Abstract
The desymmetrization of p-peroxyquinols using a Brønsted acid catalyzed acetalization/oxa-Michael cascade was achieved in high yields and selectivities for a variety of aliphatic and aryl aldehydes. Mechanistic studies suggest that the reaction proceeds through a dynamic kinetic resolution of the peroxy hemiacetal intermediate. The resulting 1,2,4-trioxane products were derivatized and show potent cancer cytotoxicity.
Trioxanes are important scaffolds, which appear in molecules that exhibit antimalarial, anticancer and antibacterial activities.1 In particular, artemisinin, administered as a part of a combination therapy for the frontline treatment of malaria, contains a 1,2,4-trioxane as the key pharmacophore. The recent emergence of an artemisinin resistant malaria strain2 combined with the fact that artemisinin’s mode of action remains under debate3 increases the difficulty of treating malaria and makes the pursuit of novel therapeutic agents more urgent. One potential solution has been the development of new synthetic endoperoxides.4 Enantiomers of a few synthetic trioxanes have shown similar anti-malarial activities5 but stereochemistry has a demonstrated impact on anti-cancer activity.6 Current methods for the enantioselective synthesis of trioxanes are lengthy and use chiral starting materials or reagents.5,6,7,8
We envisioned that enantioenriched trioxanes could be accessed quickly and enantioselectively through a desymmetrization of p-peroxyquinols via an acetalization/oxa-Michael cascade first reported by Jefford.9,10 Cascade catalysis11,12,13 and desymmetrizations14 are powerful methods utilized by our group and others to construct complex molecules containing multiple stereocenters in a rapid and efficient manner. Both enantioselective acetalization15 and oxa-Michael16 reactions are relatively unsolved problems. We were cognizant of the potential difficulties in this approach due to the inherent reversibility of both transformations particularly under acidic conditions. Nevertheless, we were emboldened by recent successes in this area.17
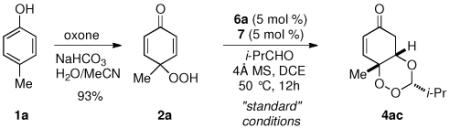
We began our investigation by studying the desymmetrization of p-peroxyquinol 2a, trivially accessed from cresol, using chiral Brønsted acid catalyst 5 (TRIP) which afforded the desired trioxane in good yield as a single diastereomer in 86% ee (entry 5, Table 1). Switching to the bis(2,4,6-triisopropylphenyl)spirobiindane phosphoric acid 6a developed by List15e,18 improved the enantioselectivity to 96%. Other biindane Brønsted acids were screened but the parent acid 6a gave the best results. Lowering the catalyst loading from 10 mol % gave decreased reactivity, which could be restored through the use of thiourea 7 as a co-catalyst. Catalyst loadings as low as 2 mol% may be used at the expense of a longer reaction time (entry 7, Table 1). The use of thiourea 7 alone leads to no product.
Table 1.
Reaction optimization.
| entry | variation from “standard” conditions | yield (%)b | ee (%)c |
|---|---|---|---|
| 1 | none | 92 | 96 |
| 2 | no 4Å MS | 90 | 88 |
| 3 | no 7 | 46 | 95 |
| 4 | no 6a | <5 | - |
| 5 | 5, instead of 6a | 93 | 86 |
| 6 | no 7, 6a (10 mol%) | 93 | 96 |
| 7 | 6a (2 mol%), 72h | 88 | 98 |
| 8 | 6b, instead of 6a | 65 | 95 |
| 9 | 23 °C, 48h, instead of 50 °C, 24 | 85 | 96 |
|
| |||
| |||
Reactions were performed on 0.1 mmol scale (0.25 M solution).
Isolated yields of analytically pure material.
Enantiomeric excess determined by HPLC using a chiral stationary phase.
