Abstract
Enamel fluorosis has been related to an increase in the amount of amelogenin in fluorosed enamel as compared to normal enamel in the maturation stage. In this study we tested the hypothesis that fluoride incorporated into carbonated apatite alters amelogenin hydrolysis. Recombinant human amelogenin (rh174) was allowed to bind to 0.15 mg of carbonated hydroxyapatite (CAP) or fluoride-containing carbonated hydroxyapatite (F-CAP) synthesized to contain 100, 1000 or 4000 ppm F-. After 3 h digestion with recombinant human MMP20 or KLK4, bound protein was characterized by reverse-phase HPLC. Proteolytic fragments formed after 24 h digestion of amelogenin, were identified by LC tandem mass spectrometry (LCMS/MS). The hydrolysis of amelogenin bound to F100-CAP by both MMP20 and KLK4 was significantly reduced in a dose dependent manner as compared to CAP. After 24 h hydrolysis, the number of cleavage sites in bound amelogenin by MMP20 were similar in CAP and F100-CAP, whereas there were 24 fewer cleavage sites identified for the KLK4 hydrolysis on F100-CAP as compared to CAP. These results suggest that the reduced hydrolysis of amelogenins in fluorosed enamel may be partially due to the increased fluoride content in fluoride containing apatite, contributing to the hypomineralized enamel matrix phenotype observed in fluorosed enamel.
Keywords: dental fluorosis, amelogenin, MMP20, KLK4, apatite
Enamel fluorosis refers to fluoride-related alterations in enamel, which occur during enamel development. These alterations become more severe with increasing fluoride intake, and duration of exposure. The severity of fluorosis is related to the concentration of fluoride in the plasma, considered to be in equilibrium with the tissue fluid that bathes the enamel organ (1,2). Plasma fluoride levels are influenced by many factors, including total fluoride intake, type of intake (i.e., ingested vs. inhaled), renal function, rate of bone metabolism, metabolic activity etc. (3), but generally range from 1 μM F (with 1 ppm F in drinking water) to 10 μM F (5-10 ppm F in drinking water) (4). In addition to these variables, genetic factors have been shown to dictate the severity of enamel fluorosis in mice (5).
Fluorosed enamel is hypomineralized, with hypomineralized defects increasing in width from the outer to the inner enamel surface with increased severity of fluorosis (6,7). This increased hypomineralization in fluorosed enamel has been linked to a delay in the removal of amelogenin in maturation stage fluorosed enamel (8-10). One possible cause for a delay in the removal of amelogenins during enamel maturation is an effect of fluoride on the enamel matrix proteinases, MMP20 and KLK4, which remove enamel matrix proteins as mineralization progresses (11). Previous studies showed a small but significant effect of fluoride on MMP20 expression or activity in vitro (12,13) and in vivo (14). Though direct effects of fluoride on MMP20 activity may contribute to the formation of fluorosed enamel, another more indirect mechanism may be an alteration in matrix/mineral/proteinase interaction related to the fluoride content of enamel crystals (15).
The fluoride content of fluorosed enamel is greater than that of normal enamel, as fluoride substitutes for hydroxyl groups in carbonated hydroxyapatite (HAP) crystals, potentially altering the crystalline structures and surface characteristics. We previously found that the initial rate of amelogenin binding, as well as the total amount of amelogenin bound to fluoride-containing carbonated hydroxyapatite, is greater than in carbonated hydroxyapatite formed without the addition of fluoride (16), suggesting that fluoride incorporation into the crystal lattice alters the crystal surface to enhance amelogenin binding.
In this study, we investigated the possibility that fluoride incorporation into apatite alters the hydrolysis of amelogenins bound to apatites by MMP20 or KLK4.
