Abstract
Increased spending and reduced funding for health care is forcing decision makers to prioritize procedures and redistribute funds. Decision making is based on reliable data regarding the costs and benefits of medical and surgical procedures; such a study design is known as an economic evaluation. The onus is on the plastic surgery community to produce high-quality economic evaluations that support the cost effectiveness of the procedures that are performed. The present review focuses on the cost-utility analysis and its role in deciding whether a novel technique/procedure/technology should be accepted over one that is prevalent. Additionally, the five steps in undertaking a cost-utility (effectiveness) analysis are outlined.
Keywords: Cost-effectiveness analysis, Cost-utility analysis, Economic analysis, Economic evaluation, Quality-adjusted life year
Abstract
L’augmentation des dépenses et la réduction du financement dans le milieu de la santé forcent les décideurs à prioriser les interventions et à redistribuer les fonds. La prise de décision se fonde sur des données fiables au sujet des coûts et des avantages des interventions médicales et chirurgicales. Une telle méthodologie d’étude est une évaluation économique. La communauté de la chirurgie plastique a la responsabilité de produire des évaluations économiques de qualité qui appuient le rapport coût-efficacité des interventions exécutées. La présente analyse s’attarde sur l’analyse coût-utilité et son rôle dans la décision d’accepter une nouvelle technique, intervention ou technologie par rapport à une autre déjà prévalente. De plus, les cinq étapes pour entreprendre une analyse coût-utilité (efficacité) sont exposées.
It has been estimated that 25% of health care dollars in Canada are wasted (1). In the United States (US), this is estimated to be closer to 50% (2). On a global basis, this figure is estimated to be somewhere between 20% and 40% (3). The Ministry of Health and Long-Term Care in Ontario, the largest province according to population in Canada, spends $8 billion on physician payments (2009/2010) – equivalent to 20% of total health care costs ($40 billion) (4). In 2009, total health expenditures in Canada were $182.1 billion, with the most spending going toward hospital expenditures (29.1% [$58.4 billion]), drug costs (16.0% [$32.0 billion]) and physician payments (14% [$28.1 billion]) (Figure 1) (5).
Figure 1.
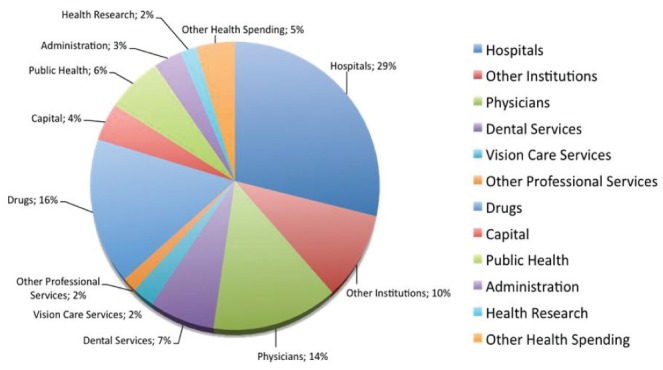
Canadian national health expenditures in 2009. Adapted from reference 5
Health care costs have been escalating in both Canada and the US over the past 30 years. Between 1975 and 2011, the cost of health care in Canada per capita increased from $527.10 to $5,811.20. This represents an increase from $12 billion to $200 billion annually, representing 7.9% of the gross domestic product in 1975 and 11.5% of the gross domestic product in 2011 (5).
This increase is not sustainable. Governments in all jurisdictions are beginning to ask for accountability from their health care providers. We can no longer continue to squander precious health care resources that could be better spent on other sectors of society.
How does waste of 25% to 50% occur? Setting aside bureaucratic inefficiency, in our case as heath care providers, it occurs when we have options to manage a surgical problem but we opt not to use the optimal approach. This happens when we do not apply evidence-based surgery principles to our surgical practice (6–9) and, more importantly, we are not familiar with the levels of evidence (10).
As health care dollars shrink, wait lists for surgery become longer and longer (11). Governments have already started to prioritize certain surgeries. In 2004, the Canadian federal government published the 10-Year Plan to Strengthen Health Care (12) and identified five priority clinical areas in which it was decided wait times must be reduced. The five priority areas included cancer, heart, diagnostic imaging, joint replacement and sight restoration (13). We must wonder, however, why these areas were prioritized over many of the reconstructive procedures that we perform as plastic surgeons. It is intuitive that surgery for cancers, which may save lives, should be prioritized. Why does cataract surgery, however, take precedence over breast reduction surgery? Surely not all cataract surgery patients require immediate surgery; not all of them are close to blindness and in need to ‘jump’ other specialties’ wait lists, such as our breast reduction cases. So what makes governments decide? The answer is simple: the health burden associated with cataracts has been well characterized (14–16), whereas the health burden of breast hypertrophy has only recently been measured (17).