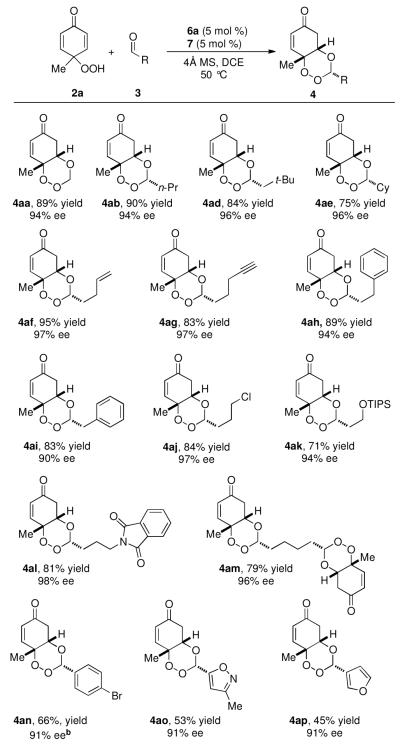
With our optimized reaction conditions in hand, we explored the aldehyde scope of the reaction (Figure 1). Paraformaldehyde and a variety of sterically hindered aliphatic aldehydes work well. Aldehydes containing alkyl halides, protected alcohols and protected amines are tolerated affording trioxanes in excellent selectivities. Aromatic aldehydes also participate in the reaction with high enantioselectivity but slightly decreased yields (Figure 1).
Figure 1.
Aldehyde substrate scopea
aConditions: 2a (0.3 mmol), 3 (1.25 equiv). All products formed as single diastereomers (>20:1). Enantiomeric excess determined by HPLC using a chiral stationary phase. bAbsolute configuration established by X-ray analysis. The rest were assigned by analogy. cent-6a used as catalyst.
The reaction also proved tolerant of substitution on the p-peroxyquinol. Products with esters, ethers, and multiple tetrasubstituted stereocenters are all isolated in good yields and selectivities (Figure 2).
Figure 2.
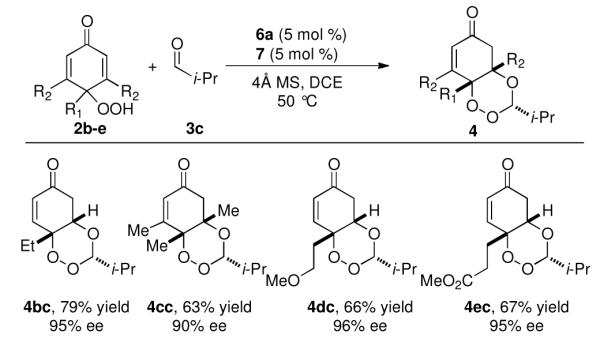
Peroxyquinol substrate scopea
aSee footnote a, Figure 1.
The enantiodetermining step is likely the oxa-Michael event based on the high enantioselectivity of the product formed from paraformaldehyde.19 We propose that the reaction proceeds via a dynamic kinetic resolution of peroxy hemiacetal (+/−)8a (Equation 1). To further test this hypothesis, racemic peroxy-hemiacetal 8ac was formed by heating p-peroxyquinol 2a with isobutyraldehyde. After excess aldehyde was removed, unpurified (+/−) 8ac was subjected to chiral acid 6a. To our delight, trioxane 4ac was formed in good yield as a single diastereomer in 94% ee. This suggests that peroxyhemiacetal 8ac is resolved through a dynamic kinetic resolution (Equation 1). Additionally, monitoring the reaction under standard conditions with catalyst 6a by HPLC, we note that the peroxyhemiacetal remains as a racemate throughout the course of the reaction. A crossover experiment subjecting 4ac to n-butyraldehyde showed that the oxa-Michael is not reversible under the reaction conditions (Equation 2).20
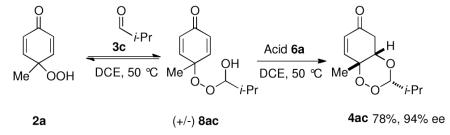 |
(1) |
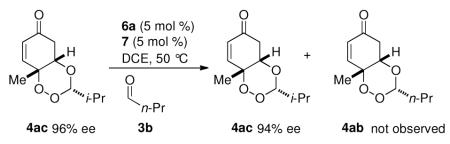 |
(2) |
The 1,2,4 trioxane products of the desymmetrization have a variety of synthetic handles for subsequent derivatization. A Luche reduction of 4ac forms the allylic alcohol in 4:1 dr and subsequent directed epoxidation delivers highly oxygenated cyclohexane 9 (Figure 3). Bromination of 4ac followed by elimination forms vinyl bromide 10 which allows for the incorporation of a variety of functional groups though cross coupling. Chemoselective reduction of the olefin in the presence of the peroxide may be achieved under the aegis of Rh/Al2O3 and Adams’ catalyst. This product can be further reduced under acidic conditions to reveal previously unreported diol 12.
