Abstract
Cancer immunotherapy attempts to harness the power and specificity of the immune system to treat tumours. The molecular identification of human cancer-specific antigens has allowed the development of antigen-specific immunotherapy. In one approach, autologous antigen-specific T cells are expanded ex vivo and then re-infused into patients. Another approach is through vaccination; that is, the provision of an antigen together with an adjuvant to elicit therapeutic T cells in vivo. Owing to their properties, dendritic cells (DCs) are often called ‘nature’s adjuvants’ and thus have become the natural agents for antigen delivery. After four decades of research, it is now clear that DCs are at the centre of the immune system owing to their ability to control both immune tolerance and immunity. Thus, DCs are an essential target in efforts to generate therapeutic immunity against cancer.
Immunity results from a complex interplay between the innate immune system (which is antigen-nonspecific) and the adaptive immune system (which is antigen-specific). The cells and molecules of the innate immune system use non-clonal recognition receptors, including lectins, Toll-like receptors (TLRs), NOD-like receptors (NLRs) and helicases. B cells and T cells of the adaptive immune system use clonal receptors that recognize antigens, or their derived peptides, in a highly specific manner. In 2011, the Nobel Prize for Medicine or Physiology was awarded for the discovery of molecular and cellular sensors of microbes. Jules Hoffman and Bruce Beutler received the award for their seminal contributions to the discovery of TLRs in the 1990s. Ralph Steinman received the award for the discovery in 1973 of DCs, a rare cell type that is one of the key cellular sensors of microbes. DCs are linked to their environment through a wealth of molecular sensors that allow them to capture invading microbes and to transmit the resulting information to lymphocytes. Thus, DCs provide an essential link between the innate and adaptive immune responses.
The immune system has the potential to eliminate neoplastic cells. Perhaps the most compelling evidence of tumour immunosurveillance in humans is provided by paraneoplastic diseases, which are neurological disorders that are a consequence of an anti-tumour immune response. Onconeural antigens, which are normally expressed on neurons, can also be expressed in breast cancer cells1. Some patients with paraneoplastic disease develop a strong antigen-specific CD8+ T cell-mediated response that controls tumour expansion but that concomitantly results in autoimmune cerebellar degeneration2, which causes a severe neurological disease. However, tumour cells themselves are poor antigen-presenting cells (APCs), which raises the question of how such potent immunity can be generated. Mouse models demonstrate that the generation of protective anti-tumour immunity depends on the presentation of tumour antigens by dendritic cells (DCs)3,4.
Vaccination strategies involving DCs have been developed owing to the special properties of these cells in coordinating innate and adaptive immune responses. The aim of DC vaccination is to induce tumour-specific effector T cells that can reduce the tumour mass specifically and that can induce immunological memory to control tumour relapse. In this process, the first step is to provide DCs with tumour-specific antigens. This can be achieved either by culturing ex vivo DCs that have been derived from patients with an adjuvant (that induces DC maturation) and the tumour-specific antigen, and then injecting these cells back into the patient, or by inducing DCs to take up the tumour-specific antigen in vivo. To improve the therapeutic use of DC vaccination strategies it is important to understand the biology of DCs and how they regulate the innate and the adaptive immune systems — particularly in the context of the tumour microenvironment. This Review discusses our current knowledge of DC biology, and the progress and future challenges of DC vaccination strategies.
Basics of DC biology
DCs are bone marrow-derived cells that are seeded in all tissues (reviewed in REFS 5–7). DCs are poised to sample the environment and to transmit the gathered information to cells of the adaptive immune system (T cells and B cells)5–7. DCs initiate an immune response by presenting the captured antigen, which is in the form of peptide–major histocompatibility complex (MHC) molecule complexes, to naive (that is, antigen inexperienced) T cells in lymphoid tissues5–7. When compared with other APCs, such as macrophages, DCs are extremely efficient and can elicit very low numbers of T cells to respond7–9, thus explaining their nickname of ‘professional APCs’. Mice and humans have two major subsets of DCs: myeloid DCs (mDCs; also known as conventional DCs and classical DCs) and plasmacytoid DCs (pDCs). We focus our discussion on human DC subsets and refer readers to excellent reviews on mouse DCs10–13.
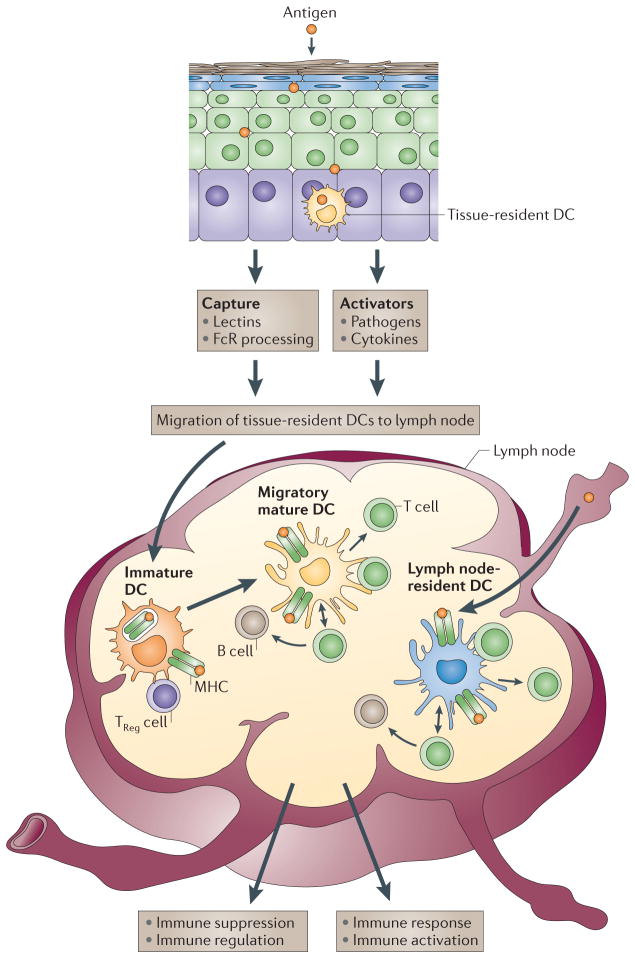
In peripheral tissues, DCs capture antigens through several complementary mechanisms14. Antigen-loaded DCs then migrate into the draining lymph nodes through the afferent lymphatics (FIG. 1). Meanwhile, they process the proteins into peptides that bind to both MHC class I molecules and MHC class II molecules. Lipid antigens are processed differently and are loaded onto non-classical MHC molecules of the CD1 family15. Antigens can also directly reach lymph node-resident DCs through the lymph16 (FIG. 1). Distinct T cell responses are generated depending on whether antigen is captured by DCs in peripheral tissues or directly in lymph nodes16.
Figure 1. Launching the immune response.
Antigens can reach lymph nodes through two pathways: via lymphatics, where the antigen is captured by lymph node-resident dendritic cells (DCs), or via tissue-resident DCs. These immature DCs capture antigens, and DC activation triggers their migration towards secondary lymphoid organs and their maturation. DCs display antigens in the context of classical major histocompatibility (MHC) class I and MHC class II molecules or in the context of non-classical CD1 molecules, which allow the selection of rare antigen-specific T lymphocytes. Activated T cells drive DCs towards their terminal maturation, which induces further expansion and differentiation of T lymphocytes into effector T cells. If DCs do not receive maturation signals, they will remain immature and antigen presentation will lead to immune regulation and/or suppression. TReg cell, regulatory T cell.
Non-activated (immature) DCs can present self-antigens to T cells17–19, which leads to immune tolerance either through T cell deletion or through the differentiation of regulatory or suppressor T cells. By contrast, activated (mature), antigen-loaded DCs can launch the differentiation of antigen-specific T cells into effector T cells with unique functions and cytokine profiles (FIG. 1). Lymph node-resident DCs that acquired antigen directly from the lymph are the first to present peptides to naive CD4+ T cells, which results in T cell priming and interleukin-2 (IL-2) production, which in turn facilitates T cell proliferation and clonal expansion. Subsequently, tissue-resident DCs that captured antigen in peripheral tissues migrate into the lymph node and present peptides to the already activated CD4+ T cells, which facilitates the generation of effector T cells.
On interaction with DCs, naive CD4+ T cells and CD8+ T cells can differentiate into antigen-specific effector T cells with different functions. CD4+ T cells can become T helper 1 (TH1) cells, TH2 cells, TH17 cells or T follicular helper (TFH) cells that help B cells to differentiate into antibody-secreting cells, as well as regulatory T (TReg) cells that downregulate the functions of other lymphocytes. Naive CD8+ T cells can give rise to effector cytotoxic T lymphocytes (CTLs). The type of T cell response — for example, CD4+ helper T cells or CD8+ CTLs — is at least partly linked to the subset of DCs that presents the antigen5. DCs can also interact with cells of the innate immune system, including natural killer (NK) cells, phagocytes and mast cells5–7.
