.2 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 697 (M+ + Na, 100), 675 (M+ + H, 80); HRMS (ESI) m/z calculated for C37H34N4O4F4Na 697.2438, found 697.2428.
rac-(4R,6aR,3bR)-5-Benzoyl-4-(4-fluorobenzyl)-1-(5-methylfuran-2-ylmethyl)-2-(pyrrolidine-1-carbonyl)-3b,4,5,6,6a,7-hexahydro-1H-1,5-diazacyclopenta[a]pentalene-4-carboxylic acid methyl ester (17{2,2,26})
mp 96-98 °C; IR 2950, 2873, 1736, 1639, 1604, 1509, 1446, 1399 cm-1; 1H NMR (CDCl3) δ 7.44-7.39 (m, 5 H), 7.30 (dd, 2 H, J = 8.6, 5.5 Hz), 7.03 (app. t, 2 H, J = 8.7 Hz), 6.17 (s, 1 H), 6.01 (d, 1 H, J = 3.0 Hz), 5.81 (dd, 1 H, J = 3.0, 1.0 Hz), 5.35 (d, 1 H, J = 15.5 Hz), 5.44 (d, 1 H, J = 15.5 Hz), 4.19 (d, 1 H, J = 13.8 Hz), 3.80 (d, 1 H, J = 7.4 Hz), 3.64-3.56 (m, 4 H), 3.57 (s, 3 H), 3.37 (d, 1 H, J = 13.9 Hz), 3.35 (dd, 1 H, J = 10.3, 8.4 Hz), 3.20 (dd, 1 H, J = 10.3, 8.5 Hz), 2.55 (dd, 1 H, J = 15.8, 7.2 Hz), 2.32 (app. quind, 1 H, J = 7.2, 1.7 Hz), 2.25 (d, 1 H, J = 16.0 Hz), 2.17 (s, 3 H), 1.97-1.85 (m, 4 H); 13C (CDCl3) δ 172.1, 169.8, 162.1, 161.9 (d, JCF = 243.8 Hz), 151.6, 149.5, 140.6, 137.5, 132.7 (d, JCF = 3.1 Hz), 132.3 (d, JCF = 7.7 Hz), 129.6, 129.5, 128.4, 126.1, 122.3, 115.0 (d, JCF = 20.9 Hz), 108.7, 108.6, 106.2, 72.9, 56.3, 52.1, 52.0, 50 (br), 46 (br), 45.5, 42.9, 38.5, 28.5, 27 (br), 24 (br), 13.5; 19F NMR (CDCl3) δ –116.5 (tt, 1 F, J = 8.5, 5.6 Hz); MS (ESI) m/z (rel. intensity) 632 (M+ + Na, 100), 610 (M+ + H, 72), 530 (14), 516 (30); HRMS (ESI) m/z calculated for C36H37N3O5F 610.2717, found 610.2714.
Footnotes
Supporting Information Available
Spectroscopic data for compounds 15, 16 and selected 17, HPLC-MS analyses, LC-MS chromatograms, and Qik Prop analyses for all library members 17 are given in the Supporting Information. This material is available free of charge via internet at http://pubs.acs.org.
References
- 1.For further information on the UPCMLD see http://ccc.chem.pitt.edu/UPCMLD.
- 2.a Lipinski C. Drug Discov. Today Technologies. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]; b Lipinski C, Hopkins A. Nature. 2004;432:855–861. doi: 10.1038/nature03193. [DOI] [PubMed] [Google Scholar]
- 3.a Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv. Drug Delivery Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]; b Waterbeend H. van de, Gifford E. Nat. Rev. Drug Discovery. 2003;2:192–204. doi: 10.1038/nrd1032. [DOI] [PubMed] [Google Scholar]; c Lajiness MS, Vieth M, Erickson J. Curr. Opin. Drug Discovery Dev. 2004;7:470–477. [PubMed] [Google Scholar]
- 4.Di L, Kerns EH. Curr. Opin. Chem. Biol. 2003;7:402–408. doi: 10.1016/s1367-5931(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 5.a Rishton GM. Drug Discov. Today. 1997;2:382–384. doi: 10.1016/s1359644602025722. [DOI] [PubMed] [Google Scholar]; b Rishton GM. Drug Discov. Today. 2002;8:86–96. doi: 10.1016/s1359644602025722. [DOI] [PubMed] [Google Scholar]
- 6.The term tool-like has been invoked to set the goals of this research apart from the goal of developing a drug where drug-like properties are the primary concern. For example, tool-like compounds may not obey the rule-of-five or other criteria for oral bioavailability but they are important for target validation in drug discovery.
