Do detrimental effects on the vasculature of a high dietary sodium intake precede the development of hypertension?
Keywords: blood pressure, fetal programming, nitric oxide, salt, vessel development
Abstract
Background
High salt intake causes hypertension, adverse cardiovascular outcomes and potentially also blood pressure (BP)-independent target organ damage. Excess salt intake in pregnancy is known to affect BP in the offspring. The present study was designed to assess whether high salt intake in pregnancy affects BP and vascular morphology in the offspring.
Methods
Sprague–Dawley rats were fed a standard rodent diet with low–normal (0.15%) or high (8.0%) salt content during pregnancy and lactation. After weaning at 4 weeks of age, offspring were maintained on the same diet or switched to a high- or low-salt diet, respectively. Vascular geometry was assessed in male offspring at 7 and 12 weeks postnatally.
Results
Up to 12 weeks of age, there was no significant difference in telemetrically measured BP between the groups of offspring. At 12 weeks of age, wall thickness of central (aorta, carotid), muscular (mesenteric) and intrapulmonary arteries was significantly higher in offspring of mothers on a high-salt diet irrespective of the post-weaning diet. This correlated with increased fibrosis of the aortic wall, more intense nitrotyrosine staining as well as elevated levels of marinobufagenin (MBG) and asymmetric dimethyl arginine (ADMA).
Conclusions
High salt intake in pregnant rats has long-lasting effects on the modeling of central and muscular arteries in the offspring independent of postnatal salt intake and BP. Circulating MBG and ADMA and local oxidative stress correlate with the adverse vascular modeling.
Introduction
There is evidence from observational epidemiological studies [1], small intervention studies [2] and controlled intervention trials [3, 4] as well as from studies in primates [5] that dietary salt intake is one causal factor responsible for elevated blood pressure (BP) in adults. This has also been shown in children [6].
In grown experimental animals, high salt intake causes cardiac fibrosis and vascular remodeling [7, 8] partially independent of BP. This observation led to the hypothesis that salt loading promotes structural and functional derangements in the heart, vessels and kidney partially by BP-independent mechanisms [9].
One recently recognized pathomechanism of salt-induced hypertension and target organ damage is the release of salt-induced endogenous cardiotonic steroids [10]. Specifically, the cardiotonic steroid marinobufagenin (MBG) is found in mammalian plasma and urine [11]. Its plasma concentration is elevated in patients with hypertension and chronic kidney disease [12]. It has also been postulated that in salt-sensitive animals, salt loading causes paradoxical stimulation of local renin–angiotensin–aldosterone systems in the kidney, heart and vessels [13]. Conversely, angiotensin type 1 receptor blockers prevent target organ damage even at doses which fail to reduce BP [7]. Both high salt intake [14] and elevated MBG concentrations [15] have been associated with epithelial-to-mesenchyme transition which plays a role in organogenesis.
In humans, salt loading causes an acute increase in oxidative stress, particularly in individuals with salt-sensitive BP [16]. Salt loading not only increases BP in salt-sensitive individuals but in addition increases oxidative stress [17] and reduces nitric oxide availability by increasing the concentration of the endothelial nitric oxide synthase (eNOS) inhibitor asymmetric dimethyl arginine (ADMA) [18].
Following the work of Barker [19], the importance of fetal programming in determining the adult risk profile has been firmly established by clinical observations and experimental models. Contreras et al. [20] showed that high salt intake of dams during pregnancy and high salt intake throughout lactation and weaning caused persistent hypertension in adult rats.
The present study was performed to clarify whether
high salt intake of dams during pregnancy affects BP and morphology of central and muscular arteries in the offspring,
high salt intake post-weaning affects the same parameters and whether
post-weaning salt intake modifies the long-term effects of prenatal high salt intake.
Materials and methods
Animals
All animals were handled according to the written approval from the local authority for animal experiments (Regierungspraesidium Karlsruhe). The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Pregnant Sprague–Dawley rats were obtained from Charles River (Sulzfeld, Germany) at Day 1 after conception (verified by vaginal smear). The dams were randomized to receive ad libitum a diet with modified salt content: 0.15% NaCl (low salt, LS; n = 28) or 8.0% NaCl (high salt, HS; n = 29). All diets were based on a standard rodent diet containing 19.3% protein, 39.1% carbohydrates and 3.3% fat, 1.00% calcium, 0.70% phosphate and 0.68% potassium (Ssniff, Soest, Germany). In the low-salt arm, the NaCl concentration of 0.15% was deliberately chosen because salt-deficient diets in pregnancy cause low birth weight and hypertension in the offspring [21]. The diet was administered from the first day of pregnancy until weaning. Twenty-one litters of dams on LS and 22 on HS were included in the study. The male offspring were investigated further; they were weaned at 4 weeks of age and continued on the diet of the mother (LL offspring on low salt intake from dams on low salt intake and HH offspring on high salt intake from dams on high-salt intake, respectively). Alternatively, offspring were switched from low to high (LH offspring) or from high to low (HL offspring) -salt diet. Equal numbers of offspring from each litter were continued on the dam's diet (LL and HH) or switched to the alternative diet (LH and HL). All animals were housed at constant room temperature (21 ± 1°C) and humidity (75 ± 5%). They were exposed to a 12-h light-on and 12-h light-off cycle. The animals had free access to deionized water and food. The offspring were observed until 12 weeks of age. Body weight was measured weekly. Food consumption was monitored in consecutive one-week periods and water intake was monitored daily (standard cages).
BP measurement
At the age of 8 weeks, five offspring were randomly chosen from each group (one offspring per dam) for the telemetric BP measurement performed as previously described [22].
In a separate subset of seven dams per group, intra-aortic systolic BP (SBP) was measured at term (gestation Day 21) after sedation [100 mg/kg ketamine hydrochloride (Ketamin; Essex Tierarznei, Germany) and 3.0 mg/kg xylazine (Xylazin; Ceva Tiergesundheit, Germany)] using a semiautomatic system (TSE Systems, Germany). The catheter was placed directly in the abdominal aorta inferior to the renal arteries. The BP was allowed to stabilize for 5 min and subsequently, 15 consecutive measurements were taken. The average of these measurements was used for further analysis.
Blood analyses
Blood samples were collected from the abdominal aorta at the time of sacrifice. Serum and urine parameters [sodium, potassium, creatinine, total and high-density lipoprotein (HDL) cholesterol, triglycerides] and blood cells were analyzed using standard laboratory methods. Urinary albumin was measured by a rat-specific enzyme-linked immunosorbent assay (ELISA) (Bethyl). Serum ADMA was measured at 12 weeks using an ELISA kit (Immundiagnostik, Bensheim, Germany). Plasma MBG was measured following extraction with C-18 columns as described previously [23]. ELISA plates were coated with MBG–bovine serum albumin conjugate at a dose of 5 ng/well. Anti-MBG mouse monoclonal antibody (4G4, titer 1:1000) was used (100 μL/well) followed by Europium-labeled anti-mouse antibody (PerkinElmer). If necessary, C-18 column extracts were diluted so that all absorbance values could be read on the linear part of an MBG concentration curve which typically ranged from 10 pM to 1 nM.