DCs also have an important role in controlling humoral immunity. They do so both directly by interacting with B cells and indirectly by inducing the expansion and differentiation of CD4+ helper T cells20,21. The mechanism by which DCs route antigens into a non-degrading recycling compartment that results in the presentation of unprocessed antigens to B cells is poorly characterized22,23. These key properties of DCs, which allow the activation of both arms of the adaptive immune system (that is, cellular and humoral) and which launch the immune response, render DCs the central candidates for antigen delivery and therapeutic vaccination against cancer.
DC plasticity
In addition to discovering DCs, Ralph Steinman and colleagues demonstrated that DCs are able to mature; that is, they acquire novel functions following microbe encounters (reviewed in REF. 14. Normally, DCs in peripheral tissues are immature. These immature DCs have the ability to efficiently capture antigens; they can accumulate MHC class II molecules in the late endosome-lysosomal compartment; they can express low levels of co-stimulatory molecules; they can express a unique set of chemokine receptors (for example, CCR7) that allow their migration to lymphoid tissues; and they have a limited capacity for secreting cytokines14. Such immature DCs induce immune tolerance either through T cell deletion or through the expansion of regulatory or suppressor T cells24.
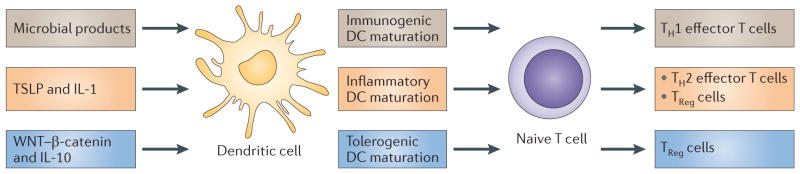
DCs promptly respond to environmental signals and differentiate into mature DCs that can efficiently launch immune responses. Maturation is associated with the downregulation of antigen-capture activity, the increased expression of surface MHC class II molecules and co-stimulatory molecules, the ability to secrete cytokines14, as well as the acquisition of CCR7, which allows migration of the DC into the draining lymph node14. The ligation of the co-stimulatory receptor CD40 (also known as TNFRSF5) is an essential signal for the differentiation of immature DCs into fully mature DCs that are able to launch adaptive T cell-mediated immunity25,26. However, DC maturation alone does not result in a unique DC phenotype (FIG. 2). Instead, the different signals that are provided by different microbes either directly or through the surrounding immune cells induce DCs to acquire distinct phenotypes that eventually contribute to different immune responses. Indeed, DC maturation varies according to different microbes because microbes express different pathogen-associated molecular patterns (PAMPs) that trigger distinct DC molecular sensors, which are called pattern recognition receptors (PPRs) (reviewed in REFS 6,27,28). Strikingly, although most microbes activate DCs, a few can block DC maturation through various pathways (reviewed in REFS 6,27,28). Tissue-localized DCs can also be polarized into distinct phenotypes by the products released from surrounding immune cells that respond to injury. For example, γδ-T cells and NK cells release interferon-γ (IFNγ), mast cells release pre-formed IL-4 and TNF, pDCs secrete IFNα, stromal cells secrete IL-15 and thymic stromal lymphopoietin (TSLP), and so on (reviewed in REFS 29,30). These cytokines induce the differentiation of progenitor cells or of precursor cells such as monocytes into distinct inflammatory DCs that yield unique types of T cells (FIG. 2).
Figure 2. DC maturation.
Dendritic cell (DC) maturation is a simple concept that is rendered complex by the likelihood that not all mature (or activated) DCs are equivalently immunogenic. For example, under steady state conditions, particularly in lymphoid tissue, DC populations that display at least some of the features of mature DCs (for example, elevated expression of surface co-stimulatory molecules) can be found despite the absence of overt inflammation or infection. The functional importance of these cells is unknown but it is not unreasonable to suspect that tolerogenic DCs may have to acquire the capacity to present antigen, migrate and interact with T cells (characteristics of mature DCs) in order to induce antigen-specific regulatory T (TReg) cells or to induce anergy or T cell apoptosis at high efficiency. The priming of TReg cells either in the thymus or in the periphery may require activation by endogenous mediators, such as thymic stromal lymphopoietin (TSLP) or WNT, respectively155,156. Whether these mediators induce morphologically recognizable maturation in vivo is likely but not known. However, it is clear that resting or immature DCs can or must be activated in some way to induce T cell tolerance; thus, it is inaccurate to assume that the relevant steady state DCs are ‘immature’ or resting. IL, interleukin; TH, T helper.
It is now accepted that the adjuvant component of vaccines primarily acts by triggering DC maturation. As described above with microbes, different adjuvants activate DCs through distinct molecular pathways, leading to various types of T cell responses31,32. For example, alum mediates its adjuvant effects partly through the activation of the DC inflammasome33, as well as through the sensing of lipids in the plasma membrane of DCs34.
The recent progress that has been made in genomics and systems immunology35 is expected to uncover the pathways that determine the type of immune responses that DCs elicit. Approaches that combine transcriptional profiling, genetic and small-molecule screening together with phosphoproteomics have unravelled molecules that regulate TLR signalling in DCs36. These strategies will help us to understand the molecular paths of DC maturation and thereby enable the discovery of novel adjuvants. The crucial issues related to cancer that need to be overcome include the lack of necessary DC co-stimulation signals in the tumour microenvironment and the presence of inappropriate signals that lead to DCs that are unable to induce protective anti-tumour immune responses.
DC subsets
Subsets of human DCs in the blood can be distinguished by the differential expression of three cell-surface molecules: CD303 (also known as BDCA2 and CLEC4C), CD1C (also known as BDCA1) and CD141 (also known as BDCA3 and thrombomodulin)37 (FIG. 3). CD303+ pDCs represent a front line of anti-viral immunity owing to their ability to secrete large amounts of IFNα in response to virus encounters38. Their presynthesized stores of MHC class I molecules39 may allow a rapid initial CD8+ T cell response to viral infections. pDC-derived IFNα may also promote the immunogenic maturation of other subsets of DCs, thus helping to activate novel T cell clones. Furthermore, activated pDCs can induce the maturation of activated B cells into plasma cells through both cytokines and surface signalling40,41. In their resting state, pDCs are considered to have an important role in immune tolerance, including in oral tolerance42.
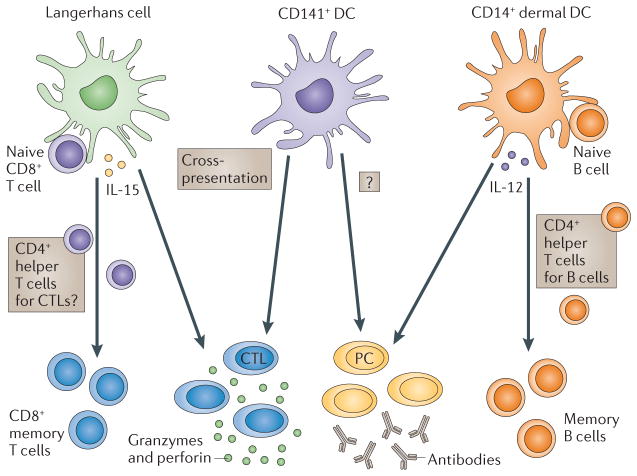
Figure 3. Subsets of DCs.
The two arms of the adaptive immune response — humoral and cellular — are regulated by different subsets of dendritic cells (DCs) in humans. Humoral immunity is preferentially regulated by CD14+ dermal DCs, which produce interleukin-12 (IL-12). IL-12, in turn, acts directly on B cells and promotes the development of T follicular helper (TFH) cells. Cellular immune responses in the skin are preferentially regulated by Langerhans cells. Among the candidate mediators is IL-15. It is also possible that Langerhans cells can preferentially activate a dedicated subset of CD4+ T cells that are specialized to help CD8+ cytotoxic T lymphocytes (CTLs). Given their capacity to cross-present antigens to CD8+ T cells, CD141+ DCs might be involved in the development of CTL-mediated responses. CD141+ DCs might also be involved in the development of humoral responses through IL-12 secretion. PC, plasma cell.
Two subsets of mDCs in the blood can be distinguished by reciprocal expression of CD1C and CD141. Human CD141+ DCs share with mouse CD8+ DCs the high capacity to capture exogenous antigens for presentation on MHC class I molecules (known as cross-presentation). CD141+ DCs express XCR1, which is the receptor for the chemokine XCL1 (also known as lymphotactin) that is produced by NK cells and activated CD8+ T cells43,44. Thus, mouse CD8+ DCs and human CD141+ DCs are equipped for the generation of CD8+ T cell-mediated immune responses. In mice, gene ablation studies have shown that the CD8+ subset of DCs have an important role in crosspresentation11. However, other human DCs, such as epidermal Langerhans cells45, also cross-present antigens. Whether CD141+ blood mDCs are related to subsets of DCs in peripheral tissues remains to be determined. The unique functions of CD1C+ DCs also continue to be analysed.
