This study makes an important contribution by being one of the first to define the burden of clinically silent myocardial infarctions in the CKD community.
Keywords: chronic kidney disease, coronary artery disease, mortality
Abstract
Background
Unrecognized myocardial infarctions (UMIs) are common in the general population but have not been well studied in patients with chronic kidney disease (CKD). The purpose of this study was to determine the prevalence and prognosis for mortality of UMI among adults with CKD.
Methods
The current study included 18 864 participants in the population-based REasons for Geographic And Racial Differences in Stroke (REGARDS) study who completed a baseline examination including a 12-lead electrocardiogram (ECG). UMI was defined as the presence of myocardial infarction (MI) by Minnesota ECG classification in the absence of self-reported or recognized MI (RMI). Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation and albuminuria using albumin-to-creatinine ratio from a spot urine sample. All-cause mortality was assessed over a median 4 years of follow-up.
Results
The prevalence of UMI was 4, 6, 6 and 13% among participants with eGFR levels of ≥60, 45–59.9, 30–44.9 and <30 mL/min/1.73m2, respectively, and 4, 5, 7 and 10% among participants with albuminuria levels of <10, 10–29.9, 30–299.9 and ≥300 mg/g, respectively. Compared to those with no MI, the multivariable adjusted hazard ratio for all-cause mortality associated with UMI and RMI was 1.65 [95% confidence interval (CI): 1.09–2.49] and 1.65 (95% CI: 1.20–2.26), respectively, among individuals with an eGFR <60 mL/min/1.73m2 and 1.49 (95% CI: 1.03–2.16) and 1.88 (95% CI: 1.40–2.52) among individuals with albuminuria ≥30 mg/g.
Conclusion
UMIs are common among individuals with an eGFR <60 mL/min/1.73m2 and albuminuria and associated with an increased mortality risk.
Introduction
Since unrecognized myocardial infarctions (UMIs) were first described in 1912 [1], several epidemiological studies have assessed its prevalence in different populations [2–7]. An estimated 20–40% of all myocardial infarctions (MIs) are unrecognized [2–4, 6, 7]. Data from prior studies suggest that individuals with UMIs have a similar mortality risk as their peers with recognized MIs (RMIs) [2].
Chronic kidney disease (CKD), defined by a reduced estimated glomerular filtration rate (eGFR) or high levels of albuminuria, is common [8] and associated with a high burden of cardiovascular disease (CVD) [9]. Among participants in the National Health and Nutrition Examination Survey 1999–2004, individuals with CKD were 3.7 times more likely to report a history of MI than their counterparts without CKD [10]. While the presence of RMI is common and associated with an increased mortality risk among individuals with CKD [11, 12], few data on the prevalence and implications of UMI among patients with CKD have been reported.
The primary goal of the present analysis was to assess the prevalence of UMI by level of eGFR and albuminuria. We also evaluated the association of UMI with mortality among individuals with reduced eGFR and albuminuria. Finally, we identified factors associated with UMI among individuals with reduced eGFR or albuminuria. To do so, we analyzed data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study, a population-based prospective cohort study of African Americans and whites ≥45 years of age [13].
Materials and methods
Population
REGARDS study participants were recruited between January 2003 and October 2007. The study was designed to oversample African Americans and provide approximately equal representation of men and women. By design, 56% (goal 50%) of the sample was recruited from the eight southern US states commonly referred to as the ‘stroke buckle’ (coastal NC, SC and GA) and the ‘stroke belt’ (remainder of NC, SC and GA as well as AL, MS, TN, AR and LA) with the remaining 44% of the sample recruited from the other 40 contiguous US states and the District of Columbia. The study protocol was reviewed and approved by the Institutional Review Boards of all participating centers. All participants provided verbal informed consent at the time of their telephone interview and in writing at the time of their in-home study visit prior to participation in REGARDS.
