Abstract
Background
We showed in a randomized double-blinded placebo-controlled clinical trial that octreotide long-acting repeatable depot.® (OctLAR®) for 12 months reduces kidney and liver growth in autosomal dominant polycystic kidney patients with severe polycystic liver disease (PLD) and liver growth in patients with severe isolated PLD. We have now completed an open-label extension for one additional year to assess safety and clinical benefits of continued use of OctLAR for 2 years (O→O) and examined drug effect in the placebo group who crossed over to OctLAR in Year 2 (P→O).
Methods
The primary end point was change in total liver volume (TLV) measured by magnetic resonance imaging (MRI); secondary end points were changes in total kidney volume (TKV) measured by MRI, glomerular filtration rate (GFR), quality of life (QOL), safety, vital signs and laboratory parameters.
Results
Forty-one of 42 patients received OctLAR (n = 28) or placebo (n = 14) in Year 1 and received OctLAR in Year 2 (maximum dose 40 mg). Patients originally randomized to placebo (P→O) showed substantial reduction in TLV after treatment with OctLAR in Year 2 (Δ% −7.66 ± 9.69%, P = 0.011). The initial reduction of TLV in the OctLAR group (O→O) was maintained for 2 years (Δ% −5.96 ± 8.90%), although did not change significantly during Year 2 (Δ% −0.77 ± 6.82%). OctLAR inhibited renal enlargement during Year 1 (Δ% +0.42 ± 7.61%) in the (O→O) group and during Year 2 (Δ% −0.41 ± 9.45%) in the (P→O) group, but not throughout Year 2 (Δ% +6.49 ± 7.08%) in the (O→O) group. Using pooled analyses of all individuals who received OctLAR for 12 months, i.e. in Year 1 for O→O patients and Year 2 for P→O patients, average reduction in TLV was −6.08 ± 7.58% (P = 0.001) compared to net growth of 0.9 ± 8.35% in the original placebo group. OctLAR-treated individuals continued to experience improvements in QOL in Year 2, although overall physical and mental improvements were not significant during Year 2 compared to Year 1. Changes in GFR were similar in both groups.
Conclusion
Over 2 years, OctLAR significantly reduced the rate of increase in TLV and possibly the rate of increase in TKV.
Keywords: chronic kidney disease, kidney volume, liver cyst, polycystic kidney disease, somatostatin analogs
Introduction
Preclinical studies in the PCK rat (a recessive model of polycystic kidney and liver disease) showed that the somatostatin analog octreotide reduces 3′, 5′-cyclic adenosine monophosphate (cAMP) levels in kidney and bile ducts and slows the progression of hepatorenal cystogenesis [1]. We previously completed a double-blinded, randomized placebo-controlled clinical trial (NCT00426153) using octreotide long-acting repeatable depot® (OctLAR) over 1 year in 42 patients with severe polycystic liver disease (PLD) due to autosomal dominant PLD (ADPLD) or autosomal dominant polycystic kidney disease (ADPKD) [2]. Two other prospective clinical trials have shown similar positive effects of somatostatin analogs in ADPLD and ADPKD [3–5]. A few other published reports relating to the use of somatostatin analogs further substantiate these observations [6–8]. All these studies are limited by small numbers of enrolled patients with a short follow-up of 6–12 months. In order to evaluate whether somatostatin analog therapy retards liver and kidney growth beyond 1 year, our protocol design included a second year of open-label active therapy with OctLAR. Here, we report the effects of OctLAR in patients who were randomized to the placebo group during 1 year and the effects of two continuous years of OctLAR treatment.
Materials and methods
Study design
This was a single-center (Mayo Clinic, Rochester, MN), placebo-controlled double-blinded trial with a 2:1 randomization which continued into the second year as an open-label extension study. All participants signed informed consents. The rationale, design and implementation of this trial (NCT00426153) have been described in detail [2]. It conformed to the principles of the Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board. Patients were randomly assigned to receive OctLAR or placebo in the first year of the study and all participants were offered OctLAR in Year 2. The primary end point (percent change in liver volume from baseline) as measured by magnetic resonance imaging (MRI) [or computed tomography (CT) in three patients, see below] at 12 and 24 months, and secondary end points were changes in total kidney volume (TKV), glomerular filtration rate (GFR), quality of life (QOL SF-36v2TM) and safety ascertained by reported adverse events, vital signs and clinical laboratory tests. Novartis US supplied OctLAR and partially funded the study.
Novartis provided logistical support during the trial. The investigators prepared the statistical analysis plan and all analyses were performed by the Mayo Clinic. The manuscript was prepared by Drs Hogan and Masyuk. Novartis was permitted to review the manuscript and suggest changes, but the final decision on content was exclusively retained by the authors. The CONsolidated Statement Of Reporting Trials guidelines were adhered to for all aspects of the conduct and manuscript writing of this clinical trial (www.consort-statement.org).
