Abstract
Background
Whether peritoneal dialysis (PD) treatment leads to greater weight gain than with hemodialysis (HD) and if this limits access of obese end-stage renal disease patients to renal transplantation has not been examined. We undertook this study to determine the interrelationship between body size and initial dialysis modality on transplantation, mortality and weight gain.
Methods
Time to transplantation, time to death and weight gain were estimated in a 1:1 propensity score-matched cohort of incident HD and PD patients treated in facilities owned by DaVita Inc. between 1 July 2001 through 30 June 2006 followed through 30 June 2007 (4008 pairs) in four strata of body mass index (BMI) (<18.5, 18.5–24.99, 25.00–29.99 and ≥30 kg/m2).
Results
Transplantation was significantly more likely in PD patients [adjusted hazards ratio (aHR) 1.48, 95% confidence interval (95% CI) 1.29–1.70]; the probability of receiving a kidney transplant was significantly higher in each strata of BMI >18.5 kg/m2, including with BMI ≥30 kg/m2 (aHR 1.45, 95% CI 1.11–1.89). PD patients had significantly lower all-cause mortality for patients with BMI 18.50–29.99 kg/m2. Both these findings were confirmed on analyses of the entire unmatched incident cohort (PD 4008; HD 58 471). The effect of dialysis modality on weight gain was tested in 687 propensity score-matched pairs; the odds of >2, >5 or >10% weight gain were significantly lower in PD patients.
Conclusion
Treatment with PD is less likely to be associated with a significant weight gain and does not limit the access of obese patients to renal transplantation.
Keywords: hemodialysis, mortality, obesity, peritoneal dialysis, renal transplantation
Introduction
Renal transplantation is the treatment of choice for patients with end-stage renal disease (ESRD) but is limited by shortage of donor organs [1, 2]. Hence, staying or becoming eligible for transplantation is of high importance for many dialysis patients. Several studies have shown that in the USA, patients treated with peritoneal dialysis (PD) are significantly more likely to receive a renal transplant than those treated with hemodialysis (HD) [3, 4]. Whether this premise holds true for all strata of body size has heretofore not been examined. The interrelationship between body size and initial dialysis modality on the probability of receiving a kidney transplant is critically important since the body mass index (BMI) is used by many transplant programs to determine the eligibility of dialysis patients for renal transplantation; many programs will not consider offering renal transplantation to individuals with BMI >30–40 kg/m2 [5]. There is concern that obligatory absorption of glucose will lead to greater weight gain in PD patients than similar patients treated with HD and is one of the reasons why large body size is considered to be a relative contraindication to PD. However, glucose absorption is not the only reason for weight gain in incident PD patients. Anorexia is a cardinal manifestation of uremia and amelioration of uremic anorexia is potentially a significant contributor to weight gain after the initiation of dialysis. Several studies have demonstrated that initiation of HD is associated with significant weight gain in the subsequent few months [6, 7]. There is a paucity of data examining the effect of initial dialysis modality on weight gain and subsequent transplantation. Only one earlier study has compared weight changes in incident PD and HD patients (118 and 132 patients, respectively) and found no significant difference between changes in BMI in patients treated with either dialysis modality over 2 years of follow-up [8]; there are no data on whether initial dialysis modality limits access of obese PD patients to transplantation. We undertook this study to bridge this gap in our knowledge and to test the hypothesis that initial treatment with PD does not limit access to transplantation for incident dialysis patients irrespective of body size, using a large, contemporary and nationally representative cohort. Additional analyses were undertaken to determine the association of dialysis modality with all-cause mortality and weight gain.
Materials and methods
Data source
The electronic records of all ESRD patients who received dialysis treatment in one of the 580 facilities owned by DaVita Inc. between 1 July 2011 through 30 June 2006 constituted the primary data source. These data were merged with those from the United States Renal Data System (USRDS). Information on date of first dialysis treatment, baseline body weight, BMI and comorbidities was obtained from the MEDEVID file of the USRDS—the file contains information from Medical Evidence form 2728, a form that is completed at the time of first dialysis treatment for all patients in the USA. This form also supplied information on race/ethnicity, marital status and primary insurance. The presence of diabetes, baseline laboratory data and follow-up body weights were ascertained using data from DaVita Inc. Follow-up body weights represented averages of all values available for a 13-week period. The policy on how to measure the body weight of PD patients—with or without intra-abdominal dialysate—was set by each individual facility. Data on events (transplant and death) were obtained from the USRDS through 30 June 2007.
The study was approved as exempt by the Institutional Research Board of the Los Angeles Biomedical Research Institute at Harbor-UCLA.
Patients
Between 1 July 2011 and 30 June 2006, 162 032 patients received dialysis treatment in facilities owned by DaVita Inc. Of these, 12 725 patients were excluded either because the recorded age was <18 or >99 years or data on their age were missing. Furthermore, patients without data on dialysis modality on Day 90 and patients with a renal transplant before dialysis initiation were excluded (n = 15 435). From among these 133 892 patients, 63 921 were identified as being incident patients (entry into the study cohort within 10 days of start of dialysis). Subjects with missing data on baseline BMI, BMI <12 or >61 or with data missing for any one of the variables used for propensity matching (see below) were excluded (n = 1274; 2%). The first dialysis modality on or after Day 90 with which the patient received continuous treatment for at least 60 days was defined as the initial dialysis modality; of the 62 647 subjects, the initial dialysis modality was defined as PD for 4028 and HD for 58 619.