Material and Methods
Preparation of proteins and apatites
Recombinant human amelogenin (amelogenin, rh174) and recombinant human MMP20 were expressed and purified as previously described (12,17). Recombinant human KLK4 was purchased from R&D Systems (Minneapolis, MN, USA). KLK4 was activated with thermolysin for 2 h at 37°C and the reaction terminated by addition of thermolysin inhibitor phosphoramidon based on the manufacturer's guidelines. Carbonated hydroxyapatite (CAP) and fluoride-containing carbonated hydroxyapatite (F-CAP) were synthesized as previously described (16). F-CAP was synthesized to contain 100, 1000 or 4000 ppm fluoride is referred to as F100-CAP, F1000-CAP and F4000-CAP respectively. Fluoride concentrations were confirmed using the diffusion method described by Taves (18) and crystal structure was analyzed by X-ray diffraction as described previously (16). Apatite crystals with mesh size between 60 μM and 30 μM were selected for these studies.
To allow an equal amount of protein to be adsorbed to the different apatites, 25 μg rh174 was incubated with 0.15 mg apatite (CAP, F100-CAP, F1000-CAP and F4000-CAP) in 20 mM Tris buffer, pH 7.5. This amount is less than that previously determined to saturate the apatite crystals (16). After 1 h of constant shaking at room temperature and washing, the protein-apatite complex was centrifuged at 5000 rpm for 5 min. A Bradford assay (BioRad, Hercules, CA, USA) was performed to confirm that equivalent amounts of protein were bound to the apatite crystals prior to the hydrolysis experiments.
Amelogenin digestion in solution and on apatite
To investigate the effect of free fluoride on amelogenin digestion, CAP-bound and unbound rh174 were hydrolyzed by MMP20 or KLK4 at enzyme/substrate ratios of 1:50 in reaction buffers with or without 100 or 1000 ppm NaF. The reaction buffer used for KLK4 hydrolysis contained 50 mM Tris-HCl, 150 mM NaCl, 0.5 mM CaCl2, pH 7.5. The reaction buffer for MMP20 activity was similar except for an additional component (10 μM ZnCl2 ), because MMP20 belongs to the class of zinc-dependent endopeptidases (19). Each digestion was carried out with constant shaking at 37°C. After 3 h, the reactions were terminated by boiling for 10 min subsequently analyzed by reversed-phase high performance liquid chromatography (RP-HPLC). The influence of fluoride incorporation into carbonated hydroxyapatite fluoride was analyzed by comparing the digestion of the equal amounts of amelogenin bound to CAP- and FAP-CAP, under the same conditions as described above.
HPLC analysis of amelogenin digestion
Trifluoroacetic acid (TFA) was added to the all the digested samples to final concentrations of 0.1%, prior to HPLC separation. The addition of TFA dissolved the apatite and released the retained proteins, which could then be separated by HPLC. HPLC separation was done on a C4 column (Varian, Walnut Creek, CA, USA), by means of a linear gradient of 30 to 60% acetonitrile at flow-rate of 1 ml/min over 20 min. Proteins and peptides were monitored by absorbance at 280 nm. The absorbance peak areas (mAU/ml) corresponding to separated protein or peptide fragments were measured and compared. The results were expressed as mean standard error of triplicate experiments. A paired Student's t-test was used to assess the statistical significance between the two groups.
Comparison of proteolytic cleavage patterns by LC-MS/MS
The proteolytic fragments of amelogenin digested for 24 h by MMP20 and KLK4 on CAP and F100-CAP were identified by liquid chromatography-mass spectrometer (LC-MS/MS) to determine and compare their cleavage sites. Prior to separation and analysis by LC-MS/MS all samples were dissolved in 0.1%TFA. LC/MALDI/MS-MS was carried out on an Ultimate 3000 (Dionex, Sunnyvale, CA, USA) LC system equipped with two Monolithic columns (200 μm I.D., 5 cm length) packed with reverse phase chromatography resin. MS and MS/MS data were acquired in the batch mode using 4000 Series Explorer Software (Applied Biosystems, Foster City, CA, USA) and analyzed using ProteinPilot software (Version 3.0, Revision 114732, AB) with Paragon search engine for protein identification. A Uniprot human database (UniProt release 14.7, January 20, 2009) plus common contaminants was used for searching the MS/MS data. To rule out non-specific cleavage during the purification and digestion process, CAP and F100-CAP bound amelogenins without addition of MMP20 or KLK4 were treated in parallel. Non-specific fragments were excluded from the mass spectrometry analysis of the digested samples.