The human skin hosts two main subsets of mDCs: epidermal Langerhans cells and dermal interstitial DCs (dermal DCs)46 (FIG. 3). The dermal DCs can be further subdivided into CD1a+ DCs and CD14+ DCs46,47. Human CD14+ DCs can directly help activated B cells, as well as induce naive T cells to differentiate into cells with the properties of TFH cells45,48. CD14+ DCs may thus be specialized for the development of humoral responses45,49. Langerhans cells are more efficient in cross-presenting peptides from protein antigens to CD8+ T cells and can prime the differentiation of CD8+ T cells into effector CTLs.
The development and homeostasis of tissue-resident DCs subsets in steady state conditions (that is, when there is no infection or activation of the immune system) is dependent on the activation of the receptor tyrosine kinase FLT3 and of the macrophage colony-stimulating factor 1 receptor (M-CSFR; also known as CSF1R)12. However, inflammatory processes such as those initiated by microbial invasion substantially alter the populations of DC subsets. The origin of DCs that are recruited to sites of inflammation is still under investigation, although it is clear that monocytes can give rise to inflammatory DCs in vivo50. Human studies depend on the in vitro exposure of monocytes to different cytokine combinations based on granulocyte–macrophage colony-stimulating factor (GM-CSF; also known as CSF2) (FIG. 3). Together with IL-4 (REF. 51), IFNα52, TNF53 or IL-15 (REF. 54) GM-CSF can induce the differentiation of inflammatory DCs that can activate T cells. The cytokine combination is crucial as it results in DCs with different phenotypes and functions. Several of these combinations have been administered as vaccines to cancer patients. Tolerogenic DCs can be generated by culturing monocytes with IL-10 (REF. 55) or with vitamin A56 or vitamin D3 (REF. 57). The activation of DCs through E-cadherin-mediated signalling58 also induces tolerogenic DCs.
We still need to establish how these distinct subsets of DCs are related, how they contribute to disease pathogenesis and how they can be used to design efficient vaccines.
Molecules expressed by DCs
DCs sense their environment through both surface and intracellular receptors, which comprise several families, including cell surface C-type lectins (CLRs), surface and intracellular TLRs, and intracellular helicases59. The helicases are a very large family of molecules, including retinoic acidinducible gene I (RIGI), which recognize nucleic acids. Activation of these helicases can differentially modulate DC functions, which thus yields distinct immune responses60,61. Some of the CLRs, which are receptors for both PAMPs and endogenous ligands, have signalling motifs in their cytoplasmic regions that deliver either activation or suppression signals62. For example, macrophage-inducible C-type lectin (MINCLE; also known as CLEC4E) detects small nuclear ribonucleoproteins released from damaged cells, whereas CLEC9A detects a ligand that is exposed on necrotic cells that has not yet been identified63. NLRs, such as NLRP1 (also known as NALP1), NLRP3 (also known as NALP3), NLRC4 (also known as IPAF) and NAIP5 (also known as BIRC1E), are components of the inflammasome, which cleaves substrates such as pro-IL-1β and pro-IL-18 to generate the active cytokines. The RNA helicase complex, DDX1–DDX21–DHX36, is a cytosolic sensor of double-stranded RNA that uses the TRIF pathway to activate type I IFN responses in mDCs59. Thus, DCs are endowed with a complex set of molecules that helps them to respond to the bewildering complexity of the external molecular and cellular world.
These properties of DCs can be harnessed for the benefit of tumour immunotherapy through several means. One strategy is to target the cell-surface molecules of DCs with antibodies. This could be used to modulate DC function using naked antibodies — for example, by inducing their activation through CD40 — or to link activation signalling with antigen delivery to DCs in vivo using antibodies linked with antigens (known as in vivo DC targeting)64. Another strategy is to exploit DC receptors for in vivo capture of dead tumour cells. Regarding this exploitation of DC receptors, immunogenic cancer cell death, which is triggered by some forms of chemotherapy, allows the exposure of ligands for DC sensors and results in the capture of dead cells by DCs in situ65. These DCs can then present the captured antigens to T cells, which thereby elicits immune responses.
An important molecular component of DCs that can be targeted for immunotherapy includes receptors that recognize microbes. Indeed, the activation of DCs is likely to contribute to therapeutic effects that are associated with the nonspecific activation of antitumour immunity by PAMPs66,67, which was pioneered with Coley’s toxin. Since then, the Bacillus Calmette-Guerin (BCG) vaccine has been used for the local therapy of bladder carcinoma67. Medicinal chemists have made progress in identifying chemicals that activate immune responses. Imiquimod, which is a ligand for TLR7 and TLR8, is used for the treatment of superficial basal cell carcinoma67. Several other TLR ligands are currently being tested in clinical trials, including CpG oligodeoxynucleotides (ODNs)68 (which target TLR9) and polyinosinic-polycytidylic acid (polyI:C), which — depending on the size of this PAMP — targets either TLR3 or helicases69.
DCs in cancer immunotherapy
Mouse models of cancer demonstrate that DCs can capture tumour antigens that are released from tumour cells, either alive or dying, and cross-present these antigens to T cells in tumour-draining lymph nodes. This results in the generation of tumour-specific CTLs that contribute to tumour rejection3,4. Thus, DCs represent important targets for therapeutic interventions in cancer.
DCs in the tumour microenvironment
DCs are found in most tumours in humans and mice. DCs can sample tumour antigens through the capture of dying tumour cells and through the ‘nibbling’ of live tumour cells (reviewed in REF. 70).
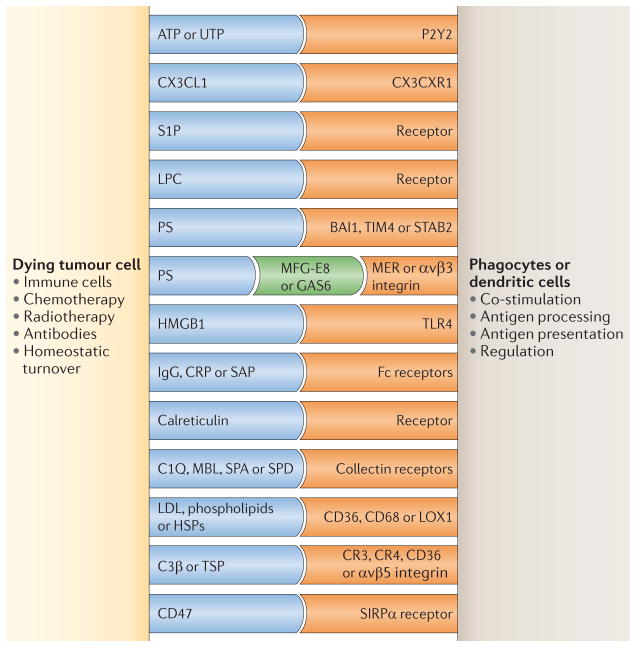
Dying tumour cells mobilize at least three types of signal when interacting with DCs and other phagocytes, including ‘find me’, ‘eat me’ and ‘do not eat me’ signals (reviewed in REF. 71) (FIG. 4). There are currently four sets of molecules that are known to be released by apoptotic cells that function as ‘find me’ signals: lipid lysophosphatidylcholine (LPC), sphingosine 1-phosphate (S1P), CX3CL1 (also known as fractalkine), and the nucleotides ATP and UTP71. The eat me signals are membrane-bound and serve as markers for phagocytes for recognizing and internalizing dying cells. These signals include phosphatidylserine, alterations in cell-surface charge, αvβ5 integrin and CD36. The eat me signals also include molecules such as milk fat globule-EGF factor 8 (MFG-E8; also known as lactadherin), which bridge the phosphatidylserine of apoptotic cells with the integrin αvβ3 of DCs71. Opsonizing antibodies can promote the capture of dying tumour cells through Fc receptors and complement component receptors that are expressed on DCs. The do not eat me signals serve as negative regulators for the capture of cancer cells by DCs and other phagocytes. These signals include lactoferrin and CD47, the interaction of which with signal-regulatory protein-α (SIRPα; also known as SHPS1) on phagocytes provides inhibitory signals that prevent phagocytosis. Accordingly, combining a CD47-blocking antibody with rituximab (a CD20 antibody that depletes B cells) results in enhanced phagocytosis of dead lymphoma cells and improved tumour eradication in mice72.
Figure 4. DC interaction with tumour cells: antigen capture.