Data collection
Trained interviewers conducted computer-assisted telephone interviews to collect information about demographics, socioeconomic status, health insurance, having a regular source of health care, cigarette smoking and prior diagnoses of medical conditions including diabetes mellitus, hypertension, MI, stroke and prior cardiovascular interventions. Following the telephone interview, trained staff conducted an in-home visit during which a physical examination and resting electrocardiogram (ECG) were performed, prescription and over the counter pill bottles were reviewed and blood and urine samples were collected using standardized methods. Height and weight were measured during the study visit and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Systolic and diastolic blood pressure (BP) were estimated based on the average of two measurements. Hypertension was defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg or use of anti-hypertensive medications. Diabetes was defined as serum glucose ≥126 mg/dL for participants who had fasted ≥8 h prior to their study visit, a non-fasting serum glucose ≥200 mg/dL or self-report of a prior diagnosis of diabetes with current use of insulin or oral hypoglycemic medications. Dyslipidemia was defined as total serum cholesterol ≥240 mg/dL, a low-density lipoprotein cholesterol ≥160 mg/dL, high-density lipoprotein cholesterol <40 mg/dL or current lipid-lowering medication use. Cognitive function was assessed using a six-item test of global cognitive function that includes recall and temporal orientation items. Individuals incorrectly answering three or more questions were categorized as having cognitive impairment [14].
Using isotope dilution mass spectrometry traceable serum creatinine, eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [15]. eGFR was divided into four levels: ≥60.0, 45.0–59.9, 30.0–44.9 and <30.0 mL/min/1.73m2. Urinary albumin and creatinine concentrations were measured using spot samples collected during the baseline examination. Albuminuria was calculated as the urinary albumin-to-creatinine ratio (ACR) and divided into four strata <10.0, 10.0–29.9, 30.0–299.9 and ≥300.0 mg/g [16].
Definition of UMI and RMI
The 12-lead ECGs performed during the in-home examination were read and coded at The Epidemiological Cardiology Research Center (EPICARE) at Wake Forest University. The research staff reading the ECG were blinded to the other REGARDS study data collected. Electrocardiographic diagnosis of prior MI was defined using the Minnesota code (MC) as presence of a major Q wave abnormality (MC 1.1.× or 1.2.×) or minor Q/QS waves with major ST-T abnormalities (MC 1.3.× with 4.1.×, or 4.2, or 5.1, or 5.2) [17]. Participants were considered to have an RMI if they responded affirmatively to the question: ‘Has a doctor or other health professional ever told you that you had a MI or heart attack?’ UMIs were defined by the presence of ECG criteria for an MI in the absence of an RMI.
Assessment of mortality outcomes
Mortality, subsequent to the REGARDS study in-home examination and through 31 March 2010, was assessed through contact with proxies provided by the participant upon recruitment or during follow-up. If a proxy reported a participant had died, an interview was conducted with the next of kin listed on study forms. The REGARDS study confirmed dates of death through the social security death index, death certificates or the National Death Index. Follow-up time was recorded as the number of days from the REGARDS study baseline in-home examination, when the presence of UMI was determined, to a participant's confirmed date of death and last follow-up date or 31 March 2010 for non-deceased participants.
Statistical analysis
Characteristics of REGARDS study participants were calculated by MI status: no MI, UMI and RMI. The statistical significance of differences in participant characteristics with UMI versus no MI and UMI versus RMI, separately, was determined using chi-square tests for categorical variables and t-tests for continuous variables. Among all participants, the prevalence of UMI was calculated by eGFR and albuminuria levels. Next, for participants with eGFR <60 mL/min/1.73m2 and those with albuminuria (≥30 mg/g), cumulative mortality curves by MI status (none, UMI and RMI) were generated using the Kaplan–Meier method. Additionally, the multivariable adjusted hazard ratios (HRs) for all-cause mortality associated with UMI and RMI, versus no MI, were calculated for participants with an eGFR <60 mL/min/1.73m2 and for participants with an ACR ≥30 mg/g. Multivariable adjustment included age, race, sex, region of residence, education, income, current smoking status, health insurance, having a regular source of health care, cognitive impairment, marital status, systolic BP, use of anti-hypertensive medication, dyslipidemia and diabetes. Finally, we pooled together REGARDS participants with an eGFR <60 mL/min/1.73m2 or ACR ≥30 mg/g and determined factors associated with UMI. Specifically, age, race, sex and multivariable-adjusted odds ratios for UMI were calculated using logistic regression models. For the multivariable model, we included variables associated with UMI (P < 0.10) in the age-, race- and sex-adjusted analyses. The proportional hazards assumption was evaluated by Schoenfeld residuals and was not violated. Analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC).