A logistic regression model with PD as initial modality as the outcome and age, gender, race, diabetic status, day of first dialysis and state of residence as predictor variables was built. This model was used to calculate probability of each patient being treated with PD (propensity scores). The entire cohort was divided into four groups based upon the baseline BMI (<18.50, 18.50–24.99, 25.00–29.99 and ≥30). Within each strata of BMI, propensity scores were used to identify one HD patient for each PD patient. The propensity score matching was limited to 4008 PD patients who had information on date of renal transplantation available. The 4008 propensity-matched pairs constituted the primary study cohort. A sensitivity analysis was performed in the entire unmatched cohort.
For the secondary analysis, only patients with at least one follow-up body weight in quarters 3, 4 or 5 were included (PD 696; HD 37 635). The change in body weight from baseline to the first available body weight between 9 and 15 months of start of dialysis was calculated and expressed as an annualized percentage of baseline body weight. Nine PD patients with <−40 or >40% annualized change in body weight were excluded. A second propensity-matched cohort was built by matching one HD patient for each of the 687 PD patients in the same strata of body size. A sensitivity analysis was performed in the entire cohort with follow-up body weights available (PD 687; HD 36 994).
Statistics
Missing covariate data were imputed by the mean or median of the existing values, as appropriate. Intent-to-treat time-to-event survival analyses were performed to determine the association of initial dialysis modality with subsequent transplantation in each of the four strata of body size and in the entire propensity score-matched cohort (4008 pairs). Similar time-to-event survival analyses were performed for all-cause mortality as the outcome. A sensitivity analysis was performed to determine the association of initial dialysis modality with transplantation or death, in which the cohort was divided into five strata of BMI (<18.50, 18.50–24.99, 25.00–29.99, 30.00–34.99 and ≥35). Logistic regression analyses were performed to determine the odds of >2, >5 or >10% annualized change in body weight in the 687 PD patients (reference: propensity score-matched HD cohort).
For each analysis, three separate models were examined:
Minimally adjusted model that included initial dialysis modality and entry calendar quarter (q1–q20);
Case-mix adjusted model that included the above plus age, gender, race and ethnicity (whites, blacks, Asians, Hispanics and others), nine pre-existing comorbid states (congestive heart failure, diabetes, hypertension, cancer, chronic obstructive pulmonary disease, peripheral vascular disease, cerebrovascular disease, atherosclerotic heart disease and other cardiac disease), history of tobacco smoking, primary insurance (Medicare, Medicaid and other) and marital status (married, single, divorced, widowed and other);
Case-mix and laboratory-adjusted model which included all mentioned covariates as well as serum levels of total iron-binding capacity, albumin, ferritin, creatinine, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, white blood cell count, lymphocyte percentage and hemoglobin. In the logistic regression analysis, baseline BMI was also included as an additional covariate.
All analyses were carried out with SAS, version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Patient characteristics
Table 1 summarizes the baseline characteristics of the 4008 propensity score-matched pairs of incident HD and PD patients stratified by BMI group and dialysis modality. In general, HD patients were more likely to be black, have Medicare or Medicaid as their primary insurance, have chronic obstructive pulmonary disease and peripheral vascular disease but less likely to be Asian or have hypertension. In subjects with baseline BMI <18.5 kg/m2, the prevalence of cardiovascular disease was lower among HD patients; in all other strata of body size, cardiovascular comorbidity was more frequent in HD patients. HD patients generally had a higher serum creatinine, ferritin, phosphorus and white blood cell count but a slightly lower total iron-binding capacity and hemoglobin compared to those treated with PD. In patients with BMI >30 kg/m2, the baseline body weight and BMI were higher for HD patients than for PD patients.