Eligibility
Men and women ≥18 years with a diagnosis of ADPKD (meeting Ravine's criteria) or ADPLD (defined by the criteria described by Reynolds et al.), severe PLD defined as a liver volume >4000 mL or symptomatic disease due to mass effects from hepatic cysts, and who were not candidates or declining surgical intervention were eligible (Figure 1) [9, 10]. Patients had to be willing to travel to Mayo Clinic, Rochester, MN. Criteria for exclusion were inability to provide informed consent, women of childbearing potential unwilling to employ adequate contraception, serum creatinine concentration >3 mg/dL or dialysis dependency, symptomatic gallstones or biliary sludge, uncontrolled hypertension (systolic blood pressure >160 mmHg and diastolic blood pressure >100 mmHg) or diabetes mellitus, cancer or major systemic diseases that could prevent completion of the planned follow-up or interfere with data collection or interpretation and current or prior use of somatostatin analog within 6 months of enrollment or history of significant adverse reaction from a somatostatin analog.
Fig. 1.
Study flow diagram: 41 patients were enrolled in the second year (open-label extension). One individual withdrew voluntarily due to steatorrhea associated with OctLAR after completing the first year.
Study protocol
Enrollment took place from 1 January 2007 to 19 May 2008. This study was an open-label extension through Year 2, preceded by a 2:1 randomized, double-blinded placebo-controlled trial. After 12 months, all 41 patients continued (O→O) or crossed over to OctLAR (P→O). The drug was dispensed at 4-month intervals at the Mayo Clinic Research Pharmacy. Phone follow-up by a study coordinator was done monthly, and adverse events were reported to the study coordinator at each call or as needed. The study coordinator contacted patients monthly to confirm drug and dose administration. If there were drug side effects during a phone visit, a decision was made by the study team to reduce next injection dose by 10 or 20 mg (only needed in one case in the P→O group). All patients were evaluated by the principal investigator (MCH) or co-investigator (VET) at initial evaluation, and following randomization, then at 1 month and every 4 months, except for the Month 20 visit as there were few medical issues and all participants had safety assessments which included a physical examination, vital signs and clinical laboratory parameters (aspartite aminotransferase, alanine aminotransferase, alkaline phosphatase, bilirubin, electrolytes, blood urea nitrogen, creatinine, fasting glucose, complete blood count, activated partial thromboplastin time, prothrombin time). MR or CT imaging of liver and kidneys was obtained at the baseline, 1 and 2 years visits. All medications commenced were checked for drug interactions by the study team using Micromedex®.
At baseline, all women of child-bearing age had a pregnancy test, and all patients were required to use contraception or were post-menopausal. They were given a subcutaneous test dose of 100 mcg of short-acting octreotide (Sandostatin®) and observed (vital signs) over a 4-h period at time zero, except one patient who had previously received OctLAR. The following day, after confirming tolerability of the test dose, they were given 40 mg of OctLAR in two 20 mg intramuscular injections (one in each buttock) by nursing staff. Side effects were classified according to the Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria [11].
Outcome measures
The primary goals of the second year of follow-up in our study were to analyze the change in total liver volume (TLV) during the second year of continued OctLAR and to analyze the effects of OctLAR in the placebo group after crossing over to OctLAR in the second year. The secondary outcomes were to assess in a similar manner the effects of OctLAR on total kidney volume TKV, GFR measured by iothalamate clearance and QOL, as well as its safety and toxicity over 2 years of treatment.
The primary end point (percent change in TLV from baseline) as measured by MRI (or CT in four subjects, see below) and the secondary end points [changes in TKV, GFR, QOL (QOL SF-36v2TM)] were compared at Years 1 and 2 to that at baseline. We also assessed the safety ascertained by reported adverse events, vital signs and clinical laboratory tests.
MRI scans and volumetric measurements
All volumetric measurements were performed by the same blinded independent investigators and were compared with the baseline and Year 1 data as previously described. Repeat MRIs of liver and kidneys (or CT scan where MRI contraindicated or not possible) to reassess organ volume were performed in all participants at the end of Year 2. Absolute and percent changes in TLV and TKV at baseline, 1 and 2 years were assessed. To ensure that these two different imaging techniques are in agreement, liver and kidney volumes were calculated from CT and MRI in 10 patients who had CT and MRI performed within 15 days of one another. Measurements were calculated with Analyze® software and results were compared using Pearson's correlation coefficient. Excellent correlation was found between the volumes measured from CT and MRI images (r = 0.998). Of the original enrolled group, one individual in the OctLAR treatment group was withdrawn after Year 1; therefore his data were excluded in the second year analysis, and another had incomplete coverage of liver volume—therefore their TLV could not be used for the second year analysis, leaving 40 patients with analyzable TLV data at the end of Year 2. In four cases, non-contrast CT was used for the analyses because MRI could not be performed—one claustrophobic individual, one oversized patient, a third individual with a metallic ocular foreign body and a fourth, who was hospitalized with abdominal pain. Both initial and second year follow-up, CTs were performed on a multi-detector CT scanner using 5-mm thickness slices.