Results
Participants' characteristics
Overall, 30 239 individuals were enrolled in the REGARDS study. The current analysis was restricted to participants who were recruited after April 2004, when 12-lead ECG assessments were initiated (n = 21 152). After excluding participants receiving dialysis (n = 76), missing serum creatinine (n = 910) or urinary albumin or creatinine measurements (n = 850), with poor quality ECG recordings (n = 99), and missing follow-up data for all-cause mortality (n = 353), the current analysis included 18 864 participants. Among individuals with 12-lead ECGs, those included in the current analyses were similar to those excluded with respect to age (64.0 versus 64.1 years, respectively; P = 0.617). A higher percentage of women versus men (11.0 versus 9.5%, respectively) and blacks versus whites (13.5 versus 8.3%, respectively) were excluded due to missing data. However, a similar percentage of individuals without MI (10.3%) and with UMI and RMI (11.7 and 11.8%, respectively) were excluded from the current analyses.
Of the 18 864 REGARDS study participants included in this analysis, 852 (4.5%) had UMI and 1365 had RMI (7.2%). UMI represented 38% of all MI. Characteristics of participants with no MI, UMI and RMI are presented in Table 1. Compared to those with no MI, participants with UMI were older and more likely to be men. They were less likely to be married but more likely to have less than a high school education, cognitive impairment, an annual household income of <$20 000, be a current smoker and have diabetes, hypertension and dyslipidemia. Additionally, those with UMI had higher BMI and systolic BP than their counterparts without MI. When compared to those with RMI, participants with UMI were younger, less likely to be men, married, have less than a high school education, diabetes, hypertension or dyslipidemia. Also, those with UMI versus RMI had higher BMI and lower diastolic BP levels.
Table 1.
Characteristics of REGARDS study participants by MI statusa
| MI status |
P-value |
||||
|---|---|---|---|---|---|
| None (n = 16647) | UMI (n = 852) | RMI (n = 1365) | UMI versus none | UMI versus RMI | |
| Age, years | 63.5 (9.6) | 67.2 (9.7) | 68.1 (9.2) | <0.001 | 0.032 |
| Men, % | 35.9 | 41.3 | 57.1 | 0.001 | <0.001 |
| African American, % | 39.8 | 40.0 | 36.2 | 0.898 | 0.070 |
| Married, % | 59.8 | 53.1 | 60.4 | <0.001 | <0.001 |
| Less than HSb education, % | 10.4 | 14.1 | 18.5 | <0.001 | 0.008 |
| Health insurance, % | 92.7 | 94.1 | 95.8 | 0.120 | 0.074 |
| Cognitive impairment, % | 6.1 | 8.9 | 10.7 | 0.001 | 0.176 |
| Household income <$20 000, % | 17.8 | 26.9 | 27.4 | <0.001 | 0.689 |
| Regular source for health care, % | 83.0 | 85.3 | 83.9 | 0.107 | 0.400 |
| Current smoking, % | 13.8 | 16.7 | 18.3 | 0.010 | 0.323 |
| BMI, kg/m2 | 29.4 (6.3) | 29.6 (6.0) | 28.9 (6.6) | 0.047 | 0.017 |
| Diabetes, % | 18.5 | 24.5 | 32.2 | <0.001 | <0.001 |
| Systolic BP, mmHg | 126.0 (16.2) | 129.2 (17.6) | 129.7 (17.3) | <0.001 | 0.479 |
| Diastolic BP, mmHg | 76.2 (9.6) | 75.5 (10.1) | 76.7 (9.9) | 0.108 | 0.007 |
| Anti-hypertensive medication use, % | 48.7 | 62.1 | 70.7 | <0.001 | <0.001 |
| Dyslipidemia, % | 54.8 | 61.2 | 79.7 | <0.001 | <0.001 |
aNumbers in table are mean (standard error) or row percent.