Results
Fluoride incorporated into apatite delayed hydrolysis of apatite-bound amelogenins by both MMP20 and KLK4
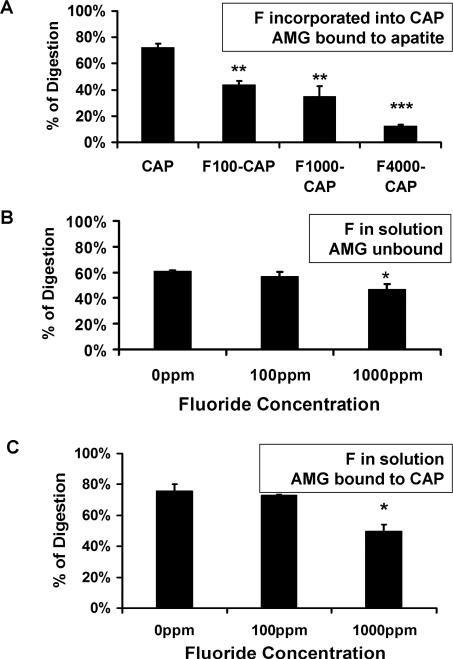
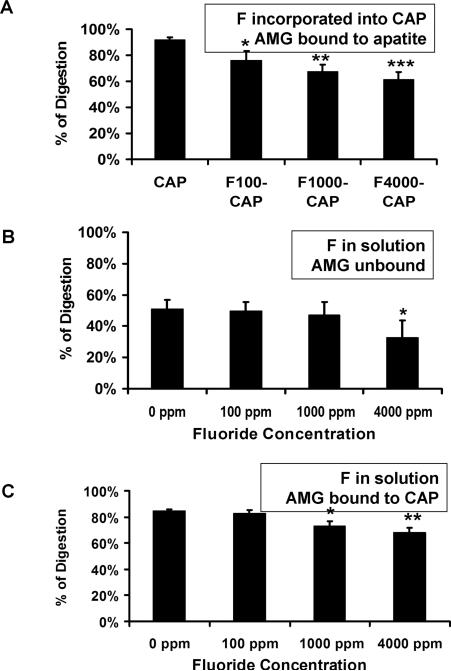
As shown in Figs. 1A and 2A, fluoride incorporation into apatite significantly delayed both MMP20 and KLK4 hydrolysis of the bound amelogenin proteins in a concentration-dependent manner. This significant inhibitory effect was observed at fluoride concentration as low as 100 ppm. In marked contrast, when 100 ppm unincorporated fluoride was added into the reaction buffer containing MMP20 or KLK4, there was no obvious delay in the digestion of both unbound (Figs. 1B, 2B) and CAP-bound amelognein (Figs. 1C, 2C). MMP-20 inhibition by fluoride in solution occurred only at 1000 ppm fluoride, possibly related to precipitation of calcium from solution at this high concentration. (Figs. 1B,C). KLK-4 hydrolysis was inhibited only when fluoride concentration reached up to 4000 ppm in the solution (Fig. 2C).
Fig. 1.
The relative percent of amelogenin (AMG) digested by MMP-20. A) Digestion of AMG bound to apatite decreased as increased fluoride fluoride was incorporation into apatite, with a significant effect even at 100 ppm F. B) When fluoride was added into solution with amelogenin, either without apatite, or C) when amelogenin was bound to CAP, digestion was reduced only at 1000 ppm. AMG; CAP, carbonated hydroxyapatite; F, fluoride; F100-CAP, F1000-CAP, fluoride-containing carbonated hydroxyapatite (F-CAP) synthesized to contain 100, or 1000 ppm fluoride, respectively. The p-value was derived from a paired t-test. * P<0.05, ** P<0.01, *** P<0.001.
Fig. 2.