Image analysis
TLVs and TKVs were measured at enrollment, Year 1 and Year 2. The volumes of transplanted kidney and atrophic native kidneys were excluded from measurement in a total of four patients (three receiving OctLAR and one receiving placebo) who underwent renal transplantation. Eight patients with ADPLD (four receiving OctLAR and four receiving placebo) were excluded from the GFR and kidney volume analyses. One other ADPKD patient was excluded from the TKV analysis due to incomplete imaging coverage.
Image analysis was performed by one of three image analysis specialists using a stereology approach implemented in the Mayo Clinic Analyze® software program http://www.mayo.edu/bir/Software/Analyze/Analyze.html [12, 13].
After completing each patient study, the marked images were verified by one of two radiologists who are specialized in abdominal MR imaging (B.F.K. and B.J.K.). The radiologists were also blinded to patient treatment arm and timing of the scan for each subject (baseline or 1-year follow-up). Intrahepatic and intrarenal major vessels and porta hepatis vessels were included in all analyses. TKVs and TLVs were obtained in one sitting for each individual case. In some cases, the organ boundary of the liver and kidneys was difficult to delineate from that of the stomach, spleen, pancreas and small and large bowel. In these cases, careful further correlation was made with the other sequences, including single-shot fast-spin echo and steady state free precession. Image analysis of CT images performed in four patients was similarly done.
Patient genotypes
Mutation analysis was performed as previously described [2]. Thirty-two patients had ADPKD: of these 25 had a PKD1 mutation, 6 had a PKD2 mutation and in 1 patient, no mutation was detected. Eight patients had ADPLD: of these, four had a PRKCSH mutation, one had a SEC63 mutation and in three patients, no mutation was detected. ADPKD and ADPLD genotypes and phenotypes were equally distributed between the OctLAR and placebo groups.
Statistical analysis
Statistical analyses were performed using paired t-test to compare differences within groups. All reported P-values were two-sided, and P-values <0.05 were considered statistically significant. Non-parametric tests gave similar conclusions (not shown). For continuous parametric variables, values were reported as mean ± SD and range. All analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC) and PRISM 5 used for graphical data.
Results
Baseline characteristics
A total of 42 patients were enrolled and randomized in this trial, of these: 28 in the OctLAR treatment arm and 14 in the placebo arm (Figure 1). Baseline demographic, socioeconomic and medical characteristics were similar between the two randomized groups [2].
Retention
Forty-one of 42 patients participated in the Year 2 open-label extension study (Figure 1). One patient withdrew from the study after the first year due to steatorrhea. Twenty-seven of the original OctLAR group (n = 28 in Year 1) received OctLAR at 40 mg every 28 days or maximum tolerated dose (O→O). The placebo group (n = 14) was crossed over to receive the same regimen of OctLAR (P→O). All forty-one patients completed the second year open-label extension. One patient from the original OctLAR group had incomplete coverage of liver volume by the end of Year 2; therefore, TLV was analyzed in 40 patients.
Main outcome
Liver volumes
In patients that were randomized to placebo (n = 14), TLV increased by 0.9 ± 8.35% compared to baseline during first year of the clinical trial (Table 1). In Year 2, after these patients were crossed over to OctLAR, TLV decreased from 5360 ± 3331 mL (assessed by the end of Year 1) to 4952 ± 3344 mL (assessed by the end of Year 2) or by −7.66 ± 9.69% (P = 0.01) (Table 1). Among these patients, several individuals experienced more substantial (10–25%) cumulative reductions of TLV after treatment with OctLAR (Figures 2 and 3).
Table 1.
Changes in liver and kidney volumes and renal function in subjects randomized to placebo (P) for Year 1, all whom crossed over to octreotide (O) for Year 2a
| Duration of treatment (year) |
Change on P | Change on O | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | Δ Year 0, Year 1 | Δ Year 1, Year 2 | |
| Total liver volume, cc | |||||
| N | 14 | 14 | 14 | ||
| Mean (SD) | 5374 (3565) | 5360 (3331) | 4952 (3344) | −13.31 (603.28) | −408.40 (775.04) |
| % Change (SD) | 0.90 (8.35) | −7.66 (9.69) | |||
| P-value | 0.69 | 0.011 | |||
| TKV, cc w/o ADPLD | |||||
| N | 8 | 8 | 8 | ||
| Mean (SD) | 803 (269) | 874 (306) | 885 (355) | 70.50 (84.15) | 11.55 (82.38) |
| % Change (SD) | 8.61 (10.07) | 0.41 (9.45) | |||
| P-value | 0.046 | 0.90 | |||
| GFR, mL/min/1.73BSA w/o ADPLD | |||||
| N | 9 | 9 | 9 | ||
| Mean (SD) | 70.8 (28.1) | 65.7 (26.4) | 62.6 (29.0) | −5.11 (7.56) | −3.11(8.42) |
| % Change (SD) | −7.2 (13.2) | −6.3 (13.0) | |||
| P-value | 0.14 | 0.19 | |||
aP-values for Year 2 versus Year 1 in liver volume, renal volume, GFR (assessed as a percent change) are 0.061, 0.16 and 0.89, respectively. Paired t-test was used for statistical analysis within group, e.g. for TLV, 14 individual percent changes were used, not the overall mean percent change at each time point. There was one incomplete kidney volume at 0 and 1 year. Wilcoxon non-parametric tests revealed similar P-values.
