Abstract
The CXC chemokine receptor 2 (CXCR2) on neutrophils, which recognizes chemokines produced at the site of infection, plays an important role in antimicrobial host defenses such as neutrophil activation and chemotaxis. Staphylococcus aureus is a successful human pathogen secreting a number of proteolytic enzymes, but their influence on the host immune system is not well understood. Here, we identify the cysteine protease Staphopain A as a chemokine receptor blocker. Neutrophils treated with Staphopain A are unresponsive to activation by all unique CXCR2 chemokines due to cleavage of the N-terminal domain, which can be neutralized by specific protease inhibitors. Moreover, Staphopain A inhibits neutrophil migration towards CXCR2 chemokines. By comparing a methicillin-resistant S. aureus (MRSA) strain with an isogenic Staphopain A mutant, we demonstrate that Staphopain A is the only secreted protease with activity towards CXCR2. Although the inability to cleave murine CXCR2 limits in-vivo studies, our data indicate that Staphopain A is an important immunomodulatory protein that blocks neutrophil recruitment by specific cleavage of the N-terminal domain of human CXCR2.
Keywords: CXCR2, immune evasion, proteases, Staphylococcus aureus
Introduction
Chemokine receptors are critical to the innate immune response since their activation results in the directed migration of inflammatory cells to the site of infection (Murdoch and Finn, 2000). Chemokine receptors are membrane-bound G protein-coupled receptors (GPCRs) that sense a wide variety of chemokines produced at the site of inflammation. The extracellular N-terminal regions of chemokine receptors are key to their activation: chemokines first bind to the N-termini and induce a conformational change thereby interacting with the transmembrane domains to activate the receptor. An intracellular signal induces the exchange of GDP for GTP of the G protein, inducing an intracellular signalling cascade that leads to many effector functions such as the mobilization of intracellular calcium and actin polymerization both needed for morphological changes of cellular chemotaxis (Allen et al, 2007).
During bacterial infections, CXCR2 is important for the rapid recruitment of neutrophils to the site of infection (Sekido et al, 1993). Neutrophils are specialized in killing bacteria through multiple mechanisms including production of oxygen radicals and granule proteases. CXCR2 can be activated by multiple chemokines that are produced by local cells in response to the invading bacterium (Tsai et al, 2000; Tateda et al, 2001). Some chemokines uniquely bind to CXCR2 (CXCL1 (Growth Related Oncogene (GRO)-α), CXCL2 (GRO-β), CXCL3 (GRO-γ), CXCL5 (Epithelial cell-derived Neutrophil-Activating peptide (ENA)-78) and CXCL7 (Neutrophil Activating Protein (NAP)-2)) while others bind both CXCR2 and the highly homologous receptor CXCR1 (76% amino-acid identity) (Holmes et al, 1991) (CXCL6 (Granulocyte Chemotactic Protein (GCP)-2) and CXCL8 (Interleukin (IL)-8)). CXCR2 is highly expressed on neutrophils and lower on monocytes, lymphocytes and NK cells (Chuntharapai et al, 1994). The crucial role of CXCR2 in the host defense against bacteria is well defined in mouse models. Mice lacking functional CXCR2 have impaired neutrophil influx towards the site of infection, increased bacterial burden and in some cases even increased mortality compared to wild-type or untreated mice (Tsai et al, 2000; Herbold et al, 2010; Eisele et al, 2011).
Staphylococcus aureus is a bacterial pathogen that causes a broad range of acute and chronic infections in humans (Lowy, 1998). Since the introduction of antibiotic therapies, S. aureus has evolved resistance mechanisms causing the increased prevalence of Staphylococcal infections including methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VRSA). The pathogenic success of S. aureus is due in part to the large number of factors that promote adhesion to human extracellular matrices, colonization, biofilm formation and resistance to the host immune system. Among these factors are secreted proteases, which initially were thought to be important only for nutrient acquisition. However, evidence is emerging that they are involved in immune evasion by interacting with neutrophils (Smagur et al, 2009a, 2009b), antimicrobial peptides (Sieprawska-Lupa et al, 2004) and plasma proteins (Prokesova et al, 1992; Laarman et al, 2011). Also, it has been shown that S. aureus proteases are associated with diseases such as the exfoliative toxins in Staphylococcal Scalded Skin Syndrome and the cysteine proteases in vascular leakage causing sepsis (Amagai et al, 2000; Imamura et al, 2005).
Most S. aureus strains secrete at least 10 proteases, 2 of which are cysteine proteases also called ‘Staphopains’. Staphopain A (ScpA) is secreted as a zymogen and activated by autolytic cleavage, resulting in the removal of a 23-kDa N-terminal propeptide (Nickerson et al, 2010). S. aureus protects itself from proteolytic degradation by producing, within the same operon of Staphopain A, a cytoplasmic inhibitor called Staphostatin A (ScpB) (Filipek et al, 2003). This inhibitor is specific for Staphopain A (Rzychon et al, 2003) and prevents premature autocatalytic activation by stabilizing the proStaphopain A zymogen. Staphopain A, highly conserved among S. aureus isolates (Golonka et al, 2004), is known to cleave a number of human proteins including elastin, collagen, fibrinogen and kininogen and has been suggested to play a role in bacterial migration and sepsis (Potempa et al, 1988; Imamura et al, 2005; Ohbayashi et al, 2011).
Here, we discover a role of Staphopain A in modulation of neutrophil responses. Staphopain A specifically cleaves the N-terminus of CXCR2 on human neutrophils and effectively inhibits important steps in neutrophil recruitment towards sites of inflammation.
Results
Staphopain A inhibits antibody binding to CXCR2 on neutrophils
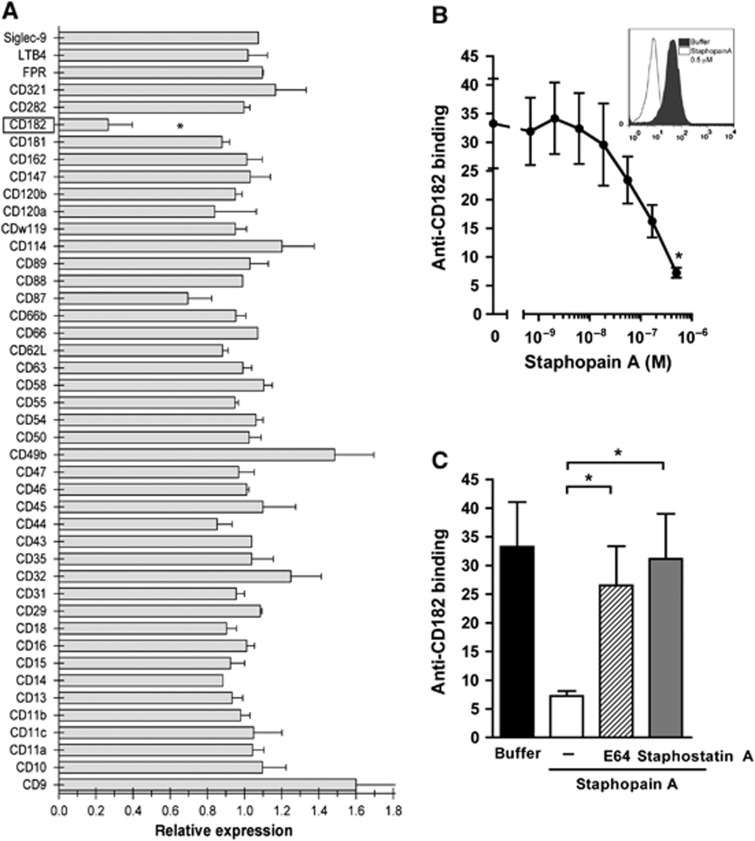
To test whether Staphopain A interacts with neutrophils, we used a multi-screening assay for surface-expressed receptors on human neutrophils. Neutrophils were incubated with Staphopain A for 15 min at 37°C, washed and subsequently incubated with a select panel of 44 blocking mAbs directed against various receptors involved in chemotaxis, activation, signalling, adhesion and phagocytosis. Staphopain A selectively inhibited the binding of an antibody directed against the N-terminus of CD182 (CXCR2) (Figure 1A; Supplementary Figure 1a), while other receptor–antibody interactions were not affected. Staphopain A (at 0.5 μM) reduced the binding of the CXCR2 antibody with 73%. Furthermore, Staphopain A caused a dose-dependent decrease of antibody binding to CXCR2 on neutrophils (Figure 1B). To investigate whether Staphopain A inhibited CXCR2 antibody binding via proteolysis, we blocked its activity using two different inhibitors: Staphostatin A and E64. Staphostatin A is a 13-kDa protein produced by S. aureus (Rzychon et al, 2003). The epoxysuccinate inhibitor E64 is an irreversible cysteine protease inhibitor that specifically targets the active site cysteine thiol (Otto and Schirmeister, 1997). This low molecular weight molecule was previously described to block Staphopain A (Potempa et al, 1988). Both inhibitors abolished the Staphopain A-mediated inhibition of antibody binding to neutrophils (Figure 1C), indicating that the reduced antibody binding is caused by proteolytic cleavage. For the other S. aureus cysteine protease Staphopain B, it was previously reported that it could induce cell death in monocytes and neutrophils (Smagur et al, 2009b). To study whether Staphopain A can induce similar effects, neutrophils were incubated with Staphopain A or Staphopain B for 75 min at 37°C and binding of Annexin V (apoptosis marker) or Propidium Iodide (cell death marker) was monitored. In contrast to Staphopain B, Staphopain A did not induce binding of Annexin V or Propidium Iodide (Supplementary Figure 1b). Overall, Staphopain A specifically cleaves the CXCR2 on human neutrophils.
Figure 1.
Staphopain A inhibits antibody binding to CXCR2 on neutrophils. (A) Neutrophils were incubated with 0.5 μM Staphopain A or buffer for 15 min at 37°C. After washing, cells were stained with a panel of blocking antibodies against surface-expressed receptors. The relative expression was calculated by dividing the mean fluorescence of Staphopain-treated cells by Buffer-treated cells. (B) Staphopain A blocks antibody binding in a dose-dependent manner. Neutrophils were incubated with various concentrations of Staphopain A for 15 min at 37°C and stained with an antibody recognizing the N-terminal domain of human CXCR2. Inlay: Representative histogram of the CXCR2 expression on neutrophils treated with 0.5 μM Staphopain A compared to buffer. (C) Protease activity is required for antibody inhibition. Neutrophils were treated with 0.5 μM Staphopain A with or without 1 μM Staphostatin A or 10 μM E64. All figures represent the mean±s.e. of three separate experiments using different donors. Background was determined using an isotype control. (A, B) *P<0.05 for Staphopain A versus buffer (two-tailed Student’s t-test); (C) *P<0.05 for Staphopain A plus inhibitor versus Staphopain A (two-tailed Student’s t-test).