- 7.a Schreiber SL. Science. 2000;287:1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]; b Burke MD, Schreiber SL. Angew Chem. Int. Ed. 2004;43:46–58. doi: 10.1002/anie.200300626. [DOI] [PubMed] [Google Scholar]; c Young SS, Ge N. Curr. Opin. Drug Descovery Dev. 2004;7:318–324. [PubMed] [Google Scholar]
- 8.a Brummond KM, Mitasev B. Org. Lett. 2004;6:2245–2248. doi: 10.1021/ol0492391. [DOI] [PubMed] [Google Scholar]; b Brummond KM, Chen H, Mitasev B, Casarez AD. Org. Lett. 2004;6:2161–2163. doi: 10.1021/ol049390a. [DOI] [PubMed] [Google Scholar]; c Brummond KM, Chen D. Organic Lett. 2005;7:3473–3475. doi: 10.1021/ol051115g. [DOI] [PubMed] [Google Scholar]
- 9.a Trautwein AW, Suessmuth RD, Jung G. Bioorg. Med. Chem. Lett. 1998:2381–2384. doi: 10.1016/s0960-894x(98)00430-2. [DOI] [PubMed] [Google Scholar]; b Attanasi OA, De Crescentini L, Filippone P, Mantellini F, Tietze LF. Tetrahedron. 2001;57:5855–5863. [Google Scholar]; c Marcotte FA, Rombouts FJR, Lubell WD. J. Org. Chem. 2003;68:6984–6987. doi: 10.1021/jo034684f. [DOI] [PubMed] [Google Scholar]
- 10.For a review see: Hoffmann H, Lindel T. Synthesis. 2003;12:1753–1783.
- 11.D'Ambrosio M, Guerriero A, Ripamonti M, Debitus C, Waikedre J, Pietra F. Helv. Chim. Acta. 1996;79:727–735. [Google Scholar]
- 12.Cafieri F, Carnuccio R, Fattorusso E, Taglialatela-Scafati O, Vallefuoco T. Bioorg. Med. Chem. Lett. 1997;7:2283–2288. [Google Scholar]
- 13.a Meijer L, Thunnissen AMWH, White AW, Garnier M, Nikolic M, Tsai LH, Walter J, Cleverley KE, Salinas PC, Wu YZ, Biernat J, Mandelkow EM, Kim SH, Pettit GR. Chem. Biol. 2000;7:51–63. doi: 10.1016/s1074-5521(00)00063-6. [DOI] [PubMed] [Google Scholar]; b Wan Y, Hur W, Cho CY, Liu Y, Adrian F,J, Lozach O, Bach S, Mayer T, Fabbro D, Meijer L, Gray NS. Chem. Biol. 2004;11:247–259. doi: 10.1016/j.chembiol.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Keifer PA, Schwartz RE, Koker MES, Hughes RG, Jr., Rittschof D, Rinehart KL. J. Org. Chem. 1991;56:2965–2975. [Google Scholar]
- 15.Palermo JA, Rodriguez Brasco MF, Seldes AM. Tetrahedron. 1996;52:2727–2734. [Google Scholar]
- 16.a Bailly C, Chaires JB. Bioconjugate Chem. 1998;9:513–538. doi: 10.1021/bc980008m. [DOI] [PubMed] [Google Scholar]; b Dyatkina NB, Roberts CD, Keicher JD, Dai Y, Nadherny JP, Zhang W, Schmitz U, Kongpachith A, Fung K, Novikov AA, Lou L, Velligan M, Khorlin AA, Chen MS. J. Med. Chem. 2002;45:805–817. doi: 10.1021/jm010375a. [DOI] [PubMed] [Google Scholar]; c Bürli RW, Ge Y, White S, Baird EE, Touami SM, Taylor M, Kaizerman JA, Moser HE. Bioorg. Med. Chem. Lett. 2002;12:2591–2594. doi: 10.1016/s0960-894x(02)00515-2. [DOI] [PubMed] [Google Scholar]
- 17.Lombardi P, Crisanti A. Pharmacol. Ther. 1997;76:125–133. doi: 10.1016/s0163-7258(97)00095-8. [DOI] [PubMed] [Google Scholar]