Fig. 2.
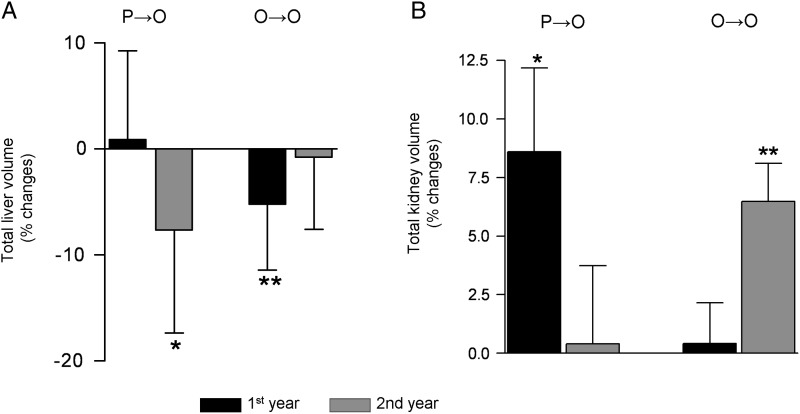
Effects of OctLAR therapy on the TLV and TKVs as represented by percentage changes (mean ± SD) during Year 1 (from baseline to Month 12) and Year 2 (mean ± SD) (from Month 12 to Month 24). (A) Percentage change in TLV (mL) (mean ± SD) in patients who received placebo (P) for 1 year and then crossed over to OctLAR during Year 2 (O) (P→O) or receiving continuous OctLAR treatment for 2 years (O→O). *P = 0.011 compared to TLV assessed by the end of Year 1; **P = 0.002 compared to baseline. (B) Percentage change (mean ± SD) in TKV on placebo and then crossed over to OctLAR during Year 2 (P→O) or receiving continuous OctLAR treatment for 2 years (O→O). *P = 0.05 compared to baseline; **P = 0.008 compared to TKV assessed by the end of 1 year.
Fig. 3.
Reduction of liver cyst burden in patients receiving continuous OctLAR therapy. Representative serial coronal abdominal and pelvic MRI views of three participants who underwent OctLAR therapy for 2 years. The images are standardized to visualize interval change in large cysts attributable to OctLAR therapy. Patient 1 had a decrease of 25%, Patient 2 of 10% and Patient 3 of 13% in TLV.
In patients that received OctLAR during Year 1 (n = 26), TLV decreased from 5984 ± 2961 mL (assessed at enrollment) to 5628 ± 2720 mL at the end of Year 1 and 5649 ± 2870 mL at the end of Year 2 (Table 2) or −5.23 ± 6.22% in Year 1 (P = 0.0002) and −0.77 ± 6.82% (P = 0.57) in Year 2. Therefore, reduction was maintained but did not change significantly during Year 2 (Figure 2A and Table 2). When we analyzed pooled data from the first year of OctLAR therapy in all 40 individuals who received OctLAR (during their first year in the 26 O→O patients or second year in the 14 P→O patients), we detected a −6.08 ± 7.58% (P = 0.001) reduction in TLV (Table 3) compared to a growth rate of 0.90 ± 8.35% during the year of placebo treatment in the P→O group (n = 14).
Table 2.
Changes in liver and kidney volumes and renal function in subjects randomized to octreotide (O) for Year 1, all whom remained on octreotide for Year 2a
| Duration of treatment (year) |
Change on O | Change on O | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | Δ Year 0, Year 1 | Δ Year 1, Year 2 | |
| Total liver volume, cc | |||||
| N | 26 | 26 | 26 | ||
| Mean (SD) | 5984 (2961) | 5628 (2720) | 5649 (2870) | 355.35 (471.24) | 20.75 (440.52) |
| % Change | −5.23 (6.22) | −0.77 (6.82) | |||
| P-value | 0.0002 | 0.57 | |||
| TKV, cc w/o ADPLD | |||||
| N | 19 | 19 | 19 | ||
| Mean (SD) | 1152 (869) | 1139 (838) | 1214 (884) | −13.20 (74.87) | 75.97 (124.46) |
| % Change | 0.42 (7.61) | 6.49 (7.08) | |||
| P-value | 0.81 | 0.0008 | |||
| GFR, mL/min/1.73BSA w/o ADPLD | |||||
| N | 21 | 21 | 20 | ||
| Mean (SD) | 68.1 (26.5) | 64.6 (25.7) | 57.7 (23.5) | −3.52 (10.53) | −5.65 (8.44) |
| % Change | −5.1 (15.5) | −7.9 (13.8) | |||
| P-value | 0.15 | 0.019 | |||
aP-values for Year 2 versus Year 1 in liver volume, renal volume, GFR and creatinine (assessed as percent changes) are 0.0022, 0.013, 0.73 and 0.77, respectively. Paired t-test was used for statistical analysis within group. Using Wilcoxon signed rank test, the only change found to be significant was GFR while on OctLAR in the first year (0.045).