Staphopain A inhibits neutrophil activation
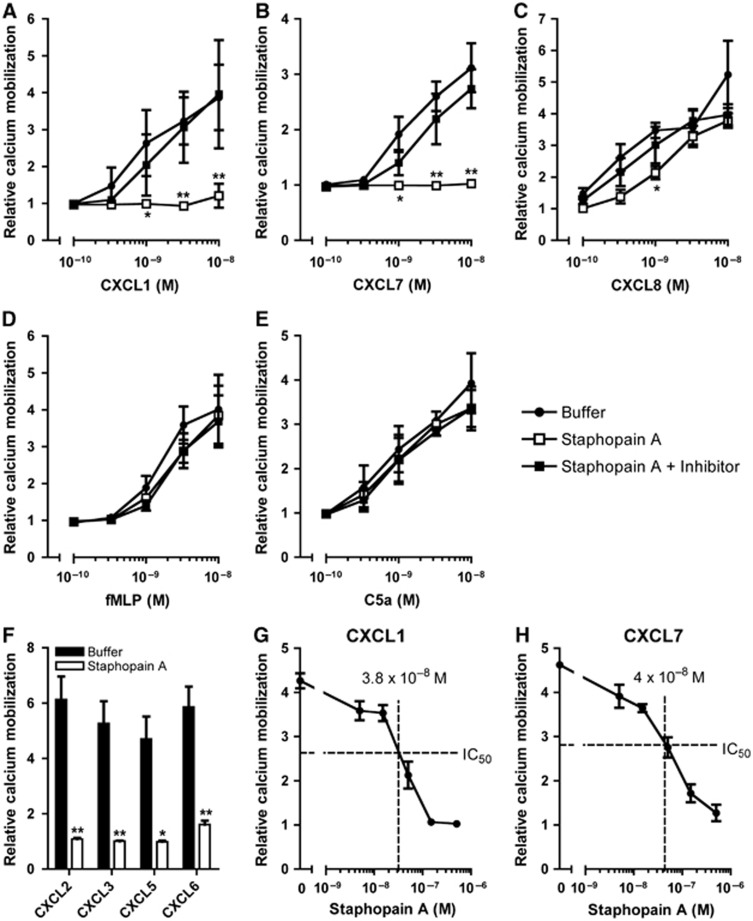
Activation of many GPCRs results in a rapid and transient release of intracellular calcium stores which can be measured by monitoring the calcium mobilization of neutrophils: the CXCR1 can be stimulated with CXCL6 and CXCL8; the CXCR2 with CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7 and CXCL8; the formyl peptide receptor (FPR) with fMLF; and the C5a receptor with C5a. To investigate whether Staphopain A inhibits CXCR2-mediated neutrophil activation by different stimuli, we incubated Fluo-3-labelled neutrophils with Staphopain A for 15 min at 37°C. Cells were washed and tested for activation by measuring the calcium mobilization (Figure 2). Staphopain A efficiently blocked calcium mobilization upon stimulation with CXCL1 and CXCL7, two potent chemoattractants that specifically activate the CXCR2 (Figure 2A and B). At 10 nM CXCL1 and CXCL7, the inhibition by Staphopain A was 92 and 99%, respectively. Again, the inhibitory effect of Staphopain A could be reversed by the addition of Staphostatin A (Figure 2A and B) and E64 (Supplementary Figure 2a), supporting that proteolytically active Staphopain A is required for the inhibition. Staphopain A did not inhibit neutrophil activation via CXCL8, which activates both the CXCR1 and CXCR2 (Figure 2C). Also, Staphopain A did not block activation via fMLF and C5a (Figure 2D and E), supporting that the protease is specific for CXCR2 and not toxic to cells. Staphopain A also inhibited neutrophil activation by the four other known CXCR2 chemokines (CXCL2, CXCL3, CXCL5 and CXCL6) with >95% (Figure 2F). The fact that CXCL6 is inhibited by Staphopain A is surprising, since CXCL6 is known to activate both CXCR1 and CXCR2 (like CXCL8) (Wuyts et al, 1997). However, we did find that the inhibition was reduced at higher concentrations of CXCL6 (34% inhibition at 1 × 10−8 M CXCL6; Supplementary Figure 2b). Finally, we show that Staphopain A blocks CXCR2 activation in a dose-dependent manner (Figure 2G and H). When neutrophils were stimulated with 3 nM CXCL1 or 10 nM CXCL7, the IC50 of Staphopain A was 38 and 40 nM, respectively. To test whether Staphopain A also blocks non-human CXCR2, murine neutrophils were treated with Staphopain A and stimulated with KC, the murine CXCL1 analogue. In contrast to human neutrophils, Staphopain A did not inhibit the calcium mobilization of murine neutrophils stimulated with different concentrations of KC (Supplementary Figure 3a). This was observed for neutrophils of both CD1 and C57Bl/6 mice. Also, treatment of murine neutrophils with Staphopain A did not alter the binding of a monoclonal antibody directed against the N-terminus of mouse CXCR2 (Supplementary Figure 3b). Thus, Staphopain A effectively blocks calcium mobilization of human neutrophils by CXCR2 ligands while it only partially inhibits chemokines that activate both CXCR1 and CXCR2.
Figure 2.
Staphopain A inhibits CXCR2-mediated calcium mobilization of neutrophils. Fluo-3-labelled neutrophils were preincubated with buffer or 0.5 μM Staphopain A with or without 1 μM Staphostatin A, for 15 min at 37°C. After washing, cells were stimulated with different concentrations of CXCL1 (A), CXCL7 (B), CXCL8 (C), fMLF (D), C5a (E) or a fixed concentration (3 × 10−9 M) of CXCL2, CXCL3, CXCL5 and CXCL6 (F). To determine the IC50 (50% of inhibition), Fluo-3-labelled neutrophils were preincubated with different concentrations of Staphopain A for 15 min at 37°C and subsequently stimulated with 3 × 10−9 M CXCL1 (G) or 1 × 10−8 M CXCL7 (H). The IC50 for CXCL1 and CXCL7 was calculated with the formulas y=−4E+10−7x+4.1314 and y=−3E+10−7x+4.0248, respectively. All figures represent the mean±s.e. of three separate experiments using different donors. The relative calcium mobilization was calculated by dividing the fluorescence after stimulation by the baseline fluorescence. *P<0.05 versus buffer; **P<0.01 for Staphopain A versus buffer (two-tailed Student’s t-test).
Staphopain A inhibits U937-CXCR2 transfected cells
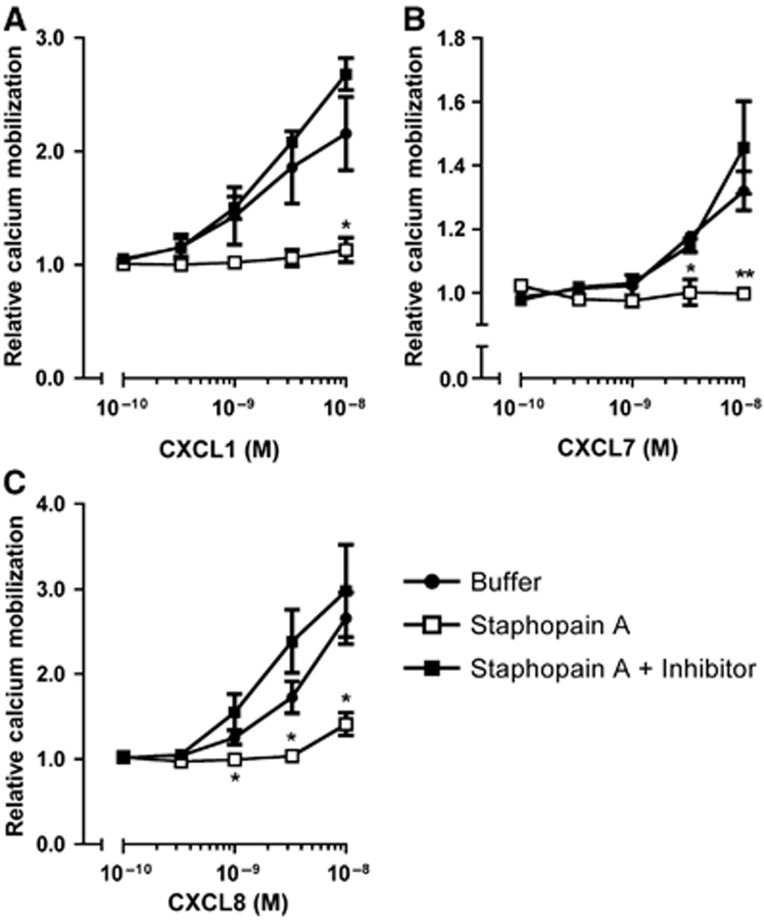
Because neutrophils normally express a variety of chemokine receptors, we employed a heterologous system to express CXCR2 in a U937 promonocytic cell line. In an undifferentiated state, these cells do not express chemoattractant receptors allowing a more focused analysis of CXCR2 (Kew et al, 1997). U937 cells stably transfected with CXCR2 (U937-CXCR2; Kew et al, 1997) were labelled and used in the calcium mobilization assay as described above for neutrophils. Activation of U937-CXCR2 cells with CXCR2 ligands caused a transient calcium mobilization (Figure 3). Pretreatment of cells with Staphopain A efficiently blocked the activation of U937-CXCR2 upon stimulation with CXCL1 and CXCL7 (85 and 100% at 1 × 10−8 M, respectively; Figure 3A and B). Also, Staphopain A potently blocked CXCL8-mediated activation (75% for CXCL8 at 1 × 10−8 M; Figure 3C) on U937-CXCR2, which is in contrast to previous results using isolated neutrophils. This strongly suggests that Staphopain A is specific for CXCR2, as U937-CXCR2 cells do not express CXCR1.
Figure 3.
Staphopain A inhibits calcium mobilization of U937-CXCR2 cells. Fluo-3-labelled U937-CXCR2 cells were preincubated with buffer, or 0.5 μM Staphopain A with and without 1 μM Staphostatin A for 15 min at 37°C. After washing, cells were stimulated with different concentrations of CXCL1 (A), CXCL7 (B) and CXCL8 (C). All figures represent the mean±s.e. of three separate experiments. The relative calcium mobilization was calculated by dividing the fluorescence after stimulation by the baseline fluorescence. *P<0.05 versus buffer; **P<0.01 for Staphopain A versus buffer (two-tailed Student’s t-test).