The relative percent of amelogenin (AMG) digested by KLK-4. A) KLK4 hydrolysis of AMG bound to apatite decreased relative to increasing amounts of fluoride incorporated into the apatite, with a significant effect even at 100 ppm F B) Fluoride in solution altered KLK4 hydrolysis of AMG only at 4000 ppm F.C) 1000 ppm F in solution significantly inhibitied KLK4 digestion of CAP bound AMG. CAP, carbonated hydroxyapatite; F, fluoride; F100-CAP, F1000-CAP, and F4000- CAP, fluoride-containing carbonated hydroxyapatite (F-CAP) synthesized to contain 100, 1,000, or 4,000 ppm fluoride, respectively. The p-value was derived from a paired t-test. * P<0.05, ** P<0.01, *** P<0.001.
Fluoride incorporation into apatite altered amelogenin cleavage sites by both MMP20 and KLK4
LC-MS/MS spectra of MMP20 hydrolysis of amelogenin after 24 h, revealed that MMP20 generated 53 identical cleavages in CAP- and F100-CAP bound amelogenin (Fig. 3A). Amelogenin hydrolyzed by MMP-20 on CAP showed one unique cleavage at the site of L45/H46. In contrast, amelogenin adsorbed onto F100-CAP were cleaved at one other particular site L142/P143.
Fig. 3.
Cleavage sites of MMP20 (A) and KLK4 (B) in amelogenin adsorbed into CAP or F100-CAP. Cleavage sites identified for both CAP and F100-CAP bound amelogenins are indicated by a slash (/). Cleavage sites not detected in F100-CAP bound amelogenin are shown by dashed arrows. The one MMP-20 cleavage site identified only in F100-CAP bound amelogenin is shown by a solid arrow.
KLK4 hydrolysis for 24 h showed CAP-bound amelogenin was cleaved at 72 sites, creating 134 proteolytic fragments. In comparison, there were 17 fewer cleavage sites identified for the amelogenin hydrolyzed on F100-CAP. Most of these 17 sites were located in the central region of the amelogenin protein (Fig. 3B). The reduction of proteolytic cleavage of rh174 by KLK4 generated 22 fewer proteolytic fragments. Some segments, such as VLSQQHPPTH, were not cleaved and remained intact after hydrolysis.
Discussion
The fluoride concentrations of the carbonated apatite crystals were similar to those found in human enamel. In normal (nonfluorosed enamel) fluoride concentrations are reported to range from 10 ppm near the DEJ, to several thousand ppm at the enamel surface (20). In teeth with minimal fluorosis, fluoride concentrations are 100 ppm in the inner enamel (300 μm from the surface) of human teeth (20). The mid layer of severely fluorosed human enamel (150 μm from the surface), is found to contain approximately 2000 ppm fluoride (20). Extremely high levels of fluoride (up to 4000 ppm) can be found in the external enamel of unerupted teeth exhibiting severe fluorosis (21). In comparison, the relatively low concentrations of fluoride found in the enamel fluid surrounding the ameloblasts (22), is likely to contain no more than 10 μM (0.19 ppm) fluoride, similar to serum levels of individuals ingesting 5 to 10 ppm fluoride in drinking water (4). This means that the different amounts of incorporated fluoride in the synthetic apatites used in the present study were similar to those found in various types of human enamel. However, the same levels of unincorporated fluoride contained in the reaction solution were much higher than the physiological situations.
The findings from this study showed that in comparison with amelogenin either in solution or bound to CAP, hydrolysis of amelogenin absorbed to F-CAP was significantly reduced. This result suggests that fluoride incorporated into the apatite delayed amelogenin degredation, even at concentrations as low as 100 ppm. When amelogenin was suspended in solution or adsorbed onto CAP, a similar decrease in protein hydrolysis occurred only at free fluoride concentrations much beyond the physiological situation.
Although we previously found that fluoride could inhibit MMP20 activity at micromolar concentrations, this only occurred at a reduced pH and in a buffer with an ionic strength similar to that found in the enamel matrix (12). Taken together, these results suggest that inhibition of protease activities by the fluoride in free ionic form is not the prevailing mechanism causing a delay in amelogenin degradation in fluorosed enamel. Our data from LC-MS/MS analyses indicates that fluoride incorporation into apatite also alters cleavage sites of the bound amelogenin by KLK-4, with most of the altered cleavage sites were located in the central region of amelogenin.