Figure 3.
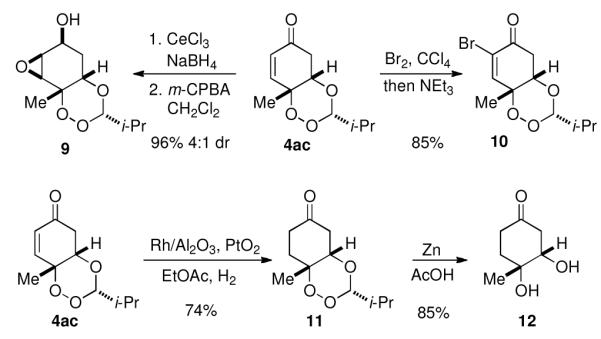
Trioxane derivatizations.
In addition to serving as the frontline antimalarial agent, artemisinin is cytotoxic toward cancer cells and the 1,2,4-trioxane is believed to play an important role.1 Our products and their derivatives were screened for cytotoxicity against a variety of cancer cell lines. Compounds 4ac, 4an, and 11 show promising activity toward bone and lung cancer cell lines with in vitro IC50’s from 3-25 μM (Figure 4). Importantly, the significant cytotoxicity of the semi-reduced trioxane 12 demonstrates that their activity is not due solely to the presence of the Michael acceptor.
Figure 4.
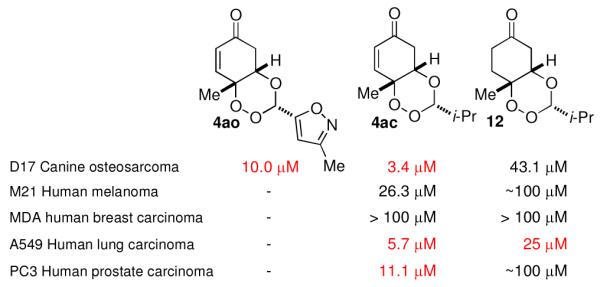
Anticancer activity of 1,2,4-trioxane products.
In conclusion we report the first catalytic enantioselective synthesis of trioxanes using a desymmetrization of p-peroxyquinols via an acetalization/oxa-Michael cascade. We propose that the reaction proceeds via a dynamic kinetic resolution of a peroxy-hemiacetal intermediate. The 1,2,4-trioxane products are easily derivatized and show promising cancer cytotoxicity. Further development of this reaction, antimalarial testing of these trioxanes and investigation of the cytotoxicity mode of action are currently underway.
Supplementary Material
ACKNOWLEDGMENT
We thank NIGMS (GM72586) for support and Kevin Oberg for X-ray analysis of 4an. TR thanks Amgen and Roche for unrestricted support.
Footnotes
Experimental procedures, crystallographic data, and characterization of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1)(a).Efferth T. Current Drug Targets. 2006;7:407. doi: 10.2174/138945006776359412. [DOI] [PubMed] [Google Scholar]; (b) Chaturvedi D, Goswami A, Saikia PP, Barua NC. Chem. Soc. Rev. 2010;39:435. doi: 10.1039/b816679j. [DOI] [PubMed] [Google Scholar]; (c) Morrissey C, Gallis B, Solazzi JW, Kim BJ, Gulati R, Vakar-Lopez F, Goodlett DR, Vessella RL, Sasaki T. Anticancer Drugs. 2010;21:423. doi: 10.1097/CAD.0b013e328336f57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2)(a).Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NPJ, Lindegardh N, Socheat D, White NJ. New Engl. J. Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) O’Brien C, Henrich PP, Passi N, Fidock DA. Curr. Opin. Infect. Dis. 2011;24:570. doi: 10.1097/QCO.0b013e32834cd3ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3)(a).Jefford CW. Curr. Med. Chem. 2001;8:1803. doi: 10.2174/0929867013371608. [DOI] [PubMed] [Google Scholar]; (b) Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Int. J. Parasitol. 2006;36:1427. doi: 10.1016/j.ijpara.2006.07.011. [DOI] [PubMed] [Google Scholar]; (c) O’Niell PM, Barton VE, Ward SA. Molecules. 