Table 1.
Baseline patient characteristics of the propensity-matched study cohort, stratified by BMI and dialysis modalitya
| BMI (kg/m2) |
<18.5 |
18.5–24.99 |
25–29.99 |
>30 |
||||
|---|---|---|---|---|---|---|---|---|
| Modality (n) | PD | HD | PD | HD | PD | HD | PD | HD |
| Number of subjects | 118 | 118 | 1419 | 1419 | 1304 | 1304 | 1167 | 1167 |
| Age (years) | 54 ± 17 | 53 ± 20 | 58 ± 17 | 58 ± 18 | 59 ± 15 | 59 ± 16 | 56 ± 13 | 57 ± 14 |
| >65 years old (%) | 30 | 34 | 41 | 40 | 38 | 38 | 26 | 29 |
| Gender (% female) | 68 | 69 | 45 | 45 | 41 | 42 | 51 | 51 |
| Diabetes mellitus (%) | 26 | 28 | 43 | 42 | 58 | 59 | 69 | 70 |
| Race and/or ethnicity (%) | ||||||||
| Caucasians | 55 | 53 | 55 | 55 | 56 | 55 | 56 | 56 |
| Blacks | 14 | 28 | 16 | 22 | 17 | 22 | 26 | 26 |
| Hispanics | 10 | 10 | 14 | 14 | 15 | 16 | 11 | 13 |
| Asians | 12 | 3 | 8 | 4 | 4 | 2 | 2 | 1 |
| Others | 8 | 5 | 7 | 5 | 7 | 5 | 5 | 4 |
| Primary insurance (%) | ||||||||
| Medicare | 52 | 58 | 55 | 58 | 51 | 58 | 49 | 55 |
| Medicaid | 8 | 7 | 3 | 8 | 3 | 7 | 2 | 6 |
| Others | 39 | 35 | 41 | 34 | 46 | 35 | 49 | 39 |
| Comorbidities (%) | ||||||||
| Alcohol dependence | 2 | 3 | 0 | 2 | 0 | 2 | 0 | 1 |
| Atherosclerotic heart disease | 8 | 14 | 17 | 18 | 17 | 22 | 18 | 19 |
| Malignant neoplasm (cancer) | 4 | 3 | 5 | 5 | 4 | 6 | 4 | 3 |
| Congestive heart failure | 19 | 28 | 18 | 25 | 18 | 27 | 20 | 34 |
| Chronic obstructive pulmonary disease | 6 | 8 | 4 | 6 | 4 | 6 | 3 | 6 |
| Cerebrovascular disease | 6 | 5 | 6 | 7 | 6 | 7 | 5 | 6 |
| History of hypertension | 81 | 68 | 81 | 79 | 84 | 81 | 86 | 81 |
| Inability to ambulate | 4 | 3 | 2 | 3 | 1 | 3 | 1 | 5 |
| Other cardiac disease | 6 | 1 | 5 | 7 | 5 | 5 | 4 | 7 |
| Peripheral vascular disease | 5 | 11 | 8 | 11 | 9 | 12 | 10 | 11 |
| Current smokers | 11 | 8 | 6 | 7 | 4 | 5 | 5 | 4 |
| Weight (kg) | 48 ± 7 | 48 ± 6 | 64 ± 9 | 63 ± 9 | 78 ± 10 | 78 ± 11 | 98 ± 17 | 103 ± 20 |
| BMI (kg/m2) | 17 ± 1 | 17 ± 1 | 22 ± 2 | 22 ± 2 | 27 ± 1 | 27 ± 1 | 35 ± 5 | 37 ± 6 |
| Serum albumin (g/dL) | 3.5 ± 0.5 | 3.5 ± 0.6 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.5 ± 0.5 | 3.6 ± 0.5 | 3.6 ± 0.4 | 3.6 ± 0.5 |
| Creatinine (mg/dL) | 6.1 ± 2.8 | 6.6 ± 3.0 | 6.6 ± 2.8 | 7.1 ± 3.1 | 6.9 ± 2.7 | 7.2 ± 3.1 | 7.1 ± 3.0 | 7.1 ± 3.0 |
| Ferritin (ng/mL) | 434 ± 804 | 439 ± 634 | 283 ± 343 | 369 ± 409 | 274 ± 394 | 371 ± 458 | 250 ± 256 | 325 ± 336 |
| TIBC (mg/dL) | 237 ± 51 | 203 ± 47 | 245 ± 50 | 214 ± 47 | 251 ± 48 | 219 ± 49 | 253 ± 51 | 221 ± 48 |
| Calcium (mg/dL) | 9.1 ± 0.7 | 9.0 ± 0.6 | 9.1 ± 0.7 | 9.1 ± 0.7 | 9.1 ± 0.7 | 9.1 ± 0.7 | 9.2 ± 0.7 | 9.1 ± 0.7 |
| Phosphorus (mg/dL) | 5.0 ± 1.4 | 5.4 ± 1.5 | 4.8 ± 1.2 | 5.6 ± 1.4 | 4.9 ± 1.2 | 5.6 ± 1.4 | 5.2 ± 1.3 | 5.6 ± 1.4 |
| PTH (pg/mL) | 367 ± 591 | 386 ± 398 | 351 ± 343 | 288 ± 280 | 367 ± 326 | 322 ± 317 | 390 ± 336 | 343 ± 311 |
| Alkaline phosphatase (U/L) | 148 ± 156 | 140 ± 142 | 113 ± 77.8 | 116 ± 78 | 109 ± 69 | 116 ± 78 | 107 ± 48 | 116 ± 84 |
| Hemoglobin (g/dL) | 12.3 ± 1.7 | 12.1 ± 1.5 | 12.5 ± 1.5 | 12.2 ± 1.4 | 12.5 ± 1.4 | 12.3 ± 1.4 | 12.1 ± 1.4 | 12.0 ± 1.4 |
| WBC (×103/μL) | 7.4 ± 2.3 | 8.0 ± 4.5 | 7.3 ± 2.6 | 7.6 ± 2.6 | 7.4 ± 2.4 | 7.7 ± 2.5 | 8.0 ± 2.5 | 8.0 ± 2.6 |
| % Lymphocyte | 20 ± 8 | 20 ± 8 | 19 ± 8 | 20 ± 8 | 19 ± 7 | 20 ± 8 | 19 ± 7 | 20 ± 8 |
aPTH, parathyroid hormone; TIBC, total iron-binding capacity; WBC, white blood cell count.
Relationship of initial dialysis modality on time to transplantation in strata based upon baseline BMI
Events were ascertained through 30 June 2007. In the propensity score-matched cohort, PD patients were significantly more likely to receive a renal transplant [adjusted hazards ratio (aHR) 1.48, 95% confidence interval (95% CI) 1.29–1.70] (Table 2). The significantly higher probability of PD patients to receive a renal transplant was seen in every strata of body size, except among individuals with a baseline BMI of <18.5 kg/m2 (Table 2 and Figure 1). The same trend for a significantly higher probability of renal transplantation was seen in the analyses using the entire unmatched cohort (Table 2).
Table 2.