Morphologic investigations
Tissues were collected from offspring at 7 (analogous to puberty) and 12 (adulthood) weeks of age. The abdominal aorta was catheterized under anesthesia (100 mg/kg ketamine hydrochloride and 3.0 mg/kg xylazine). Blood samples were taken, the vasculature was flushed with 0.9% NaCl solution, arteries were pre-dilated (procaine hydrochloride 1.0 g/L) and fixed with glutaraldehyde (9–10 animals per group, 1 offspring per dam) or collected for molecular investigations (9–10 animals per group, 1 offspring per dam). To assess vascular geometry, samples were taken from the descending thoracic aorta (Th6–Th8), carotid arteries, iliac arteries and the small to medium size arteries in mesenteric tissue (second and third order) as well as small to medium size arteries in lungs. Wall and lumen areas were analyzed planimetrically using a semiautomatic image analysis system (Image Pro Plus 6.0; Media Cybernetics Inc., Bethesda, MD). The collagen content of the aortic wall was quantitated in sections stained with 0.1% Sirius red F3BA saturated with picric acid; the elastin content was similarly quantitated in samples stained with Elastica-van Gieson (EVG) and analyzed with the same semiautomatic image analysis system.
Immunohistochemistry
Immunohistochemical analysis was performed on paraffin sections using antibodies against Proliferating cell nuclear antigen (PCNA; Immunotech, France), nitrotyrosine, serum- and glucocorticoid-regulated kinase (SGK) and phospho-SGK (Santa Cruz Biotechnology, Heidelberg, Germany). The sections were analyzed using the semiautomatic image analysis system (see above) and expressed as numbers of positively stained cells (PCNA) or stained area (SGK, p-SGK, nitrotyrosine). All analyses were performed by an observer unaware of the protocol.
Western blotting
Western blotting of aortic tissue was performed as previously described [24]. Aortic tissue samples were taken from seven randomly chosen animals per group (one offspring per dam). Primary antibody against caldesmon, calponin, transforming growth factor (TGF)-β1, TGF-β receptor 1, TGF-β receptor 2, angiotensin type 2 receptor (sc-9040 [25]), soluble guanyl cyclase subunit β 1 (sGC-β1), sGC-β2, (Santa Cruz Biotechnology), eNOS (Affinity BioReagents), angiotensin-converting enzyme (ACE; Chemicon, Germany), smooth muscle myosin heavy chain 1 and 2, angiotensin type 1 receptor (Abcam, UK) and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) were used. Peroxidase labeling was detected with enhanced chemiluminescence detection (GE Healthcare, UK) according to the manufacturer's recommendations. To control for variations in protein loading or transfer, membranes were washed and re-incubated with anti-β-actin antibody (Abcam). Exposed films were scanned and data were analyzed with the computer software (ImageJ 1.41; NIH, Bethesda, MD).
Statistical analysis
Data are given as mean ± SD or median and range as appropriate. Two-way analysis of variance was used with pre- and post-weaning diets as independent variables followed by Tukey's post-hoc test. The results are labeled: PreW—the effect of pre-weaning diet, PostW—the effect of post-weaning diet, PreW × PostW—the effect of interaction between pre- and post-weaning diet. For the analysis of BP and heart rate, only one value per rat (average) was used for comparison of groups. The results were considered significant when the probability of error (P) was < 0.05.
Results
Mother animals
There was no difference in body weight or body weight gain between the groups of dams, but the food intake during pregnancy was significantly higher in dams on high-salt compared with dams on low-salt diet (Table 1). At the end of pregnancy, there was no difference in intra-aortic SBP between the groups of dams.
Table 1.
Data of the dams
| Group | Body weight |
Food intake in pregnancy (g) | SBP at end of pregnancy (mmHg) | ||
|---|---|---|---|---|---|
| At start of pregnancy (g) | At end of pregnancy (g) | Gain in pregnancy (g) | |||
| Low salt | 192 ± 28 | 302 ± 27 | 109 ± 27 | 425 ± 97 | 142 ± 8 |
| High salt | 186 ± 24 | 282 ± 25 | 96 ± 27 | 533 ± 71 | 130 ± 11 |
| t-test | P = NS | P = NS | P = NS | P < 0.05 | P = NS |
Animal data
The average litter size [12.5 ± 2.6 in low salt (LS) dams and 12.7 ± 1.6 in high salt (HS) dams] as well as birth weight (6.13 ± 0.61 g in offspring of LS dams and 6.19 ± 0.61 g in offspring of HS dams) was not significantly different between groups.
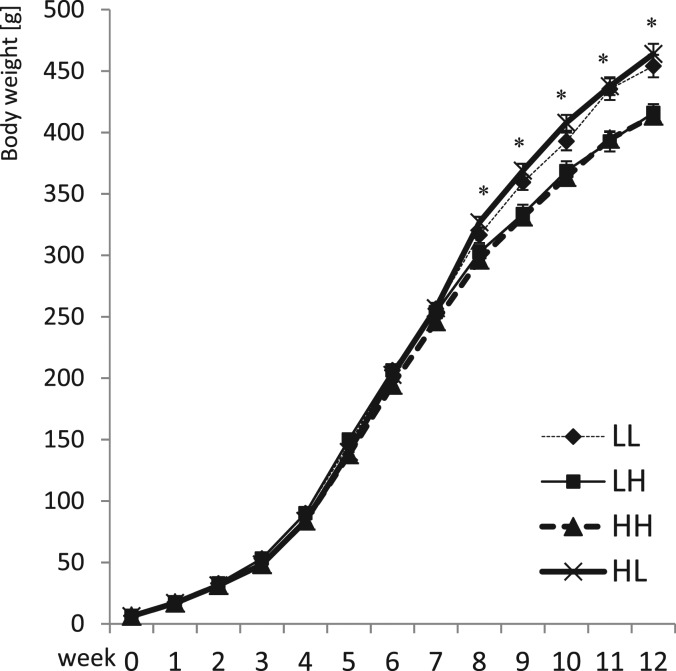
Starting from postnatal Week 3, the body weight of offspring of HS dams was significantly lower (Figure 1). Between Weeks 5 and 8 in HL animals (offspring of dams on high salt switched postnatally to low salt), food consumption was lower (179.2 ± 14.7 g/week), but weight gain was higher compared to their HH littermates (offspring of dams on high salt continued postnatally on high salt; weekly food consumption 199.5 ± 9.1 g; P < 0.001 compared to HL). Similarly, LH offspring (food consumption 198.1 ± 16.8 g/week) gained less weight than their LL littermates (food consumption 170.1 ± 16.1 g/week; P < 0.01). As a result, after postnatal Week 8, body weight was significantly lower in LH and HH offspring compared to LL and HL offspring (Figure 1). Water consumption was significantly (P < 0.001) higher in offspring on high salt intake (average for Weeks 8–12: HH 87 ± 5 mL/day and LH 76 ± 7mL/day) compared to low salt intake (LL 30 ± 4 mL/day and HL 31 ± 3mL/day).
Fig. 1.
Body weight development in the male offspring until 12 weeks of age. Two-way analysis of variance: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; *P < 0.05 LH versus LL and HH versus HL, †P < 0.05 HH versus LH and HL versus LL.
The heart weight was significantly higher in offspring of dams on high-salt diet irrespective of their post-weaning salt intake (Table 2). Heart to body weight ratio was significantly higher in offspring on high salt intake post-weaning and in offspring of dams on high-salt diet (Table 2).
Table 2.