Staphopain A blocks neutrophil activation and migration
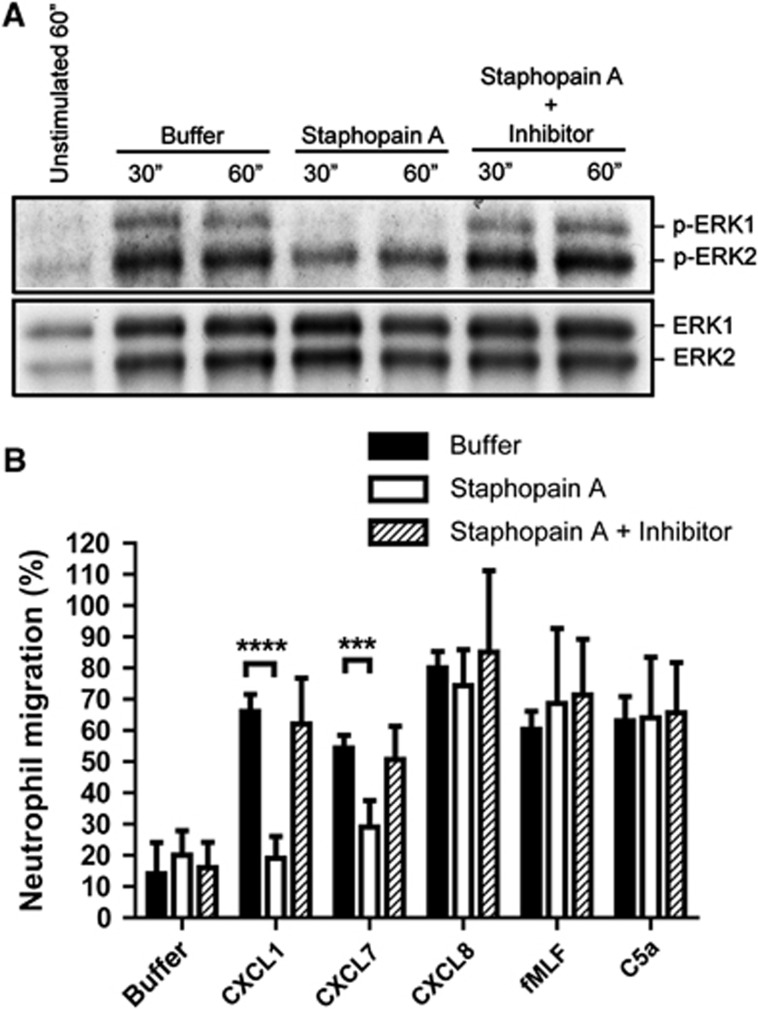
Once neutrophils are activated, they sense the chemoattractant gradient with their chemokine receptors and move towards the site of inflammation. Since Staphopain A blocks the calcium mobilization via CXCR2 receptor, we postulated that CXCR2-mediated intracellular signalling and subsequent neutrophil migration would be affected as well. First, we tested for intracellular activation of the Extracellular signal-Regulated Kinases (ERK) pathway, by detecting total ERK (ERK1/2) and phosphorylated ERK (p-ERK1/2) of neutrophils stimulated with CXCL1. In our hands, the optimal time for detecting ERK phosphorylation by CXCL1 was around 30–60 s. Pretreatment of neutrophils with Staphopain A resulted in a 50% reduction of pERK1 and a 25% reduction of pERK2 while leaving total ERK unaltered (representative blot in Figure 4A). Densitometry analysis of three independent experiments revealed this was statistically significant: mean grey values of 93±19 for buffer versus 47±13 for Staphopain A for pERK1 at 30″ (P=0.01) and 136±22 for buffer versus 85±24 for Staphopain A for pERK2 at 30″ (P=0.01). Staphostatin A inhibited this effect of Staphopain A. At this point, it is not clear why we observed differential inhibition of pERK1 and pERK2, possibly this is caused by higher background levels of pERK2 in unstimulated cells.
Figure 4.
Staphopain A inhibits ERK phosphorylation and migration of neutrophils. (A) Staphopain A inhibits phosphorylation of ERK after CXCL1 stimulation. Neutrophils treated with buffer, 0.5 μM Staphopain A with or without 1 μM Staphostatin A were stimulated with 1 × 10−7 M CXCL1 at 37°C. Samples were taken after 30 and 60 s, subjected to SDS–PAGE, and p-ERK and total ERK were detected using western blotting. Blot is a representative of three separate experiments. (B) Staphopain A inhibits neutrophil migration towards CXCL1 and CXCL7. Calcein-labelled neutrophils treated with buffer, 0.5 μM Staphopain A or 0.5 μM Staphopain A plus 1 μM Staphostatin A, were added to each upper well of the ChemoTX transmembrane system. The lower compartment was filled with 1 × 10−8 M CXCL1, CXCL7, CXCL8, fMLF or 1 × 10−9 M C5a, and the transmembrane system was placed at 37°C for 30 min. The fluorescence of the lower compartment was measured and the percentage of chemotaxis was calculated relative to the fluorescence value of cells added directly to the lower well. The figure represents the mean±s.e. of three separate experiments using different donors. ***P<0.005; ****P<0.001 for Staphopain A versus buffer (two-tailed Student’s t-test).
Next, we tested the effect of Staphopain A on neutrophil migration using a 96-multiwell transmembrane system and optimal chemotactic concentrations of several chemoattractants. After 30 min, we observed that Staphopain A inhibited neutrophil migration towards CXCL1 and CXCL7 with 71 and 46%, respectively (Figure 4B). Inhibition could be prevented by addition of Staphostatin A. No inhibition was observed for neutrophil migration towards CXCL8, fMLF and C5a, showing that Staphopain A specifically inhibits CXCR2-mediated neutrophil migration. Thus, Staphopain A delays CXCR2-mediated intracellular activation and cellular migration.
Staphopain A specifically cleaves the N-terminus of CXCR2
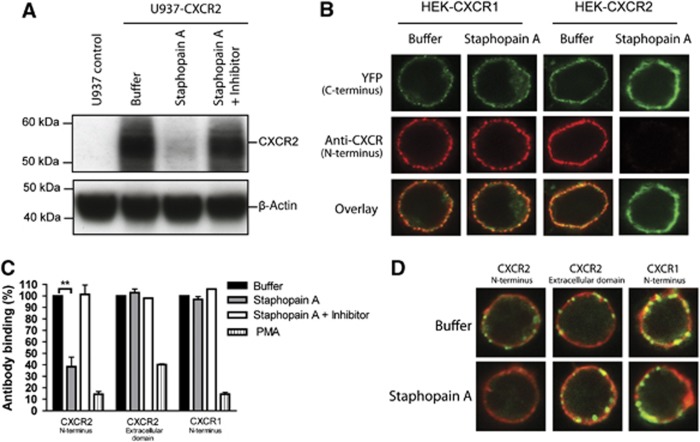
Activation of CXCR2 on neutrophils is initiated by binding of the chemokine to the N-terminal extracellular region (for CXCL1 and CXCL8) and to the first extracellular loop (for CXCL7 and CXCL8) (Katancik et al, 1997). The critical role of the N-terminus in neutrophil activation was demonstrated in studies using antibodies that specifically target the N-terminal domain of CXCR2 (Norgauer et al, 1996). Since treatment of neutrophils with proteolytically active Staphopain A prevented binding of the anti-CXCR2 mAb that recognizes the N-terminus, we hypothesized that Staphopain A activity cleaves and removes the N-terminal domain of CXCR2. To test this, we first probed whole-cell lysates of U937-CXCR2 cells for CXCR2 by western blotting (Figure 5A) using an antibody against the N-terminus. When U937-CXCR2 cells were treated with Staphopain A, we observed a reduced signal for detection by this antibody. Densitometry analysis of three independent experiments revealed that Staphopain A reduced the signal by 60% (mean grey values of 207±13 for buffer versus 83±22 for Staphopain A-treated cells, P=0.008). To study whether Staphopain A specifically cleaves the N-terminal domain of CXCR2, we used Human Embryonic Kidney (HEK) cells expressing the human CXCR2 receptor modified with a C-terminal fusion to Yellow Fluorescent Protein (YFP; Wilson et al, 2005). HEK-CXCR2-YFP cells were incubated with Staphopain A and immunostained with the monoclonal antibody directed against the N-terminus. Confocal analyses showed that Staphopain A-treated cells were no longer recognized by the antibody against the N-terminus of human CXCR2, while the YFP-labelled C-terminus was still present at the cell surface (Figure 5B). This strongly suggests that Staphopain A specifically removes the N-terminus of CXCR2, leaving the rest of the receptor intact. As expected, this cleavage could be reversed in the presence of Staphostatin A (data not shown). Further, using similar constructs for the human CXCR1 receptor, we confirmed that Staphopain A does not cleave human CXCR1 (Figure 5B). The specificity of Staphopain A for the N-terminus of human CXCR2 was also observed on human neutrophils: Staphopain-treated neutrophils were incubated with a polyclonal antibody raised against a peptide mapping within the third extracellular domain of CXCR2 (amino acids 160–210). Using flow cytometry, we showed that Staphopain A treatment did not affect the binding of this antibody to neutrophils (Figure 5C). Using an antibody against the N-terminus of human CXCR1, we confirmed that Staphopain A does not cleave human CXCR1 on neutrophils (Figure 5C). As a control, we stimulated cells with PMA, which causes receptor internalization and a lack of detection by all antibodies. Finally, we used confocal microscopy to confirm these results. Staphopain A-treated neutrophils were stained with the three different antibodies and the membrane was stained using the lipophilic fluorescent carbocyanine membrane dye Did. In our buffer-treated controls, we observed that both CXCR2 and CXCR1 receptors are expressed in clusters on the neutrophil membrane, as described previously (Gray et al, 1997). In Staphopain A-treated cells, the N-terminus of CXCR2 was no longer detected, while the extracellular loop was intact (Figure 5D). The cleavage could be reversed by treatment with Staphostatin A. As expected, we did not observe Staphopain A cleavage of the CXCR1.
Figure 5.