HR of renal transplantation in PD patients (reference: incident HD patients), stratified by BMIa
| PD |
HD |
HR (95% CI) (ref. HD) |
|||||
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | n | % Transplant | n | % Transplant | Minimally adjusted | Case-mix adjusted | Case-mix and laboratory adjusted |
| Propensity score-matched cohort | |||||||
| <18.50 | 118 | 15 | 118 | 11 | 1.54 (0.72–3.72) | 1.27 (0.39–4.10) | 1.34 (0.20–8.95) |
| 18.50–24.99 | 1419 | 19 | 1419 | 12 | 1.53 (1.26–1.85) | 1.34 (1.11–1.63) | 1.41 (1.13–1.77) |
| 25.00–29.99 | 1304 | 20 | 1304 | 11 | 1.81 (1.47–2.23) | 1.64 (1.31–2.04) | 1.60 (1.25–2.04) |
| ≥30.00 | 1167 | 15 | 1167 | 11 | 1.49 (1.18–1.87) | 1.41 (1.11–1.79) | 1.45 (1.11–1.89) |
| Total | 4008 | 18 | 4008 | 11 | 1.59 (1.42–1.79) | 1.46 (1.29–1.65) | 1.48 (1.29–1.70) |
| Unmatched incident cohort | |||||||
| <18.50 | 118 | 15 | 2460 | 5 | 2.44 (1.48–4.04) | 1.08 (0.63–1.84) | 0.98 (0.55–1.76) |
| 18.50–24.99 | 1419 | 19 | 21 358 | 8 | 2.36 (2.08–2.69) | 1.33 (1.17–1.52) | 1.28 (1.12–1.48) |
| 25.00–29.99 | 1304 | 20 | 16 849 | 8 | 2.37 (2.08–2.71) | 1.39 (1.21–1.59) | 1.34 (1.16–1.56) |
| ≥30.00 | 1167 | 15 | 17 804 | 7 | 2.05 (1.75–2.39) | 1.39 (1.18–1.62) | 1.40 (1.18–1.65) |
| Total | 4008 | 18 | 58 471 | 8 | 2.29 (2.11–2.47) | 1.38 (1.27–1.49) | 1.32 (1.21–1.44) |
aMinimally adjusted model included initial dialysis modality and entry calendar quarter (q1–q20); case-mix adjusted model included the above plus age, gender, race and ethnicity (whites, blacks, Asians, Hispanics and others), nine pre-existing comorbid states (congestive heart failure, diabetes, hypertension, cancer, chronic obstructive pulmonary disease, peripheral vascular disease, cerebrovascular disease, atherosclerotic heart disease and other cardiac disease), history of tobacco smoking, primary insurance (Medicare, Medicaid and other) and marital status (married, single, divorced, widowed and other); case-mix and laboratory-adjusted model which included all mentioned covariates as well as serum levels of total iron-binding capacity, albumin, ferritin, creatinine, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, white blood cell count, lymphocyte percentage and hemoglobin.
Fig. 1.
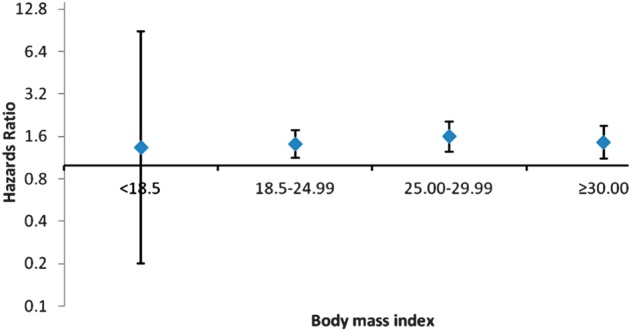
HRs for receiving a renal transplant for PD patients (n = 4008) stratified by BMI, with propensity score-matched HD patients as a reference group. Shown are the fully adjusted HRs, with a 95% CI.
Relationship of initial dialysis modality on time to death in strata based upon baseline BMI
In the propensity score-matched cohort, the HR of all-cause mortality was significantly lower in PD patients (aHR 0.88, 95% CI 0.81–0.95) (Table 3). In analyses stratified by body size, the HR of all-cause mortality was significantly lower in individuals with baseline BMI of 18.50–29.99 kg/m2 (Table 3 and Figure 2). There was no significant difference in the risk for death in patients with baseline BMI <18.50 or >29.99 kg/m2 treated with either dialysis modality. The same trend was seen in the analysis using the entire unmatched cohort (Table 3).
Table 3.
HR of death in PD patients (reference: incident HD patients), stratified by BMIa
| PD |
HD |
HR (95% CI) (ref. HD) |
|||||
|---|---|---|---|---|---|---|---|
| n | % Death | n | % Death | Minimally adjusted | Case-mix adjusted | Case-mix and laboratory adjusted | |
| Propensity score-matched cohort | |||||||
| <18.50 | 118 | 42 | 118 | 42 | 0.83 (0.55–1.27) | 0.71 (0.45–1.15) | 0.56 (0.30–1.04) |
| 18.50–24.99 | 1419 | 37 | 1419 | 46 | 0.81 (0.72–0.91) | 0.87 (0.77–0.98) | 0.80 (0.69–0.92) |
| 25.00–29.99 | 1304 | 34 | 1304 | 44 | 0.78 (0.69–0.89) | 0.85 (0.74–0.97) | 0.81 (0.70–0.94) |
| ≥30.00 | 1167 | 35 | 1167 | 42 | 0.93 (0.81–1.06) | 1.05 (0.91–1.21) | 1.08 (0.92–1.25) |
| Total | 4008 | 36 | 4008 | 39 | 0.83 (0.78–0.89) | 0.91 (0.85–0.98) | 0.88 (0.81–0.95) |
| Unmatched incident cohort | |||||||
| <18.50 | 118 | 42 | 2460 | 59 | 0.60 (0.45–0.79) | 0.84 (0.63–1.12) | 0.79 (0.59–1.06) |
| 18.50–24.99 | 1419 | 37 | 21 358 | 50 | 0.67 (0.61–0.73) | 0.85 (0.77–0.92) | 0.77 (0.71–0.85) |
| 25.00–29.99 | 1304 | 34 | 16 849 | 44 | 0.74 (0.67–0.81) | 0.90 (0.81–0.99) | 0.87 (0.79–0.97) |
| ≥30.00 | 1167 | 35 | 17 804 | 38 | 0.91 (0.82–1.00) | 1.10 (1.00–1.22) | 1.02 (0.92–1.13) |
| Total | 4008 | 36 | 58 471 | 45 | 0.75 (0.71–0.79) | 0.92 (0.87–0.97) | 0.86 (0.81–0.91) |
aMinimally adjusted model included initial dialysis modality and entry calendar quarter (q1–q20); case-mix adjusted model included the above plus age, gender, race and ethnicity (whites, blacks, Asians, Hispanics and others), nine pre-existing comorbid states (congestive heart failure, diabetes, hypertension, cancer, chronic obstructive pulmonary disease, peripheral vascular disease, cerebrovascular disease, atherosclerotic heart disease and other cardiac disease), history of tobacco smoking, primary insurance (Medicare, Medicaid and other) and marital status (married, single, divorced, widowed and other); case-mix and laboratory-adjusted model which included all mentioned covariates as well as serum levels of total iron-binding capacity, albumin, ferritin, creatinine, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, white blood cell count, lymphocyte percentage and hemoglobin.