Table 3.
Pooled analysis of changes in liver and kidney volumes and renal function (GFR and creatinine) during 1 year of octreotide (O) therapya
| Duration of treatment | |
|---|---|
| Δ Year 1 on OctLAR | |
| Total liver volume, cc | |
| N | 40 |
| Mean (SD) | −373.92 (585.86) |
| % Change | −6.08 (7.58) |
| P-value | 0.001 |
| Total kidney volume, cc w/o ADPLD | |
| N | 27 |
| Mean (SD) | −5.86 (76.43) |
| % Change | 0.42 (8.01) |
| P-value | 0.79 |
| GFR, mL/min/1.73BSA w/o ADPLD | |
| N | 30 |
| Mean (SD) | −3.4 (9.8) |
| % Change | −5.4 (14.5) |
| P-value | 0.0501 |
| Creatinine, mg/dL w/o ADPLD | |
| N | 30 |
| Mean (SD) | 0.05 (0.14) |
| % Change | 4.5 (12.5) |
| P-value | 0.0597 |
aPooled analysis was performed for all individuals who received OctLAR for 1 year (during either Year 1 in the O→O and Year 2 in the P→O group). Wilcoxon signed rank test revealed the same P-values for TLV and TKV, creatinine (0.0506) GFR (P = 0.02).
Secondary outcomes
Kidney volumes
For the final analysis of TKV, we excluded individuals with ADPLD (n = 8) and renal transplant recipients (n = 4); therefore, 8 participants in the placebo group who crossed over to OctLAR in Year 2 and 19 enrollees in the original OctLAR group who continued OctLAR during second year were evaluated (Table 1 and Figure 2B).
The eight patients initially randomized to placebo (P→O) showed no significant change in TKV during treatment with OctLAR in Year 2 (Δ% +0.41 ± 9.45%, P = 0.90) compared to a significant increase of 8.61 ± 10.07% (P = 0.046) in Year 1. The group of 19 patients receiving OctLAR for 2 years in this clinical trial (O→O) showed an increase in TKV from 1152 ± 869 to 1214 ± 884 mL (or by 6.49 ± 7.08%, P = 0.0008) during the second year (Table 2).
During the first year of OctLAR treatment in 27 patients who received one full year of OctLAR therapy (first year in the 19 O→O patients or second year in the 8 P→O patients), TKV increased by 0.42 ± 8.01% (P = 0.79) compared to a growth rate of 8.61 ± 10.07% during the year of placebo treatment in the P→O group (Tables 1 and 3). Thus, OctLAR therapy stalls kidney growth during the first year of treatment, but the effect does not appear to be sustained during Year 2.
Glomerular filtration rate
Changes in GFR (limited to ADPKD patients) in the placebo group (n = 9) in Year 1 (−7.2 ± 13.2% from baseline) and in Year 2 after crossing over to OctLAR (−6.3 ± 13% compared to Year 1) were not significantly different (Table 1). There were also no differences in the serum creatinine levels. In individuals who received OctLAR in their first year, GFR decreased by −5.1 ± 15.5% (n = 20, P = 0.15) and continued to decline (−7.9 ± 13.8%, P = 0.019) during the second year (Table 2). Pooled analysis of GFR in the 30 individuals who received 1 year of OctLAR revealed a decline of 5.4 ± 14.5% during the first year of treatment (P = 0.05) (Table 3).
Laboratory parameters
Prothrombin time was 9.6 (range 8.4–11.0) at baseline and at reassessment 10.6 (range 9.6–11.8) in individuals receiving OctLAR (P = 0.38) without any detectable clinical consequence. One patient taking warfarin was asked to hold the medication for 2 days prior to her monthly injection so as to avoid gluteal hematoma at the injection sites. There was a non-significant trend toward a reduction in aspartate transaminase (normal range 8–48 U/L) in the OctLAR group from Year 1 to Year 2 (32.5 U/L at baseline to 29.9 U/L by end of Year 1 and 27.1 U/L at completion of Year 2, P = 0.12). There were no significant differences in albumin, bilirubin or alkaline phosphatase, all of which remained within normal limits.
Fasting plasma glucose levels increased by 8% from baseline after commencing OctLAR treatment compared with a 2% increase in placebo group (P = 0.33) during the first year. No patient developed diabetes during the study. We did not observe any significant fluctuations in cyclosporin levels in the three renal transplant recipients receiving both medications throughout Year 2.