Staphopain A specifically cleaves the N-terminus of CXCR2. (A) Western blot analysis of U937-CXCR2 cells incubated with buffer, 0.5 μM Staphopain A, 0.5 μM Staphopain A plus 1 μM Staphostatin A for 15 min at 37°C. Whole-cell lysates were analysed by western blotting using an mAb against the N-terminus of CXCR2. Figure represents three independent experiments. (B) Confocal images of HEK cells transfected with human CXCR1 or CXCR2 fused to a C-terminal YFP tag. Cells were incubated with buffer or 0.5 μM Staphopain A for 30 min at 37°C. The N-termini were stained with mAbs and subsequent Alexa633-labelled anti-mouse antibodies. YFP was artificially coloured green. Images are representatives of three independent experiments. (C) Flow cytometry analysis of human neutrophils treated with 0.5 μM Staphopain A or 40 nM PMA for 15 min at 37°C. Cells were first stained with antibodies against the N-terminus of CXCR2, the extracellular loop of CXCR2 or the N-terminus of CXCR1 and subsequently with FITC-labelled secondary antibodies. Antibody binding is expressed in percentages, calculated by dividing the fluorescence of treated cells by fluorescence of buffer-treated cells and multiplying with 100. Figure represents the mean±s.e. of three separate experiments using different donors. **P<0.01 versus buffer (two-tailed Student’s t-test). (D) Confocal images of human neutrophils stained with antibodies against CXCR2 and CXCR1 (green). Cells were treated as described above for (C) after which the membranes were stained with Did (red). Images are representative for three independent experiments using different donors.
Identification of the Staphopain A cleavage site in CXCR2
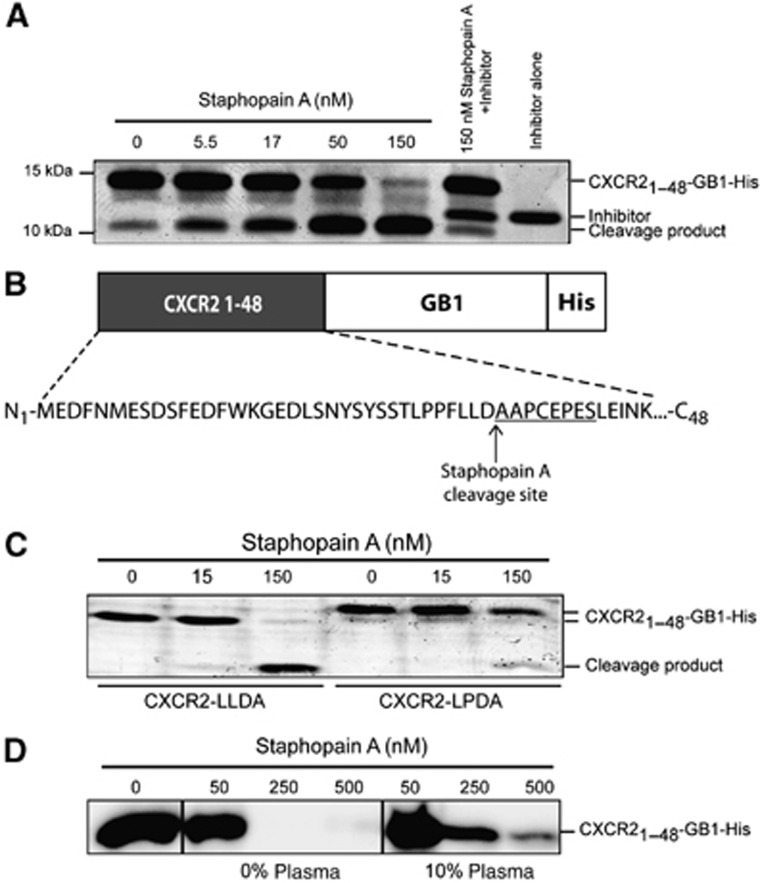
To identify the cleavage site of Staphopain A in the CXCR2 N-terminus, we recombinantly produced the extracellular region (amino acids 1–48) of CXCR2 (CXCR21–48) in E. coli. The CXCR2 peptide was fused to the N-terminus of the B1 domain of protein G (GB1) for improved expression (Huth et al, 1997) and provided with a C-terminal histidine (His) tag to allow purification. The 13-kDa CXCR21–48-GB1-His protein was incubated with different concentrations of Staphopain A for 15 min at 37°C and analysed by SDS–PAGE. We observed that Staphopain A efficiently cleaved CXCR21–48-GB1-His protein (Figure 6A) to a smaller fragment of around 9 kDa and this cleavage could be blocked by Staphostatin A and E64 (data not shown). N-terminal sequencing revealed that the cleavage product starts with the amino-acid sequence NH2-AAP*EPES, indicating that Staphopain A cleaves between Aspartate (D35) and Alanine (A36): LLD↓A (Figure 6B; Supplementary Figure 4). In a recent study, the Staphopain A substrate binding site was mapped using a bacterial cell-surface display system (CLiPS; Kalińska et al, 2012). This CLiPS study indicated that Staphopain A prefers a Leucine residue for the P2 site of the substrate, corresponding with the Leucine at position 34 in CXCR2. The murine CXCR2 receptor contains a Proline instead of a Leucine at this position. We studied whether the inability to cleave murine CXCR2 is caused by this single amino-acid difference. Therefore, we produced a modified version of the human CXCR21–48-GB1-His protein with a Proline at position 34 (LPDA). Indeed, we observed less potent cleavage of this protein by Staphopain A (Figure 6C) but at higher enzyme concentrations there was still significant cleavage. Since Staphopain A did not inactivate the native receptor on murine cells, this suggests that other parts of the receptor than this Proline probably determine the cleavage differences between human and murine CXCR2. An alternative explanation is that the structure of the receptor in the context of a fusion partner might be different from the native N-terminus expressed on the cell. Since it is known that Staphopain A also cleaves human fibrinogen (Ohbayashi et al, 2011), an abundant plasma protein, we tested whether Staphopain A can still cleave CXCR2 in the presence of blood plasma. Therefore, CXCR21–48-GB1-His protein was incubated with Staphopain A in the presence of human plasma. Although plasma slightly competes with the cleavage, we found that Staphopain A can still cleave CXCR2 in 10% plasma (Figure 6D).
Figure 6.
Determination of the Staphopain A cleavage site in CXCR2. (A) Staphopain A cleaves the CXCR21–48-GB1-His protein. Different concentrations of Staphopain A were incubated with 6.75 μM CXCR21–48-GB1-His protein for 15 min at 37°C. Samples were analysed by SDS–PAGE and Instant blue staining. Lane 6: CXCR21–48-GB1-His plus 150 nM Staphopain A plus 1 μM Staphostatin A; lane 7: Staphostatin A alone. (B) N-terminal sequencing of the CXCR2 cleavage product generated by incubation of 6.75 μM CXCR21–48-GB1-His protein with 150 nM Staphopain A for 15 min at 37°C. Sequencing was performed once. (C) Staphopain A cleavage of CXCR21–48-GB1-His (left, LLDA) or CXCR21–48-GB1-His mutated with a Proline at position 34 (right, LPDA). CXCR2 protein at 6.75 μM, 15 min at 37°C; proteins were stained using Instant Blue. (D) Staphopain A cleaves CXCR2 in the presence of plasma. CXCR21–48-GB1-His (1.5 μM) was incubated with Staphopain A with or without 10% human plasma for 30 min at 37°C. CXCR2 was visualized by immunoblotting using anti-CXCR2 (N-terminus specific) antibodies. (A, C and D) Representatives of three separate experiments are shown.
Staphopain A production in bacterial supernatants
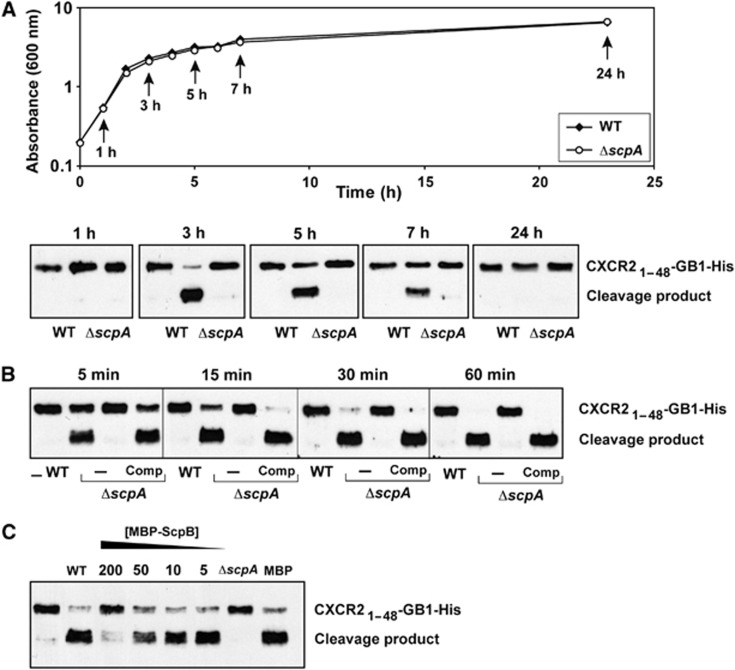
In order to study the growth phase-dependent production of Staphopain A, we first used allelic exchange mutagenesis to create a ΔscpA mutant in S. aureus USA300, a predominant isolate of community-acquired MRSA (Diep et al, 2006). Wild-type and mutant bacteria were grown in liquid cultures and supernatants were collected at different time points of the growth curve, incubated with CXCR21–48-GB1-His protein, and analysed for proteolytic cleavage by immunoblotting. By comparing wild-type and ΔscpA mutant supernatants, we observed Staphopain A-dependent cleavage of the CXCR2 fusion protein (Figure 7A). Surprisingly, we observed maximal levels of Staphopain A activity in supernatants of 3 h cultures that were entering late logarithmic growth (Figure 7A), and after this time point, Staphopain A activity levels declined as USA300 cells entered stationary phase (5 and 7 h) and by 24 h activity was not detectable. Considering extracellular enzymes of S. aureus usually accumulate over time and maximal activity is detected in overnight cultures (Kiedrowski et al, 2011), this narrow window of Staphopain A activity was unexpected and the reason for the loss of activity at late time points is unknown. Supernatants of the ΔscpA mutant did not cleave the CXCR2 protein at any of the time points, showing that Staphopain A is the only protease secreted by S. aureus that can cleave CXCR2. Based on the coordinated regulation of the Aureolysin, V8 protease, Staphopain A and Staphopain B extracellular proteases (Shaw et al, 2004), these other enzymes should be present during the 24-h time course. Indeed, our analysis of wild-type and ΔscpA supernatants in various substrate assays indicated that other proteases like V8 protease, Staphopain B and SplB are present in the supernatants of later time points (Supplementary Figure 5). Since the SplB substrate specifically detects SplB in the supernatant, we used this to estimate the concentration of SplB in the supernatants to be between 40 to 50 nM in 24 h supernatant.
Figure 7.