Blood and urine analysis and heart weight in offspring at Week 12a
| Group | Heart weight (g) | Heart/body weight (g/kg) | Serum sodium (mmol/L) | Serum potassium (mmol/L) | Plasma ADMA (μmol/L) | Plasma MBG (nmol/L) | Urinary sodium excretion (mmol/24 h) | Urinary albumin excretion (mg/24 h) |
|---|---|---|---|---|---|---|---|---|
| LL (n = 10) | 1.72 ± 0.17 | 3.8 ± 0.3 | 141 ± 3 | 3.9 ± 0.2 | 0.64 ± 0.13 | 0.29 ± 0.15 | 0.6 ± 0.3 | 0.62 ± 0.29 |
| LH (n = 10) | 1.77 ± 0.24 | 4.3 ± 0.5* | 138 ± 2 | 4.2 ± 0.4 | 0.77 ± 0.10* | 0.34 ± 0.09 | 22.1 ± 8.5* | 3.21 ± 1.21* |
| HH (n = 10) | 1.85 ± 0.18† | 4.5 ± 0.4* | 140 ± 3 | 4.0 ± 0.6 | 0.82 ± 0.16 | 0.62 ± 0.32† | 17.2 ± 6.7* | 4.73 ± 1.75*,† |
| HL (n = 10) | 1.89 ± 0.24† | 4.2 ± 0.3† | 140 ± 2 | 4.0 ± 0.3 | 0.77 ± 0.09† | 0.55 ± 0.16† | 1.1 ± 0.6 | 2.04 ± 0.66† |
| 2-way ANOVA | ||||||||
| PreW | P < 0.01 | P < 0.005 | P = NS | P = NS | P < 0.05 | P < 0.01 | P = NS | P < 0.001 |
| PostW | P = NS | P < 0.001 | P = NS | P = NS | P < 0.05 | P = NS | P < 0.001 | P < 0.001 |
| PreW × PostW | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS | P < 0.05 | P = NS |
aTwo-way ANOVA: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; ANOVA, analysis of variance; NS, not significant.
*P < 0.05 LH versus LL and HH versus HL.
†P < 0.05 HH versus LH and HL versus LL.
Blood and urine analysis
At age 12 weeks, serum sodium and potassium (Table 2) concentrations were not significantly different depending on pre- or post-weaning diet.
At age 12 weeks, no differences in hematocrit and hemoglobin concentration were observed between the groups. Serum concentrations of creatinine, total and HDL cholesterol as well as triglycerides were not significantly different between the groups (data not shown).
Serum ADMA was significantly higher in offspring of dams on high salt (Table 2) compared with offspring of dams on low salt. High salt intake post-weaning also caused a significant increase of serum ADMA, while pre-weaning salt intake had no impact on serum ADMA.
Plasma MBG was significantly higher in HH and HL compared with LH and LL offspring, respectively (Table 2).
At 12 weeks, urinary sodium excretion was significantly higher in offspring receiving a high-salt diet post-weaning irrespective of the dam's diet (Table 2). However, the increase in sodium excretion on high-salt diet was higher in offspring of dams on low-salt diet.
Urinary albumin excretion was significantly higher in offspring of dams on high-salt versus low-salt diet. Urinary albumin excretion was also higher in offspring on high versus low salt intake post-weaning (Table 2).
BP is not increased in offspring of dams on high salt
Average (24 h) systolic, diastolic and mean BPs were not different between the groups of offspring (Table 3). There was no difference in variability of BP and heart rate during the light-on and light-off periods (Tables 3 and 4).
Table 3.
Blood pressure (BP) and heart rate (HR)—24 h means and variability (SD) in the offspringa
| Group | SBP (mmHg) | Diastolic BP (mmHg) | Mean BP (mmHg) | Mean BP variability (24-h SD) (mmHg) | Mean BP difference light-on/light-off (mmHg) | HR (1/min) | HR variability (24-h SD) (1/min) | Mean HR difference light-on/light-off (1/min) |
|---|---|---|---|---|---|---|---|---|
| LL (n = 10) | 114 ± 7 | 81 ± 7 | 96 ± 7 | 7.4 ± 0.8 | 5.0 ± 1.4 | 380 ± 13 | 51 ± 8 | 58 ± 12 |
| LH (n = 10) | 114 ± 4 | 77 ± 4 | 93 ± 4 | 7.7 ± 1.0 | 5.1 ± 2.0 | 395 ± 15* | 48 ± 4 | 54 ± 10* |
| HH (n = 10) | 116 ± 3 | 82 ± 3 | 97 ± 4 | 8.3 ± 1.1 | 5.6 ± 2.5 | 386 ± 20 | 46 ± 7 | 50 ± 10* |
| HL (n = 10) | 113 ± 5 | 81 ± 3 | 95 ± 4 | 7.5 ± 0.8 | 5.5 ± 0.8 | 404 ± 15*,† | 52 ± 5 | 63 ± 8 |
| Two-way ANOVA | ||||||||
| PreW | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS |
| PostW | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS | P < 0.01 |
| PreW × PostW | P = NS | P = NS | P = NS | P = NS | P = NS | P < 0.005 | P = NS | P = NS |
aTwo-way ANOVA: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; ANOVA, analysis of variance; NS, not significant.
*P < 0.05 LH versus LL and HH versus HL.
†P < 0.05 HH versus LH and HL versus LL.
Table 4.
Daytime (light-on) and nighttime (light-off) blood pressure and heart rate in the offspringa
| Group | Daytime (light-on) |
Nighttime (light-off) |
||||||
|---|---|---|---|---|---|---|---|---|
| SBP (mmHg) | Diastolic BP (mmHg) | Mean BP (mmHg) | HR (1/min) | SBP (mmHg) | Diastolic BP (mmHg) | Mean BP (mmHg) | HR (1/min) | |
| LL | 111 ± 7 | 78 ± 7 | 93 ± 7 | 352 ± 15 | 116 ± 7 | 83 ± 7 | 98 ± 7 | 410 ± 14 |
| LH | 112 ± 4 | 74 ± 4 | 91 ± 4 | 368 ± 14* | 117 ± 5 | 80 ± 5 | 96 ± 5 | 422 ± 17 |
| HH | 113 ± 6 | 79 ± 3 | 94 ± 4 | 360 ± 21 | 119 ± 7 | 85 ± 5 | 100 ± 5 | 410 ± 20 |
| HL | 110 ± 4 | 78 ± 3 | 92 ± 3 | 372 ± 16*,† | 116 ± 5 | 84 ± 3 | 98 ± 4 | 435 ± 15*,† |
| Two-way ANOVA | ||||||||
| PreW | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS |
| PostW | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS | P = NS |
| PreW × PostW | P = NS | P = NS | P = NS | P < 0.001 | P = NS | P = NS | P = NS | P < 0.001 |
aTwo-way ANOVA: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; ANOVA, analysis of variance; NS, not significant.
*P < 0.05 LH versus LL and HH versus HL.
†P < 0.05 HH versus LH and HL versus LL.
The effect of post-weaning high salt intake on heart rate differed depending on the dam's salt intake: the heart rate was higher with high compared to low salt intake post-weaning. It was higher if the dam had been on low salt intake and it was lower if the dam had been on high salt intake (Tables 3 and 4).