Fig. 2.
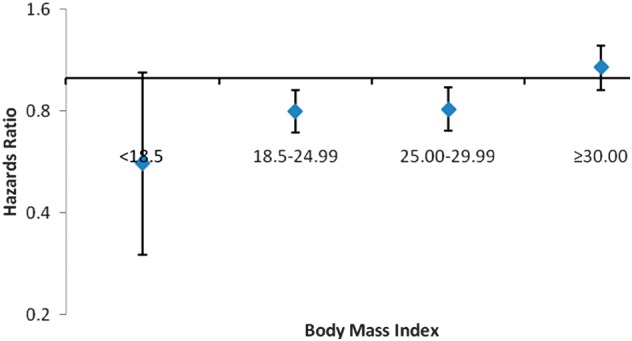
HRs for death for PD patients (n = 4008) stratified by BMI, with propensity score-matched HD patients as a reference group. Shown are the fully adjusted HRs, with a 95% CI.
Relationship of initial dialysis modality on probability of weight gain
This analysis was performed in 687 propensity score-matched HD–PD pairs in whom at least one follow-up body weight was available between 9 and 15 months of first dialysis treatment (third, fourth or fifth quarter). Table 4 summarizes the baseline characteristics of HD and PD patients in this second propensity-matched cohort and compares them with PD patients that were excluded due to missing data (baseline body weight, follow-up weight in quarters 3, 4 or 5 or because of extreme values of annualized weight change). PD patients excluded from the analysis were less frequently diabetic, more frequently white and more likely to suffer from an unspecified cardiac disease than PD patients included in the analysis. The baseline BMI, serum creatinine and phosphorus of excluded patients were lower and hemoglobin higher than in patients included in the analysis.
Table 4.
Baseline patient characteristics of the propensity-matched cohort of HD and PD patients with follow-up weights available and of PD patients with no follow-up weight availablea
| With follow-up weight |
Without follow-up weight | ||
|---|---|---|---|
| HD | PD | PD | |
| Number of subjects | 687 | 687 | 3402 |
| Age (years) | 57 ± 16 | 58 ± 15 | 58 ± 15 |
| >65 years old (%) | 36 | 34 | 35 |
| Gender (% female) | 41 | 47 | 46 |
| Diabetes mellitus (%) | 63 | 61 | 54 |
| Race and/or ethnicity (%) | |||
| Caucasians | 49 | 52 | 57 |
| Blacks | 28 | 23 | 19 |
| Hispanics | 15 | 14 | 13 |
| Asians | 3 | 4 | 6 |
| Others | 6 | 6 | 6 |
| Primary insurance (%) | |||
| Medicare | 58 | 51 | 52 |
| Medicaid | 7 | 3 | 3 |
| Others | 35 | 46 | 45 |
| Comorbidities (%) | |||
| Alcohol dependence | 2 | 1 | 0 |
| Atherosclerotic heart disease | 21 | 17 | 17 |
| Malignant neoplasm (cancer) | 4 | 4 | 4 |
| Congestive heart failure | 28 | 19 | 18 |
| Chronic obstructive pulmonary disease | 5 | 3 | 4 |
| Cerebrovascular disease | 7 | 6 | 6 |
| History of hypertension | 79 | 84 | 83 |
| Inability to ambulate | 2 | 1 | 1 |
| Other cardiac disease | 4 | 3 | 6 |
| Peripheral vascular disease | 10 | 8 | 9 |
| Current smokers | 6 | 5 | 5 |
| Weight (kg) | 80 ± 22 | 80 ± 19 | 78 ± 20 |
| BMI (kg/m2) | 28 ± 7 | 28 ± 6 | 27 ± 6 |
| Serum albumin (g/dL) | 3.6 ± 0.5 | 3.5 ± 0.5 | 3.5 ± 0.5 |
| Creatinine (mg/dL) | 7.2 ± 2.9 | 7.1 ± 3.0 | 6.7 ± 2.8 |
| Ferritin (ng/mL) | 322 ± 287 | 279 ± 302 | 273 ± 371 |
| TIBC (mg/dL) | 219 ± 46 | 242 ± 53 | 251 ± 49 |
| Calcium (mg/dL) | 9.1 ± 0.7 | 9.1 ± 0.8 | 9.1 ± 0.7 |
| Phosphorus (mg/dL) | 5.6 ± 1.5 | 5.2 ± 1.4 | 4.9 ± 1.2 |
| PTH (pg/mL) | 319 ± 296 | 370 ± 356 | 368 ± 343 |
| Alkaline Phosphatase (U/L) | 121 ± 93 | 112 ± 85 | 111 ± 68 |
| Hemoglobin (g/dL) | 12.2 ± 1.3 | 12.1 ± 1.5 | 12.4 ± 1.5 |
| WBC (×103/μL) | 7.7 ± 2.4 | 7.6 ± 2.6 | 7.5 ± 2.5 |
| % Lymphocyte | 20 ± 8 | 19 ± 8 | 19 ± 7 |
aPTH, parathyroid hormone; TIBC, total iron-binding capacity; WBC, white blood cell count.