bHS, high school.
eGFR, albuminuria and UMI
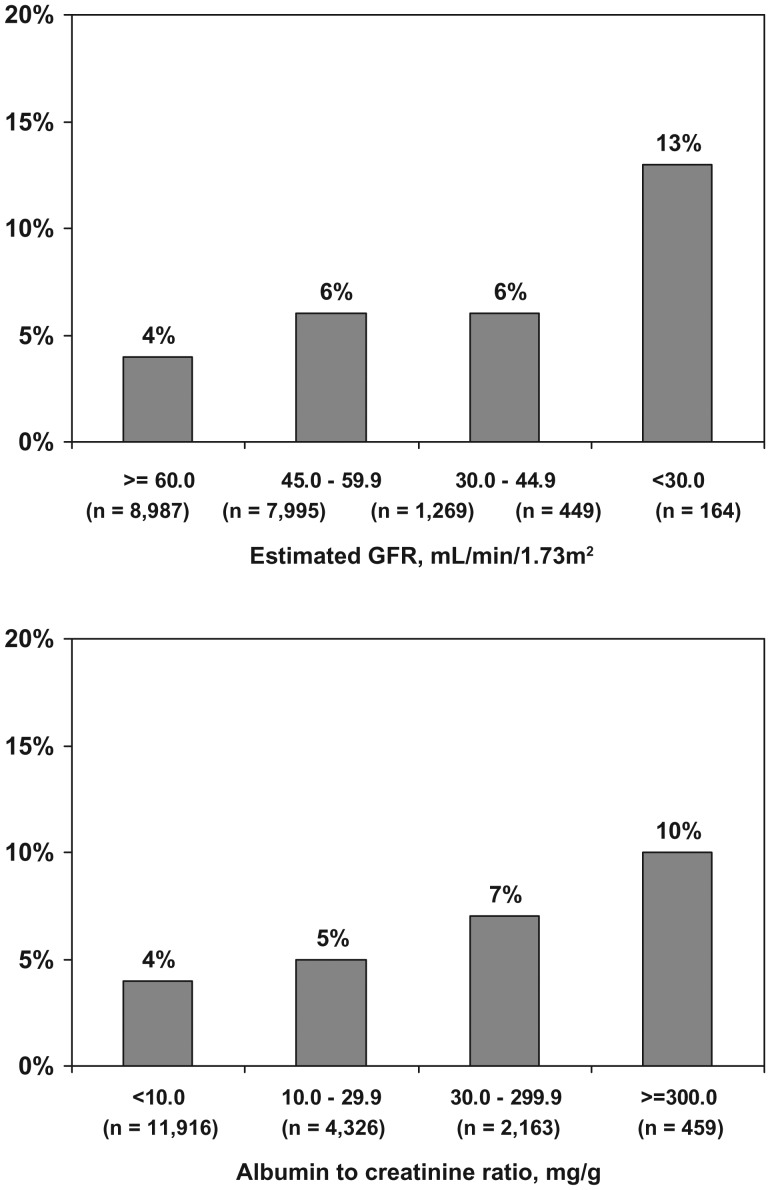
The prevalence of UMI was higher at lower eGFR levels (Figure 1, top panel). Also, UMI was more prevalent among REGARDS study participants with higher albuminuria levels (Figure 1, bottom panel). The prevalence of UMI was 4.3% of participants with eGFR ≥60 mL/min/1.73m2 and ACR <30 mg/g, 5.9% among participants with eGFR <60 mL/min/1.73m2 and ACR <30 mg/g, 6.0% among participants with eGFR ≥60 mL/min/1.73m2 and ACR ≥30 mg/g and 13.4% for those with both eGFR <60 mL/min/1.73m2 and ACR ≥30 mg/g.
Fig. 1.
Prevalence of UMI by level of eGFR (top panel) and ACR (bottom panel).