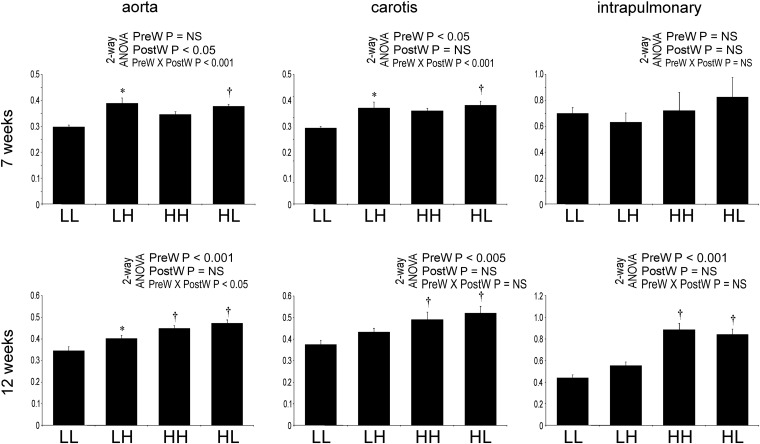
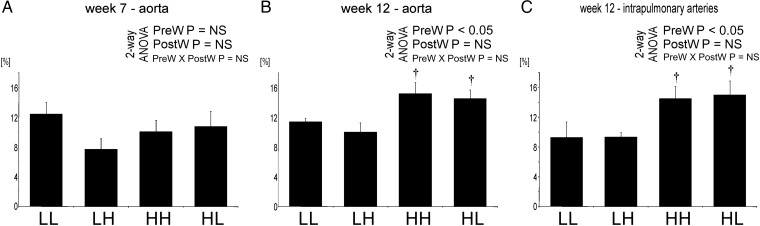
Arterial wall is thicker in offspring of dams on high salt
At age 7 weeks, the wall/lumen ratio of aorta and carotid arteries was significantly higher in LH compared to LL and was also higher in HL compared to LL offspring (Figure 2).
Fig. 2.
Wall to lumen ratio (μm2/μm2) in the offspring at 7 and 12 weeks of age in the aorta, carotid and pulmonary arteries. Two-way analysis of variance: PreW, the effect of pre-weaning diet; PostW, the effect of Post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; *P < 0.05 LH versus LL and HH versus HL, †P < 0.05 HH versus LH and HL versus LL.
At age 12 weeks, the wall/lumen ratio of aorta, carotids, mesenteric and intrapulmonary arteries was significantly higher in HH and HL compared to LH and LL offspring, respectively (Figure 2). The wall/lumen ratio of the aorta was also higher in LH offspring compared with LL group.
The luminal areas of aorta, carotid, mesenteric and pulmonary arteries were not significantly different between the groups (data not shown).
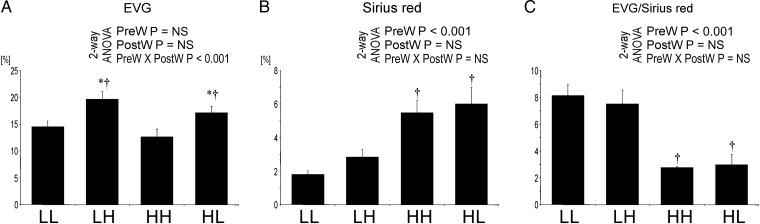
The aortic wall is more fibrotic in offspring of dams on high salt
At age 12 weeks, the proportion of the wall of the aorta stained by EVG was highest in LH offspring; the area stained positively for collagen was significantly higher in HH and HL compared with LH and LL offspring, respectively (Figure 3). Consequently, the ratio of EVG- to Sirius red-positive areas, reflecting collagen content, was significantly higher in the aortas of HH and HL compared with LH and LL offspring, respectively (Figures 3 and 4).
Fig. 3.
Areas with positive staining for EVG (A) and Sirius red (B) in the aorta and ratio EVG/Sirius red-positive areas (C) in the offspring at 12 weeks. Two-way analysis of variance: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; *P < 0.05 LH versus LL and HH versus HL, †P < 0.05 HH versus LH and HL versus LL.
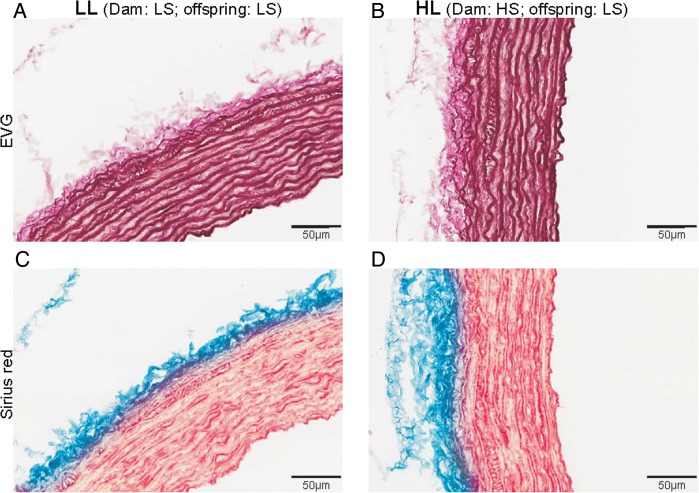
Fig. 4.
Representative microphotographs of EVG stain in LL (A) and HL (B) and Sirius red stain in LL (C) and HL (D) offspring.
Cell proliferation is more pronounced in the aorta of offspring of dams on high salt
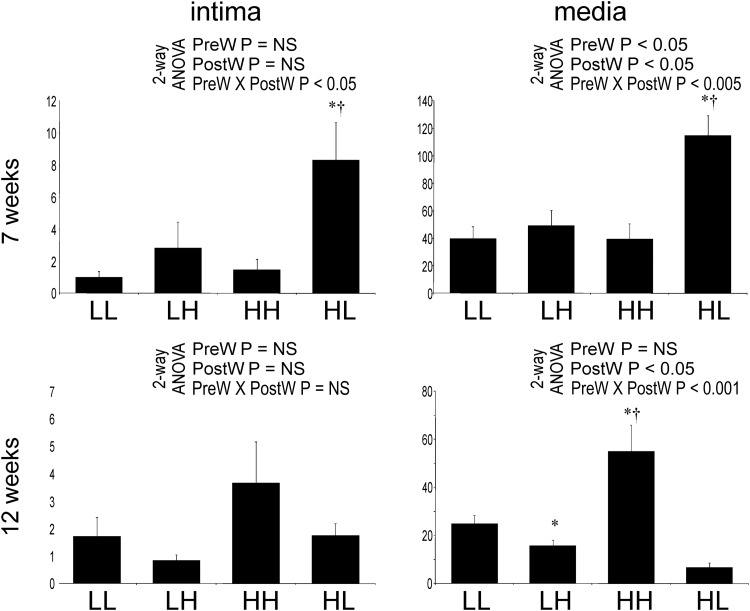
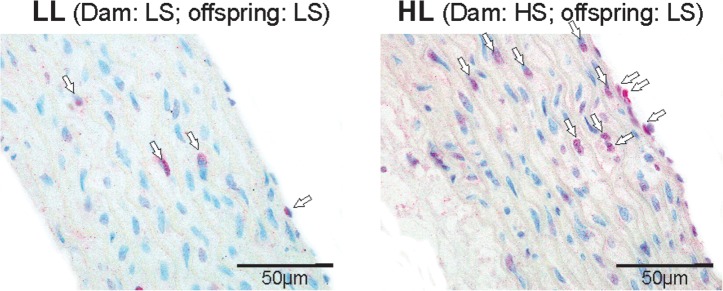
At Week 7, significantly more endothelial and vascular smooth muscle cells (VSMCs) in the aorta stained positive for PCNA in HL offspring compared with the other groups (Figure 5). At Week 12, there was no difference in the number of aortic endothelial cells staining positive for PCNA between the groups. Significantly more PCNA-positive VSMCs, however, were observed in HH offspring compared with other groups (Figure 6).
Fig. 5.
Number of cells staining positive for PCNA in aortic endothelium (%) in the offspring at 7 (A) and 12 (B) weeks of age and in the media (1/mm2) at 7 (C) and 12 (D) weeks of age. Two-way analysis of variance: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; *P < 0.05 LH versus LL and HH versus HL, †P < 0.05 HH versus LH and HL versus LL.
Fig. 6.