In the matched HD and PD cohort, the former were more often male, primarily insured with Medicare and black. HD patients were also more likely to suffer from atherosclerotic heart disease, congestive heart failure and peripheral vascular disease but less likely to suffer from hypertension. The serum ferritin and phosphorus were higher, but the total iron-binding capacity and parathyroid hormone levels were lower for HD patients.
Patients whose initial dialysis modality was PD were significantly less likely to have significant annualized weight gain than whose initial dialysis modality was HD (Table 5). In the analysis using the unmatched cohort, the odds of weight gain over 2% were significantly lower for patients treated with PD; however, the odds ratios for weight gain over 5 or 10% were no longer significant (Table 5).
Table 5.
Odds ratio of significant weight gain in PD patients (reference: incident HD patients)a
| PD |
HD |
Odds ratio (95% CI) (ref. HD) |
|||||
|---|---|---|---|---|---|---|---|
| Weight gain | n | % | n | % | Minimally adjusted | Case-mix adjusted | Case-mix and laboratory adjusted |
| Propensity score-matched cohort (687 pairs) | |||||||
| >2% | 170 | 25 | 211 | 31 | 0.74 (0.58–0.94) | 0.73 (0.57–0.95) | 0.69 (0.52–0.91) |
| >5% | 115 | 17 | 150 | 22 | 0.71 (0.54–0.94) | 0.70 (0.53–0.94) | 0.63 (0.46–0.88) |
| >10% | 51 | 7 | 82 | 12 | 0.61 (0.42–0.88) | 0.61 (0.42–0.91) | 0.58 (0.37–0.89) |
| Unmatched incident cohort (PD 687; HD 36 994) | |||||||
| >2% | 170 | 25 | 10 957 | 30 | 0.78 (0.66–0.93) | 0.78 (0.65–0.93) | 0.82 (0.69–0.99) |
| >5% | 115 | 17 | 7322 | 20 | 0.82 (0.67–1.00) | 0.83 (0.68–1.02) | 0.88 (0.72–1.09) |
| >10% | 51 | 7 | 3575 | 10 | 0.75 (0.57–1.00) | 0.78 (0.58–1.04) | 0.82 (0.61–1.10) |
aMinimally adjusted model included initial dialysis modality and entry calendar quarter (q1–q20); case-mix adjusted model included the above plus age, gender, race and ethnicity (whites, blacks, Asians, Hispanics and others), BMI, nine pre-existing comorbid states (congestive heart failure, diabetes, hypertension, cancer, chronic obstructive pulmonary disease, peripheral vascular disease, cerebrovascular disease, atherosclerotic heart disease and other cardiac disease), history of tobacco smoking, primary insurance (Medicare, Medicaid and other) and marital status (married, single, divorced, widowed and other); Case-mix and laboratory-adjusted model which included all mentioned covariates as well as serum levels of total iron-binding capacity, albumin, ferritin, creatinine, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, white blood cell count, lymphocyte percentage and hemoglobin.
Sensitivity analysis
When the cohort was divided into five strata, PD patients with BMI between 18.50 and 34.99 kg/m2 were significantly more likely to receive a transplant in each subgroup; in individuals with BMI <18.50 or ≥35 kg/m2, there was no relationship between dialysis modality and time to transplantation (Table 6). Furthermore, there was no difference in death risk in patients with BMI <18.5 or >30 kg/m2.
Table 6.
HR of renal transplantation or death in PD patients (reference: propensity score-matched incident HD patients), divided into five strata of BMIa
| Number of PD–HD pairs | Transplantation | Death | |
|---|---|---|---|
| <18.50 | 118 | 1.34 (0.20–8.95) | 0.56 (0.30–1.04) |
| 18.50–24.99 | 1419 | 1.41 (1.13–1.77) | 0.80 (0.69–0.92) |
| 25.00–29.99 | 1304 | 1.60 (1.25–2.04) | 0.81 (0.70–0.94) |
| 30.00–34.99 | 725 | 1.77 (1.29–2.44) | 0.91 (0.73–1.12) |
| >35.00 | 440 | 1.10 (0.65–1.86) | 0.97 (0.74–1.27) |
| Total | 4008 | 1.48 (1.29–1.70) | 0.88 (0.81–0.95) |
aData are derived from case-mix and laboratory-adjusted models which included initial dialysis modality, entry calendar quarter (q1–q20), age, gender, race and ethnicity (whites, blacks, Asians, Hispanics and others), BMI, nine pre-existing comorbid states (congestive heart failure, diabetes, hypertension, cancer, chronic obstructive pulmonary disease, peripheral vascular disease, cerebrovascular disease, atherosclerotic heart disease and other cardiac disease), history of tobacco smoking, primary insurance (Medicare, Medicaid and other) and marital status (married, single, divorced, widowed and other), serum levels of total iron-binding capacity, albumin, ferritin, creatinine, calcium, phosphorus, alkaline phosphatase, parathyroid hormone, white blood cell count, lymphocyte percentage and hemoglobin.