The figure illustrates some phagocyte surface receptors and their putative ligands that are implicated in the recognition of dying tumour cells. Receptors shown in blue represent molecules expressed by dying tumour cells, receptors shown in orange represent molecules expressed by dendritic cells (DCs) and the receptors shown in green function as a ‘bridge’ between the two cells. BAI1, brain-specific angiogenesis inhibitor 1; C1Q, complement C1q subcomponent; C3β, complement C3β; CRP, cysteine-rich protein; GAS6, growth arrest-specific protein 6; HMGB1, high mobility group protein B1; HSP, heat shock protein; LDL, low-density lipoprotein; LOX1, lectin-like oxidized LDL receptor 1; LPC, lipid lysophosphatidylcholine; MBL, mannose binding lectin; MFG-E8, milk fat globule-EGF factor 8 (also known as lactadherin); P2Y2, P2Y purinoceptor 2; PS, phosphatidylserine; S1P, sphingosine 1-phosphate; SAP, sphingolipid activator protein 1; SIRPα, signal-regulatory protein-α; STAB2, stabilin 2; TIM4, T cell membrane protein 4; TLR4, Toll-like receptor 4; TSP, thrombospondin.
Tumours can prevent antigen presentation and the establishment of tumour-specific immune responses through a variety of mechanisms. By switching the differentiation of monocytes to macrophages, which is mediated by the interplay of IL-6 and macrophage colony-stimulating factor (M-CSF; also known as CSF1)73, rather than DCs, tumours can prevent the priming of tumour-specific T cells by DCs. Alternatively, the tumour glycoproteins carcinoembryonic antigen (CEA; also known as CEACAM5) and mucin 1 (MUC1) that are endocytosed by DCs can be confined to early endosomes, which thus prevents efficient processing and presentation to T cells74.
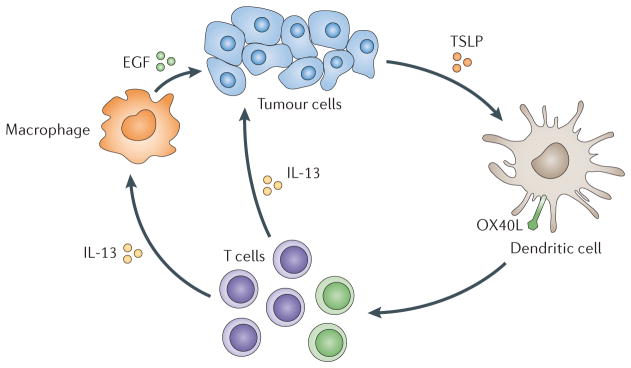
Tumours also interfere with DC maturation. First, they can inhibit DC maturation through the secretion of IL-10 (REFS 75,76), which leads to antigen-specific anergy. Second, tumour-derived factors can alter the maturation of mDCs and so yield cells that indirectly promote tumour growth (‘pro-tumour’ DCs) (FIG. 5). For example, TSLP, which is produced by tumour cells, induces DCs to express OX40 ligand (OX40L; also known as TNFSF4), which directs the generation of TH2 cells. These skewed CD4+ T cells accelerate breast tumour development through the secretion of IL-4 and IL-13 (REF. 77). These cytokines prevent tumour cell apoptosis and indirectly promote the proliferation of tumour cells by stimulating tumour-associated macrophages to secrete epidermal growth factor (EGF). A similar pathway operates in pancreatic cancer78, and studies in mice corroborate the pro-tumorigenic effect of TH2 cells79.
Figure 5. The interaction of DCs with tumour cells: modulation of DC maturation.
Cancer cells attract immature dendritic cells (DCs), possibly through chemokines such as CCL20 or CXCL12. DCs can then be exposed to cancer-derived factors — for example, thymic stromal lymphopoietin (TSLP) — which skews their maturation towards T helper 2 (TH2)-type inflammation. In this environment, TH2 cells promote tumour development either directly or via macrophages. EGF, epidermal growth factor; IL-13, interleukin-13; OX40L, OX40 ligand.
The mDCs are not the only culprit in tumour progression, as pDCs that infiltrate breast carcinomas produce little IFNα on ligation of TLRs80. This inhibition results from the ligation of ILT7 (also known as LILRA4) on pDCs with bone marrow stromal antigen 2 (BST2) on tumour cells80. These pDCs induce naive CD4+ T cells to differentiate into IL-10-producing T cells with immunosuppressive functions. The inhibition of IFNα secretion might also affect the generation of effector CTLs, as DCs require type I IFN signalling to cross-present tumour antigens3,4. Whether this mechanism explains why pDCs are associated with poor prognosis81 remains to be determined. Finally, DCs can have direct pro-tumour effects: mDCs directly promote the survival and clonogenicity of multiple myeloma tumour cells82,83. In ovarian cancer, pDCs contribute to tumour angiogenesis by secreting pro-angiogenic cytokines84,85.
Therefore, understanding the functions of DCs in the tumour bed might represent a rich field of investigation. Ultimately, rewiring the pro-tumour DCs into anti-tumour DCs might represent a novel approach to cancer immunotherapy (BOX1).
Box 1. Endogenous vaccination and combination therapies.
The classical cancer chemotherapies seem to engage the immune system, and part of their efficacy might result from this. For example, chemotherapeutic agents, such as anthracyclines and oxaliplatin, induce tumour cells to undergo apoptosis, which is associated with cell surface exposure of calreticulin. The expression of calreticulin on the tumour cell surface might contribute to the capture of apoptotic bodies by dendritic cells (DCs) and thus might elicit tumour-specific CD8+ T cell-mediated immune responses. These T cells might contribute to the elimination of tumour cells138 that have not responded to chemotherapy.
There is some evidence that the immune system might also partly contribute to the beneficial effects of radiotherapy139. Less surprisingly, there is now strong evidence that antibody therapy — for example, anti-CD20 and anti-HER2 agents140 — involve the adaptive immune system beyond the elicitation of antibody-dependent cell-mediated cytotoxicity (ADCC). Indeed, antibodies against HER2 can enhance cross-presentation of tumour antigens, leading to a break in immune tolerance against this antigen140. Accordingly, patients responding to trastuzumab (a HER2 antibody) showed enhanced CD8+ T cell-mediated immune responses to HER2 (REF. 140). The use of ‘armed’ antibodies against cancer cells141 is a promising strategy to improve antibody therapy. These antibodies can be fused with effector molecules, such as toxins or radionuclides, which enables the therapeutic modulation of the tumour microenvironment.
The negative immunomodulatory cues of the tumour microenvironment can be counteracted by a series of therapeutic modalities. These include antibodies that neutralize cytokines, such as interleukin-10 (IL-10)142, IL-13 (REF. 143) and transforming growth factor-β (TGFβ)144,145. Antibodies targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA4)146,147 and programmed cell death 1 ligand 1 (PDL1)148–150, which block co-inhibitory signalling of the immune checkpoint in lymphocytes represent important contributors to cancer vaccines. Likewise, antibodies151,152 that promote co-stimulation of effector T cells, such as those that target CD137 (REFS 153,154), should be tested in combination with vaccination. Just as oncologists currently use different combinations of cytostatic drugs and targeted therapies to treat cancer patients, we foresee the development of clinical protocols combining DC vaccines with individualized adjuvant therapies. This development, however, is almost entirely dependent on partnership and collaboration between academia and industry. Indeed, it has been hampered not by the lack of ideas and candidate targets and vaccines in academia but mainly by the lack of access to drugs available in industry.
Therapeutic vaccination via DCs
The goal of cancer vaccinologists is to elicit tumour-specific CD8+ T cell-mediated immune responses that will be sufficiently robust and long-lasting to generate durable tumour regression and/or eradication. This goal is encouraged by clinical studies showing that the infusion of autologous tumour-specific CD8+ T cells can eventually lead to the rejection of large metastatic tumours in patients86–88. The aim is to identify vaccination protocols that will result in the generation of potent T cell responses in vivo. Ideally, vaccine-elicited CD8+ T cells should be of high avidity and able to recognize peptide–MHC class I complexes on tumour cells; be able to express high levels of granzyme and perforin — molecules that are essential for cytotoxic activity against cancer cells; be able to enter the tumour microenvironment; and be able to overcome immunomodulatory mechanisms that are present in the tumour45,89. At least four components of the immune response are necessary for this ideal response to happen: the presence of appropriate DCs; the quality of induced CD4+ T helper cells; the elimination and/or non-activation of TReg cells; and the breakdown of the immunosuppressive tumour microenvironment. Several of these elements have been discussed above and in recent excellent reviews90,91. Here, we briefly elaborate on the fate of CD8+ T cells in the context of DC vaccination.
