Abstract
EMBO J (2012) 31 17, 3513–3523 doi:; DOI: 10.1038/emboj.2012.183
Molecular mechanisms that enable cancer cells to recruit endothelial cells are intensely studied. Zhuang et al (2012) in this issue of The EMBO Journal describe a new mode of communication between cancer cells and endothelial cells that drives endothelial migration. The authors characterize a small non-coding RNA (microRNA-9) that transfers information from cancer to endothelial cells. This microRNA is transported to endothelial cells in microvesicles, functionally facilitating angiogenesis and tumour growth.
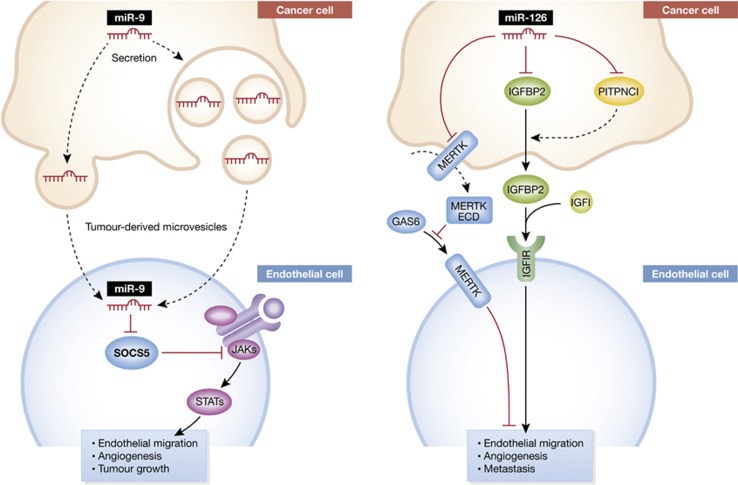
Tumours require the establishment of vasculature for their increasing nutrient, energy, and oxygen requirements as well as for removal of metabolic waste. Cancer cells within a tumour generate such pathologic vasculature by recruiting endothelial cells to the tumour site (Hanahan and Weinberg, 2011). This is accomplished by secreting molecular factors—such as the well-known vascular endothelial growth factor (VEGF)—into the extracellular space (Kim et al, 1993; Carmeliet and Jain, 2011). VEGF binding to VEGF receptors on endothelial cells results in the migration and recruitment of endothelial cells. In this way, proteins expressed by cancer cells can regulate the cellular and structural content of tumours—giving rise to continued tumour growth. Recent work has revealed a major role for another class of genes—known as small non-coding RNAs (microRNAs)—in the regulation of endothelial recruitment and tumour angiogenesis. One member of this family (miR-126) was recently found to inhibit endothelial recruitment by suppressing a set of cancer genes that activate endothelial migration (Png et al, 2011). In this way, a non-coding RNA expressed by cancer cells could shape the tumour and metastatic microenvironment (Figure 1). In this issue of The EMBO Journal, Zhuang, Ferrara, and colleagues uncover a novel role for microRNAs in cancer–endothelial biology (Zhuang et al, 2012). The authors describe the remarkable ability of cancer cells to recruit endothelial cells by releasing microvesicles that contain a miRNA (miR-9). Taken up by endothelial cells, the released miR-9 targets SOCS5—an inhibitor of JAK-STAT signalling to promote endothelial migration (Figure 1). Consistent with their model, systemic inhibition of miR-9 (through the use of an antagomir) inhibits colorectal as well as lung cancer growth due to reduced angiogenesis.
Figure 1.
Regulation of endothelial migration and cancer angiogenesis by microRNAs. Left, A small non-coding RNA, miR-9, expressed and secreted by cancer cells promotes endothelial migration by targeting endothelial SOCS5, an inhibitor of JAK-STAT signalling. SOCS5 suppression by miR-9 drives endothelial migration. Right, Another small non-coding RNA, miR-126, suppresses endothelial migration by targeting a set of genes in cancer cells that drive endothelial migration. These genes drive endothelial migration by activating endothelial IGF1 receptor, a promoter of endothelial migration, and inhibiting endothelial MERTK receptor, a suppressor of endothelial migration. Mertk ECD, Mertk extracellular domain.
These findings, when integrated together, reveal microRNAs as regulators of cancer expression programs that dictate tumour endothelial biology as well as regulators of endothelial expression programs that govern tumour endothelial biology (Figure 1). MicroRNAs thus add a new layer of versatility in the interplay among tumour and endothelial cells.
The work of Zhuang and colleagues is also of translational interest since the in-vivo inhibition of JAK-STAT signalling with a novel small-molecule inhibitor (e.g., antagonizing the biological activity of miR-9) reduces tumour angiogenesis and growth. The importance of miR-9 in cancer progression, and therefore its potential therapeutic value, is further emphasized by its described role as a promoter of metastasis (Ma et al, 2010).
These compelling findings, while revealing unconventional roles for microRNAs in the regulation of the tumour microenvironment, raise a number of additional questions for future study: Are there other microRNAs that regulate recruitment of additional cell types such as macrophages, fibroblasts, or neutrophils? What are the cell-biological mechanisms responsible for selective microRNA release by cancer cells and/or uptake by endothelial cells? How far can this cancer-secreted microRNA reach? What are the spectrum of roles for secreted microvesicles and exosomes (Valadi et al, 2007; Peinado et al, 2012)? Are there instructive roles for circulating microRNAs not secreted by microvesicles?
While these questions will take time and toil to address, one thing is for certain: we are far from a complete understanding of the fascinating cellular and cell-biological roles of microRNAs in cancer, endothelial biology, and beyond.
Footnotes
The authors declare that they have no conflict of interest.
References
- Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473: 298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674 [DOI] [PubMed] [Google Scholar]
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Philipps HS, Ferrara N (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362: 841–844 [DOI] [PubMed] [Google Scholar]
- Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA (2010) miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 12: 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Alecković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar CM, Nitadori-Hoshino A, Hoffman H, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS et al. (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18: 883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Png KP, Halberg N, Yoshida M, Tavazoie SF (2011) A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature 481: 190–194 [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and miRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9: 654–659 [DOI] [PubMed] [Google Scholar]
- Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, Oeh J, Modrusan Z, Bais C, Sampath D, Ferrara N (2012) Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J 31: 3513–3523 [DOI] [PMC free article] [PubMed] [Google Scholar]