UMI/RMI and mortality
Over a median of 4 years of follow-up, there were 236 deaths among individuals with eGFR <60 mL/min/1.73m2 and 189 deaths among individuals with ACR ≥30 mg/g. Cumulative mortality curves for participants with no MI, UMI and RMI are presented in the top panel of Figure 2 for those with eGFR <60 mL/min/1.73m2 and in the bottom panel for those with ACR ≥30 mg/g. After multivariable adjustment for age, race, sex, region of residence, education, income, current smoking status, health insurance, having a regular source of health care, cognitive impairment, marital status, systolic BP, use of anti-hypertensive medication, dyslipidemia and diabetes, the HRs for all-cause mortality associated with UMI and RMI, compared to no MI, were 1.65 [95% confidence interval (CI): 1.09–2.49] and 1.65 (95% CI: 1.20–2.26), respectively, in individuals with eGFR <60 mL/min/1.73m2 and 1.49 (95% CI: 1.03–2.16) and 1.88 (1.40–2.52), respectively, for individuals with ACR ≥30 mg/g.
Fig. 2.
Kaplan–Meier cumulative mortality curves associated with MI (none, UMI and RMI) for REGARDS participants with an eGFR <60 mL/min/1.73m2 (top panel) and ACR ≥30 mg/g (bottom panel).
Risk factors associated with UMI among CKD participants without RMI
Among REGARDS study participants with an eGFR <60 mL/min/1.73m2 or ACR ≥30 mg/g, older age and male sex were associated with higher odds ratios for UMI (Table 2). Additionally, after adjustment for age, race, and sex, a household income <$20 000, current smoking status and hypertension were associated with an increased odds ratio for having a UMI. Also, the age-, race- and sex-adjusted odds ratio for UMI was increased for individuals with eGFR <30 mL/min/1.73m2 and ACR levels ≥30 mg/g. These associations remained present after further multivariable adjustment (Table 2).
Table 2.
Odds ratios for UMI among REGARDS participants with eGFR <60 mL/min/1.73m2 or ACR ≥30 mg/ga
| Age, race, sex-adjusted odds ratios (95% CI) | P-value | Multivariable-adjusted odds ratios (95% CI) | P-value | |
|---|---|---|---|---|
| Age, 10 years | 1.42 (1.24–1.63) | <0.001 | 1.53 (1.32–1.77) | <0.001 |
| Men | 1.53 (1.18–1.98) | 0.001 | 1.47 (1.12–1.91) | 0.005 |
| African American | 1.17 (0.90–1.52) | 0.242 | 0.90 (0.68–1.19) | 0.451 |
| Married | 0.80 (0.60–1.05) | 0.105 | b | |
| Less than HS education | 0.79 (0.56–1.10) | 0.166 | b | |
| Health insurance | 0.95 (0.52–1.73) | 0.865 | b | |
| Cognitive impairment | 1.44 (0.98–2.09) | 0.060 | 1.33 (0.91–1.95) | 0.141 |
| Household income <$20 000 | 1.63 (1.22–2.17) | <0.001 | 1.45 (1.08–1.94) | 0.013 |
| Regular source for health care | 1.03 (0.71–1.50) | 0.879 | b | |
| Current smoking | 2.00 (1.43–2.80) | <0.001 | 1.96 (1.39–2.76) | <0.001 |
| Obesity | 1.09 (0.83–1.44) | 0.523 | b | |
| Diabetes | 1.21 (0.92–1.59) | 0.176 | b | |
| Hypertension | 1.66 (1.17–2.36) | 0.005 | 1.67 (1.17–2.39) | 0.005 |
| Dyslipidemia | 1.11 (0.84–1.46) | 0.471 | b | |
| eGFR category | ||||
| ≥60 | 1 (ref) | 1 (ref) | ||
| 45–59 | 0.75 (0.55–1.03) | 0.071 | 0.90 (0.60–1.35) | 0.617 |
| 30–44 | 0.76 (0.49–1.19) | 0.232 | 0.79 (0.48–1.31) | 0.366 |
| <30 | 2.14 (1.30–3.52) | 0.003 | 1.85 (1.07–3.19) | 0.0272 |
| ACR, mg/g | ||||
| <10 | 1 (ref) | 1 (ref) | ||
| 10–29 | 1.70 (1.03–2.82) | 0.038 | 1.62 (0.98–2.69) | 0.062 |
| 30–299 | 1.75 (1.18–2.60) | 0.006 | 1.55 (0.96–2.52) | 0.074 |
| ≥300 | 3.25 (2.01–5.23) | <0.001 | 2.42 (1.42–4.15) | 0.001 |
aParticipants with RMI were excluded from this analysis.