2010;15:1705. doi: 10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4)(a).Tang Y, Dong Y, Vennerstorm JL. Med. Res. Rev. 2004;24:425–448. doi: 10.1002/med.10066. [DOI] [PubMed] [Google Scholar]; (b) Kumar N, Sharma M, Rawat DS. Curr. Med. Chem. 2011;18:3889. doi: 10.2174/092986711803414340. [DOI] [PubMed] [Google Scholar]; (c) Jefford CW. Drug Discov. Today. 2012;12:487. doi: 10.1016/j.drudis.2007.04.009. [DOI] [PubMed] [Google Scholar]; (d) Jefford CW. Curr. Top. Med. Chem. 2012;12:373. doi: 10.2174/156802612799362940. [DOI] [PubMed] [Google Scholar]; (e) Slack RD, Jacobine AM. Posner G. H. Med. Chem. Commun. 2012;3:281. [Google Scholar]
- (5).O’Niell PM, Rawe SL, Borstnik K, Miller A, Ward SA, Bray PG, Davies J, Oh CH, Posner GH. Chem. Bio. Chem. 2005;6:2048. doi: 10.1002/cbic.200500048. [DOI] [PubMed] [Google Scholar]
- (6).Beekman AC, Barentsen ARW, Woerdenbag HJ, Van Uden W, Pras N, Konings AWT, El-Feraly FS, Galal AM, Wikström HV. J. Nat. Prod. 1997;60:325. doi: 10.1021/np9605495. [DOI] [PubMed] [Google Scholar]
- (7)(a).Jefford CW, Kohmoto S, Jaggi D, Timári G, Rossier J-C, Rudaz M, Barbuzzi O, Gérard D, Burger U, Kamalaprija P, Mareda J, Bernardinelli G. Helv. Chim. Acta. 1995;78:647. [Google Scholar]; (b) Hamzaoui M, Provot O, Riche C, Chiaroni A, Gay F, Moskowitz H, Mayrargue J. Tetrahedron: Asymmetry. 1997;8:2085. [Google Scholar]; (c) Zouhiri F, Desmaële D, d’Angelo J, Riche C, Gay F, Cicéron L. Tetrahedron Lett. 1998;39:2969. [Google Scholar]
- (8)(a).For the catalytic asymmetric synthesis of peroxides, see: Lu X, Liu Y, Cindric B, Deng L. J. Am. Chem. Soc. 2008;130:8134–8135. doi: 10.1021/ja802982h.. Reisinger CM, Wang X, List B. Angew. Chem. Int. Ed. 2008;47:8112–8115. doi: 10.1002/anie.200803238.. Lifchits O, Reisinger CM, List B. J. Am. Chem. Soc. 2010;132:10227–10229. doi: 10.1021/ja1037935.. Zheng W, Wojtas L, Antilla JC. Angew. Chem. Int. Ed. 2010;49:6589–6591. doi: 10.1002/anie.201002972..
- (9)(a).Jefford CW, Jaggi D, Kohmoto S. Boukouvalas Helv. Chim. Acta. 1984;67:2254. [Google Scholar]; (b) Jefford CW, Rossier JC, Kohmoto S, Boukouvalas J. Synthesis. 1985:29. [Google Scholar]; (c) Redondo MC, Ribagorda M, Carreño MC. Org. Lett. 2010;12:568. doi: 10.1021/ol902763g. [DOI] [PubMed] [Google Scholar]; (d) Jefford CW, Jaggi D, Boukouvalas J, Kohmoto S. J. Am. Chem. Soc. 1983;105:6497. [Google Scholar]; (e) Jefford CW, Kohmoto S, Boukouvalas J, Burger U. J. Am. Chem. Soc. 1983;105:6498. [Google Scholar]
- (10)(a).Two asymmetric acetalization/oxa-Michael reactions have been reported using λ-hydroxy aryl enones or λ-hydroxy α,β-unsaturated thioesters and aldehydes as partners catalyzed by a chiral thiourea, proceeding in excellent enantioselectivity but modest diastereoselectivity; see:Asano K, Matsubara S. Org. Lett. 2012;14:1620. doi: 10.1021/ol3003755.. Okamura T, Asano K, Matsubara S. Chem. Commun. 2012;48:5076–5078. doi: 10.1039/c2cc31602a.