Naive CD8+ T cells initiate a CTL differentiation programme on encountering DCs that present tumour-derived peptides92. A complex system of signals drive the subsequent CD8+ T cell expansion and differentiation, which includes co-stimulatory pathways mediated by CD80 (REF. 93), CD70 and 4-1BB (also known as TNFRSF9)94, as well as DC-derived cytokines such as IL-12 and IL-15 (REFS 90,91,95). The quality of CD8+ T cell differentiation is further regulated by CD4+ T cells96, as they influence the differentiation and expansion of tumour antigen-specific CTLs97 and the induction of long-term memory CD8+ T cells98. CD4+ T cells also contribute to the eradication of the tumours through the activation of macrophages at tumour sites99, and they actively kill tumour cells100. Furthermore, the expression of CD103 (also known as integrin αE) on CTLs mediates the adherence of these cells to E-cadherin, which seems to be an important pathway in tumour cell lysis and tumour rejection101.
Unfortunately, CD4+ T cells can also suppress CTL differentiation. Thus, TReg cells can inhibit CTLs through the secretion of IL-10 (REF. 102), and/or transforming growth factor-β (TGFβ)103. TReg cells can also compete with CD8+ T cells for IL-2 through constitutive expression of CD25 (also known as IL2Rα)104. TH2 cells can inhibit the generation of CTLs by secreting IL-4, which instead leads to the generation of a subpopulation of CTLs, termed TC2 cells, which have limited killing capacity owing to their low expression of granzymes and perforin105.
Antigen-specific CTLs must also traffic into the tumour bed, the details of which are not clearly understood89,106. Deregulation of chemokine homeostasis might prevent CTLs from entering the tumour bed; indeed, tumours might actively repel the infiltration of CTLs101,107. Finally, the tumour-infiltrating myeloid-derived suppressor cells108 and TReg cells109 might inhibit CTL functions.
Ex vivo-generated DC vaccines
The therapeutic use of cancer vaccines has recently been revived owing to a series of clinical trials that have yielded encouraging clinical outcomes. First, treatment of metastatic prostate cancer with sipuleucel-T (also known as APC 8015), which is a cellular product based on enriched blood APCs that are briefly cultured with a fusion protein of prostatic acid phosphatase (PAP) and GM-CSF, resulted in an approximately 4-month-prolonged median survival in Phase III trials110,111. Sipuleucel-T has been approved by the US Food and Drug Administration (FDA) for the treatment of metastatic prostate cancer, thereby paving the clinical development and regulatory path for the next generation of cellular immunotherapy products. Second, a Phase III trial in metastatic melanoma that tested peptide vaccine in combination with high-dose IL-2 versus IL-2 alone showed significant improvement in overall response rate and progression-free survival in patients who received the vaccine112. Third, a Phase III trial in patients with follicular lymphoma showed that an idiotype vaccine therapy significantly prolongs the duration of chemotherapy-induced remission113. Furthermore, a randomized Phase II trial of a poxvirus-based vaccine targeting prostate-specific antigen (PSA) (PROSTVAC) in men with metastatic castration-resistant prostate cancer showed an improved overall survival in patients when compared with patients receiving control vectors (an observed difference in median survival of 8.5 months)114.
DCs that are generated ex vivo by culturing haematopoietic progenitor cells or monocytes with cytokine combinations have been tested as therapeutic vaccines in cancer patients for more than a decade29. These studies concluded that DC-based vaccines are safe and can induce the expansion of circulating CD4+ T cells and CD8+ T cells that are specific for tumour antigens (TABLE 1). Objective clinical responses have been observed in some patients. The clinical response takes time to build up but remissions can be very long-lasting (TABLE 1) (reviewed in REFS 115,116).
Table 1.
Examples of clinical trials testing vaccination with ex vivo DCs
| Vaccine and antigen | Indication | Key observations | Refs |
|---|---|---|---|
| GM-CSF–IL-4 DCs with or without HLA-A*0201-restricted peptides or peptides alone | Metastatic prostate cancer | One of the first studies that tested the immunogenicity of DCs | 157 |
| GM-CSF–IL-4 DCs with peptides, tumour lysates or autologous tumour-eluted peptides | Stage IV melanoma, renal cell carcinoma and malignant glioma |
|
158–160 |
| Blood DCs and idiotype antigens | Multiple myeloma |
|
161,162 |
| Mature GM-CSF–IL-4 DCs and peptides | Stage IV melanoma |
|
163 |
| CD34+ HPC-derived DCs and peptides | Stage IV melanoma |
|
164,165 |
| FLT3 ligand-expanded blood DCs and altered peptides | Advanced CEA+ cancer |
|
166 |
| Immature GM-CSF–IL-4 DCs | Healthy volunteers | Antigen-specific inhibition of effector T cell function after injection of immature DCs | 167 |
| GM-CSF–IL-4 DCs and tumour lysates | Refractory paediatric solid tumours |
|
168 |
| Mature cryopreserved GM-CSF–IL-4 DCs | Stage IV melanoma | Immunogenicity | 169 |
| DCs loaded with autologous tumour RNA | Colon cancer |
|
170 |
| DCs loaded with killed allogeneic tumour cells | Stage IV melanoma |
|
171,172 |
| Monocyte-derived DCs loaded with the NK T cell ligand α-galactosylceramide | Advanced cancer | Adjuvant effect of NK cell activation on CD8+ T cell-mediated immune response | 173 |
| Monocyte-derived DCs | Melanoma | In vivo identification of antigen-specific immune response by PET imaging in patients | 174 |
| Route of DC administration affects T cell activation, with intra-dermal administration showing better responses than intra-nodal administration | 175 | ||
| Comparative study of CD34+ HPC-derived Langerhans cells versus monocyte-derived DCs | Melanoma | Langerhans cell-based vaccines stimulated significantly greater tyrosinase-HLA-A*0201 tetramer reactivity than the monocyte-derived DC vaccines | 176 |
| Type 1-polarized monocyte-derived DCs | Glioma | Combination of DC vaccination with polyICLC to trigger systemic inflammation driven by type I interferon family members | 177 |
CEA, carcinoembryonic antigen; DC, dendritic cell; IL-4, interleukin-4; GM-CSF, granulocyte–macrophage colony-stimulating factor; HLA, human leukocyte antigen; HPC, haematopoietic progenitor cell; NK cell, natural killer cell; PET, positron emission tomography; polyICLC, polyinosinic–polycytidylic acid stabilized with poly-L-lysine and carboxymethylcellulose.
The selection of tumour antigens for loading the DCs is an important parameter. Candidate tumour antigens include unique (mutated) antigens and shared nonmutated self-antigens117–120. To generate broadly applicable vaccines, non-mutated self-antigens have often been selected. These, however, have potential shortcomings: the range of high-avidity clones might be depleted through negative selection89,121, and the existing memory T cells often include TReg cells.
Using mutated antigens might avoid these drawbacks. Cancer vaccines that are designed to elicit strong immune responses against these mutated antigens will require a fully personalized approach. This represented a considerable challenge a few years ago. Fortunately, however, the development of RNA sequencing (RNA–Seq) technologies will allow us to determine the complete range of mutated antigens from the primary tumour and metastases of a patient, thereby allowing us to tailor therapeutic vaccines to the patient’s tumour.
Targeting antigen to DCs in vivo
Antigens can be directly delivered to DCs in vivo using chimeric proteins that are comprised of an antibody that is specific for a DC receptor fused to a selected antigen. Ralph Steinman and colleagues demonstrated that the specific targeting of antigens to DCs in vivo elicits potent antigen-specific CD4+ and CD8+ T cell-mediated immunity122–124. The induction of immunity also requires the provision of DC maturation signals122–124. Otherwise, this strategy induces antigen-specific tolerance — a finding of substantial value in the context of autoimmunity. The use of vaccines that target surface molecules that are expressed on different subsets of DCs allowed Steinman and Nussenzweig to demonstrate that distinct subsets of DCs elicit distinct immune responses64. This confirmed earlier studies that were carried out in vitro or in vivo with purified DC subsets31,32. In this regard, CD8+ DCs that express the cell-surface protein CD205 (also known as Ly-75) present delivered antigens in the context of both MHC class I and MHC class II molecules; whereas, CD8− DCs, which express the DC marker antigen recognized by the monoclonal antibody 33D1, are specialized for antigen presentation on MHC class II molecules64. Furthermore, targeting antigen to these distinct receptors on DCs leads to the generation of T cell-mediated responses through independent pathways. Thus, DCs expressing CD205 generate a TH1 cell-mediated immune response in an IL-12-independent, CD70-dependent mechanism; whereas, CD8−33D1+ DCs generate TH1 cell-mediated immune responses through the classic IL-12 pathway125.