Representative microphotographs of PCNA staining in the aorta from LL (left) and HL (right) offspring at Week 7. PCNA-positive cells are marked with arrows.
Altered differentiation in the aorta of offspring of dams on high salt VSMC differentiation
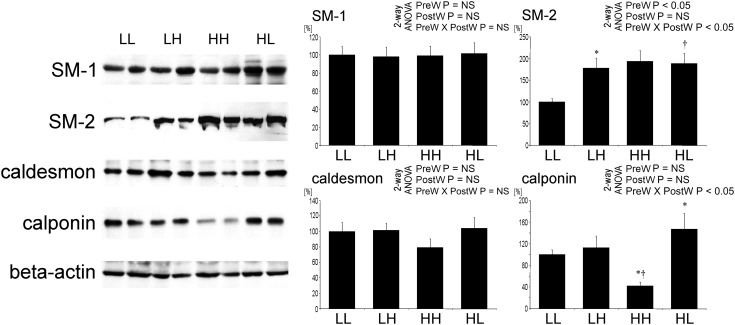
At age 12 weeks, the expression of smooth muscle actin heavy chain 1 was not different between the groups. In contrast, the expression of smooth muscle actin heavy chain 2 was significantly higher in offspring of dams on HS versus LS (Figure 7). The expression of smooth muscle actin heavy chain 2 was significantly increased on high post-weaning salt intake only in offspring of dams on LS.
Fig. 7.
Expression of smooth muscle myosin heavy chain 1 (SM2) and 2 (SM2), caldesmon and calponin in the aorta of offspring at 12 weeks of age. Representative western blots and analysis of blots optical density. Two-way analysis of variance: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; *P < 0.05 LH versus LL and HH versus HL, †P < 0.05 HH versus LH and HL versus LL.
The expression of caldesmon, characteristic for differentiated VSMC, tended to be lower in HH offspring, but the difference was not significant. The expression of calponin, also characteristic for differentiated VSMC, was significantly lower in HH offspring compared with other groups (Figure 7). This went in parallel with increased proliferation of VSMCs in this group.
Marked oxidative stress in the aorta of offspring of dams on high salt
At age 7 weeks, the area stained positive for nitrotyrosine in the aortic wall was not different between the groups. At age 12 weeks, the aortic wall area stained for nitrotyrosine was significantly larger in HH and HL compared to LH and LL groups, respectively (Figure 8). At that age, staining for nitrotyrosine in the wall of intrapulmonary arteries was also increased in HH and HL compared to LH and LL offspring, respectively.
Fig. 8.
Quantification of staining for nitrotyrosine in the aorta and intrapulmonary arteries. Two-way analysis of variance: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; *P < 0.05 LH versus LL and HH versus HL, †P < 0.05 HH versus LH and HL versus LL.
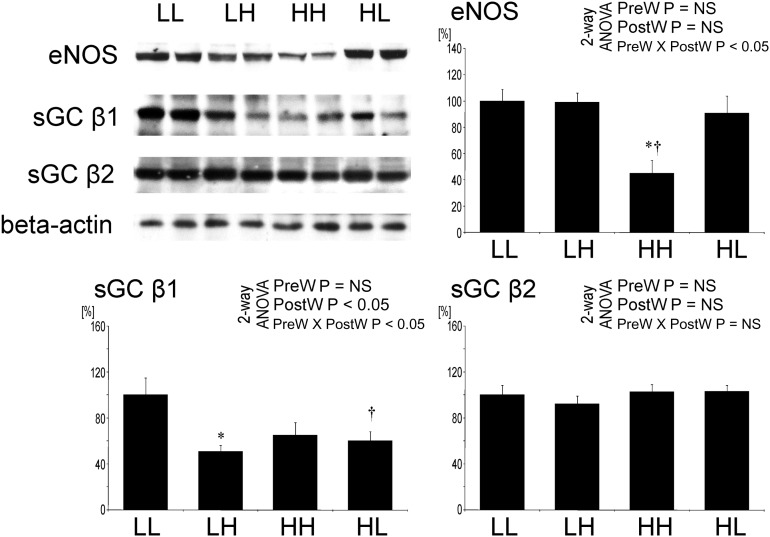
Downregulation of proteins involved in NO signaling in offspring of dams on high salt
The expression of eNOS in the aorta by western blot was significantly lower in HH offspring compared with the other groups (Figure 9).
Fig. 9.
Expression of eNOS, sGC-β1 and sGC-β2 in the aorta of offspring at 12 weeks of age. Representative western blots and analysis of blots optical density. Two-way analysis of variance: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; *P < 0.05 LH versus LL and HH versus HL, †P < 0.05 HH versus LH and HL versus LL.
By western blot, the expression of sGC-β1 in the aorta was significantly lower in LH and HL compared with LL offspring (Figure 9). There was no significant difference in the expression of sGC-β2 in the aorta between the groups.
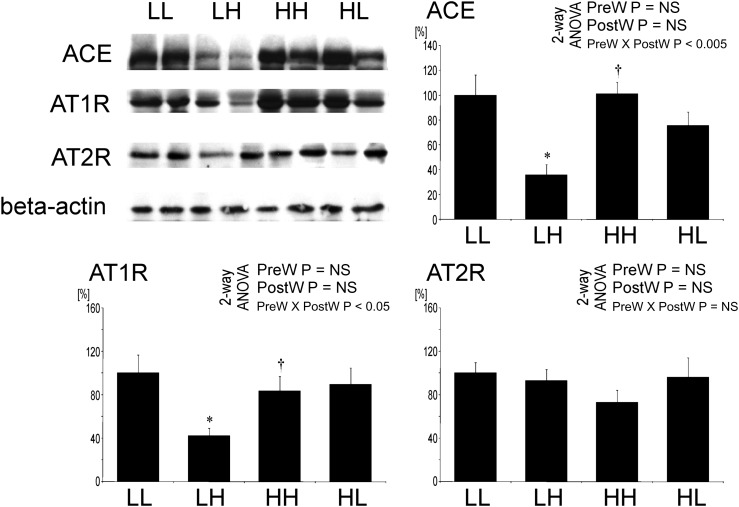
Expression of renin–angiotensin system in the aorta is not suppressed by salt in offspring after prenatal high salt
By western blot, the expression of ACE and angiotensin II type 1 receptor (AT1R) in the aorta was significantly lower in LH versus LL offspring, but it was not different in HH versus HL offspring (Figure 10). No significant difference of angiotensin II type 2 receptor (AT2R) expression in the aorta was observed between the groups (Figure 10).
Fig. 10.
Expression of ACE, angiotensin II type 1 (AT1R) and type 2 (AT2R) receptors in the aorta of offspring at 12 weeks of age. Representative western blots and analysis of blots optical density. Two-way analysis of variance: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; *P < 0.05 LH versus LL and HH versus HL, †P < 0.05 HH versus LH and HL versus LL.