One of the ‘sins’ of academic plastic surgery is that we still rely on before-and-after photographs in our publications and scientific conferences as the outcome of our interventions.
The use of before-and-after photographs and physiological measures, such as range of motion, grip and pinch strength, are proxies of the improvement of the quality of life of patients, which may or may not be true. Our failure to use correct methodology, such as health-related quality of life scales that use the patient’s perspective, leads to uncertainty or erroneous conclusions about the true value of surgical interventions. This, subsequently, may lead us to choose the wrong approach to solving a surgical problem when different choices are available. For example, in breast reconstruction we have several choices to reconstruct a postmastectomy defect: case in point, the need for unilateral breast reconstruction in a 50-year-old woman who has redundant skin and fat in the lower abdomen and is a candidate for autogenous tissue reconstruction. In such a case, we have multiple choices: unipedicled tranverse rectus abdominus myocutaneous (TRAM), free TRAM, muscle-sparing TRAM, deep inferior epigastric perforator (DIEP) flap or superficial inferior epigastric artery flap. If there are no unique circumstances precluding one technique over another (eg, upper abdominal transverse scar that may preclude a unipedicled TRAM flap), all are candidate flaps for reconstruction. Theoretically there is one ‘ideal’ flap for this purpose. Using another option will have an opportunity cost, which is defined as the cost of an alternative that must be forgone to pursue a certain action. Put another way, the benefits you could have received by taking an alternative action.
In general, as surgeons, when we choose between a novel surgical procedure and a prevailing one, we compare them side by side and if the new technique provides better outcomes we adopt it. We need to recognize, however, that both techniques are also associated with different costs. To make our specialty relevant to third-party payers and society in general, this is exactly the issue we must devote attention to.
WHY THE NEED FOR ECONOMIC EVALUATIONS IN PLASTIC SURGERY?
The specialty of plastic surgery is prolific in its introduction of new technologies and new techniques, all claiming to provide results superior to the prevailing strategy. When we compare a novel surgical technique with a prevailing one, there are nine possibilities. Figure 2 illustrates the nine potential scenarios, and shows the incremental cost and effectiveness when we compare a novel with a prevailing technology.
Figure 2.
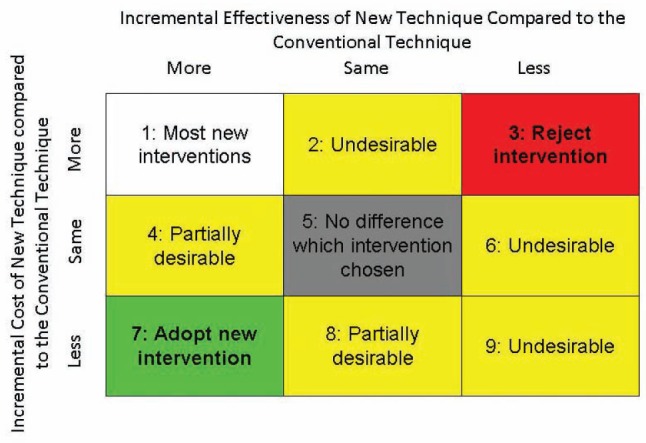
Possible results of a cost-effectiveness analysis
The new technique may be more, equal or less effective than the old one. It can also be more, the same or less costly. While it is clear that less expensive and more effective interventions (cell 7) should supersede a prevailing intervention, and more expensive and less effective interventions (cell 3) should be rejected, the decision-making process to either adopt or reject an intervention that falls into some of the other seven categories is not so easy.
Another way of representing these choices is the cost-effectiveness plane shown in Figure 3. If a new plastic surgery technique falls in the right lower quadrant, we accept it because it is more effective and less costly (point 4: ‘win-win’ scenario). If it falls in the left upper quadrant, we reject it because it less effective and more costly (point 1: ‘lose-lose’ scenario). In general, most novel interventions fall in the right upper quadrant (point 2) or cell 1 in Figure 2, in which the new technology is more effective but also more costly. It is here that we need to perform an economic evaluation.