DC targeting is not purely confined to the delivery of an antigenic cargo, as the engagement of some surface molecules, such as dendritic cell-associated C-type lectin 1 (dectin 1; also known as CLEC7A), dendritic cell-specific ICAM3-grabbing non-integrin 1 (DCSIGN; also known as CD209) and CD40, by targeting antibodies also provides activation signals. It remains important to determine whether the activation signals might actually polarize the DCs in the desired manner. For example, engaging dendritic cell asialoglycoprotein receptor (DCASGPR; also known as ASGPR1)126 induces DCs to secrete IL-10, which polarizes T cells into IL-10-secreting suppressor T cells, which in turn might negatively affect tumour-specific effector T cells. Thus, the challenge will be to match the DC surface target and the selected adjuvant with the desired immune outcome, all in the context of an altered immune system. This will undoubtedly require considerable studies over the coming years.
Immune and clinical efficacy
Most Phase I and II studies have stumbled on two crucial issues: how to assess the clinical efficacy of cancer immunotherapy; and how to define the correlates (biomarkers) of clinical efficacy127. Initially, the Response Evaluation Criteria In Solid Tumours (RECIST) had been designed to assess chemotherapy-based trials and were considered to be crucial for assessing clinical efficacy in vaccine and immunomodulation trials127,128. However, this has recently been challenged. A randomized Phase III clinical trial testing anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA4) in patients with stage IV melanoma, showed a twofold improved overall survival in patients who received the drug129. No early tumour shrinkage was observed in treated patients, which reflects the slow build-up of anti-tumour immunity. In fact, in the early phase of treatment, tumours might increase in size and new lesions might appear; these are findings that normally call for removing the patients from the trial. However, the enlarged tumour size might instead reflect the inflammatory process that is associated with active immune responses and lymphocyte infiltration.
Thus, overall survival might be the only objective parameter of clinical efficacy. However, surrogate markers are needed, because clinical trials that are based on overall survival might be exceedingly long and costly, which could result in the loss of potentially valuable treatments. Several cancer vaccine studies have suggested that the therapeutic vaccination outcome — success or failure — correlates with the vaccine-induced expansion of antigen-specific effector T cells130,131. However, the quality, more than the quantity, of the antigen-specific immune response remains one of the key parameters of efficacy. A better understanding of how effective vaccines stimulate protective immune responses132,133 might contribute to a better selection of immune parameters that indicate the efficacy of cancer vaccines. Systems immunology approaches that combine transcriptional profiling with multi-parameter flow cytometry, proteomics and transcriptomics might enable the identification of novel biomarkers of immune efficacy35,134.
Final remarks
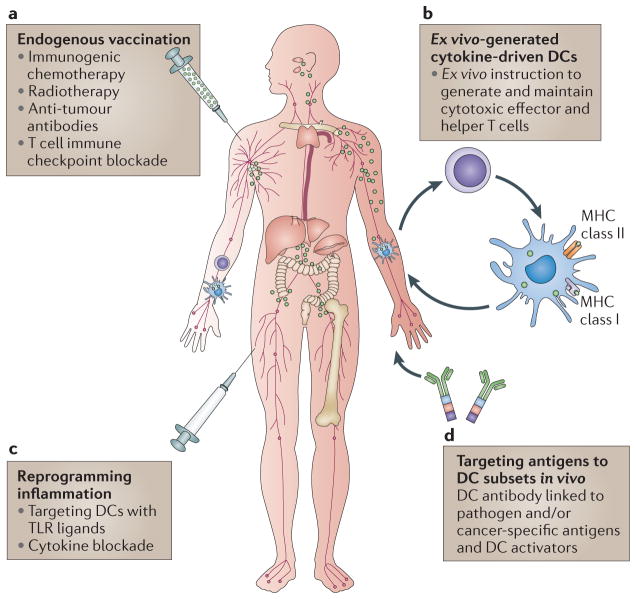
Nearly 40 years after their discovery, the importance of DCs has been recognized by the award to Ralph Steinman of the Nobel Prize for Medicine or Physiology in 2011. However, it has not been a smooth road, and many in the scientific community were sceptical at the time DCs were discovered. Ralph, however, pursued his studies of DCs relentlessly. He used to tell his laboratory members and colleagues “just do the experiment”! Ultimately, the data have not only erased the scepticism but have also created a new field in immunobiology135. It is impossible not to draw parallels with the cancer immunotherapy field, which was initially met with scepticism and which now, finally, is coming of age136,137. Our views on the potential role of DCs in cancer immunotherapy have expanded remarkably, moving from the early trials with ex vivo DC vaccines to a whole new array of therapeutic options that include rewiring DC molecular pathways (FIG. 6). Just as immunotherapy is moving to the forefront of cancer therapy, DC-based therapy is moving to the forefront of cancer immunotherapy.
Figure 6. DCs and cancer immunotherapy.
a | Random targeting of dendritic cells (DCs) in ‘endogenous’ vaccination results from in vivo antigen release owing to immunogenic cell death in response to chemotherapy, radiotherapy and immunomodulation approaches that are targeted at T cells. b | Vaccines can be based on ex vivo-generated tumour antigen-loaded DCs that are injected back into patients. c | Specific in vivo DC targeting with DC antibodies fused with antigens and with DC activators is shown. d | Targeting DCs in the tumour microenvironment to reprogramme pro-tumour inflammation towards tumour rejection is shown. MHC, major histocompatibility complex; TLR, Toll-like receptor.
At a glance.
The molecular identification of human cancer antigens has allowed the development of antigen-specific immunotherapy. In one approach, autologous antigen-specific T cells are expanded ex vivo and then re-infused into patients. Another approach is through vaccination; that is, the provision of an antigen together with an adjuvant to elicit therapeutic T cells in vivo. Cancer vaccines aim to induce tumour-specific effector T cells that can reduce the tumour mass and to induce tumour-specific memory T cells that can control tumour relapse.
Owing to their properties, dendritic cells (DCs) are often called ‘nature’s adjuvants’ and thus have become the natural targets for antigen delivery. DCs provide an essential link between the innate and the adaptive immune responses. DCs are at the centre of the immune system owing to their ability to control both tolerance and immune responses. These key properties of DCs render them the central candidates for antigen delivery and vaccination, including therapeutic vaccination against cancer.
The immune system has the potential to eliminate neoplastic cells. However, tumour cells alone are poor antigen-presenting cells (APCs). Studies with mouse models demonstrate that the generation of protective anti-tumour immune responses depends on the presentation of tumour antigens by DCs. When compared with other APCs, such as macrophages, DCs are extremely efficient at antigen presentation and inducing T cell immunity, thus explaining their nickname of ‘professional APCs’.
Mice and humans have distinct functional subsets of DCs that generate different types of immune response. DCs are also able to mature; that is, to acquire novel functions following microbe encounters. Under steady state conditions, DCs in peripheral tissues are ‘immature’. These immature DCs induce tolerance either through T cell deletion or through inducing the expansion of regulatory and/or suppressor T cells. DCs promptly respond to environmental signals and differentiate into mature DCs that can efficiently launch immune responses. It is now accepted that the adjuvant component of vaccines primarily acts by triggering DC maturation.
DCs are important targets for therapeutic interventions in cancer. Two themes of research are growing: first, how cancer cells alter DC physiology; and second, how we can build on the powerful properties of DCs to generate novel cancer immunotherapies (including vaccines).
Acknowledgments
We dedicate this Review to our long-time friend and colleague, Ralph M. Steinman, who compelled many of us to study dendritic cells and their role in disease pathophysiology and medicine. We thank all of the patients and volunteers who participated in our studies and clinical trials. We thank former and current members of the Baylor Institute for Immunology Research for their contributions to their progress. Our studies have been supported by the US National Institutes of Health (P01 CA084514, U19 AIO57234, R01 CA089440, CA078846 and CA140602), the Dana Foundation, the Susan Komen Foundation, the Baylor Health Care System; the Baylor Health Care System Foundation, the ANRS and the INSERM. K.P. holds the Michael A. Ramsay Chair for Cancer Immunology Research. Owing to space limits we could cite only a small proportion of the vast number of publications.