Proteins usually alter their conformation when bound to solid surfaces (23), which may further affect the protein hydrolysis by the enzymes. This is supported by our observation that CAP bound amelogenin was hydrolysed at a faster speed as compared with the amelogenin without binding (Figs. 1A,B, 2A,B). In apparent contrast, a study from the Moradian-Oldak group (24) found that apatite reduced amelogein proteolysis in vitro. However, this difference can be explained by the fact that they used a different system in which the apatite was added to buffer containing amelogenin and proteinases. In their model, amelogenin and its hydrolyzed fragments would both bind to the apatite crystals, possibly resulting in inhibition of proteolytic activity. In contrast, we added proteinase to the buffer after the uncleaved amelogenin protein was bound to apatite. This means that any conformational changes resulting from amelogenin binding to apatites would affect hydrolysis of the full-length amelogenin by proteinases.
When fluoride is incorporated into apatite an elevated level of fluoride is found in the immediate vicinity of mineralized tissue, making the local concentration of fluoride much higher than those in tissue fluid in general. Fluoride incorporation into enamel apatite also generates a surface modification of the crystals, such as increasing their degree of crystallinity, and relative mechanical and acid resistance. It has been suggested that increased surface roughness of fluorosed rat enamel may be a surface restructuring secondary to the incorporation of fluoride into mineral (25). Because of such fluoride-induced changes in crystal chemistry and morphology, we assume that fluoride incorporated into the crystal lattice may cause conformational changes of amelogenin following its binding to the apatite. A change in protein structure could affect protease accessibility, resulting in reduced degradation and altered cleavage pattern, found in this in vitro study.
The KLK4 cleave sites altered by binding to F-CAP were on either side of the hydrophobic central portion of the amelogenin protein. Previously we had suggested that the effect of fluoride containing apaitites on amelognin hydrolysis by matrix proteinases, may be related to a potentially reduced apatite crystal size, secondary to fluoride incorporation into the apatite lattice. In this possible scenario, calcium-binding regions of the amelogenin protein at the N and C terminus would bind to the apatite, and the smaller size of the fluoride containing apatite crystals would then alter the configuration of hydrophobic central region. This could then affect amelogenin cleavage sites, particularly in the central portion of the molecule.
In a previous study, we did find that amelogenin binds more tightly to fluoride containing apatite as compared to apatite without added fluoride. It is therefore possible that this tighter binding could inhibit the release of cleaved amelogenin peptides from the apatite surface. The finding that fluoride containing apatites enhance both the binding of amelogenin to the apatite surface as well as hydrolysis by MMP20 and KLK4, point to a probable mechanism contributing to the formation of fluorosed enamel. A delay in amelogenin hydrolysis would results in a delay in final enamel matrix mineralization, consistent with the hypomineralization that is observed in fluorosed enamel (26-28). We propose the following multi-stage effect of fluoride containing apatite on amelogenin hydrolysis and enamel formation, though it is likely that there are also additional mechanisms:
Increased fluoride incorporation into the forming enamel matrix crystals in the secretory stage of forming enamel results in enhanced amelogenin/apatite binding.
Hydrolysis of amelogenins bound to the growing secretory stage apatite crystals by MMP20 is delayed in fluoride containing apatites, resulting in a relative increase in amelogenins at the transition/early maturation stage of enamel formation.
At the transition stage fluoride is rapidly deposited into the porous enamel matrix, resulting in increased formation of fluoride containing apatite. This increases fluoride content of apatite further delays hydrolysis of apatite bound amelogenins by both MMP20 and KLK4 throughout the transition stage.
Final hydrolysis of amelogenins bound to fluoride containing apatite crystals by KLK4 is delayed in the maturation stage.