Figure 3.
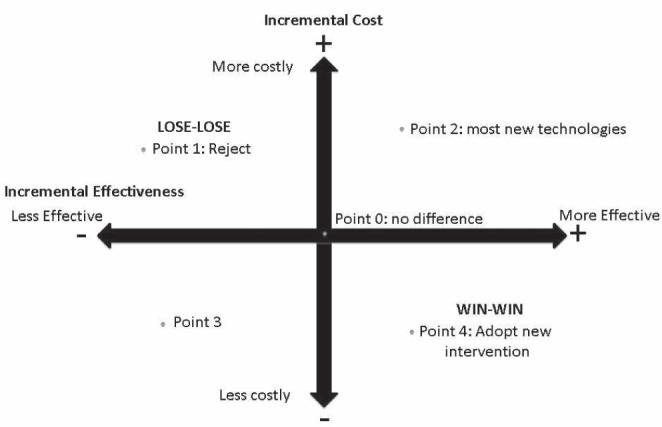
Incremental effectiveness and cost of new versus conventional surgical interventions
TYPES OF ECONOMIC EVALUATIONS
There are four types of economic evaluations that are commonly reported in the literature. They include cost analysis, cost-benefit analysis, cost-effectiveness analysis and cost-utility analysis (18) (Table 1). The cost-utility analysis is the most appropriate type of analysis because it uses quality-adjusted life years (QALYs) as the outcome measure (19). The use of the QALY as a unit of measurement allows for comparisons between disparate interventions/programs. QALYs incorporate both the quality and the quantity of life gained by a particular intervention (20). QALYs will be discussed in greater detail in the ‘Outcomes’ section. By using a common metric, we can compare the cost effectiveness of various procedures in the spectrum of medicine. For example, a third-party payer can compare the incremental benefit of a cataract surgery innovation with a new technique in breast reconstruction, which we cannot perform with a cost-effectiveness analysis. Cost analyses are typically not appropriate because they ignore the differences in effectiveness and evaluate the cost alone. As such, they are considered to be ‘partial economic evaluations’. Cost-benefit analyses, while incorporating both cost and the effectiveness, present a number of problems because the outcomes are converted into a monetary value. Valuing parameters, such as patients’ time and burden of disease, can be problematic, not only because it is difficult to monetize but also because patients have different thresholds for ‘willingness-to-pay’ and value their time and burden of disease differently (18). Because of equity issues, cost-benefit analyses are considered to be discriminatory against the poor and, therefore, are not commonly used in the assessment of health interventions. They are, however, commonly used in other sectors of society such as education and transportation.
TABLE 1.
Types of economic evaluation
| Cost analysis | A straight comparison of the costs of two or more procedures |
| Cost-benefit analysis | A comparison of costs and benefits of two procedures, whereby both the costs and benefits are expressed in dollars |
| Cost-effectiveness analysis | A comparison of the costs and outcome(s) of two or more procedures, providing a value of dollars per natural unit (ie, dollars per successful reconstruction, dollars per life saved, etc) |
| Cost-utility analysis | A comparison of the costs and utility of two or more procedures, providing a value of dollars per quality-adjusted life year gained |
A recent systematic review by Ziolkowski et al (21) identified 92 articles published between 1986 and 2011 that compared two plastic surgery interventions and included an economic evaluation as a component of their publication. The economic evaluations in these 92 articles were comprised of 73 cost analyses, 12 cost-effectiveness analyses, six cost-utility analyses and one cost-benefit analysis. Partial economic evaluations (cost analyses) were found to comprise the majority of analyses (79.3%). This implies that in plastic surgery, we fail to perform (and report) correct economic evaluations in 79% of cases! We should not be surprised, therefore, that our specialty is not regarded as relevant when health resource allocation is decided by third-party payers. This is why we need to devote particular attention to this type of study design.
PERFORMING AN ECONOMIC EVALUATION
Drummond et al (18) define an economic evaluation as the comparative analysis of alternative courses of action in terms of both their costs and consequences. There are five steps to performing an economic evaluation (Table 2). The following section will elaborate on each of the steps.
TABLE 2.