- 18.Pinna GA, Pirisi MA, Chelucci G, Mussinu JM, Murineddu G, Loriga G, D'Aquila PS, Serra G. Bioorg. Med. Chem. 2002;10:2485–2496. doi: 10.1016/s0968-0896(02)00118-9. [DOI] [PubMed] [Google Scholar]
- 19.Silvestri R, La Regina G, De Martino G, Artico M, Befani O, Palumbo M, Agostinelli E, Turini P. J. Med. Chem. 2003;46:917–920. doi: 10.1021/jm0256124. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, Cui J, Liang C, Zhou Y, Nematalla A, Wang X, Chen H, Tang C, Wei J. Bioorg. Med. Chem. Lett. 2002;12:2153–2157. doi: 10.1016/s0960-894x(02)00364-5. [DOI] [PubMed] [Google Scholar]
- 21.Shoda M, Harada T, Kogami Y, Tsujita R, Akashi H, Kouji H, Stahura FL, Xue L, Bajorath J. J. Med. Chem. 2004;47:4286–4290. doi: 10.1021/jm040103i. [DOI] [PubMed] [Google Scholar]
- 22.Manley JM, Kalman MJ, Conway BG, Ball CC, Havens JL, Vaidyanathan R. J. Org. Chem. 2003;68:6447–6450. doi: 10.1021/jo034304q. [DOI] [PubMed] [Google Scholar]
- 23.Brummond KM, Curran DP, Mitasev B, Fischer S. J. Org. Chem. 2005;70:1745–1753. doi: 10.1021/jo0481607. [DOI] [PubMed] [Google Scholar]
- 24.a Rose S. Drug Discovery Today. 2002;7:133–138. doi: 10.1016/s1359-6446(01)02093-1. [DOI] [PubMed] [Google Scholar]; b Jamois E,A. Curr. Opin Chem. Biol. 2003;7:326–330. doi: 10.1016/s1367-5931(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 25.The commercial suppliers were Acros, Aldrich, Lancaster and TCI.
- 26.Castelhano AL, Horne S, Taylor GJ, Billedeau R, Krantz A. Tetrahedron. 1988;44:5451–5466. [Google Scholar]
- 27.Werner S, Iyer PS. Org. Lett. 2005;9:1405–1408. [Google Scholar]
- 28.Popa-Burke IG, Issakova O, Arroway JD, Bernasconi P, Chen M, Coudurier L, Galasinski S, Jadhav AP, Janzen WP, Lagasca D, Liu D, Lewis RS, Mohney RP, Sepetov N, Sparkman DA, Hodge CN. Anal. Chem. 2004;76:7278–7287. doi: 10.1021/ac0491859. [DOI] [PubMed] [Google Scholar]
- 29.a Kibbey CE, Poole SK, Robinson B, Jackson JD, Durham D. J. Pharm Sci. 2001;90:1164–1175. doi: 10.1002/jps.1070. [DOI] [PubMed] [Google Scholar]; b Kerns E. J. Pharm. Sci. 2001;90:1838–1858. doi: 10.1002/jps.1134. [DOI] [PubMed] [Google Scholar]
- 30.a QikProp 2.1. Schroedinger Inc.; New York: 2003. [Google Scholar]; b Duffy EM, Jorgensen WL. J. Am. Chem. Soc. 2000;122:2878–2888. [Google Scholar]; c Jorgensen WL, Duffy EM. Adv. Drug Delivery Rev. 2002;54:355–366. doi: 10.1016/s0169-409x(02)00008-x. [DOI] [PubMed] [Google Scholar]; d Jorgensen WL. Science. 2004;303:1813–1818. doi: 10.1126/science.1096361. [DOI] [PubMed] [Google Scholar]
- 31.The Radleys GreenHouse rack was temporarily modified by removing the top plate of the rack to fit the Emrys Process Vials 0.5-2 mL.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.