The net result of these fluoride related effects in the secretory and transition stages is retention of amelogenins in the maturation stage, resulting in a delay in mineral removal and the formation of hypomineralized enamel at subsurface layers in fluorosed enamel. However, in a previous in vivo study, we found that the total enzymatic activity contained within fluorosed maturation stage enamel is reduced as compared to maturation stage enamel of rats (12), indicating that additional mechanisms also contribute to the eitology of the fluorosed enamel matrix. Further studies of the effects of fluoride on cell/matrix interactions are necessary to finally understand the multifactorial mechanisms that can result in the formation of fluorosed enamel.
Acknowledgements
These studies were supported by grants R01-DE13508 and R01-DE015821 from the National Institute of Dental and Craniofacial Research.
Footnotes
Conflicts of interest – The authors declare no conflicts of interest.
References
- 1.Angmar-Mansson B, Ericsson Y, Ekberg O. Plasma fluoride and enamel fluorosis. Calcif Tissue Res. 1976;22:77–84. doi: 10.1007/BF02010348. [DOI] [PubMed] [Google Scholar]
- 2.Angmar-Mansson B, Whitford GM. Enamel fluorosis related to plasma F levels in the rat. Caries Res. 1984;18:25–32. doi: 10.1159/000260743. [DOI] [PubMed] [Google Scholar]
- 3.Angmar-Mansson B, Lindh U, Whitford GM. Enamel and dentin fluoride levels and fluorosis following single fluoride doses: a nuclear microprobe study. Caries Res. 1990;24:258–262. doi: 10.1159/000261279. [DOI] [PubMed] [Google Scholar]
- 4.Singer L, Ophaug R. Ionic and nonionic fluoride in plasma (or serum). Crit Rev Clin Lab Sci. 1982;18:111–140. doi: 10.3109/10408368209083493. [DOI] [PubMed] [Google Scholar]
- 5.Everett ET, McHenry MA, Reynolds N, Eggertsson H, Sullivan J, Kantmann C, Martinez-Mier EA, Warrick JM, Stookey GK. Dental fluorosis: variability among different inbred mouse strains. J Dent Res. 2002;81:794–798. doi: 10.1177/0810794. [DOI] [PubMed] [Google Scholar]
- 6.Fejerskov O, Silverstone LM, Melsen B, Moller IJ. Histological features of fluorosed human dental enamel. Caries Res. 1975;9:190–210. doi: 10.1159/000260157. [DOI] [PubMed] [Google Scholar]
- 7.Fejerskov O, Thylstrup A, Larsen MJ. Clinical and structural features and possible pathogenic mechanisms of dental fluorosis. Scand J Dent Res. 1977;85:510–534. doi: 10.1111/j.1600-0722.1977.tb02110.x. [DOI] [PubMed] [Google Scholar]
- 8.DenBesten PK. Effects of fluoride on protein secretion and removal during enamel development in the rat. J Dent Res. 1986;65:1272–1277. doi: 10.1177/00220345860650101401. [DOI] [PubMed] [Google Scholar]
- 9.DenBesten PK, Crenshaw MA. The effects of chronic high fluoride levels on forming enamel in the rat. Arch Oral Biol. 1984;29:675–679. doi: 10.1016/0003-9969(84)90171-7. [DOI] [PubMed] [Google Scholar]
- 10.Wright JT, Chen SC, Hall KI, Yamauchi M, Bawden JW. Protein characterization of fluorosed human enamel. J Dent Res. 1996;75:1936–1941. doi: 10.1177/00220345960750120401. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Papagerakis P, Yamakoshi Y, Hu JC, Bartlett JD, Simmer JP. Functions of KLK4 and MMP-20 in dental enamel formation. Biol Chem. 2008;389:695–700. doi: 10.1515/BC.2008.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DenBesten PK, Yan Y, Featherstone JD, Hilton JF, Smith CE, Li W. Effects of fluoride on rat dental enamel matrix proteinases. Arch Oral Biol. 2002;47:763–770. doi: 10.1016/s0003-9969(02)00117-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Yan Q, Li W, DenBesten PK. Fluoride down-regulates the expression of matrix metalloproteinase-20 in human fetal tooth ameloblast-lineage cells in vitro. Eur J Oral Sci. 2006;114(Suppl 1):105–110. doi: 10.1111/j.1600-0722.2006.00303.x. discussion 127-109, 380. [DOI] [PubMed] [Google Scholar]