Steps to performing an economic evaluation
|
Identify all relevant treatment options
First, all reasonable treatment options for a particular problem must be identified. This may include an exhaustive literature search in the form of a systematic review and/or assembling a multidisciplinary team to ensure that all treatment options are included. While a surgeon may identify several surgical treatments for a particular problem, another specialist may add some other treatments to this list. It would be inappropriate to perform an economic evaluation between a unipedicled TRAM and free TRAM and ignore the DIEP flap. In general, we perform an economic evaluation between a prevailing (ie, commonly used) technology and a novel one that has entered the main stream of plastic surgery.
Identify a perspective
When planning to conduct an economic evaluation, it is important to consider the purpose of the study and who will benefit from the evaluation. Perhaps you are evaluating two competing techniques for breast reduction, hypothesizing that one technique requires less operating room (OR) time. If the shorter procedure is universally adopted, this would lead to savings from the hospital’s perspective. From the surgeon’s point of view, this may be of benefit because now one may be able to add another case in the OR block. From the patient’s point of view, it may not be of any additional benefit. Perhaps you are evaluating two techniques for surgical management of carpometacarpal arthritis, hypothesizing that patients undergoing one of the techniques requires fewer follow-up appointments. If the technique requiring fewer follow-up visits was universally adopted, this would lead to savings from the third-party payer (ie, Ministry of Health) perspective (as a result of fewer patient visits billed) as well as savings from the patient’s perspective (as a result of savings in fuel, parking and, potentially, time off work). Some perspectives are listed in Table 3. Alternatively, an economic evaluation could be performed from a societal perspective, incorporating all costs from the perspective of the hospital, third-party payer, patient, etc. Choosing a perspective is important because the benefits of a particular procedure are often not the same across all beneficiaries. Consider a patient with a stiff digit following replantation. From the surgeon’s perspective, the procedure was a success because the digit is viable. From the patient’s perspective, however, the procedure is a failure because their digit is stiff and painful, and renders the patient unable to work. From a societal perspective, the procedure is a failure because the patient will be living on social assistance. By delineating the benefits and costs of a procedure for each party, a clearer understanding of how the procedure affects each party involved can be obtained. The Panel on Cost-Effectiveness in Health and Medicine convened by the US Public Health Service in 1993 (19) recommended that we strive to use the societal perspective because this will ultimately assist in the prudent allocation of scarce health care resources.
TABLE 3.
Perspectives of an economic evaluation
| Hospital, clinic, practice |
| Third-party payer (Ministry of Health, Workers’ Compensation Board, Medicare, Health Maintenance Organization or National Health Service) |
| Patient |
| Society (incorporates costs and outcomes of all above perspectives) |
Measure the costs and outcomes of the relevant techniques
Costs:
Costs can typically be divided into direct costs and indirect (productivity) costs. Direct costs are the resources required for the procedure including the cost of running the surgical facility, cost of diagnostic and support services, professional fees (surgeons, anesthetists, nursing staff, physiotherapy), cost of supplies, cost of medication, etc. Indirect (ie, productivity) costs are typically those associated with lost time (either from work or from child care). Indirect costs can include lost wages due to time off work (from the surgery, recovery, follow-up appointments, etc), cost of medication, transportation of patient to and from appointments, etc. Indirect costs are typically borne by the patient and, as a result, are under-reported in the literature (18). How the costs are collected will depend largely on how precise you would like the values to be. For example, the cost to run a surgical operating room could be measured using an average per diem cost (in which the total yearly costs to run the OR are divided by the number of days the OR is in use). A more precise way of calculating this is to provide an average disease-specific per diem (ie, average cost of running the OR for a breast reduction surgery). The most precise way to measure this cost would be to microcost for all potential treatment options, ie, calculate exact OR time, an itemized list of medical supplies used, etc. The choice of how precisely the costs should be collected will depend on the perspective chosen, the research question you are posing and, as always, what information is available. If your hypothesis lies around differential costs to patients undergoing two similar procedures, microcosting the OR costs may not be necessary if there is no reason to believe there will any differences observed.
Outcomes:
As mentioned before, the purpose of the study will help direct the choice of outcome(s). For example, a study examining different options for breast reconstruction may be interested in the cost of the procedure per unit of OR time (ie, the cost per each successful reconstruction). Typically, these types of analyses are conducted when the research question is based on a specific hypothesis centred on one or two aspects of a procedure.