Tolerability
Repeated administration of OctLAR resulted in a low incidence of treatment-related adverse events. One individual was withdrawn from the study after Year 1 due to steatorrhea and weight loss, which were considered due to the medication. There were three serious adverse events in Year 2—one individual had chest pain, another developed an infected liver cyst(s) and a third was hospitalized with urosepsis. None of these were considered to be due to the study medication. The target dose (40 mg) was reached in 35/41 (85%) patients. The dose was reduced to 30 mg in four (10%) and to 20 mg in two individuals (5%), mostly because of diarrhea/loose stools, especially in the first 2 weeks after an injection. As was seen in the first year, the most common adverse events observed in Year 2 were injection site pain or granuloma (n = 16/28, 57%) and diarrhea (n = 8, 28%).
After completing the study, one individual developed abdominal pain and underwent surgery for a presumptive diagnosis of acute cholecystitis (this occurred 1 month after completion of the study). A CT obtained immediately prior to her surgical procedure was used for her final TLV and TKV assessments. It was felt that the complication was not drug related, as the gallstone disease predated study enrollment. Another patient was hospitalized following a fall that was complicated by acute renal failure. This hospitalization was not considered to be related to the study medication.
Quality of life
For those individuals receiving continued OctLAR treatment for 2 years, statistically significant improvements of QOL in three subdomains were observed: (i) physical role (P = 0.0031), (ii) bodily pain (P = 0.0035) and (iii) vitality (P = 0.0022) compared with their baseline status (assessed at enrollment). While mean scores in the patients randomized to placebo did not significantly improve following 1 year of OctLAR treatment, non-significant trends towards improvement over 2 years were observed in seven of eight QOL subdomains although only one (general health perception) was close to significance (P = 0.056).
Discussion
Several studies have reported the substantial positive effects of somatostatin analogs in decreasing liver and kidney growth in PKD and ADPLD over a treatment period of 6 months. Our study extends information on the safety and efficacy of OctLAR in polycystic liver and kidney disease utilizing data from three patient groups: (i) 14 individuals with ADPKD and ADPLD who crossed over to OctLAR in their second year following 1 year of placebo (P→O), (ii) 26 patients who completed 2 years of continued OctLAR therapy (O→O) and (iii) a pooled analysis of a total of 40 patients who received at least 1 year of OctLAR therapy during the first year of treatment. In patients initially randomized to placebo and then crossed over to receive OctLAR (P→O), we observed even more substantial reductions in TLV (−7.66 ± 9.69%) compared to those seen in our original treated cohort (−4.95 ± 6.77%) [2]. Moreover, during the first year of active OctLAR treatment, TKV did not change (i.e. 0.42 ± 8.01%; Table 3) compared to the 8.61 ± 10.07% growth in the patients on placebo. These data suggest that OctLAR therapy may also have a beneficial effect on kidney growth at least for the initial first year of treatment. The principal finding of this study, however, is that continuous OctLAR therapy in the 26 patients with PLD (O→O) sustained liver growth arrest over 2 years of treatment and that this treatment is relatively well-tolerated. From the pooled analyses of 40 patients who received 1 year of OctLAR therapy, we observed an overall 6.08% reduction in TLV. We will continue to follow these individuals for a further 2 years.
While OctLAR inhibited renal enlargement within the first year of treatment, it appeared to lose effectiveness during Year 2. Several reasons may account for the reduction of effect seen in Year 2. First, somatostatin receptors may undergo receptor downregulation/desensitization (probably by endocytosis of the receptor–ligand complexes) after prolonged exposure to OctLAR [14–16]. Second, we did not monitor OctLAR levels in plasma, its metabolism, cAMP concentration, or whether levels of the effectors IGF, GH or EGF were modulated by therapy. In the preclinical animal studies, liver and kidney effects were dose-dependent; therefore, higher doses of somatostatin analogs may be required [1]. Third, OctLAR is known to bind to three of five known somatostatin receptors (SSTRs)—SSTR2, SSTR3 and SSTR5. At this point, we do not know what SSTRs are present in liver and kidneys of our patients, and how their expression is different from normal hepatic and renal epithelia. Thus, the use of more potent somatostatin analogs binding to a broader range of SSTRs might be more efficient. Alternatively, their use in combination with other anti-proliferative and anti-secretory agents, e.g. low-dose mammalian target of rapamycin mTOR inhibitors (a pathway known to be implicated in the pathogenesis of progressive liver and kidney enlargement in PKD), may be another feasible strategy and the combination of octreotide and everolimus is currently being evaluated in the extended low-intensity anticoagulation for thromboembolism trial for PLD (NCT01157858) [17].
While the results of OctLAR therapy on TLV are clearly positive and results on TKV may show some benefit, we did not detect any positive effect of OctLAR on GFR. Possible reasons for this may be (i) the increase in TKV was not inhibited during Year 2 of OctLAR treatment, (ii) the small number of patients evaluated for this secondary outcome (21 O→O and 9 P→O patients), (iii) the relatively short duration of the study, (iv) somatostatin-induced renal vasoconstriction causing a reduction in renal blood flow and GFR [18] and most importantly, (v) the non-linearity of GFR decline in ADPKD. For example in the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) longitudinal study of 241 patients with normal GFR at entry, GFR remained stable from baseline to Year 3 but decreased markedly at Year 6 and Year 8 pointing to a phase of accelerated rate of GFR decline after many years of GFR stability [19]. Of the 30 ADPKD patients in our study, 12 had established chronic kidney disease 3 (CKD3) and 1 had CKD4 at enrollment. Given these limitations, larger placebo-controlled studies of longer duration will be necessary to determine the effect of somatostatin analogs on the progression of CKD in ADPKD.