Discussion
Obesity is often considered a relative contraindication to PD, in part, because of concern about weight gain and its effect on patients' subsequent eligibility for renal transplantation. However, to our knowledge, there are no previous studies that have tested the interrelationship of body size and initial dialysis modality to the probability of transplantation. In this analysis of a contemporary and nationally representative propensity score-matched cohort of incident patients, PD patients had a significantly higher probability of transplantation in most strata of body size and the HR for obese patients was of the same magnitude as seen for the entire PD cohort. Moreover, contrary to conventional wisdom, HD patients were significantly more likely to gain significant body weight after initiation of dialysis than a propensity score-matched cohort of PD patients. These findings have significant implications for informing patients about selection of dialysis modality, particularly those with obesity.
For medically eligible patients, renal transplantation is the treatment of choice for patients with ESRD [1, 2]. However, shortage of organs has resulted in progressively longer waiting times and in some parts of the USA, the waiting times approach 8–10 years. It is, thus, important to ensure that interested patients maintain their eligibility for transplantation. The prevalence of obesity is increasing more rapidly in the incident US dialysis population than in the population-at-large and the incidence of wound complications increases with increasing body size [9, 10]. Hence, most transplant programs would not offer renal transplantation to patients if their BMI exceeds 30–40 kg/m2 [5]. Thus, whether the pre-transplant dialysis modality affects access to transplantation is a very relevant but previously unanswered clinical question. Previous studies from the USA have consistently shown that PD patients have a higher probability of transplantation than those treated with HD [3, 4]. The underlying reasons for a higher transplantation rate in incident PD patients may, in part, be related to the lower likelihood of medical eligibility of incident HD patients. While we were unable to determine the medical eligibility of individual patients, we sought to minimize the differences in patients treated with the two different modalities by using a propensity score-matched cohort. The 46% higher adjusted probability of transplantation in the incident PD cohort herein is of the same magnitude as seen in the entire national incident cohort. This higher probability was seen in every strata of body size such that PD patients with baseline BMI ≥30 kg/m2 had a 48% higher probability of receiving a transplant than similar patients treated with HD. Our sensitivity analysis indicates that treatment with PD did not disadvantage even the largest individuals starting dialysis therapy (BMI >35.00 kg/m2). Even though we cannot exclude residual confounding, it seems reasonable to conclude that treatment with PD does not limit the access of obese patients to transplantation any more than treatment with HD.
It is the concern about the contribution of obligatory glucose absorption from the peritoneal dialysate to the weight gain that makes some questions about the appropriateness of PD for obese incident dialysis patients. Surprisingly, the probability of significant weight gain within the first year after initiation of dialysis was significantly higher in the propensity score-matched HD patients than those treated with PD. This finding is consistent with our observation of no effect of body size on the higher probability of renal transplantation in patients treated with PD. Weight gain in dialysis patients could be secondary to either accumulation of fluid in the extracellular space or increase in body fat or edema-free, fat-free mass. Our study was not designed to elucidate which of these reasons was the dominant cause for a higher probability of weight gain in HD patients. Observational studies have indicated that the residual renal function declines faster in patients treated with HD than those with PD [11, 12]; it is conceivable that differential loss of residual renal function may have led to a greater degree of volume expansion and thus, weight gain in HD patients. Alternatively, delay in gastric emptying and/or suppression of appetite seen with the intra-peritoneal instillation of dialysate may limit the weight gain in PD patients despite a similar amelioration of uremic anorexia and the effect of obligatory glucose absorption in these patients [13, 14]. It is also conceivable that PD patients started dialysis at a higher level of native renal function, and thus, were less uremic than HD patients. However, in the USA, the estimated renal function at the time of start of dialysis is similar for PD and HD patients [4]. Differences in trajectory of weight change may have been accounted by differences in achieved solute clearances. However, we did not have access to adequacy data for PD patients and are unable to confirm this possibility. Notwithstanding the underlying reasons, our study does not support the notion that treatment with PD is associated with a significantly greater weight gain than with HD. In the only other multi center comparative study, albeit with a significantly smaller sample size, there was no significant difference in the change in BMI over two years after the start of dialysis in patients treated with either dialysis modality [8].
A large number of studies have evaluated the relationship between body size and mortality in dialysis patients. While the death risk in HD patients is inversely related to body size, the results are less consistent for PD patients [15]. Very few studies have evaluated the interrelationship between body size, dialysis modality and all-cause mortality. Analyses of data from the Australia and New Zealand registry (ANZDATA) have consistently shown a higher risk for death in obese patients treated with PD [16]. Similarly, analyses of data of patients treated between 1995 and 1997 in the USA showed a higher risk for death for patients with the highest quintile of BMI treated with PD [17]. However, since the 1990s, the improvements in outcomes of PD patients have outpaced those seen with HD in the USA and in the most recent cohorts, there is no significant difference in overall mortality of incident HD and PD patients [18, 19]. As distinct from the studies using the data only from the USRDS, this study of a contemporary cohort of patients allowed us to adjust effectively not only for case-mix but also for a variety of laboratory measures. The overall death risk was lower for patients treated with PD and there was no significant difference in the death risk for obese patients treated with either dialysis modality. It, thus, appears reasonable to conclude that in contemporary cohorts, the survival of obese patients treated with PD is similar to that seen in those treated with HD.