A broader measure of the benefits of two or more interventions/procedures is the health utility. The health utility is simply a value that describes a patient’s current health state on a scale from 0 to 1, whereby 0 indicates the worst possible health state (death) and 1 represents the best possible health state (perfect health). There are several ways to collect health utilities, including rating scales, time trade-off, standard gamble and quality of life questionnaires (18,20).
A rating scale, such as a visual analogue scale or ‘feeling thermometer’, is the simplest (Figure 4). To ascertain an individual’s health state, one would ask the patient, “Based on how you are feeling today, please indicate on this scale how good or bad your own health is today”.
Figure 4.
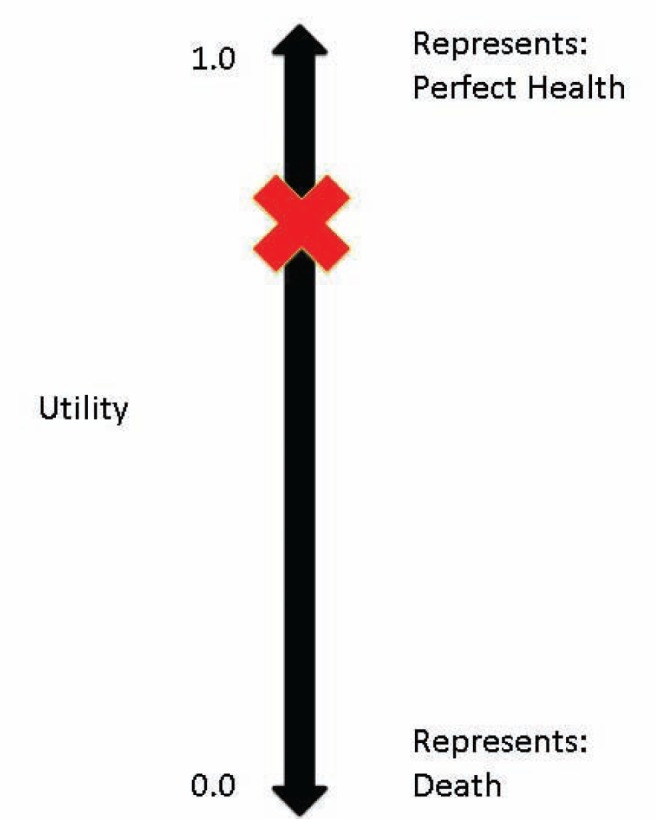
Health utility determined using a ‘feeling thermometer’
The time trade-off method is used to determine quality of life by asking the individual to consider hypothetical scenarios in which their life is shorter, but healthier. The individual’s long life in a poor health state is compared with shorter and shorter life spans in a better health state until the individual becomes indifferent between the two choices.
In a standard gamble, the individual considers hypothetical scenarios in which they can undergo a medical intervention that will either restore them to perfect health or result in their death. The probabilities of the intervention restoring the individual to perfect health or resulting in their death are methodically adjusted until the individual is indifferent between continuing their life in their current health state, and choosing to gamble on receiving the medical intervention.
Another commonly used method to obtain utilities is validated health utility questionnaires, such as the EuroQol 5D (EQ-5D) (22), the Health Utilities Index (23), and the Quality of Well Being Index (24). These questionnaires ask the patient specific questions about key components of quality of life including mobility, self-care, pain, emotion, etc. The result is a health utility ranging from 0 to 1. Health utility refers to the general preference of individuals for a particular health state. There may be variation in preferences among the population as we see frequently in our emergency rooms. A young female patient may plead with us to reattach her amputated finger tip knowing that she will require hospitalization, whereas a 70-year-old man may simply be happy to undergo a revision amputation and be discharged home from the emergency department. In this example, the female patient would attach a low utility to finger stump amputation (eg, 0.7) whereas the older male a higher one (eg, 0.92).
Health utilities are useful because they capture all of the advantages and disadvantages of a procedure. Therefore, the benefits of a successful procedure, such as fast recovery and little time away from work, will be reflected in the health utility as well as the burden of any adverse events such as extended hospital stays and frequent follow-up appointments. Utilities are also valuable because they can be converted into QALYs. The equation for calculating QALYs can be found in Figure 5. QALYs are interpreted as the number of years a given patient will gain in perfect health. This wording can be tricky, because patients with a net gain of QALYs may not actually gain years of life. Consider a woman who undergoes reduction mammoplasty. In 2007, Thoma et al (17) published a series of 50 patients who underwent the inferior pedicle technique. Patients’ mean health utility increased from 0.74 before surgery to 0.89 after surgery (a difference of 0.13). Considering the average age of study patients was 38 years and this demographic is projected to live to an average of 79 years, an average reduction mammoplasty patient will live 41 years with an additional utility of 0.13 for each year of life following the procedure. The multiplication of these two values amounts to gaining 5.32 years of life in perfect health.