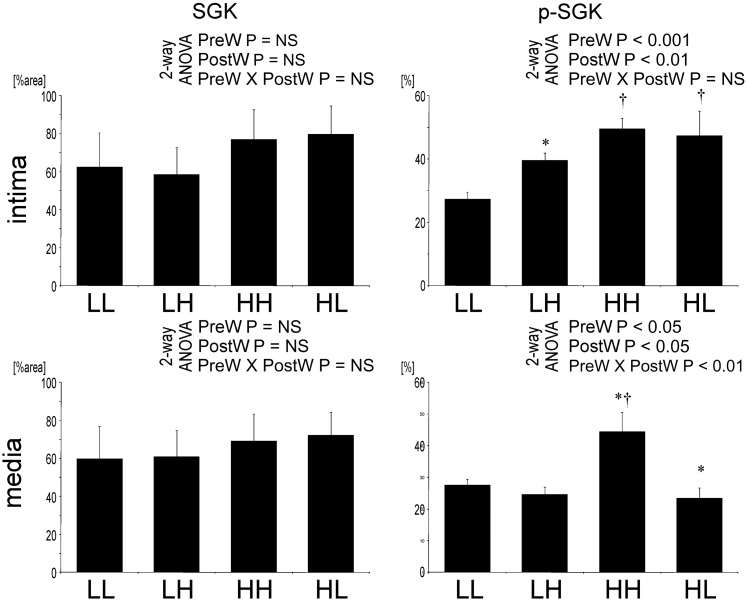
SGK is activated in offspring of dams on high salt
While staining for the total SGK in the aorta was not different between the groups of offspring, staining for the phosphorylated SGK was more intense in aortic endothelial cells of LH and HL compared to LL offspring (Figure 11). In the VSMCs, the staining for phosphorylated SGK was higher in HH compared with other offspring.
Fig. 11.
Quantification of staining for total SGK and phosphorylated SGK in the aorta. Two-way analysis of variance: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; *P < 0.05 LH versus LL and HH versus HL, †P < 0.05 HH versus LH and HL versus LL.
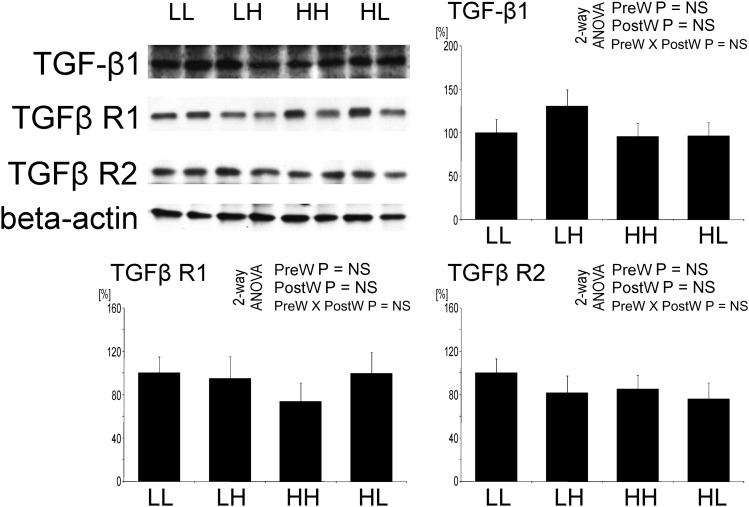
The TGF-β system is not influenced by salt intake
Furthermore, in the aorta, no significant differences in the expression of TGF-β1 (Figure 12), TGF-β receptor 1 and TGF-β receptor 2 were observed between the groups.
Fig. 12.
Expression of transforming growth factor β 1 (TGF-β1), TGF- β type 1 (TGFβ R1) and type 2 (TGFβ R2) receptors in the aorta of offspring at 12 weeks of age. Representative western blots and analysis of blots optical density. Two-way analysis of variance: PreW, the effect of pre-weaning diet; PostW, the effect of post-weaning diet; PreW × PostW, the effect of interaction between pre- and post-weaning diet; *P < 0.05 LH versus LL and HH versus HL, †P < 0.05 HH versus LH and HL versus LL.
Discussion
The main result of this study is the observation that high maternal salt intake during pregnancy has a progressive long-lasting effect on modeling of both central and muscular arteries of male offspring independent of postnatal salt intake and systemic BP. These changes of wall thickness without change in luminal area in the central and muscular arteries of the systemic (and remarkably also in the pulmonary) circulation were accompanied by differences in proliferation and differentiation of cells and expression of the components of the renin–angiotensin system (RAS) in the vascular wall. Furthermore, there were differences in eNOS and sGC expression, signs of oxidative stress and differences in the plasma concentrations of ADMA and MBG. Elevated MBG concentrations were found in the groups with increased wall thickness and fibrosis of the wall of the different arteries.
Although direct short-term effects of increased salt intake on elevation of BP are more marked in females [26], we deliberately studied the arteries in male offspring, which are generally more susceptible to structural vascular damage.
The observation that salt intake in dams affects the characteristics of arteries in the offspring is in agreement with the ‘fetal programming’ hypothesis proposed by Barker who postulated that factors operative during organogenesis cause long-lasting effects persisting during adult life [19]. Animal models, designed to study the impact of fetal programming on adult BP, were mostly models characterized by low birth weight caused by maternal undernutrition, hyperinsulinemia or uterine underperfusion. In humans, low birth weight is associated in adulthood with elevated BP and cardiovascular disease [27, 28] as well as with albuminuria [29] and susceptibility to end-stage renal disease [30]. Developmental programming of the aortic structure has been documented in offspring of rats on high-fat diet [31]. Our results show that another component of maternal diet, i.e. salt, influences the vascular structure in the offspring as well.
High prenatal salt intake caused in the male offspring vascular hypertrophy, increased arterial collagen content and nitrotyrosine deposition as well as elevated MBG concentration. In adults, this constellation is indicative of increased vascular stiffness and increased cardiovascular risk in the long term. Lack of direct measurement of vascular compliance is a limitation of this study.
A potential confounder in the study is food intake, which was higher in mothers as well as in offspring with a high salt intake. Interestingly, despite higher food consumption, weight gain was less in offspring on high salt. High salt intake has previously been shown to be associated with increased food intake and decreased weight gain [32]. The small difference in sodium excretion between LL versus HL offspring is consistent with the small difference in food intake. It is also possible that animals previously on high-sodium diets continued to excrete sodium from osmotically inactive stores [33]. The difference in sodium excretion between LH and HH offspring is most probably due to differences in intestinal absorption. All animals on a high-sodium diet produced visibly more feces than animals on a low-sodium diet. This was also reflected by lower weight gain despite higher food intake in these groups.
Our results are in contrast with the observation of the development of increased adiposity in offspring of dams, which had been on low salt intake, but in this study, the fetuses had also been exposed to intrauterine growth restriction [34], which was avoided in our study.
This may also explain why until Week 12 telemetrically measured baseline BP was not higher in offspring on a high-salt diet. This does not exclude an increased BP response to stress as documented by Porter [35]. Furthermore, pregnant dams on high-salt diet had no elevation of baseline BP; this does not exclude higher BP responses to stimuli as observed in pregnant dams on saline [36].
Contreras et al. [20] documented that high salt intake in the perinatal period, i.e. throughout lactation and weaning, caused persistent hypertension in adult rats. A similar effect of salt exposure at an early age has also been seen in humans [37]. In agreement with the findings of other authors, a high-salt diet during pregnancy did not cause intrauterine growth restriction in our study [20, 35]. Porter et al. [35] had limited high salt intake to the period of pregnancy and discontinued high salt intake during the lactation period: this protocol caused an increased BP response to stress in the offspring despite no elevation of baseline BP. In our study, although there was no increase of telemetrically measured BP in animals age 7–12 weeks, abnormal modelling of vascular walls was seen in offspring of dams on high salt. Even at 7 weeks of age, minor differences in the geometry of the aorta of offspring were noted. By Week 12, pronounced changes in vascular geometry were seen in offspring of dams on high salt, although the offspring had been switched to low salt after weaning at 4 weeks. This finding suggests that vascular modeling had been programmed in utero.