As our primary study end point focused on TLV, we selected ADPLD and ADPKD patients with severely enlarged livers. Therefore, the patients in this study represent a minority of PLD patients with more severe liver disease than the ‘average’ ADPKD patient and we restrict the use of somatostatin analogs to this patient subgroup (i.e. >4000 mL liver volume with symptomatic PLD).
Potential limitations of this study include missing imaging in one patient and reliance on CT instead of MRI for image acquisition in four individuals. Despite finding an excellent correlation between these two different imaging techniques, they could result in a potential source of measurement bias. Thus, we used the same image acquisition technique at each patient visit to minimize the potential for measurement error. Average intra-observer and inter-observer variability are 1.12 and 3.2%, respectively. Variability was similar regardless of whether CT or MRI was used.
The majority of individuals tolerated the 40 mg OctLAR dose. Only one individual was discontinued from the study after 12 months because of drug-related side effects (steatorrhea, despite initiation on pancreatic enzyme supplements)—all the other 41 individuals receiving OctLAR had an acceptable side effect profile. One individual with Stage 4 CKD and another with Stage 3 CKD needed reduction in dose to 30 mg. Dose adjustments are advised for patients with severe renal impairment. Cases of hyperkalemia (due to the drug's impact on insulin-mediated potassium transport) have been reported in patients on dialysis [20–22]. Therefore, the starting dose for patients on dialysis should not exceed 10 mg every 4 weeks according to the product information sheet for Sandostatin LAR (octreotide). We restricted the trial to patients with creatinine <3 mg/dL for safety considerations. The most common adverse events were injection site pain and granuloma, although no participant discontinued therapy for that reason. Patients undergoing long-term OctLAR treatment should be monitored for cholelithiasis symptoms or signs, since this is a known complication [23–26]. Prothrombin time should be monitored before surgical procedures while patients are receiving OctLAR.
It is our opinion that a 6% reduction in massive PLD is clinically significant in terms of patient comfort and this is supported by the persistent improvement in QOL in the patients treated with octreotide for 2 years and possibly by a non-significant trend in the smaller group of placebo-treated patients switched to octreotide treatment during the second year. We continue to use this medication in patients with highly symptomatic PLD and severe hepatomegaly who are not candidates for or wish to avoid or delay (the majority being women engaged in home and family life or mid-career) surgical management. Furthermore, most patients chose to continue the therapy after their second year in an open-label extension and have averted or delayed surgical management. More precise definition of the role of somatostatin analogs and the optimal timing for initiation of therapy will require studies of longer duration in a larger number of patients.
In conclusion, while this is a small study, it further substantiates the positive effects of somatostatin analogs in reducing TLV, demonstrating they are safe and efficacious over a 2-year period in individuals with ADPKD or ADPLD, many of whom had chronic renal insufficiency. It remains to be seen if more potent somatostatin analogs or the combination of somatostatin analog with other growth inhibitor pharmacotherapies will be more effective in the medical management of this disease.
Funding
This research was funded by: Mayo Clinic, Novartis USA, NIH (NIDDK P30 DK090728 and UL1 RR024150) and the NIH Roadmap for Medical Research in a research award to Dr M.H. and to the Mayo Clinic CTSA Center.
Conflict of interest statement
Dr M.H. received partial funding support for this study from Novartis USA. Drs N.L. and T.M. have filed a patent for use of somatostatin analogs in PLD. Dr V.T. has participated in an expert panel meeting sponsored by Ipsen Innovation to discuss the potential of lanreotide in the treatment of patients with PLD.
Acknowledgements
We are indebted to the patients who participated in the study, their families and physicians and thank the Angel Flight Organization for their assistance. We thank Dawn P. Bergen and Jody L. Clikeman in the Department of Internal Medicine for secretarial assistance. This study was presented in an abstract form at the American Society of Nephrology 2010 Annual Meeting in Denver Colorado.