Figure 5.
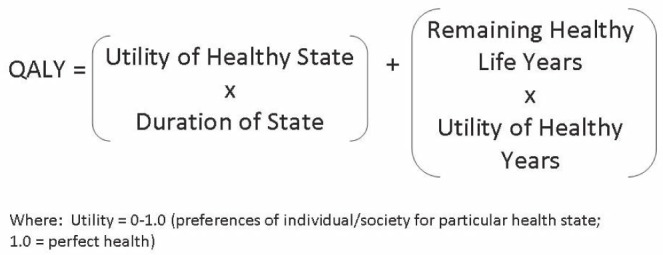
Equation for quality-adjusted life years (QALYs). Adapted from reference 18
Calculate the incremental cost-effectiveness/utility ratio:
Once costs and outcomes have been collected, each of the relevant techniques can be described as costing ‘x’ dollars for each chosen unit of benefit. In the most commonly encountered scenario, in which a novel technique is more effective and more costly than the prevailing technique, an incremental cost-effectiveness ratio (ICER) can be calculated. The formula for the incremental cost-utility ratio (ICUR) can be found in Figure 6. The ICER provides us with an idea of how much more this program will cost for each unit of benefit by adopting the new technology.
Figure 6.
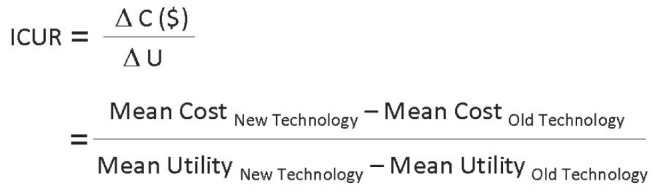
Incremental cost-utility ratio (ICUR). Adapted from reference 18
Decide whether the ICER/ICUR favours adoption:
When considering whether a new intervention should be adopted, we are basically asking how much more money are we willing to spend to achieve better outcomes? The answer depends entirely on the circumstances. When working with a budget in a hospital setting, the constraints of the budget will dictate the upper limit of what can be spent. When evaluating the use of programs on a larger scale, such as an outpatient facility for burn care proposed to the provincial Ministry of Health, it is difficult to determine an absolute threshold.
In 1992, Laupacis et al (25) proposed a threshold of $20,000/QALY based on previous societies’ decisions regarding programs that were approved and denied. Since then, higher thresholds have been proposed, including $40,000 to $100,000/QALY, meaning that anything below this value is considered to be good value for money, while anything that exceeds this threshold is not. Brauer et al (26) stated that interventions within $20,000 to $100,000/QALY gained are considered to be good value for money, while interventions >$100,000/QALY gained are not the best value, although many interventions that fall within this range are still funded. The National Institute for Health and Clinical Excellence (27) economic advisers state that a technology with an ICUR >£30,000/QALY (approximately $50,000/QALY) needs increasingly strong reasons for considering the intervention to be cost-effective.
REPORTING COSTS AND OUTCOMES
When either appraising or undertaking an economic evaluation, it is important to consider the issue of costs versus charges. The ‘true cost’ of services, equipment and supplies will vary widely among hospitals, regions, provinces and countries for a number of reasons. For example, several studies have shown that in the American health care system, the charge to the patient for a particular procedure far exceeds the cost to the hospital to perform that procedure (28,29). There is considerable debate about how these data should be expressed. Regardless of the chosen method, the details regarding analysis should be reported in detail for comparison among sites. By the same token, all components of a procedure should be reported. The performance of similar procedures may vary widely among sites; for example, carpal tunnel release could be performed in a minor procedure room or main operating suite. These details should be outlined because costs and natural units could vary among sites. For example, if we compare two techniques of breast reconstruction, we should report the OR time per procedure and the time in hospital consumed by the comparative techniques in tabular form.
From such tabulations, investigators in other countries can insert the $ cost/OR time unit and $ cost/hospital stay and calculate the costs in their setting. From these data, readers can calculate the ICUR in their health care system and decide on the acceptability of the novel plastic surgery procedure.