Staphopain A detection in bacterial supernatants. (A) S. aureus strain USA300 (WT) and its isogenic mutant (ΔscpA) were grown in TSB media and supernatants were collected at different time points. Above: OD600 measurements taken at different time points; Below: cleavage of 1 μM CXCR21–48-GB1-His protein by undiluted supernatants collected at different points (incubation for 30 min at 37°C unless otherwise noted). The CXCR21–48-GB1-His protein was detected using western blotting with an antibody against the His tag. (B) Time-dependent cleavage of CXCR21–48-GB1-His protein (1 μM) by 3 h supernatants (undiluted) of USA300 (WT), its isogenic mutant (ΔscpA) and a complemented mutant (ΔscpA Comp). (C) CXCR2 cleavage by WT supernatant is inhibited by Staphostatin A (MBP-ScpB). CXCR21–48-GB1-His protein (1 μM) was incubated with 3 h supernatants (undiluted) of USA300 (WT) in the presence of various concentrations of MBP-tagged ScpB. MBP is maltose binding protein. (A–C) Representative blots of three separate experiments are shown.
To study whether the Staphopain A-dependent CXCR2 cleavage by supernatants can go to completion, we incubated 3 h supernatants with the CXCR2 protein for several time points. Figure 7B demonstrates that within 1 h all CXCR2 protein is converted to product. Supernatants of ΔscpA mutant bacteria did not convert the substrate and complementation of the mutant restored activity to wild-type levels. Finally, we analysed the native levels of Staphopain A produced by S. aureus by titrating Staphostatin A inhibitor into the supernatant of wild-type USA300 and analysed CXCR2 cleavage. Since Staphostatin A is highly specific for Staphopain A and forms a 1:1 complex (Filipek et al, 2003), it can be used for this purpose. CXCR2 cleavage by USA300 supernatant was blocked by exogenous Staphostatin A (MBP-ScpB) addition in a dose-dependent fashion (Figure 7C). Staphopain A activity was partially inhibited at 50 nM MBP-ScpB and completely inhibited at 200 nM, suggesting that S. aureus secretes levels of ScpA that range from 50 to 200 nM.
Discussion
Neutrophils play an important role in the innate immune system since they are crucial for the clearance of bacteria during an infection. At the site of inflammation, local activation of the complement system and production of chemokines by neighbouring cells leads to the recruitment of neutrophils. CXCR2 on neutrophils, which recognizes chemoattractants, mediates activation and chemotaxis of neutrophils. S. aureus is a major human pathogen that is well known for its large arsenal of secreted immune evasion factors. For instance, S. aureus secretes specific complement inhibitory proteins that shutdown complement activation (Geisbrecht, 2008). Furthermore, a number of immune evasion factors inhibit different steps in neutrophil recruitment. Neutrophil rolling and extravasation is inhibited by Staphylococcal superantigen-like 5 (SSL5) protein which binds P-selectin glycoprotein ligand-1 (Bestebroer et al, 2007). Neutrophil chemotaxis is blocked by (1) the chemotaxis inhibitory protein of Staphylococci (CHIPS, blocking the C5a receptor and the FPR 1 (FPR1) (de Haas et al, 2004)); (2) the FPR-like 1 inhibitory proteins (FLIPr and FLIPr-like, blocking FPR1 and FPR2, respectively (Prat et al, 2006, 2009)); (3) SSL5 (inhibiting neutrophil activation by all chemokines and anaphylatoxins (Bestebroer et al, 2009)) and (4) SSL10 (blocking CXCR4 (Walenkamp et al, 2009)). Here, we identify Staphopain A as the first specific bacterial CXCR2 inhibitor. The function of Staphopain A, blocking neutrophil activation and chemotaxis, seems redundant next to the other Staphylococcal chemotaxis inhibitors. However, Staphopain A exclusively inhibits neutrophil activation via CXCR2 stimuli. Since a large number of different chemoattractants mediate neutrophil influx to the infected tissue, S. aureus benefits from an elaborate army of chemotaxis inhibitors that target different receptors. Likely, these inhibitors all act in concert to effectively downmodulate the inflammatory response and enhance the bacterial chances for survival. We predict that taking away only a few of these inhibitors would greatly reduce that bacterial virulence. This hypothesis is strongly supported by a recent study in which we mutated two S. aureus complement inhibitors and analysed bacterial virulence in various mouse models. Even though S. aureus expresses a large number of complement inhibitory proteins (Geisbrecht, 2008), taking away two of these factors significantly reduced bacterial pathogenesis (Jongerius et al, 2012). Still, our study offers limited evidence for the in-vivo importance of Staphopain A during S. aureus infections. The inability of Staphopain A to cleave murine CXCR2 makes it difficult to study its importance in conventional mouse models. Since mice are not a natural reservoir for S. aureus (Van den Berg et al, 2011), there is no need for the bacterium to cleave murine CXCR2. In analogy, we found that a vast majority of the S. aureus immune evasion proteins are highly human specific (Rooijakkers et al, 2005). These findings illustrate the high adaptation of S. aureus to the human host and also reflect the limitations of S. aureus pathogenesis studies using mouse models.
The mechanism for CXCR2 inhibition was pinpointed to cleavage of the N-terminal domain of CXCR2 (Supplementary Figure 4), a region that is necessary for proper chemokine binding and subsequent receptor activation. We identified LLD↓A as the Staphopain A cleavage site in CXCR2, a finding that largely corresponds with a recent study using CLiPS showing that Staphopain A prefers a Leucine at the P2 substrate subsite. Further, this study indicated that Staphopain A has no preferential P3 site and that Staphopain A, like other Staphopain enzymes, prefers a residue with small side chains in the P1 (Gly) and P1′ (Ala or Ser) position (Kalińska et al, 2012). The LLD↓A site also contains an Alanine at the P1′ site. Although CLiPS did not reveal an Aspartic acid at P1, it should be noted this amino acid has a relatively small side chain. The murine CXCR2 contains a Proline instead of a Leucine at the P2 site (LPDA). Despite the fact that modification of the human CXCR2 N-terminus with an LPDA site revealed less effective substrate conversion, the experiment clearly indicated that the discrepancy in the P2 site does not fully explain the lack of activity versus mouse CXCR2. However, one cannot exclude that the structure of the receptor in the context of a fusion partner might be different from the native N-terminus expressed on the cell. Since the N-termini of mouse and human CXCR2 are only 47% identical, it is also possible that other sites in the N-terminus contribute to the definition of Staphopain A specificity (Cerretti et al, 1993).
Next to Staphopain A, S. aureus secretes other proteases that are involved in the modulation of the immune system (Potempa and Pike, 2009). Aureolysin cleaves both complement protein C3 and the antimicrobial peptide LL-37 (Sieprawska-Lupa et al, 2004; Laarman et al, 2011). V8 protease cleaves all immunoglobulin classes (Prokesova et al, 1992). Staphopain B cleaves the neutrophil and monocyte receptors CD11b and CD31 inducing cell death (Smagur et al, 2009a, 2009b). Our multi-screening antibody assay indicated that the interaction of Staphopain A with neutrophils is highly specific for CXCR2. All antibodies were selected for recognizing important epitopes, however, a negative result in this antibody assay does not exclude that Staphopain A might also cleave other neutrophil receptors. To study whether Staphopain A is unique among S. aureus proteases to cleave CXCR2, we created an isogenic Staphopain A mutant in USA300. Unfortunately, due to cellular toxicity of other secreted Staphylococcal factors (Wang et al, 2007; Kennedy et al, 2010), we could not show a comparison of bacterial supernatants in cellular assays. Still, comparing supernatants of wild-type and mutant S. aureus in cleavage assays with purified CXCR2 N-terminus convincingly showed that Staphopain A is unique among S. aureus proteases to cleave CXCR2. Among S. aureus proteases, only the Staphopains seem to have activity on human neutrophils. It seems tempting to speculate that the Staphopains might work together in modulating neutrophil functions. This will be subject of further investigations. Although it is impossible to determine the levels of Staphopain A during an infection in vivo, it was previously reported that Staphopains are the most abundantly produced proteases of S. aureus (Jones et al, 2008). In corroboration with these data, our Staphostatin A titrations revealed Staphopain A concentrations of around 50–200 nM in liquid culture, higher than the determined SplB concentrations. Furthermore, these concentrations are 1.25–5 × higher than the determined IC50 for CXCR2 cleavage (40 nM). Our experiments indicated that the CXCR2-GB1-His protein is a useful tool for studying the regulation of Staphopain A in bacterial supernatants. Although the S. aureus extracellular proteases are known to be co-regulated (Shaw et al, 2004), we were somewhat surprised by the strong activity of Staphopain A early in the time course and subsequent loss of activity over time. Previous studies suggested that Staphopain A is subject to autolytic degradation (Nickerson et al, 2010), which might explain the inability of CXCR2 substrate to be cleaved efficiently at later time points. However, extracellular proteome studies of S. aureus demonstrate that Staphopain A protein remains intact at similar late time points in growth (Jones et al, 2008; Lin et al, 2011). Further investigation will be necessary to resolve this discrepancy.
In conclusion, Staphopain A is the first immune evasion factor of S. aureus that specifically cleaves the N-terminus of the neutrophil CXCR2 receptor. Secretion of Staphopain A at the site of infection is likely to inhibit neutrophil activation and recruitment, adding to the elaborate immune evasion repertoire of S. aureus.
Materials and methods
Reagents and (recombinant) proteins
The antibody screening assay on neutrophils was performed using commercial fluorophore-conjugated antibodies: Phycoerythrin (PE)-conjugated antibodies recognizing CD32 (7.3, Fitzgerald), CD35 (E11, BD), CD44 (515, BD), CD47 (B6H12, BD), CD49b (12F1, BD), CD54 (HA58, BD), CD58 (1C3, BD), CD63 (CLB-gran/12, ImmunoTech), CD87 (VIM5, BD), CD88 (S5/1, Biolegend), CD89 (Mip8a, Abd-Serotec), CD114 (LMM741, BD), CD119 (GIR-208, BD), CD162 (KPL-1, BD), CD181 (42705.111, R&D), CD182 (48311.211, BD), CD282 (T2.5, EBioscience) and CD321 (M.Ab.F11, BD); Fluorescein isothiocyanate (FITC)-labelled antibodies recognizing CD9 (M-L13, BD), CD11a (HI111, BD), CD15 (MMA, BD), CD18 (6.7, BD), CD31 (WM59, BD), CD43 (6D269, Santa Cruz), CD46 (E4.3, BD), CD62L (Dreg-56, BD), CD66b (G10F5, BD), CD66 (B1.1/CD66, BD), CD120a (16803.161, R&D), CD120b (22235.311, R&D), CD147 (HIM6, BD), LTB4R (202/7B1, Abd-Serotec); Allophycocyanin (APC)-labelled antibodies recognizing CD10 (MEM-78, Invitrogen), CD11b (ICRF44, BD), CD11c (B-ly6, BD), CD13 (WM15, BD), CD14 (M5E2, BD), CD29 (MAR4, BD), CD45 (HI30, BD), CD55 (IA10, BD), CD50 (CBR-IC3/1, BD) and anti-Siglec-9 (191240, R&D); Alexa-647-labelled anti-CD16 (3G8, BD). The FPR was detected using FITC-conjugated formyl-Nle-Leu-Phe-Nle-Tyr-Lys (abbreviated as FITC-fMLP; Invitrogen).