References
- 1.Masyuk TV, Masyuk AI, Torres VE, et al. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3',5'-cyclic monophosphate. Gastroenterology. 2007;132:1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 2.Hogan MC, Masyuk TV, Page LJ, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21:1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruggenenti P, Remuzzi A, Ondei P, et al. Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int. 2005;68:206–216. doi: 10.1111/j.1523-1755.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 4.van Keimpema L, Nevens F, Vanslembrouck R, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;137:1661–1668. doi: 10.1053/j.gastro.2009.07.052. e1-2. [DOI] [PubMed] [Google Scholar]
- 5.Caroli A, Antiga L, Cafaro M, et al. Reducing polycystic liver volume in ADPKD: effects of somatostatin analogue octreotide. Clin J Am Soc Nephrol. 2010;5:783–789. doi: 10.2215/CJN.05380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Keimpema L, de Man RA, Drenth JP. Somatostatin analogues reduce liver volume in polycystic liver disease. Gut. 2008;57:1338–1339. doi: 10.1136/gut.2008.155721. [DOI] [PubMed] [Google Scholar]
- 7.van Keimpema L, Drenth JP. Effect of octreotide on polycystic liver volume. Liver Int. 2010;30:633–634. doi: 10.1111/j.1478-3231.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- 8.Peces R, Cuesta-Lopez E, Peces C, et al. Octreotide reduces hepatic, renal and breast cystic volume in autosomal-dominant polycystic kidney disease. Int Urol Nephrol. 2011;43:565–569. doi: 10.1007/s11255-010-9748-1. [DOI] [PubMed] [Google Scholar]
- 9.Ravine D, Gibson RN, Walker RG, et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–827. doi: 10.1016/s0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds DM, Falk CT, Li A, et al. Identification of a locus for autosomal dominant polycystic liver disease, on chromosome 19p.13.2-13.1. Am J Hum Genet. 2000;67:1598–1604. doi: 10.1086/316904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS March 31, 2003 (http://ctep.cancer.gov), Publish Date: August 9, 2006. [Google Scholar]
- 12.Hanson DP, Robb RA, Aharon S, et al. New software toolkits for comprehensive visualization and analysis of three-dimensional multimodal biomedical images. J Digit Imaging. 1997;10:229–230. doi: 10.1007/BF03168711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts N, Puddephat MJ, McNulty V. The benefit of stereology for quantitative radiology. Br J Radiol. 2000;73:679–697. doi: 10.1259/bjr.73.871.11089458. [DOI] [PubMed] [Google Scholar]
- 14.Boehm S, Betz H. Somatostatin inhibits excitatory transmission at rat hippocampal synapses via presynaptic receptors. J Neurosci. 1997;17:4066–4075. doi: 10.1523/JNEUROSCI.17-11-04066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenig JA, Edwardson JM, Humphrey PPA. Somatostatin receptors in Neuro2A neuroblastoma cells: ligand internalization. Br J Pharmacol. 1997;120:52–59. doi: 10.1038/sj.bjp.0700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloch B, Dumartin B, Bernard V. In vivo regulation of intraneuronal trafficking of G protein-coupled receptors for neurotransmitters. Trends Pharmaco Sci. 1999;20:315–319. doi: 10.1016/s0165-6147(99)01360-7. [DOI] [PubMed] [Google Scholar]
- 17.Chrispijn M, Drenth JP. Everolimus and long acting octreotide as a volume reducing treatment of polycystic livers (ELATE): study protocol for a randomized controlled trial. Trials. 2011;12:246. doi: 10.1186/1745-6215-12-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt A, Pleiner J, Schaller G, et al. Renal hemodynamic effects of somatostatin are not related to inhibition of endogenous insulin release. Kidney Int. 2002;61:1788–1793. doi: 10.1046/j.1523-1755.2002.00320.x. [DOI] [PubMed] [Google Scholar]
- 19.Chapman AB, Bost JE, Torres VE, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–486. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke MT, Gray NA. Carcinoid tumour management in haemodialysis: a case report. Nephrology. 2012;17:198. doi: 10.1111/j.1440-1797.2011.01519.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee P-H, Lin C-L, Lai P-C, et al. Octreotide therapy for chylous ascites in a chronic dialysis patient. Nephrology. 2005;10:344–347. doi: 10.1111/j.1440-1797.2005.00427.x. [DOI] [PubMed] [Google Scholar]
- 22.Adabala M, Jhaveri KD, Gitman M. Severe hyperkalaemia resulting from octreotide use in a haemodialysis patient. Nephrol Dial Transplant. 2010;25:3439–3442. doi: 10.1093/ndt/gfq381. [DOI] [PubMed] [Google Scholar]
- 23.Bigg-Wither GW, Ho KK, Grunstein RR, et al. Effects of long term octreotide on gall stone formation and gall bladder function. BMJ. 1992;304:1611–1612. doi: 10.1136/bmj.304.6842.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies PH, Stewart SE, Lancranjan L, et al. Long-term therapy with long-acting octreotide (Sandostatin-LAR) for the management of acromegaly. Clin Endocrinol (Oxf) 1998;48:311–316. doi: 10.1046/j.1365-2265.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- 25.Ho KY, Weissberger AJ, Marbach P, et al. Therapeutic efficacy of the somatostatin analog SMS 201-995 (octreotide) in acromegaly. Effects of dose and frequency and long-term safety. Ann Intern Med. 1990;112:173–181. doi: 10.7326/0003-4819-112-3-173. [DOI] [PubMed] [Google Scholar]
- 26.Hussaini SH, Pereira SP, Veysey MJ, et al. Roles of gall bladder emptying and intestinal transit in the pathogenesis of octreotide induced gall bladder stones. Gut. 1996;38:775–783. doi: 10.1136/gut.38.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]