Furthermore, the costs and benefits of a given intervention studied may not occur in the same calendar year. For example, complications needing additional health resources may occur in a different calendar year than the original operation or the research project may take place over a number of years. For costs and benefits to be uniform across a number of years, discounting – the process of determining the present value of costs and benefits in the future – is used. Discounting is particularly important when measuring long-term outcomes. Furthermore, this reflects society’s preference for valuing present/near future health outcomes over those that are distant. The amount of 3% or 5% is commonly used (18).
EXAMPLES OF ECONOMIC EVALUATIONS IN PLASTIC SURGERY
Some recent economic evaluations in the field of plastic surgery can be found in Table 4. Chung et al (30) compared the costs and health utilities of unilateral hand transplantation, unilateral prosthesis, bilateral hand transplantation and bilateral prostheses. They found that unilateral prosthesis was favoured over hand transplantation (in terms of cost and quality of life), while bilateral hand transplantation was favoured over prostheses in terms of quality of life; however, the costs far exceed any accepted threshold at $381,961/QALY. Another economic analysis by Thoma et al (31) compared the use of open carpal tunnel release versus endoscopic carpal tunnel release (31). The authors found that endoscopic carpal tunnel release costs an additional $124,311/QALY over open carpal tunnel release. This value exceeds the acceptable threshold; therefore, it is not recommended that this technique be adopted (31). In 2004, an analysis by Rockwell and Thoma (32) examined prophylactic plating of a donor radius following the harvest of a radial osteocutaneous flap. Prophylactic plating was found to be less effective and more costly than nonplating and, therefore, should not be adopted.
TABLE 4.
Examples of economic evaluations in plastic surgery
| Author (reference) | Comparative procedures | Incremental cost-utility ratio | Interpretation |
|---|---|---|---|
| Chung et al (30) | Unilateral hand transplantation versus unilateral prosthesis | N/A | Unilateral prosthesis is more effective and less costly than unilateral hand transplant (‘win-win’) |
| Chung et al (30) | Bilateral hand transplantation versus bilateral prosthesis | $381,961/QALY | Bilateral hand transplantation is more effective but more costly than bilateral prostheses. The ICUR exceeds the threshold of $100,000/QALY; therefore, bilateral hand transplantation should not be adopted |
| Thoma et al (31) | ECTR versus OCTR | $124,311/QALY | ECTR is more effective but more costly than OCTR. The ICUR exceeds the threshold of $100,000/QALY; therefore, the ECTR should not be adopted |
| Rockwell and Thoma (32) | Prophylactic plating versus no prophylactic plating of donor radius following harvest of osteocutaneous flap | N/A | Not plating the donor radius was found to be more effective and less costly than when the donor radius is plated (‘win-win’) |
| Thoma et al (33) | Free TRAM flap versus unipedicled TRAM flap | $5,114/QALY | The free TRAM flap is more effective but more costly than the unipedicled TRAM flap. The ICUR is <$20,000/QALY and, thus, the free TRAM flap should be adopted |
| Thoma et al (34) | Free TRAM flap versus DIEP flap | $1,464/QALY | The DIEP flap is more effective but more costly than the free TRAM flap. The ICUR is <$20,000/QALY and, thus, the DIEP flap should be adopted |
DIEP Deep inferior epigastric perforator; ECTR Endoscopic carpal tunnel release; ICUR Incremental cost-utility ratio; N/A Not applicable; OCTR Open carpal tunnel release; QALY Quality-adjusted life year; TRAM Tranverse rectus abdominus myocutaneous
In the domain of breast reconstruction, such economic evaluations were performed to assess the novel techniques that led to less disruption of the rectus abdominis and the overlying fascia. The free TRAM technique was shown to be more cost effective than the unipedicled TRAM (33). In sequence, the DIEP flap was found to be more cost effective than the free TRAM flap (34).