bNot included in the model because variable was not associated with UMI in the age, race, gender adjusted model (P > 0.1).
Discussion
Despite a high burden of CVD among individuals with CKD [9], the prevalence and clinical implications of UMI have not been well documented in this population. While prior studies have investigated risk factors for UMI, they did not report results for individuals with CKD. In the current study, UMI was more common at lower eGFR and higher albuminuria levels. Among participants with reduced eGFR or high ACR, UMI was associated with a risk for mortality similar to that imparted by a diagnosis of RMI. Some but not all traditional cardiovascular risk factors were associated with UMI among participants with eGFR <60 mL/min/1.73m2 or ACR ≥30 mg/g.
Previous population-based studies have reported that UMIs represent ∼20–40% of all MIs [2–4, 6, 7]. This large variability in the prevalence of UMI may be due, in part, to the different populations studied but also to the different criteria used to define UMI. The Cardiovascular Heart Study [2] for example enrolled an elderly population as opposed to the Reykjavik Study that included Icelandic adults aged 35–75 years [3]. Some studies relied on patient recollection of an MI [2], whereas others involved medical chart review for the identification of prior MI [3, 4]. In the REGARDS study, a UMI was defined by the presence of well-validated ECG criteria [17] and the absence of a history of MI provided by the patient during standardized interviews. This definition has been used in previously published work [2]. In this context, UMI represented 38% of all MIs in the REGARDS study. Importantly, the prevalence of UMIs was higher at progressively lower eGFR and higher albuminuria levels. Future studies that capture data on incident UMI by level of renal function may provide insight into the reasons why a substantial proportion of MIs are unrecognized in those with CKD.
Several prior studies have compared the mortality risk for individuals with RMI and UMI. While the balance of evidence suggests that the mortality risk is similar for individuals with UMI and RMI, some studies have reported that RMIs are associated with a worse prognosis [18]. For example, the 5-year cumulative mortality in the Israeli Heart Attack Trial was higher for individuals with RMI versus UMI (3.6 versus 1.7%) [18]. In the REGARDS study population with CKD (whether based on reduced eGFR or increased albuminuria), the mortality rate was significantly higher in both those with UMI and those with RMI versus their counterparts with no MI. Our findings are in accordance with the Cardiovascular Heart Study where, over 6 years of follow-up, 25.4% of those with RMI and 21.4% of those with UMI died versus 12.0% among those with no MI (P = 0.24 comparing RMI and UMI and P < 0.01 comparing RMI and UMI, separately, to no MI) [2]. The poor prognosis that UMIs portend in terms of mortality provides further support for more aggressive screening in this high-risk population.
The high absolute rate of UMI among individuals with CKD should alert physicians that the CKD population is at increased risk for coronary events that could be silent or atypical as seen for example in the diabetic population. From a practical standpoint, ECGs are widely available and relatively cheap diagnostic tests. Medical internists are trained to interpret ECGs and therefore may be able to capture CKD patients with UMI that might otherwise miss detection. Such information on UMI can be used to guide treatment and perhaps justify instituting more aggressive strategies to modify cardiovascular risk factors. The feasibility and effectiveness of implementing widespread ECG screening for UMI in patients with CKD are currently unclear and require further study. However, given that secondary preventive strategies have been associated with improved survival in individuals with RMI, the potential benefits of increased detection of those with UMI through more vigilant screening could potentially mean improved cardiovascular outcomes in this population.