Staphopain A (purified from S. aureus culture supernatant) was purchased from BioCentrum. Recombinant mouse KC and human CXCL8 were purchased from Tebu-Bio, recombinant human CXCL2, CXCL3, CXCL5, CXCL6 and CXCL7 from R&D systems. fMLF and recombinant C5a were obtained from Sigma-Aldrich. The N-terminus of CXCR2 (CXCR21–48) was produced recombinantly in E. coli using the GB1 as a fusion partner, as described previously (Huth et al, 1997) with modifications. In short, CXCR21–48 was expressed containing a C-terminal fusion of GB1 followed by a hexahistidine tag: CXCR21–48-GB1-His. The GB1 template was produced by an overlap extension PCR in which four overlapping primers covering the complete GB1 sequence were mixed. As a target for CXCR2, we used human chromosomal DNA since CXCR2 is encoded by a single exon. CXCR21–48 and GB1 were amplified by PCR with the following overlapping primer pairs: 5′-CAAAGCGAGGTCCATATGGAAGATTTTAACATGGAGAGTG-3′/5′-TCAGAATCACTTTATACTGCTTGTTGATTTCCAGGGATTC-3′ and 5′-TCCCTGGAAATCAACAAGCAGTATAAAGTGATTCTGAACG-3′/5′-GCCGAATTCTTAATGGTGATGATGATGATGTTCGGTCACCGTAAAGGTTTTGG-3′, respectively (NdeI and EcoRI recognition sites underlined). To anneal the CXCR21–48 and GB1 PCR products, we performed a third PCR using the CXCR21–48 5′-primer and the GB1 3′-primer. The final PCR product was ligated into the expression vector pRSETB (Invitrogen) using NdeI and EcoRI and the correct sequence was verified using DNA sequencing. This plasmid was used as a template to generate the modified version of human CXCR21–48-GB1-His containing a Proline at position 34. The insert was amplified by overlap extension PCR using overlapping primers introducing the 34L>34P mutation. Expression vectors were transformed in E. coli Rosetta-Gami(DE3)pLysS according to manufacturer’s protocol (Novagen). Proteins were isolated under denaturing conditions on a Histrap column according to manufacturer’s protocol (GE Healthcare) and eluted under denaturing conditions with 100 mM EDTA. The proteins were refolded by dialysis against PBS and further purified on a MonoQ 5/50 GL (GE Healthcare) in 20 mM Tris 1 mM DTT pH 7.4. Bound protein was eluted with a gradient of 20 mM Tris 1 mM DTT 1 M NaCl pH 7.4. Finally, CXCR21–48-GB1-His was dialysed against PBS and stored at −80°C. The purity and concentration were estimated by SDS–PAGE and Bradford protein assay (Pierce). Recombinant non-tagged Staphostatin A was purchased from BioCentrum. MBP-tagged Staphostatin A (MBP-ScpB) was produced recombinantly as following. The scpB gene was amplified by PCR from S. aureus strain AH1263 using the following oligonucleotides: JMM007: 5′-GTCTACGAATTCATGGAACAAATTGAATTATTTAG-3′ and JMM008: 5′-GTCTACCTGCAGTTATGACTTATGCTTAATGAAAG-3′. The PCR product was purified, digested with EcoRI and PstI enzymes, and cloned into plasmid pMAL-c2X (New England Biolabs) cut with the same enzymes. The pMAL-scpB plasmid was transformed into strain E. coli ER2566, and MBP-ScpB protein purification was performed using Amylose Resin according to manufacturer’s instructions (New England Biolabs). Eluted fractions were analysed via SDS–PAGE and concentrated with an Amicon Ultra-15 with a 10-K cutoff (Millipore). Protein concentration was measured using the BioRad protein assay. The cysteine protease inhibitor E64 was purchased from Sigma.
Cells
Human neutrophils were isolated freshly from human blood as previously described and used on the same day (Bestebroer et al, 2007). Informed consent was obtained from all subjects and was provided in accordance with the Declaration of Helsinki. Approval was obtained from the medical ethics committee of the UMC Utrecht, The Netherlands. Mouse leukocytes were isolated from the bone marrow of 3-month-old female C57Bl/6 mice (Harlan). The femur and tibia were removed, and the bone marrow was harvested by flushing with PBS containing 2 mM EDTA and 0.5% BSA. The cells were passed through a 70-μm cell strainer (BD Biosciences) to obtain a single-cell suspension and subsequently through a CD115 MACS selection kit (Miltenyi) to remove murine monocytes, macrophages, osteoclasts and dendritic cell precursors. The erythrocytes were removed using hypotonic shock. Untransfected and CXCR2-transfected human promonocytic U937 cells, a generous gift from Dr E Prossnitz (University of New Mexico, Albuquerque, NM), were maintained in DMEM supplemented with 10% FCS and 10 μg/ml gentamycin. HEK293 cells (ATCC, Rockville, MD) were maintained in DMEM supplemented with 10% FCS, 1% pen/strep and glutamate. HEK293 cells were transfected with pcDNA3.1 (+) plasmids harbouring human CXCR1 and CXCR2 with C-terminal YFP fusions (Wilson et al, 2005). One 1 μg plasmid DNA was mixed with 9.75 μg PEI for 30 min at RT and then added to 1 × 106 cells coated in a 6-well flat bottom tissue culture plate. Transfection was allowed overnight in the absence of antibiotics. Cells were harvested using 3 mM EDTA in PBS.
Flow cytometry
The antibody screening competition assay using human neutrophils was performed as previously described (Bestebroer et al, 2007) with minor modifications. In short, neutrophils (5 × 106 cells/ml) were incubated with 0.5 μM Staphopain A or buffer for 15 min at 37°C in RPMI/0.05% HSA. After washing, cells were incubated with mixtures of fluorophore-conjugated antibodies directed against different cell surface receptors for 30 min on ice. After washing, fluorescence was measured using a FACSCalibur™ (BD). The mean fluorescence of 10 000 gated neutrophils was determined and the relative expression was calculated by dividing the mean fluorescence of Staphopain A-treated cells by buffer-treated cells. Background was determined by using various isotype controls (BD).
To analyse binding of different CXCR2 and CXCR1 antibodies, neutrophils or U937-CXCR2 cells were treated with buffer, 0.5 μM Staphopain A with or without 1 μM Staphostatin A for 15 min at 37°C. After washing, cells were incubated with mouse anti-human CXCR2 (clone 19, Abcam, directed against the N-terminus), goat anti-human CXCR2 (sc-22661, Santa Cruz, directed against the extracellular domain) or mouse anti-human CXCR1 (R&D, directed against the N-terminus) for 30 min on ice. After washing, cells were incubated with FITC-labelled goat anti-mouse (Dako) or rabbit anti-goat antibodies (Dako). CD115-negative murine leukocytes were stained with an APC-labelled rat anti-mouse CXCR2 antibody (R&D) and, to enable gating of neutrophil in flow cytometry, with PE-labelled anti-mouse Ly-6C/G (Invitrogen). The mean fluorescence of 10 000 gated neutrophils was determined and the percentage inhibition compared to buffer-treated cells was determined. To analyse apoptosis, neutrophils were incubated with buffer (RPMI/0.05% HSA), 0.5 μM Staphopain A or Staphopain B for 75 min at 37°C. After washing, cells were stained with APC-labelled Annexin V (Ebiosciences) according to manufacturer’s protocol and Propidium Iodide and cells were analysed by flow cytometry.
To determine neutrophils activation by chemoattractants, the transient increase in free intracellular calcium concentration was measured by flow cytometry (de Haas et al, 2004). Neutrophils were loaded with 2 μM Fluo-3-AM (Invitrogen), washed and resuspended in RPMI/0.05% HSA to a concentration of 5 × 106 cells/ml. Cells were then incubated with buffer, 0.5 μM Staphopain A or 0.5 μM Staphopain A plus 1 μM Staphostatin A for 15 min at 37°C in RPMI/0.05% HSA. Cells were washed and calcium mobilization was monitored by flow cytometry. Neutrophils were gated based on scatter properties. The basal fluorescence level for Fluo-3 was monitored for 10 s, the stimulus was added and the sample tube was rapidly placed back to the sample holder and the fluorescence measurement continued up to 42 s. Relative calcium mobilization was calculated by dividing the fluorescence after stimulation by that of the background.
Chemotaxis and ERK detection
Chemotaxis of human neutrophils towards several chemoattractants was measured as previously described (Prat et al, 2009) using a 96-multiwell transmembrane system (ChemoTX; Neuro Probe) with a 5-μm pore size polycarbonate membrane. Calcein-labelled cells (5 × 106 cells/ml) were incubated with 0.5 μM Staphopain A or 0.5 μM Staphopain A plus 1 μM Staphostatin A for 15 min at 37°C in HBSS containing 1% HSA. Optimal concentrations of 1 × 10−9 M (C5a) and 1 × 10−8 M (CXCL1, CXCL7, CXCL8 and fMLF) were prepared in HBSS/HSA, and placed into each well of the lower compartment of the chamber (in triplicate). Wells with control medium and cells were included to measure the spontaneous cell migration and total fluorescence, respectively. The membrane holder was assembled, and labelled cells were added as a droplet to each upper well (except for the total fluorescence wells). The plate was incubated for 30 min at 37°C in a humidified 5% CO2 atmosphere. The membrane was washed extensively with PBS and fluorescence of the wells was measured in a FlexStation fluorescent plate reader (Molecular Devices) with excitation at 485 nm and emission at 530 nm. The percentage of chemotaxis was calculated relative to the fluorescence value of cells added directly to the lower well.
For ERK detection, neutrophils (1 × 106) were treated with buffer or 0.5 μM Staphopain A in the presence or absence of 1 μM Staphostatin A for 15 min at 37°C in RPMI/0.05% HSA. Cells were washed and subsequently stimulated with 10−7 M CXCL1 for 30 and 60 s at 37°C. Ice-cold PBS containing 0.1% HSA was added to the cells to stop the reaction, and cells were washed in ice-cold PBS without HSA. Cells were resolved in Laemmli sample buffer, subjected to SDS–PAGE under reducing conditions and transferred onto a PVDF membrane. The membrane was blocked with 5% skimmed milk in TBS containing 0.1% Tween-20. p-ERK was detected using the primary rabbit monoclonal antibody against Phospo-p44/42 ERK (Cell Signaling Technology) followed by a peroxidase-labelled goat anti-rabbit (SBI) diluted in TBS containing 0.1% Tween-20 and 1% skimmed milk powder. To detect total ERK, the membrane was stripped with stripping buffer (Thermo Scientific) for 15 min at room temperature, blocked and probed again with the rabbit polyclonal antibody against anti-ERK1/2 (Millipore) as described above. ECL (GE Healthcare) was used for signal detection.