The findings of our study have to be interpreted in light of its limitations. The assignment of patients to any dialysis modality is non-random and as discussed above, despite propensity score matching, residual confounding cannot be excluded. Thus, it is as likely that the differences in outcomes are attributable to the patients who selected the dialysis modality and not the effects of any given therapy. Follow-up data on body weight were available only for <20% of the incident cohort limiting the external validity of these findings. However, the findings on weight gain were only used to corroborate the primary findings of a higher probability of transplantation in patients treated with PD, irrespective of body size. Furthermore, the sample size was not large enough for us to examine the probability of significant weight gain in each strata of body size. Finally, it is likely that the follow-up body weight in PD patients was measured with intra-peritoneal dialysate, while the baseline weight reported on Medical Evidence Form 2728 was not. This makes our findings even more significant since this would have led us to overestimate the weight gain in PD patients.
In conclusion, in this propensity-matched cohort analysis, obese incident dialysis patients treated with PD had a significantly higher probability of receiving a transplant and similar overall survival compared to a propensity score-matched cohort of HD patients. Thus, the concern that treatment with PD is more likely to lead to significant weight gain and limits the access of obese ESRD patients to transplantation does not appear to be justified. Hence, obese patients should be free to choose the dialysis modality that best fits their lifestyle.
Conflict of interest statement
None declared.
Acknowledgements
Funding source. The study was supported by research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health R21 DK 077341 (K.K.Z. and R.M.), a philanthropist grant from Mr Harold Simmons (K.K.Z.) and research grants from DaVita Clinical Research (K.K.Z. and R.M.). R.M. has received grant support and honoraria from Baxter Healthcare.
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.McDonald SP, Russ GR. Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991–2001. Nephrol Dial Transplant. 2002;17:2212–2219. doi: 10.1093/ndt/17.12.2212. [DOI] [PubMed] [Google Scholar]
- 3.Snyder JJ, Kasiske BL, Gilbertson DT, et al. A comparison of transplant outcomes in peritoneal and hemodialysis patients. Kidney Int. 2002;62:1423–1430. doi: 10.1111/j.1523-1755.2002.kid563.x. [DOI] [PubMed] [Google Scholar]
- 4.Mehrotra R, Chiu YW, Kalantar-Zadeh K, et al. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med. 2011;171:110–118. doi: 10.1001/archinternmed.2010.352. [DOI] [PubMed] [Google Scholar]
- 5.Potluri K, Hou S. Obesity in kidney transplant recipients and candidates. Am J Kidney Dis. 2010;56:143–156. doi: 10.1053/j.ajkd.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Mehrotra R, Berman N, Alistwani A, et al. Improvement of nutritional status after initiation of maintenance hemodialysis. Am J Kidney Dis. 2002;40:133–142. doi: 10.1053/ajkd.2002.33922. [DOI] [PubMed] [Google Scholar]
- 7.Pupim LB, Kent P, Caglar K, et al. Improvement in nutritional parameters after initiation of chronic hemodialysis. Am J Kidney Dis. 2002;40:143–151. doi: 10.1053/ajkd.2002.33923. [DOI] [PubMed] [Google Scholar]
- 8.Jager KJ, Merkus MP, Huisman RM, et al. Nutritional status over time in hemodialysis and peritoneal dialysis. J Am Soc Nephrol. 2001;12:1272–1279. doi: 10.1681/ASN.V1261272. [DOI] [PubMed] [Google Scholar]
- 9.Kramer HJ, Saranathan A, Luke A, et al. Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol. 2006;17:1453–1459. doi: 10.1681/ASN.2005111241. [DOI] [PubMed] [Google Scholar]
- 10.Lynch RJ, Ranney DN, Shijie C, et al. Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg. 2009;250:1014–1020. doi: 10.1097/SLA.0b013e3181b4ee9a. [DOI] [PubMed] [Google Scholar]
- 11.Lysaght MJ, Vonesh EF, Gotch F, et al. The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans. 1991;37:598–604. [PubMed] [Google Scholar]
- 12.Jansen MAM, Hart AAM, Korevaar JC, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim DJ, Kang W-H, Kim HY, et al. The effect of dialysate dwell on gastric emptying time in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 1999;19(Suppl 2):S176–S178. [PubMed] [Google Scholar]
- 14.Balaskas EV, Rodela H, Oreopoulos DG. Effects of intraperitoneal infusion of dextrose and amino acids on the appetite of rats. Perit Dial Int. 1993;13:S490–S498. [PubMed] [Google Scholar]
- 15.Khawar O, Kalantar-Zadeh K, Lo WK, et al. Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin J Am Soc Nephrol. 2007;2:1317–1328. doi: 10.2215/CJN.02550607. [DOI] [PubMed] [Google Scholar]
- 16.McDonald SP, Marshall MR, Johnson DW, et al. Relationship between dialysis modality and mortality. J Am Soc Nephrol. 2009;20:155–163. doi: 10.1681/ASN.2007111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stack AG, Murthy BV, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney Int. 2004;65:2398–2408. doi: 10.1111/j.1523-1755.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 18.Mehrotra R, Kermah D, Fried L, et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol. 2007;18:2781–2788. doi: 10.1681/ASN.2006101130. [DOI] [PubMed] [Google Scholar]
- 19.Chiu YW, Jiwakanon S, Lukowsky L, et al. An update on the comparisons of mortality outcomes of hemodialysis and peritoneal dialysis patients. Semin Nephrol. 2011;31:152–158. doi: 10.1016/j.semnephrol.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