Glossary
- Innate immune system
Comprises the cells and molecules that defend the host from infection by other organisms in a nonspecific manner. That is, cells of the innate immune system recognize and respond to pathogens in a generic way and, unlike the adaptive immune system, do not confer immune memory
- Adaptive immune system
The part of the immune system that is mediated by antigen-specific lymphocytes and antibodies; it is highly antigen-specific and includes the development of immunological memory
- Paraneoplastic diseases
Neurological diseases induced as a result of tumour burden; generally caused by the release of tumour-derived hormones, peptides and/or cytokines, or by the misguided destruction of normal tissue by immune cells targeted against malignant cells. Most commonly present with cancers of the lung, breast, ovaries or lymphatic system (lymphoma)
- CD8+ T cell
A subgroup of T lymphocytes that recognize their targets by binding to antigen that is associated with major histocompatibility class I molecules, which are present on the surface of nearly every cell. They can give rise to cytotoxic T lymphocytes, which can kill virally infected cells and tumour cells
- Antigen-presenting cells
(APCs). Cells that display foreign antigen complexes with major histocompatibility complex molecules to T cells
- Adjuvant
An agent mixed with an antigen that enhances the immune response to that antigen on vaccination
- Macrophages
Specialized monocyte-derived phagocytic cells that can capture and degrade invading microbes
- Afferent lymphatics
Vessels that enter the periphery of the lymph node and bring cells and particles from the tissue to the lymph node
- CD4+ T cells
Also known as helper T cells. A subgroup of T lymphocytes that regulate other immune cells and that are essential in B cell antibody class switching, as well as in the activation and growth of cytotoxic T cells. These cells recognize antigen that is associated with major histocompatibility complex class II molecules
- Regulatory T (TReg) cells
A subset of CD4+ T cells that maintain self-tolerance. They can express high levels of CD25 and the forkhead transcription factor FOXP3. They can secrete cytokines, such as IL-10 and TGFβ, which inhibit other T cells
- Natural killer (NK) cells
Innate immune system lymphocytes that are able to kill virally infected cells and tumour cells, particularly cells that lack the expression of major histocompatibility complex class I molecules (the presence of which inhibits NK cell cytotoxicity)
- Phagocytes
White blood cells that are able to ingest foreign particles, microbes and dying cells
- Mast cells
Tissue-resident cells that contain histamine and heparin-rich granules and that mediate allergy and anaphylaxis. They are also important in the defence against pathogens
- Humoral immunity
A component of the adaptive immune system that is mediated by secreted antibodies. Antibodies are secreted from B cells that have differentiated into plasma cells
- Opsonizing antibodies
A subclass of antibodies that can bind to a pathogen or particle and at the same time bind to an Fc receptor on a phagocyte, thereby facilitating phagocytosis and pathogen clearance
- Idiotype
Individual antigenic determinants from the variable regions of the immunoglobulin heavy and light chains are referred to as idiotopes; the sum of the individual idiotopes is referred to as the idiotype
- Memory T cells
A subgroup of antigen-specific T cells that persist long after an infection has resolved. They quickly expand to large numbers of effector T cells on re-exposure to cognate antigen, thus providing immunological ‘memory’
Footnotes
Competing interests statement
The authors declare competing financial interests. See Web version for details.
DATABASES
National Cancer Institute Drug Dictionary:
http://www.cancer.gov/drugdictionary
imiquimod | rituximab | sipuleucel-T
FURTHER INFORMATION
Karolina Palucka’s homepage: www.biir.orgALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Darnell RB. Onconeural antigens and the paraneoplastic neurologic disorders: at the intersection of cancer, immunity, and the brain. Proc Natl Acad Sci USA. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert ML, et al. Tumor-specific killer cells in paraneoplastic cerebellar degeneration. Nature Med. 1998;4:1321–1324. doi: 10.1038/3315. [DOI] [PubMed] [Google Scholar]
- 3.Diamond MS, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuertes MB, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. References 3 and 4 demonstrate that DCs are essential for the generation of anti-tumour immunity in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. References 5 and 6 are outstanding reviews that cover a decade of research on DCs starting from basic biology and moving onto pathophysiology and medicine. [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2011 Nov 17; doi: 10.1146/annurev-immunol-100311-102839. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinman RM, Cohn ZA. In: Mononuclear Phagocytes in Immunity, Infection, and Pathology. van Furth R, editor. Blackwell Scientific Publications Ltd; Oxford: 1975. pp. 95–109. [Google Scholar]
- 10.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nature Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 11.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. An outstanding review summarizing the development of DCs and the identification of transcription factors that are specific to DC lineage. [DOI] [PubMed] [Google Scholar]
- 14.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. An outstanding review that summarizes the principles of antigen capture, processing and presentation by DCs. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 16.Itano AA, Jenkins MK. Antigen presentation to naive CD4 T cells in the lymph node. Nature Immunol. 2003;4:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 17.Albert ML, Bhardwaj N. Resurrecting the dead: DCs cross-present antigen derived from apoptotic cells on MHC I. Immunologist. 1998;6:194–198. [Google Scholar]
- 18.Albert ML, et al. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Jego G, Pascual V, Palucka AK, Banchereau J. Dendritic cells control B cell growth and differentiation. Curr Dir Autoimmun. 2005;8:124–139. doi: 10.1159/000082101. [DOI] [PubMed] [Google Scholar]
- 21.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 22.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nature Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 23.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. This review describes the principles of tolerance induction by DCs. [DOI] [PubMed] [Google Scholar]
- 25.Caux C, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 28.Palucka AK, Banchereau J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr Opin Immunol. 2002;14:420–431. doi: 10.1016/s0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 29.Ueno H, et al. Harnessing human dendritic cell subsets for medicine. Immunol Rev. 2010;234:199–212. doi: 10.1111/j.0105-2896.2009.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng P, Zhou J, Gabrilovich D. Regulation of dendritic cell differentiation and function by Notch and Wnt pathways. Immunol Rev. 2010;234:105–119. doi: 10.1111/j.0105-2896.2009.00871.x. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado-Lopez R, et al. CD8α+ and CD8α-subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulendran B, et al. Distinct dendritic cell subsets differentially regulate the class of immune responsein vivo. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. References 31 and 32 demonstrate for the first time that distinct subsets of DCs induce different types of immune responses in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kool M, et al. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 34.Flach TL, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nature Med. 2011;17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 35.Pascual V, Chaussabel D, Banchereau J. A genomic approach to human autoimmune diseases. Annu Rev Immunol. 2010;28:535–571. doi: 10.1146/annurev-immunol-030409-101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chevrier N, et al. Systematic Discovery of TLR signaling components delineates viral-sensing circuits. Cell. 2011;147:853–867. doi: 10.1016/j.cell.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dzionek A, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 38.Siegal FP, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 39.Di Pucchio T, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nature Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jego G, et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 41.Shaw J, Wang YH, Ito T, Arima K, Liu YJ. Plasmacytoid dendritic cells regulate B-cell growth and differentiation via CD70. Blood. 2010;115:3051–3057. doi: 10.1182/blood-2009-08-239145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. An outstanding summary of the biology of pDCs and the production of type I interferon family members. [DOI] [PubMed] [Google Scholar]
- 43.Bachem A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crozat K, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8α+ dendritic cells. J Exp Med. 2010;207:1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151:6535–6545. [PubMed] [Google Scholar]
- 48.Caux C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor α: II. Functional analysis. Blood. 1997;90:1458–1470. The concept and the first demonstration of distinct subsets of human DCs eliciting different types of T cell immunity in vitro are presented. [PubMed] [Google Scholar]
- 49.Ueno H, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 50.Cheong C, et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209+ dendritic cells for immune T cell areas. Cell. 2010;143:416–429. doi: 10.1016/j.cell.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Romani N, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paquette RL, et al. Interferon-α and granulocyte-macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J Leukoc Biol. 1998;64:358–367. doi: 10.1002/jlb.64.3.358. [DOI] [PubMed] [Google Scholar]
- 53.Chomarat P, Dantin C, Bennett L, Banchereau J, Palucka AK. TNF skews monocyte differentiation from macrophages to dendritic cells. J Immunol. 2003;171:2262–2269. doi: 10.4049/jimmunol.171.5.2262. [DOI] [PubMed] [Google Scholar]
- 54.Mohamadzadeh M, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levings MK, et al. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 56.Zapata-Gonzalez F, et al. 9-cis-Retinoic acid (9cRA), a retinoid X receptor (RXR) ligand, exerts immunosuppressive effects on dendritic cells by RXR-dependent activation: inhibition of peroxisome proliferator-activated receptor γ blocks some of the 9cRA activities, and precludes them to mature phenotype development. J Immunol. 2007;178:6130–6139. doi: 10.4049/jimmunol.178.10.6130. [DOI] [PubMed] [Google Scholar]
- 57.Penna G, Adorini L. 1 α, 25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 58.Jiang A, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z, et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nature Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. An outstanding review that focuses on how phagocytes recognize microbes. [DOI] [PubMed] [Google Scholar]
- 62.Reis e Sousa C. Dendritic cells in a mature age. Nature Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 63.Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. The first identification of a DC receptor that is involved in the recognition of necrotic cells; the engagement of this receptor leads to the generation of immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. Targeting distinct subsets of DCs in vivo with specific antibodies generated distinct types of T cell responses through distinct antigen-processing pathways. [DOI] [PubMed] [Google Scholar]
- 65.Tesniere A, et al. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. This paper discusses how different types of cell death, including those induced by chemotherapy, might induce anti-tumour immunity. [DOI] [PubMed] [Google Scholar]
- 66.Davis ID, Jefford M, Parente P, Cebon J. Rational approaches to human cancer immunotherapy. J Leukoc Biol. 2003;73:3–29. doi: 10.1189/jlb.0502261. [DOI] [PubMed] [Google Scholar]