In the experiments of Varagic et al. [7] and Matavelli et al. [8], salt loading caused cardiac fibrosis and vascular remodeling in adult animals, partially independent of BP. Based on this observation, Frohlich [9] proposed the hypothesis that high-salt intake promotes derangements of structure and function of heart, vessels and kidney at least partially as a result of BP independent mechanisms. Our observation supports this hypothesis in a different context, i.e. altered vascular structure despite no change in BP. Moreover, our study documents that vascular modeling after weaning was abnormal in offspring of dams which had been on a high-salt diet during pregnancy; this was seen even when the offspring were switched to low salt after weaning. This observation is compatible with the concept of intrauterine programming. We emphasize that abnormal vascular geometry was not explained by differences in the growth rate of the animals.
Our results document that the adverse BP-independent effects of salt loading during pregnancy persist even when postnatal salt intake has been reduced. One hypothetical explanation for the delayed disappearance of increased PCNA staining after 7 weeks in HL offspring is an initial transient activation of proliferation by transient activation of the RAS activity after lowering sodium intake; this explanation is hypothetical in the absence of information on systemic RAS parameters.
Our study does not definitively clarify whether the effect of salt is mediated via plasma sodium concentration, positive sodium balance or indirectly via salt-induced hormonal changes e.g. cardiotonic steroids [38]. Nevertheless, some findings in our study bear on potential mechanisms underlying salt-induced changes in vascular development. The plasma levels of the cardiotonic steroid MBG were increased at Week 12 in offspring of mothers on high salt independent of the offspring's current salt intake. The MBG concentration was correlated to structural abnormalities of the vascular wall (increased wall thickness and fibrosis) as well as to increased markers of oxidative stress. Elevated MBG levels may, at least in part, be responsible for the changes of arterial wall composition observed in our study. MBG is known to cause fibrosis in organs, such as heart and kidney [15, 39].
Increased salt intake should suppress the circulating RAS. In the offspring of dams on high salt intake, the—admittedly limited—parameters measured are not indicative of suppression at least of the local RAS in the arterial wall.
The fibrosis of the arterial wall was not correlated to abnormal TGF-β signaling, in line with previous observations in the heart [40]. High salt intake has been previously shown to acutely increase TGF-β production in aortic and glomerular endothelial cells [41–43]. The discrepancy between our and those previous results may be due to the longer period of high salt intake in our study.
High salt intake is known to lower vascular nitric oxide (NO) production [44]. In our study, we also found lower expression of eNOS in the aortas of offspring of mother animals fed a high-salt diet. In addition, plasma levels of ADMA—an endogenous eNOS inhibitor—were significantly higher in offspring of mothers on high salt during pregnancy irrespective of whether after weaning they were fed a high- or low-salt diet. Moreover, the expression of the NO receptor in vasculature—sGC—was significantly lower in offspring of mothers on high salt, irrespective of salt intake post-weaning. Downregulation of sGC by high salt intake had been shown in hypertensive or DOCA-treated rats [45, 46]. We demonstrated that not only the high salt intake post-weaning but also the high maternal salt intake during pregnancy reduced the expression of sGC. The unavailability of material for more detailed analysis of the systemic NO/ROS balance is a limitation of the study.
Our observation of adverse modeling and increased oxidative stress in pulmonary arteries after prenatal exposure to high salt is in agreement with recent similar findings in a different model of fetal programming, i.e. calorie deprivation [47].
Taken together, the results indicate that high salt intake in pregnancy results in abnormal development of the vascular wall of both central and muscular arteries in the offspring at least until age 12 weeks independent of basal BP. The morphological changes of the large vessels, accompanied by heart hypertrophy, may point to increased vascular stiffness. The structural and compositional changes of the vasculature were only moderately influenced by salt intake after weaning. They were correlated with the plasma levels of MBG pointing to a potential causal role of cardiotonic steroids. If these observations can be extrapolated to humans, high salt intake in pregnancy could impact on the development of arteries in the offspring resulting in greater wall thickness and stiffness of the central and muscular arteries potentially causing lower perfusion reserve and increased cardiovascular risk.
Conflict of interest statement
None declared.
(See related article by Rotmans et al. Antenatal excessive sodium intake induces adverse vascular remodelling in offspring. Nephrol Dial Transplant 2012; 27: 3379–3381.)
Acknowledgements
The skillful technical assistance of Z. Antoni and J. Moyers is gratefully acknowledged.
Funding. The study was supported by Collegium Nephrologicum e.V., Heidelberg. G.P. and N.K. were supported by fellowships of the International Society of Nephrology. The work of A.Y.B. and O.V.F. was supported by Intramural Research Program, National Institute on Aging, National Institutes of Health.
References
- 1.He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16:761–770. doi: 10.1038/sj.jhh.1001459. [DOI] [PubMed] [Google Scholar]
- 2.Melander O, von Wowern F, Frandsen E, et al. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N-terminal atrial natriuretic peptide in plasma. J Hypertens. 2007;25:619–627. doi: 10.1097/HJH.0b013e328013cd50. [DOI] [PubMed] [Google Scholar]
- 3.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 4.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334:885. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott P, Walker LL, Little MP, et al. Change in salt intake affects blood pressure of chimpanzees: implications for human populations. Circulation. 2007;116:1563–1568. doi: 10.1161/CIRCULATIONAHA.106.675579. [DOI] [PubMed] [Google Scholar]
- 6.He FJ, MacGregor GA. Importance of salt in determining blood pressure in children: meta-analysis of controlled trials. Hypertension. 2006;48:861–869. doi: 10.1161/01.HYP.0000245672.27270.4a. [DOI] [PubMed] [Google Scholar]
- 7.Varagic J, Frohlich ED, Diez J, et al. Myocardial fibrosis, impaired coronary hemodynamics, and biventricular dysfunction in salt-loaded SHR. Am J Physiol Heart Circ Physiol. 2006;290:H1503–H1509. doi: 10.1152/ajpheart.00970.2005. [DOI] [PubMed] [Google Scholar]
- 8.Matavelli LC, Zhou X, Varagic J, et al. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H814–H819. doi: 10.1152/ajpheart.00671.2006. [DOI] [PubMed] [Google Scholar]
- 9.Frohlich ED. The salt conundrum: a hypothesis. Hypertension. 2007;50:161–166. doi: 10.1161/HYPERTENSIONAHA.107.088328. [DOI] [PubMed] [Google Scholar]
- 10.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagrov AY, Fedorova OV. Effects of two putative endogenous digitalis-like factors, marinobufagenin and ouabain, on the Na + , K + -pump in human mesenteric arteries. J Hypertens. 1998;16:1953–1958. doi: 10.1097/00004872-199816121-00015. [DOI] [PubMed] [Google Scholar]
- 12.Komiyama Y, Dong XH, Nishimura N, et al. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin Biochem. 2005;38:36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Chandramohan G, Bai Y, Norris K, et al. Effects of dietary salt on intrarenal angiotensin system, NAD(P)H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-kappaB activity in salt-sensitive and -resistant rat kidneys. Am J Nephrol. 2008;28:158–167. doi: 10.1159/000110021. [DOI] [PubMed] [Google Scholar]