- 14.Sharma R, Tye CE, Arun A, Macdonald D, Chatterjee A, Abrazinski T, Everett ET, Whitford GM, Bartlett JD. Assessment of dental fluorosis in mmp20 +/- mice. J Dent Res. 90:788–792. doi: 10.1177/0022034511398868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson C, Brookes SJ, Bonass WA, Shore RC, Kirkham J. Enamel maturation. Ciba Found Symp. 1997;205:156–170. doi: 10.1002/9780470515303.ch11. discussion 170-154. [DOI] [PubMed] [Google Scholar]
- 16.Tanimoto K, Le T, Zhu L, Chen J, Featherstone JD, Li W, DenBesten P. Effects of fluoride on the interactions between amelogenin and apatite crystals. J Dent Res. 2008;87:39–44. doi: 10.1177/154405910808700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Gao C, Yan Y, DenBesten P. X-linked amelogenesis imperfecta may result from decreased formation of tyrosine rich amelogenin peptide (TRAP). Arch Oral Biol. 2003;48:177–183. doi: 10.1016/s0003-9969(02)00170-x. [DOI] [PubMed] [Google Scholar]
- 18.Taves DR. Separation of fluoride by rapid diffusion using hexamethyldisiloxane. Talanta. 1968;15:969–974. doi: 10.1016/0039-9140(68)80097-9. [DOI] [PubMed] [Google Scholar]
- 19.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 20.Richards A, Fejerskov O, Baelum V. Enamel fluoride in relation to severity of human dental fluorosis. Adv Dent Res. 1989;3:147–153. doi: 10.1177/08959374890030021301. [DOI] [PubMed] [Google Scholar]
- 21.Richards A, Likimani S, Baelum V, Fejerskov O. Fluoride concentrations in unerupted fluorotic human enamel. Caries Res. 1992;26:328–332. doi: 10.1159/000261463. [DOI] [PubMed] [Google Scholar]
- 22.Aoba T, Moreno EC. The enamel fluid in the early secretory stage of porcine amelogenesis: chemical composition and saturation with respect to enamel mineral. Calcif Tissue Int. 1987;41:86–94. doi: 10.1007/BF02555250. [DOI] [PubMed] [Google Scholar]
- 23.Gray JJ. The interaction of proteins with solid surfaces. Curr Opin Struct Biol. 2004;14:110–115. doi: 10.1016/j.sbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z, Carpiaux W, Fan D, Fan Y, Lakshminarayanan R, Moradian-Oldak J. Apatite reduces amelogenin proteolysis by mmp-20 and klk4 in vitro. J Dent Res. 2010;89:344–348. doi: 10.1177/0022034509360660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson C, Connell S, Kirkham J, Brookes SJ, Shore RC, Smith AM. The effect of fluoride on the developing tooth. Caries Res. 2004;38:268–276. doi: 10.1159/000077766. [DOI] [PubMed] [Google Scholar]
- 26.DenBesten PK, Crenshaw MA, Wilson MH. Changes in the fluoride-induced modulation of maturation stage ameloblasts of rats. J Dent Res. 1985;64:1365–1370. doi: 10.1177/00220345850640120701. [DOI] [PubMed] [Google Scholar]
- 27.Richards A, Kragstrup J, Nielsen-Kudsk F. Pharmacokinetics of chronic fluoride ingestion in growing pigs. J Dent Res. 1985;64:425–430. doi: 10.1177/00220345850640030601. [DOI] [PubMed] [Google Scholar]
- 28.Suckling G, Thurley DC, Nelson DG. The macroscopic and scanning electron-microscopic appearance and microhardness of the enamel, and the related histological changes in the enamel organ of erupting sheep incisors resulting from a prolonged low daily dose of fluoride. Arch Oral Biol. 1988;33:361–373. doi: 10.1016/0003-9969(88)90070-2. [DOI] [PubMed] [Google Scholar]