- 67.Dunne A, Marshall NA, Mills KH. TLR based therapeutics. Curr Opin Pharmacol. 2011;11:404–411. doi: 10.1016/j.coph.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Barrat FJ, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z, et al. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity. 2011;34:866–878. doi: 10.1016/j.immuni.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhodapkar MV, Dhodapkar KM, Palucka AK. Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell Death Differ. 2008;15:39–50. doi: 10.1038/sj.cdd.4402247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–455. doi: 10.1016/j.immuni.2011.09.004. An outstanding review on the recognition of apoptotic cells by phagocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chao MP, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nature Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 74.Hiltbold EM, Vlad AM, Ciborowski P, Watkins SC, Finn OJ. The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells [In Process. Citation] J Immunol. 2000;165:3730–3741. doi: 10.4049/jimmunol.165.7.3730. [DOI] [PubMed] [Google Scholar]
- 75.Fiorentino DF, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 76.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 77.Aspord C, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Monte L, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeNardo DG, et al. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao W, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Treilleux I, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 82.Kukreja A, et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–1865. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bahlis NJ, et al. CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood. 2007;109:5002–5010. doi: 10.1182/blood-2006-03-012542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coukos G, Benencia F, Buckanovich RJ, Conejo-Garcia JR. The role of dendritic cell precursors in tumour vasculogenesis. Br J Cancer. 2005;92:1182–1187. doi: 10.1038/sj.bjc.6602476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Curiel TJ, et al. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535–5538. doi: 10.1158/0008-5472.CAN-04-1272. [DOI] [PubMed] [Google Scholar]
- 86.Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N Engl J Med. 1994;331:679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- 87.Yee C, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nature Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. An outstanding review that discusses the key features and requirements for effective anti-tumour CD8+ T cell-mediated immune responses. [DOI] [PubMed] [Google Scholar]
- 90.Araki K, Youngblood B, Ahmed R. The role of mTOR in memory CD8 T-cell differentiation. Immunol Rev. 2010;235:234–243. doi: 10.1111/j.0105-2896.2010.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang N, Bevan MJ. CD8+ T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. An outstanding review that discusses the key features of CD8+ T cell-mediated immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nature Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 93.Chen L, et al. Costimulation of antitumor immunity by the B7 counter-receptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 94.Shuford WW, et al. 4–1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nature Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 96.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 97.Antony PA, et al. CD8+ T cell immunity against a tumor/self-antigen Is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corthay A, et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 100.Quezada SA, et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Le Floc’h A, et al. α E β 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 103.Fukaura H, et al. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-β1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–77. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kastenmuller W, et al. Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction. J Immunol. 2011;187:3186–3197. doi: 10.4049/jimmunol.1101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sasaki K, Pardee AD, Okada H, Storkus WJ. IL-4 inhibits VLA-4 expression on Tc1 cells resulting in poor tumor infiltration and reduced therapy benefit. Eur J Immunol. 2008;38:2865–2873. doi: 10.1002/eji.200838334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Harlin H, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vianello F, et al. Murine B16 melanomas expressing high levels of the chemokine stromal-derived factor-1/CXCL12 induce tumor-specific T cell chemorepulsion and escape from immune control. J Immunol. 2006;176:2902–2914. doi: 10.4049/jimmunol.176.5.2902. [DOI] [PubMed] [Google Scholar]
- 108.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Menetrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69:7895–7898. doi: 10.1158/0008-5472.CAN-09-1642. [DOI] [PubMed] [Google Scholar]
- 110.Higano CS, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 111.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 112.Schwartzentruber DJ, et al. A phase III multi-institutional randomized study of immunization with the gp100:209–217 (210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol Abstr. 2009;27:CRA9011. [Google Scholar]
- 113.Schuster SJ, et al. Idiotype vaccine therapy (BiovaxID) in follicular lymphoma in first complete remission: phase III clinical trial results. J Clin Oncol Abstr. 2009;27:2. [Google Scholar]
- 114.Kantoff PW, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Palucka K, Ueno H, Roberts L, Fay J, Banchereau J. Dendritic cells: are they clinically relevant? Cancer J. 2010;16:318–324. doi: 10.1097/PPO.0b013e3181eaca83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Draube A, et al. Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PLoS ONE. 2011;6:e18801. doi: 10.1371/journal.pone.0018801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 118.Parmiani G, De Filippo A, Novellino L, Castelli C. Unique human tumor antigens: immunobiology and use in clinical trials. J Immunol. 2007;178:1975–1979. doi: 10.4049/jimmunol.178.4.1975. [DOI] [PubMed] [Google Scholar]
- 119.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. References 117–119 discuss the issue of tumour antigenicity. [DOI] [PubMed] [Google Scholar]
- 120.Finn O. Cancer Immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 121.Finn OJ. Cancer vaccines: between the idea and the reality. Nature Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 122.Bonifaz L, et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bonifaz LC, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hawiger D, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditionsin vivo. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. References 122–124 describe the seminal work demonstrating DC targeting in vivo with specific antibodies that target DC surface receptors and the consequences on T cell-mediated immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Soares H, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li D, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Exp Biol Med. 2008;233:522–534. doi: 10.3181/0708-MR-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoos A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Paczesny S, et al. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J Exp Med. 2004;199:1503–1511. doi: 10.1084/jem.20032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Welters MJ, et al. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci USA. 2010;107:11895–11899. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gaucher D, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Querec TD, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nature Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pulendran B, Li S, Nakaya HI. Systems vaccinology. Immunity. 2010;33:516–529. doi: 10.1016/j.immuni.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Greenberg PD, Ralph M. Steinman: a man, a microscope, a cell, and so much more. Proc Natl Acad Sci USA. 2011;108:20871–20872. doi: 10.1073/pnas.1119293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Topalian SL, Weiner GJ, Pardoll DM. Cancer Immunotherapy comes of age. J Clin Oncol. 2011;29:4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. An outstanding review that summarizes current concepts of cancer biology and for the first time incorporates inflammation and immune evasion into the paradigm of cancer–host interactions. [DOI] [PubMed] [Google Scholar]
- 138.Zitvogel L, et al. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100–3104. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 139.Ma Y, et al. How to improve the immunogenicity of chemotherapy and radiotherapy. Cancer Metastasis Rev. 2011;30:71–82. doi: 10.1007/s10555-011-9283-2. [DOI] [PubMed] [Google Scholar]
- 140.Taylor C, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–5143. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 141.Mitsunaga M, et al. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nature Med. 2011;17:1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 143.Terabe M, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nature Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 144.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2005;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 145.Terabe M, et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-β monoclonal antibody. Clin Cancer Res. 2009;15:6560–6569. doi: 10.1158/1078-0432.CCR-09-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 147.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 150.Hamanishi J, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nature Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 152.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 154.Maus MV, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4–1BB. Nature Biotech. 2002;20:143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 155.Watanabe N, et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 156.Manicassamy S, et al. Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996;29:371–380. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 158.Nestle FO, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nature Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 159.Holtl L, et al. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol. 1999;161:777–782. [PubMed] [Google Scholar]
- 160.Yu JS, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 161.Reichardt VL, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma: a feasibility study. Blood. 1999;93:2411–2419. [PubMed] [Google Scholar]
- 162.Timmerman JM, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 163.Thurner B, et al. Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mackensen A, et al. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34+ hematopoietic progenitor cells. Int J Cancer. 2000;86:385–392. doi: 10.1002/(sici)1097-0215(20000501)86:3<385::aid-ijc13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 165.Banchereau J, et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 166.Fong L, et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Geiger JD, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–8519. [PubMed] [Google Scholar]
- 169.Schuler-Thurner B, et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med. 2002;195:1279–1288. doi: 10.1084/jem.20012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nair SK, et al. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg. 2002;235:540–549. doi: 10.1097/00000658-200204000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Palucka AK, et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 172.Salcedo M, et al. Vaccination of melanoma patients using dendritic cells loaded with an allogeneic tumor cell lysate. Cancer Immunol Immunother. 2006;55:819–829. doi: 10.1007/s00262-005-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chang DH, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aarntzen EH, et al. Early identification of antigen-specific immune responses in vivo by [18F]-labeled 3′-fluoro-3′-deoxy-thymidine ([18F]FLT) PET imaging. Proc Natl Acad Sci USA. 2011;108:18396–18399. doi: 10.1073/pnas.1113045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lesterhuis WJ, et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res. 2011;17:5725–5735. doi: 10.1158/1078-0432.CCR-11-1261. [DOI] [PubMed] [Google Scholar]
- 176.Romano E, et al. Peptide-loaded Langerhans cells, despite increased IL15 secretion and T-cell activation in vitro, elicit antitumor T-cell responses comparable to peptide-loaded monocyte-derived dendritic cells in vivo. Clin Cancer Res. 2011;17:1984–1997. doi: 10.1158/1078-0432.CCR-10-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Okada H, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]