- (11)(a).Reviews: Enders D, Grondal C, Hüttl MRM. Angew. Chem. Int. Ed. 2007;46:1570. doi: 10.1002/anie.200603129.. Grondal1 C, Jeanty M, Enders D. Nat. Chem. 2010;2:167. doi: 10.1038/nchem.539.. Zhou J. Chem. Asian J. 2010;5:422. doi: 10.1002/asia.200900458.. Albrecht Ł, Jiang H, Jorgensen K-A. Angew. Chem. Int. Ed. 2011;50:8492. doi: 10.1002/anie.201102522.. Peng F-Z, Shao Z-H. Curr. Org. Chem. 2011;15:4144.. Pellissier H. Adv. Synth. Catal. 2012;354:237..
- (12)(a).Chiral Bronsted acid cascade examples: Rueping M, Antonchik AP, Theissmann T. Angew. Chem. Int. Ed. 2006;44:3683. doi: 10.1002/anie.200600191.. Zhou J, List B. J. Am. Chem. Soc. 2007;129:7498. doi: 10.1021/ja072134j.. Terada M, Machioka K, Sorimachi K. J. Am. Chem. Soc. 2007;129:10336. doi: 10.1021/ja0739584.. Mukherjee S, List B. J. Am. Chem. Soc. 2007;129:11336. doi: 10.1021/ja074678r.. Enders D, Narine AA, Toulgoat F, Bisschops T. Angew. Chem. Int. Ed. 2008;47:5662. doi: 10.1002/anie.200801354.. Rueping M, Antonchik AP. Angew. Chem. Int. Ed. 2008;47:5836. doi: 10.1002/anie.200801435.. Cai Q, Zaho Z-A, You S-L. Angew. Chem. Int. Ed. 2009;48:7428. doi: 10.1002/anie.200903462.. Liu H, Dagousset G, Masson G, Retailleau P, Zhu J. J. Am. Chem. Soc. 2009;131:4598. doi: 10.1021/ja900806q.. Han Z-Y, Xiao H, Chen X-H, Gong L-Z. J. Am. Chem. Soc. 2009;131:9182. doi: 10.1021/ja903547q.. Muratore ME, Holloway CA, Pilling AW, Storer RI, Trevitt G, Dixon DJ. J. Am. Chem. Soc. 2009;131:10796. doi: 10.1021/ja9024885.. Liu X-Y, Che C-M. Org. Lett. 2009;11:4204. doi: 10.1021/ol901443b.. Holloway CA, Muratore ME, Storer RI, Dixon DJ. Org. Lett. 2010;12:4720. doi: 10.1021/ol101651t.. He Y, Lin M, Li Z, Liang X, Li G, Antilla JC. Org. Lett. 2011;13:4490. doi: 10.1021/ol2018328.. Dagousset G, Zhu J, Masson G. J. Am. Chem. Soc. 2011;133:14804. doi: 10.1021/ja205891m..