Another important aspect of our study involved trying to identify factors associated with UMI in individuals with CKD defined as eGFR <60 mL/min/1.73m2 or ACR ≥30 mg/g. In this context, some traditional cardiovascular risk factors like age, male gender, smoking and hypertension were associated with a higher odds ratio of having a UMI. Interestingly, however, other risk factors like diabetes and dyslipidemia were not, raising the possibility that UMI may have a different mechanistic etiology than RMI. Non-traditional risk factors that could not be explored in the current study, include vascular calcifications for example, and may play a role in the occurrence of UMI. The prevalence of UMI increased across lower eGFR and higher albuminuria strata even when adjusted for known risk factors. A possible explanation would be that neurosensory pathways that may affect the perception of angina pain may be disturbed in more advanced CKD. Future studies should investigate these possible mechanisms.
The current study has several potential limitations. The categorization of UMI versus RMI was based on self-report and may be subject to recall bias. The prevalence of cognitive impairment was similar among individuals with UMI and RMI making this an unlikely reason for recall bias. RMI determination was not confirmed by chart review creating a potential ascertainment bias; however, prior studies have shown that self-reported MI is a reliable tool [19–21]. Our analysis included prevalent and not incident UMI. While it may be interesting to evaluate the incidence of UMI among individuals with CKD, the high prevalence of UMI in this population highlights the burden of unrecognized disease among individuals with CKD. Also, there are several reasons why the prevalence of UMI may even be higher than reported in the current study. By definition, non Q-wave MIs were not included in the diagnostic criteria for UMI. Additionally, previous studies have shown that ∼10–45% of Q-wave ECG changes resolve within 2 years of the initial event [22]. Data to assess whether these potential biases occurred at differential rates for individuals with and without CKD in the REGARDS study were not available. Finally, the current analysis only included a single ECG and laboratory values were only assessed at one time point.
Despite these limitations, the current analysis has many strengths. The REGARDS study included a very large number of participants from throughout the USA. African Americans were oversampled in the REGARDS study. This is particularly important because African Americans represent a substantial proportion of the CKD population, especially at the more advanced stages [23]. The REGARDS study data were collected through rigorous methods and UMI were identified using the MC ECG criteria, which have been validated in numerous studies, following a standardized protocol by trained readers [24]. There was a single centralized ECG reading center (EPICARE). EPICARE uses strict QT measures to monitor the reliability and reproducibility of the ECG reading done in the center. The EPICARE center has a long-standing history of reading ECG for multiple major national studies.
In conclusion, the current analysis suggests that the prevalence of UMI increases with lower eGFR and higher albuminuria. Furthermore, UMI and RMI are associated with a similar increased mortality risk in CKD. Studies are needed to determine whether increased detection of UMI in CKD patients can lead to early implementation of secondary preventive strategies that have been associated with improved outcomes in individuals with RMI.
Conflict of interest statement
None declared.
(See related article by Bansal. Clinically silent myocardial infarctions in the CKD community. Nephrol Dial Transplant 2012; 27: 3387–3391.)
Acknowledgements
Part of this data was presented in abstract format at the National Kidney Foundation Spring Clinical Meeting 2010.
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH) and Department of Health and Human Services. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Dr M.S. received support for this manuscript through NIH grant (R01 HL80477-01A1) and Dr E.B.L. received support for this manuscript through Agency for Healthcare Research and Quality (K12 HS019465).
Transparency declarations. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Amgen reviewed the manuscript but did not have any role in the design and conduct of the study, the collection, management, analysis and interpretation of the data or the preparation or approval of the manuscript.