Immunoblotting of CXCR2 on U937 cells
U937-CXCR2 cells (1 × 105 cells) were incubated with buffer, 0.5 μM Staphopain A, or 0.5 μM Staphopain A plus 1 μM Staphostatin A for 15 min at 37°C in RPMI/0.05% HSA. Cells were washed in ice-cold PBS and lysed in RIPA buffer (50 mM Tris–HCl pH 7.4, 1% Nonidet P-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 μg/ml Aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM NaF and 1 mM Na3VO4) for 30 min on ice. The lysates were centrifuged for 15 min 13 000 r.p.m. at 4°C. Supernatants were dissolved in Laemmli buffer, subjected to SDS–PAGE and transferred onto a PVDF membrane. The membrane was blocked with 2% BSA in PBS containing 0.1% Tween-20. CXCR2 was detected using mouse anti CXCR2 (clone 19, Abcam) followed by a peroxidase-labelled goat anti-mouse (BioRad) diluted in PBS containing 2% BSA and 0.1% Tween-20. ECL (GE Healthcare) was used for signal detection.
Confocal analyses
Neutrophils or transfected HEK cells (2.5 × 105) were incubated with buffer, 0.5 μM Staphopain A, or 0.5 μM Staphopain A plus 1 μM Staphostatin A for 30 min at 37°C in RPMI/0.05% HSA. Cells were washed and stained with mouse anti-human CXCR2 (clone 19, Abcam), goat anti-human CXCR2 (sc-22661, Santa Cruz) or mouse anti-human CXCR1 (R&D). Bound antibodies were detected using Alexa633-labelled goat anti-mouse antibody (on HEK cells, Molecular Probes) or FITC-labelled goat anti-mouse or rabbit anti-goat antibodies (on neutrophils, Dako). After washing, neutrophils were stained with a Did membrane dye (Invitrogen). Cells were mounted on a microscope slide and imaged using a Leica TSC SP5 confocal microscope equipped with a × 63 oil-immersion HCX PL APO CS objective lens with a numerical aperture of 1.4 (Leica Microsystems, The Netherlands). During visualization of HEK cells, YFP and Alexa633 fluorophores were excited sequentially. The excitation laser line for YFP was the 514-nm helium/neon laser with detection via a 458/514-nm double band pass filter, emission bandwidth was set at 518–610 nm. Alexa633 label was detected using a 633-nm helium/neon laser and detected via a 488/543/633 triple band pass filter; emission bandwidth was set at 660–738 nm. For neutrophils, FITC and Did were excitated simultaneously using the 488-nm argon laser for FITC and the 633-nm helium/neon laser for Did, both detected via a 488/543/633 triple band pass filter. Emission bandwidth was 512–596 nm for FITC and 646–760 nm for Did. Confocal experiments were performed three times, analysing 50 cells per experiment.
Bacteria and supernatants
S. aureus strains were maintained in Tryptic Soy Broth (TSB) and when necessary, chloramphenicol was added to 10 μg/ml for plasmid maintenance. S. aureus strain USA300 AH1263 was used as the wild-type strain in these studies (Boles et al, 2010). To construct the Staphopain A mutant in AH1263, plasmid pJB38-scpA (Wörmann et al, 2011) was transformed into AH1263 (Schenk and Laddaga, 1992) and the scpA deletion mutation was constructed using the pKOR1 protocol (Bae and Schneewind, 2006). The mutation was verified through molecular analysis. For constructing the scpA complementing clone, the scpAB genes was PCR amplified from AH1263 using the following oligonucleotides: JMM012: 5′-GTCTACCATATGATGAAAAGAAACTTTCCAAAATTAATAG-3′ and JMM013: 5′-GTCTACCTCGAGTTATGACTTATGCTTAATGAAAG-3′. The PCR product was purified, and digested by NdeI and XhoI, and ligated into plasmid pOS1-plgt (Benson et al, 2011) digested with the same enzymes. Plasmid construction was confirmed by PCR and transformed into AH1263 and the scpA deletion mutant. For preparing supernatants, wild-type and mutant S. aureus strains were cultured overnight in 5 ml TSB media at 37°C with shaking. The following day, the bacteria were subcultured in 5 ml TSB to an initial OD600 of 0.2 and grown overnight with shaking at 37°C. OD600 measurements were taken and supernatants were collected at different time points.
Cleavage of CXCR21–48-GB1-His
For N-terminal sequencing, 6.75 μM CXCR21–48-GB1-His protein was incubated with 0.15 μM Staphopain A for 15 min at 37°C in PBS. Proteins were subjected to SDS–PAGE and transferred onto a PVDF membrane that was visualized with 0.1% Coomassie blue in 40% methanol. The cleavage product was excised and analysed by N-terminal sequencing (Alphalyse, Denmark). In other experiments, cleavage was performed at different time points and using 6.75 or 1 μM CXCR21–48-GB1-His for Instant Blue staining (Gentaur) or western blotting, respectively. CXCR2 cleavage in the presence of plasma was analysed by western blotting using anti-CXCR2 (cl. 19, Abcam) antibodies combined with peroxidase-labelled goat anti-mouse antibodies (Southern). Human plasma was isolated from a single donor using lepuridin as an anti-coagulant. For cleavage by bacterial supernatants, 1 μM CXCR21–48-GB1-His protein was incubated with undiluted freshly collected supernatants for different time points at 37°C. To collect supernatants, 100 μl broth culture (prepared as described above) was filtered through a 0.22-μm Spin-X centrifuge tube filter (Costar). No reducing agents were included in the reaction mixture. Samples were incubated at 37°C for designated time periods, and the reaction was stopped by the addition of SDS–PAGE loading dye, followed by heating at 100°C for 10 min. CXCR2 was detected by western blotting and mouse anti-His antibodies. For Staphostatin titration, CXCR21–48-GB1-His protein (1 μM) was incubated with 3 h supernatants (undiluted) of USA300 (WT) in the presence of various concentrations of MBP-tagged ScpB.
Protease substrate assays
Casein cleavage was analysed by incubating bacterial supernatants with 10 μM purified β-casein (Sigma) for 3 h at 37°C in PBS. Similarly, supernatants were incubated with 1 μM human fibrinogen (Sigma) for 30 min at 37°C in PBS. Reactions were stopped by adding sample buffer and samples were analysed by SDS–PAGE and Instant Blue staining. SplB activity was measured by incubating supernatants with 0.1 mM WELQ-AMC (Dubin et al, 2008) for 30 min at 37°C in 50 mM Tris–HCl pH 8. Fluorescence (ex355/em460) was detected using the Flex station fluorometer (Molecular Devices).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 4.0 package and the differences between groups were analysed for significance using the two-tailed Student’s t-test.
Supplementary Material
Acknowledgments
We thank William Nauseef for critically reading the manuscript. We thank Jeffrey Beekman for technical assistance on ERK phosphorylation, Reindert Nijland for helping with confocal analyses and Cassandra Krone for providing CD115-negative murine leukocytes. AL, JvS and SHMR are supported by grants of the Netherlands Organization for Scientific Research (NWO-TOP and NWO-Vidi). ARH was supported by award AI078921 from the National Institute of Allergy and Infectious Diseases.