- 14.Pletinck A, Consoli C, Van Landschoot M, et al. Salt intake induces epithelial-to-mesenchymal transition of the peritoneal membrane in rats. Nephrol Dial Transplant. 2010;25:1688–1696. doi: 10.1093/ndt/gfq036. [DOI] [PubMed] [Google Scholar]
- 15.Fedorova LV, Raju V, El-Okdi N, et al. The cardiotonic steroid hormone marinobufagenin induces renal fibrosis: implication of epithelial-to-mesenchymal transition. Am J Physiol Renal Physiol. 2009;296:F922–F934. doi: 10.1152/ajprenal.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laffer CL, Bolterman RJ, Romero JC, et al. Effect of salt on isoprostanes in salt-sensitive essential hypertension. Hypertension. 2006;47:434–440. doi: 10.1161/01.HYP.0000202480.06735.82. [DOI] [PubMed] [Google Scholar]
- 17.Bayorh MA, Ganafaa AA, Soccia RR, et al. The role of oxidative stress in salt-induced hypertension. Am J Hypertens. 2004;17:31–36. doi: 10.1016/j.amjhyper.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Fang Y, Mu J-J, He L-C, et al. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt-sensitive Asians. Hypertension. 2006;48:724–729. doi: 10.1161/01.HYP.0000238159.19614.ce. [DOI] [PubMed] [Google Scholar]
- 19.Barker DJP. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contreras RJ, Wong DL, Henderson R, et al. High dietary NaCl early in development enhances mean arterial pressure of adult rats. Physiol Behav. 2000;71:173–181. doi: 10.1016/s0031-9384(00)00331-0. [DOI] [PubMed] [Google Scholar]
- 21.Battista M-C, Oligny LL, St-Louis J, et al. Intrauterine growth restriction in rats is associated with hypertension and renal dysfunction in adulthood. Am J Physiol Endocrinol Metab. 2002;283:E124–E131. doi: 10.1152/ajpendo.00004.2001. [DOI] [PubMed] [Google Scholar]
- 22.Odenwald T, Nakagawa K, Hadtstein C, et al. Acute blood pressure effects and chronic hypotensive action of calcimimetics in uremic rats. J Am Soc Nephrol. 2006;17:655–662. doi: 10.1681/ASN.2005090914. [DOI] [PubMed] [Google Scholar]
- 23.Priyadarshi S, Valentine B, Han C, et al. Effect of green tea extract on cardiac hypertrophy following 5/6 nephrectomy in the rat. Kidney Int. 2003;63:1785–1790. doi: 10.1046/j.1523-1755.2003.00914.x. [DOI] [PubMed] [Google Scholar]
- 24.Koleganova N, Piecha G, Ritz E, et al. A calcimimetic (R-568), but not calcitriol, prevents vascular remodeling in uremia. Kidney Int. 2009;75:60–71. doi: 10.1038/ki.2008.490. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K, Han GD, Miyauchi N, et al. Angiotensin II type 1 and type 2 receptors play opposite roles in regulating the barrier function of kidney glomerular capillary wall. Am J Pathol. 2007;170:1841–1853. doi: 10.2353/ajpath.2007.060484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J, Gu D, Chen J, et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27:48–54. doi: 10.1097/hjh.0b013e328316bb87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roseboom TJ, van der Meulen JHP, Osmond C, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roseboom TJ, van der Meulen JHP, Ravelli ACJ, et al. Blood pressure in adults after prenatal exposure to famine. J Hypertens. 1999;17:325–330. doi: 10.1097/00004872-199917030-00004. [DOI] [PubMed] [Google Scholar]
- 29.Keijzer-Veen MG, Schrevel M, Finken MJ, et al. Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol. 2005;16:2762–2768. doi: 10.1681/ASN.2004090783. [DOI] [PubMed] [Google Scholar]
- 30.Vikse BE, Irgens LM, Leivestad T, et al. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008;19:151–157. doi: 10.1681/ASN.2007020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armitage JA, Lakasing L, Taylor PD, et al. Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J Physiol. 2005;565:171–184. doi: 10.1113/jphysiol.2005.084947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coelho MS, Passadore MD, Gasparetti AL, et al. High- or low-salt diet from weaning to adulthood: effect on body weight, food intake and energy balance in rats. Nutr Metab Cardiovasc Dis. 2006;16:148–155. doi: 10.1016/j.numecd.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Titze J. Water-free sodium accumulation. Semin Dial. 2009;22:253–255. doi: 10.1111/j.1525-139X.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 34.Lopes KL, Furukawa LN, de Oliveira IB, et al. Perinatal salt restriction: a new pathway to programming adiposity indices in adult female Wistar rats. Life Sci. 2008;82:728–732. doi: 10.1016/j.lfs.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Porter JP, King SH, Honeycutt AD. Prenatal high-salt diet in the Sprague-Dawley rat programs blood pressure and heart rate hyperresponsiveness to stress in adult female offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R334–R342. doi: 10.1152/ajpregu.00887.2006. [DOI] [PubMed] [Google Scholar]
- 36.Auger K, Beausejour A, Brochu M, et al. Increased Na+ intake during gestation in rats is associated with enhanced vascular reactivity and alterations of K+ and Ca2+ function. Am J Physiol Heart Circ Physiol. 2004;287:H1848–H1856. doi: 10.1152/ajpheart.00055.2004. [DOI] [PubMed] [Google Scholar]
- 37.Geleijnse JM, Hofman A, Witteman JCM, et al. Long-term effects of neonatal sodium restriction on blood pressure. Hypertension. 1997;29:913–917. doi: 10.1161/01.hyp.29.4.913. [DOI] [PubMed] [Google Scholar]
- 38.Anderson DE, Fedorova OV, Morrell CH, et al. Endogenous sodium pump inhibitors and age-associated increases in salt sensitivity of blood pressure in normotensives. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1248–R1R54. doi: 10.1152/ajpregu.00782.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy DJ, Elkareh J, Shidyak A, et al. Partial nephrectomy as a model for uremic cardiomyopathy in the mouse. Am J Physiol Renal Physiol. 2008;294:F450–F454. doi: 10.1152/ajprenal.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elkareh J, Periyasamy SM, Shidyak A, et al. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: implications for uremic cardiomyopathy. Am J Physiol Renal Physiol. 2009;296:F1219–F1226. doi: 10.1152/ajprenal.90710.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-beta1. Am J Physiol Renal Physiol. 2008;295:F406–F414. doi: 10.1152/ajprenal.90294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ying WZ, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-beta1 in rat aortic endothelium. Am J Physiol. 1999;277:H1293–H1298. doi: 10.1152/ajpheart.1999.277.4.H1293. [DOI] [PubMed] [Google Scholar]
- 43.Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-beta in rats. Am J Physiol. 1998;274:F635–F641. doi: 10.1152/ajprenal.1998.274.4.F635. [DOI] [PubMed] [Google Scholar]
- 44.Giardina JB, Green GM, Rinewalt AN, et al. Role of endothelin B receptors in enhancing endothelium-dependent nitric oxide-mediated vascular relaxation during high salt diet. Hypertension. 2001;37:516–523. doi: 10.1161/01.hyp.37.2.516. [DOI] [PubMed] [Google Scholar]
- 45.Kagota S, Tamashiro A, Yamaguchi Y, et al. Downregulation of vascular soluble guanylate cyclase induced by high salt intake in spontaneously hypertensive rats. Br J Pharmacol. 2001;134:737–744. doi: 10.1038/sj.bjp.0704300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor TA, Pollock JS, Pollock DM. Down-regulation of soluble guanylyl cyclase in the inner medulla of DOCA-salt hypertensive rats. Vascul Pharmacol. 2003;40:155–160. doi: 10.1016/s1537-1891(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 47.Rexhaj E, Bloch J, Jayet PY, et al. Fetal programming of pulmonary vascular dysfunction in mice: role of epigenetic mechanisms. Am J Physiol Heart Circ Physiol. 2011;301:H247–H252. doi: 10.1152/ajpheart.01309.2010. [DOI] [PubMed] [Google Scholar]