References
- 1.Herrick JB. Clinical features of sudden obstruction of the coronary arteries. JAMA. 1983;250:1757–1765. [PubMed] [Google Scholar]
- 2.Sheifer SE, Gersh BJ, Yanez D, et al. Prevalence, predisposing factors, and prognosis of clinically unrecognized myocardial infarction in the elderly. J Am Coll Cardiol. 2000;35:119–126. doi: 10.1016/s0735-1097(99)00524-0. [DOI] [PubMed] [Google Scholar]
- 3.Sigurdsson E, Thorgeirsson G, Sigvaldason H, et al. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med. 1995;122:96–102. doi: 10.7326/0003-4819-122-2-199501150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Jonsdottir LS, Sigfusson N, Sigvaldason H, et al. Incidence and prevalence of recognised and unrecognised myocardial infarction in women. The Reykjavik Study. Eur Heart J. 1998;19:1011–1018. doi: 10.1053/euhj.1998.0980. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction. An update on the Framingham study. N Engl J Med. 1984;1:1144–1147. doi: 10.1056/NEJM198411013111802. [DOI] [PubMed] [Google Scholar]
- 6.Grimm RH, Tillinghast S, Daniels K, et al. Unrecognized myocardial infarction: experience in the Multiple Risk Factor Intervention Trial (MRFIT) Circulation. 1987;75(Suppl II):II6–II8. [PubMed] [Google Scholar]
- 7.Margolis JR, Kannel WB, Feinleib M, et al. Clinical features of unrecognized myocardial infarction—silent and symptomatic: eighteen year follow-up: the Framingham study. Am J Cardiol. 1973;32:1–7. doi: 10.1016/s0002-9149(73)80079-7. [DOI] [PubMed] [Google Scholar]
- 8.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 9.Astor BC, Hallan SI, Miller ER, 3rd, et al. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol. 2008;167:1226–1234. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 10.McCullough PA, Li S, Jurkovitz CT, et al. CKD and cardiovascular disease in screened high-risk volunteer and general populations: the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008;51:S38–S45. doi: 10.1053/j.ajkd.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Beattie JN, Soman SS, Sandberg KR, et al. Determinants of mortality after myocardial infarction in patients with advanced renal dysfunction. Am J Kidney Dis. 2001;37:1191–1200. doi: 10.1053/ajkd.2001.24522. [DOI] [PubMed] [Google Scholar]
- 12.Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339:799–805. doi: 10.1056/NEJM199809173391203. [DOI] [PubMed] [Google Scholar]
- 13.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 14.Callahan CM, Unverzagt FW, Hui SL, et al. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolton K, Coresh J, Culleton B, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2007;39:S1–S266. [PubMed] [Google Scholar]
- 17.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Bristol, UK: John Wright PSG; 1982. [Google Scholar]
- 18.Medalie JH, Goldbourt U. Unrecognized myocardial infarction: five-year incidence, mortality, and risk factors. Ann Intern Med. 1976;84:526–531. doi: 10.7326/0003-4819-84-5-526. [DOI] [PubMed] [Google Scholar]
- 19.Walker MK, Whincup PH, Shaper AG, et al. Validation of patient recall of doctor-diagnosed heart attack and stroke: a postal questionnaire and record review comparison. Am J Epidemiol. 1998;148:355–361. doi: 10.1093/oxfordjournals.aje.a009653. [DOI] [PubMed] [Google Scholar]
- 20.Okura Y, Urban LH, Mahoney DW, et al. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Yamagishi K, Ikeda A, Iso H, et al. Self-reported stroke and myocardial infarction had adequate sensitivity in a population-based prospective study JPHC (Japan Public Health Center)-based Prospective Study. J Clin Epidemiol. 2009;62:667–673. doi: 10.1016/j.jclinepi.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Lindberg HA, Berkson DM, Stamler J, et al. Totally asymptomatic myocardial infarction: an estimate of its incidence in the living population. Arch Intern Med. 1960;106:628–633. doi: 10.1001/archinte.1960.03820050040008. [DOI] [PubMed] [Google Scholar]
- 23.McClellan W, Warnock DG, McClure L, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17:1710–1715. doi: 10.1681/ASN.2005111200. [DOI] [PubMed] [Google Scholar]
- 24.Kottke TE, Daida H, Bailey KR, et al. Reliability of the Minnesota and Mayo electrocardiographic coding systems. J Electrocardiol. 1998;4:303–312. doi: 10.1016/s0022-0736(98)90015-2. [DOI] [PubMed] [Google Scholar]