Author contributions: AJL, GM, WJMvR, MR, JMM, KPMvK and ECH performed experiments. CLM and ARH provided the S. aureus strain and constructed the S. aureus isogenic mutants. RW and GM provided HEK293 constructs and technical advice. SHMR performed confocal analyses. AJL, ARH, CJCdH, JAGvS, KPMvK and SHMR contributed to important discussions and wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Allen SJ, Crown SE, Handel TM (2007) Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol 25: 787–820 [DOI] [PubMed] [Google Scholar]
- Amagai M, Matsuyoshi N, Wang ZH, Andl C, Stanley JR (2000) Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat Med 6: 1275–1277 [DOI] [PubMed] [Google Scholar]
- Bae T, Schneewind O (2006) Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55: 58–63 [DOI] [PubMed] [Google Scholar]
- Benson MA, Lilo S, Wasserman GA, Thoendel M, Smith A, Horswill AR, Fraser J, Novick RP, Shopsin B, Torres VJ (2011) Staphylococcus aureus regulates the expression and production of the staphylococcal superantigen-like secreted proteins in a Rot-dependent manner. Mol Microbiol 81: 659–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestebroer J, Poppelier MJJG, Ulfman LH, Lenting PJ, Denis CV, van Kessel KPM, van Strijp JAG, de Haas CJC (2007) Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 109: 2936–2943 [DOI] [PubMed] [Google Scholar]
- Bestebroer J, van Kessel KP, Azouagh H, Walenkamp AM, Boer IG, Romijn RA, van Strijp JA, de Haas CJ (2009) Staphylococcal SSL5 inhibits leukocyte activation by chemokines and anaphylatoxins. Blood 113: 328–337 [DOI] [PubMed] [Google Scholar]
- Boles BR, Thoendel M, Roth AJ, Horswill AR (2010) Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS ONE 5: e10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretti DP, Nelson N, Kozlosky CJ, Morrissey PJ, Copeland NG, Gilbert DJ, Jenkins NA, Dosik JK, Mock BA (1993) The murine homologue of the human interleukin-8 receptor type B maps near the Ity-Lsh-Bcg disease resistance locus. Genomics 18: 410–413 [DOI] [PubMed] [Google Scholar]
- Chuntharapai A, Lee J, Hebert CA, Kim KJ (1994) Monoclonal antibodies detect different distribution patterns of IL-8 receptor A and IL-8 receptor B on human peripheral blood leukocytes. J Immunol 53: 5682–5688 [PubMed] [Google Scholar]
- de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, van Wamel WJ, Heezius EC, Poppelier MJ, van Kessel KP, van Strijp JA (2004) Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199: 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F (2006) Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367: 731–739 [DOI] [PubMed] [Google Scholar]
- Dubin G, Stec-Niemczyk J, Kisielewska M, Pustelny K, Popowicz GM, Bista M, Kantyka T, Boulware KT, Stennicke HR, Czarna A, Phopaisarn M, Daugherty PS, Thøgersen IB, Enghild JJ, Thornberry N, Dubin A, Potempa J (2008) Enzymatic activity of the Staphylococcus aureus SplB serine protease is induced by substrates containing the sequence Trp-Glu-Leu-Gln. J Mol Biol 379: 343–356 [DOI] [PubMed] [Google Scholar]
- Eisele NA, Lee-Lewis H, Besch-Williford C, Brown CR, Anderson DM (2011) Chemokine receptor CXCR2 mediates bacterial clearance rather than neutrophil recruitment in a murine model of pneumonic plague. Am J Pathol 178: 1190–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipek R, Rzychon M, Oleksy A, Gruca M, Dubin A, Potempa J, Bochtler M (2003) The Staphostatin-staphopain complex: a forward binding inhibitor in complex with its target cysteine protease. J Biol Chem 278: 40959–40966 [DOI] [PubMed] [Google Scholar]
- Geisbrecht BV (2008) Staphylococcal complement inhibitors: biological functions, recognition of complement components, and potential therapeutic implications. Adv Exp Med Biol 632: 221–236 [PubMed] [Google Scholar]
- Golonka E, Filipek R, Sabat A, Sinczak A, Potempa J (2004) Genetic characterization of staphopain genes in Staphylococcus aureus. Biol Chem 385: 1059–1067 [DOI] [PubMed] [Google Scholar]
- Gray GD, Hasslen SR, Ember JA, Carney DF, Herron MJ, Erlandsen SL, Nelson RD (1997) Receptors for the chemoattractants C5a and IL-8 are clustered on the surface of human neutrophils. J Histochem Cytochem 45: 1461–1467 [DOI] [PubMed] [Google Scholar]
- Herbold W, Maus R, Hahn I, Ding N, Srivastava M, Christman JW, Mack M, Reutershan J, Briles DE, Paton JC, Winter C, Welte T, Maus UA (2010) Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect Immun 78: 2620–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI (1991) Structure and functional expression of a human interleukin-8 receptor. Science 253: 1278–1280 [DOI] [PubMed] [Google Scholar]
- Huth JR, Bewley CA, Jackson BM, Hinnebusch AG, Clore GM, Gronenborn AM (1997) Design of an expression system for detecting folded protein domains and mapping macromolecular interactions by NMR. Protein Sci 6: 2359–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T, Tanase S, Szmyd G, Kozik A, Travis J, Potempa J (2005) Induction of vascular leakage through release of bradykinin and a novel kinin by cysteine proteinases from Staphylococcus aureus. J Exp Med 201: 1669–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RC, Deck J, Edmondson RD, Hart ME (2008) Relative quantitative comparisons of the extracellular protein profiles of Staphylococcus aureus UAMS-1 and its sarA, agr, and sarA agr regulatory mutants using one-dimensional polyacrylamide gel electrophoresis and nanocapillary liquid chromatography coupled with tandem mass spectrometry. J Bacteriol 190: 5265–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongerius I, Köckritz-Blickwede von M, Horsburgh MJ, Ruyken M, Nizet V, Rooijakkers SHM (2012) Staphylococcus aureus virulence is enhanced by secreted factors that block innate immune defenses. J Innate Immun 4: 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalińska M, Kantyka T, Greenbaum DC, Larsen KS, Władyka B, Jabaiah A, Bogyo M, Daugherty PS, Wysocka M, Jaros M, Lesner A, Rolka K, Schaschke N, Stennicke H, Dubin A, Potempa J, Dubin G (2012) Substrate specificity of Staphylococcus aureus cysteine proteases—Staphopains A, B and C. Biochimie 94: 318–327 [DOI] [PubMed] [Google Scholar]
- Katancik JA, Sharma A, Radel SJ, De NE (1997) Mapping of the extracellular binding regions of the human interleukin-8 type B receptor. Biochem Biophys Res Commun 232: 663–668 [DOI] [PubMed] [Google Scholar]
- Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, Deleo FR (2010) Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202: 1050–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew RR, Peng T, DiMartino SJ, Madhavan D, Weinman SJ, Cheng D, Prossnitz ER (1997) Undifferentiated U937 cells transfected with chemoattractant receptors: a model system to investigate chemotactic mechanisms and receptor structure/function relationships. J Leukoc Biol 61: 329–337 [DOI] [PubMed] [Google Scholar]
- Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR (2011) Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS ONE 6: e26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laarman AJ, Ruyken M, Malone CL, van Strijp JA, Horswill AR, Rooijakkers SH (2011) Staphylococcus aureus metalloprotease aureolysin cleaves Complement C3 to mediate immune evasion. J Immunol 186: 6445–6453 [DOI] [PubMed] [Google Scholar]
- Lin Y, Anderson MJ, Kohler PL, Strandberg KL, Olson ME, Horswill AR, Schlievert PM, Peterson ML (2011) Proinflammatory exoprotein characterization of toxic shock syndrome Staphylococcus aureus. Biochemistry 50: 7157–7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339: 520–532 [DOI] [PubMed] [Google Scholar]
- Murdoch C, Finn A (2000) Chemokine receptors and their role in inflammation and infectious diseases. Blood 95: 3032–3043 [PubMed] [Google Scholar]
- Nickerson N, Ip J, Passos DT, Mcgavin MJ (2010) Comparison of Staphopain A (ScpA) and B (SspB) precursor activation mechanisms reveals unique secretion kinetics of proSspB (Staphopain B), and a different interaction with its cognate Staphostatin, SspC. Mol Microbiol 75: 161–177 [DOI] [PubMed] [Google Scholar]
- Norgauer J, Metzner B, Schraufstatter I (1996) Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol 156: 1132–1137 [PubMed] [Google Scholar]
- Ohbayashi T, Irie A, Murakami Y, Nowak M, Potempa J, Nishimura Y, Shinohara M, Imamura T (2011) Degradation of fibrinogen and collagen by staphopains, cysteine proteases released from Staphylococcus aureus. Microbiology 157: 786–792 [DOI] [PubMed] [Google Scholar]
- Otto HH, Schirmeister T (1997) Cysteine proteases and their inhibitors. Chem Rev 97: 133–172 [DOI] [PubMed] [Google Scholar]
- Potempa J, Dubin A, Korzus G, Travis J (1988) Degradation of elastin by a cysteine proteinase from Staphylococcus aureus. J Biol Chem 263: 2664–2667 [PubMed] [Google Scholar]
- Potempa J, Pike RN (2009) Corruption of innate immunity by bacterial proteases. J Innate Immun 1: 70–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat C, Bestebroer J, de Haas CJ, van Strijp JA, van Kessel KP (2006) A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J Immunol 177: 8017–8026 [DOI] [PubMed] [Google Scholar]
- Prat C, Haas PJ, Bestebroer J, de Haas CJ, van Strijp JA, van Kessel KP (2009) A homolog of formyl peptide receptor-like 1 (FPRL1) inhibitor from Staphylococcus aureus (FPRL1 inhibitory protein) that inhibits FPRL1 and FPR. J Immunol 183: 6569–6578 [DOI] [PubMed] [Google Scholar]
- Prokesova L, Potuznikova B, Potempa J, Zikan J, Radl J, Hachova L, Baran K, Porwit-Bobr Z, John C (1992) Cleavage of human immunoglobulins by serine proteinase from Staphylococcus aureus. Immunol Lett 31: 259–265 [DOI] [PubMed] [Google Scholar]
- Rooijakkers SHM, van Kessel KPM, van Strijp JAG (2005) Staphylococcal innate immune evasion. Trends Microbiol 13: 596–601 [DOI] [PubMed] [Google Scholar]
- Rzychon M, Sabat A, Kosowska K, Potempa J, Dubin A (2003) Staphostatins: an expanding new group of proteinase inhibitors with a unique specificity for the regulation of staphopains, Staphylococcus spp. cysteine proteinases. Mol Microbiol 49: 1051–1066 [DOI] [PubMed] [Google Scholar]
- Schenk S, Laddaga RA (1992) Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett 73: 133–138 [DOI] [PubMed] [Google Scholar]
- Sekido N, Mukaida N, Harada A, Nakanishi I, Watanabe Y, Matsushima K (1993) Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature 365: 654–657 [DOI] [PubMed] [Google Scholar]
- Shaw L, Golonka E, Potempa J (2004) The role and regulation of the extracellular proteases of Staphylococcus aureus. Microbiology 150: 217–228 [DOI] [PubMed] [Google Scholar]
- Sieprawska-Lupa M, Mydel P, Krawczyk K, Wojcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, Shafer W, McAleese F, Foster T, Travis J, Potempa J (2004) Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother 48: 4673–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagur J, Guzik K, Bzowska M, Kuzak M, Zarebski M, Kantyka T, Walski M, Gajkowska B, Potempa J (2009a) Staphylococcal cysteine protease staphopain B (SspB) induces rapid engulfment of human neutrophils and monocytes by macrophages. Biol Chem 390: 361–371 [DOI] [PubMed] [Google Scholar]
- Smagur J, Guzik K, Magiera L, Bzowska M, Gruca M, Thogersen IB, Enghild JJ, Potempa J (2009b) A new pathway of staphylococcal pathogenesis: apoptosis-like death induced by Staphopain B in human neutrophils and monocytes. J Innate Immun 1: 98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ (2001) Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun 69: 2017–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ (2000) CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun 68: 4289–4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg S, van Wamel WJB, Snijders SV, Ouwerling B, de Vogel CP, Boelens HA, Willems RJL, Huijsdens XW, Verreck FAW, Kondova I, Heidt PJ, Verbrugh HA, van Belkum A (2011) Rhesus macaques (Macaca mulatta) are natural hosts of specific Staphylococcus aureus lineages. PLoS ONE 6: e26170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Braughton KR, Kretschmer D, Bach T-HL, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, Deleo FR, Otto M (2007) Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med 13: 1510–1514 [DOI] [PubMed] [Google Scholar]
- Walenkamp AM, Boer IG, Bestebroer J, Rozeveld D, Timmer-Bosscha H, Hemrika W, van Strijp JA, de Haas CJ (2009) Staphylococcal superantigen-like 10 inhibits CXCL12-induced human tumor cell migration. Neoplasia 11: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Wilkinson G, Milligan G (2005) The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J Biol Chem 280: 28663–28674 [DOI] [PubMed] [Google Scholar]
- Wörmann ME, Reichmann NT, Malone CL, Horswill AR, Gründling A (2011) Proteolytic cleavage inactivates the Staphylococcus aureus lipoteichoic acid synthase. J Bacteriol 193: 5279–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuyts A, Van ON, Haelens A, Samson I, Herdewijn P, Ben-Baruch A, Oppenheim JJ, Proost P, Van DJ (1997) Characterization of synthetic human granulocyte chemotactic protein 2: usage of chemokine receptors CXCR1 and CXCR2 and in vivo inflammatory properties. Biochemistry 36: 2716–2723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.