- (13)(a).Lathrop SP, Rovis T. J. Am. Chem. Soc. 2009;131:13628. doi: 10.1021/ja905342e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Filloux CM, Lathrop SP, Rovis T. Proc. Natl. Acad. Sci. USA. 2010;107:20666. doi: 10.1073/pnas.1002830107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ozboya KE, Rovis T. Chem. Sci. 2011;2:1835. doi: 10.1039/C1SC00175B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14)(a).Imbos R, Brilman MHG, Pineschi M, Feringa BL. Org. Lett. 1999;1:623. [Google Scholar]; (b) Imbos R, Minnaard AJ, Feringa BL. J. Am. Chem. Soc. 2002;124:184. doi: 10.1021/ja017200a. [DOI] [PubMed] [Google Scholar]; (c) Hayashi Y, Gotoh H, Tamura T, Yamaguchi H, Masui R, Shoji M. J. Am. Chem. Soc. 2005;127:16028. doi: 10.1021/ja055740s. [DOI] [PubMed] [Google Scholar]; (d) Liu Q, Rovis T. J. Am. Chem. Soc. 2006;128:2552. doi: 10.1021/ja058337u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Vo NT, Pace RDM, O’Hara F, Gaunt MJ. J. Am. Chem. Soc. 2008;130:404. doi: 10.1021/ja077457u. [DOI] [PubMed] [Google Scholar]; (f) Gu Q, Rong Z-Q, You S-L. J. Am. Chem. Soc. 2010;132:4056. doi: 10.1021/ja100207s. [DOI] [PubMed] [Google Scholar]; (g) Leon R, Jawalekar A, Redert T, Gaunt MJ. [Google Scholar]; (h) Gu Q, You S-L. Chem. Sci. 2011;2:1519. [Google Scholar]; (i) Gu Q, You S-L. Org. Lett. 2011;13:5192–5195. doi: 10.1021/ol202073p. [DOI] [PubMed] [Google Scholar]; (j) Tello-Aburto R, Kalstabakken KA, Volp KA, Harned AM. Org. Biomol. Chem. 2011;9:7849. doi: 10.1039/c1ob06125a. [DOI] [PubMed] [Google Scholar]; (k) Jia M-Q, You S-L. Chem. Commun. 2012;48:6363–6365. [Google Scholar]
- (15)(a).Rowland GB, Zhang H, Rowland EB, Chennamadhavuni S, Wang Y, Antilla JC. J. Am. Chem. Soc. 2005;127:15696. doi: 10.1021/ja0533085. [DOI] [PubMed] [Google Scholar]; (b) Liang Y, Rowland EB, Rowland GB, Perman JA, Antilla JC. Chem. Commun. 2007:4477. doi: 10.1039/b709276h. [DOI] [PubMed] [Google Scholar]; (c) Li G-L, Fronczek FR, Antilla JC. J. Am. Chem. Soc. 2008;130:12216. doi: 10.1021/ja8033334. [DOI] [PubMed] [Google Scholar]; (d) Čorić I, Vellalath S, List B. J. Am. Chem. Soc. 2010;132:8536. doi: 10.1021/ja102753d. [DOI] [PubMed] [Google Scholar]; (e) Čorić I, Müller S, List B. J. Am. Chem. Soc. 2010;132:17370. doi: 10.1021/ja108642s. [DOI] [PubMed] [Google Scholar]; (f) Rauniyar V, Lackner AD, Hamilton GL, Toste FD. Science. 2011;334:1681. doi: 10.1126/science.1213918. [DOI] [PubMed] [Google Scholar]; (g) Čorić I, List B. Nature. 2012;483:315. doi: 10.1038/nature10932. [DOI] [PubMed] [Google Scholar]
- (16)(a).Christoffers J, Koripelly G, Rosiak A, Rössle M. Synthesis. 2007:1279. [Google Scholar]; (b) Yang JW, Hoffmann S, List B. Chem. Rev. 2007;107:5471. doi: 10.1021/cr0684016. [DOI] [PubMed] [Google Scholar]; (c) Nising CF, Bräse S. Chem. Soc. Rev. 2008;37:1218. doi: 10.1039/b718357g. [DOI] [PubMed] [Google Scholar]; (d) Tokoroyama T. Eur. J. Org. Chem. 2010:2009. [Google Scholar]; (e) Nising CF, Bräse S. Chem. Soc. Rev. 2012;41:988. doi: 10.1039/c1cs15167c. [DOI] [PubMed] [Google Scholar]
- (17).For recent contributions, see reference 15.
- (18).For the synthesis of the diol (SPINOL), see: Birman VB, Rheingold AL, Lam K-C. Tetrahedron: Asymmetry. 1999;10:125.
-
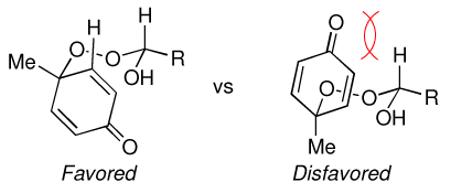
(19).The high diastereoselectivity of the desymmetrizations can be explained by the reduced 1,3-diaxial interactions in the transition state leading to the favored product.
- (20).While yields are universally improved, enantioselectivities are not appreciably impacted by the presence of the thiourea co-catalyst suggesting that it is not involved in the enantioselectivity determining event. Its exact role in this transformation is the subject of further investigations.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.