PRECISION OF COST-UTILITY ANALYSES
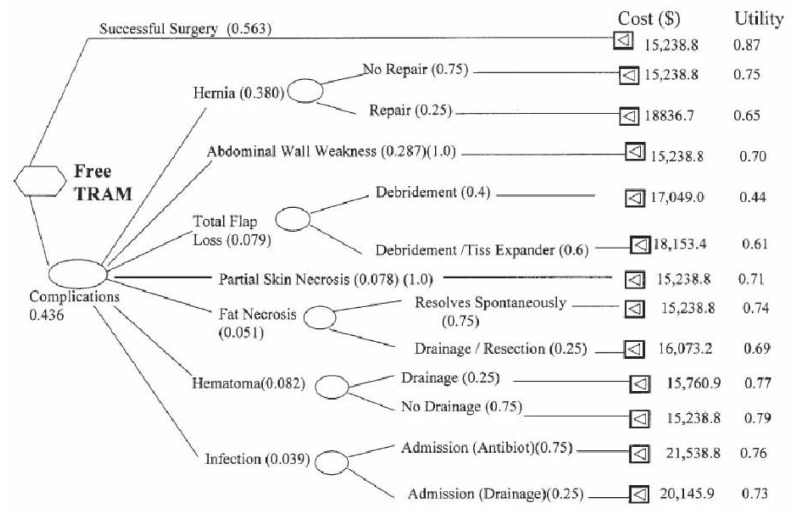
Economic evaluations can be performed as a deterministic analysis, in which primary data are lacking, or through stochastic analysis, which uses primary outcome and cost data directly from the patients studied. The stochastic method is the ideal form of analysis because it uses patient-derived data. Deterministic analysis calculates expected costs (for example, from the Canadian health care system) and components of QALY (duration and utilities of health states; future remaining life expectancy – duration of health state and utility of successful intervention) through a review of the plastic surgery literature (9). To illustrate deterministic analysis, a free versus unipedicled TRAM flap (for postmastectomy breast reconstruction) example will be used (33). In this analysis, the perspective was the Ministry of Health (Ontario), utilities were obtained from plastic surgeon ‘experts’ across Canada via a questionnaire that included various health states (complications) retrieved from published literature. Each scenario (health state) was rated on a vertical ‘feeling thermometer’ with a preference from a scale of 0 to 10 (with 10 representing perfect health). Costs were obtained from the Ontario Ministry of Health Schedule benefits and a systematic review was conducted to obtain the probabilities of various health states associated with the unipedicled and free TRAM flaps. From the information presented in a decision-analytic tree (Figure 7), QALYs were calculated for each health state and the ICUR was determined for both interventions. This method of analysis contains a wide range of assumptions (health states are determined by the quality of literature presently available), making it less ideal than directly analyzing primary outcome and cost data from the pool of patients, as is found with the stochastic method. This method was used in another published cost-utility analysis (35) comparing two techniques of carpal tunnel release: open release without and with ligament reconstruction from a societal perspective. Patients were randomized and completed the Health Utilities Index Mark 2/3 questionnaire (for utility calculation) and a case report form (for cost determination). These data were then directly converted into quality-adjusted life weeks (‘weeks’ rather than years because the follow-up was six weeks in duration). The stochastic method is favoured because it is more direct, with fewer assumptions regarding complications made and is not dependent on the quality of the literature.
Figure 7.
Decision-analytic tree illustrating probabilities, costs and utilities for each health state/pathway for the free tranverse rectus abdominus myocutaneous (TRAM) flap. A similar decision-analytic tree was also developed for outcomes of the deep inferior epigastric perforator flap. Tiss Tissue. Adapted from reference 33
CONCLUSION
In plastic surgery training, we have been taught to use physiological measures as a determinant of accepting or rejecting new technologies. The outcomes research movement and the introduction of evidence-based clinical practice philosophy in the past three decades commands, however that we also consider the cost of what we do. Level I evidence would be considered one in which two competing plastic surgery interventions are compared side by side in a randomized controlled trial and in which an economic evaluation was ‘piggybacked’ to the randomized controlled trial (35).
Because most plastic surgeons have not been trained in health research methodology, it is recommended that a health economist be involved at the inception of a cost-utility analysis to ensure that is performed correctly.
Guides also exist on how to appraise the validity of published economic evaluations before adopting their conclusions such as the Quality of Health Economic Studies instrument (36) and the Users’ Guide for Economic Analysis in Surgical Practice (8). It is only when we perform such studies and we show that as a specialty we are concerned with the prudent allocation of scarce health care resources that our specialty will be considered seriously by third-party payers, professional organizations and government.
REFERENCES
- 1.Organisation for Economic Cooperation and Development (OECD) Health Care System: Efficiency and Policy Settings. Paris: OECD Publishing; 2010. [Google Scholar]
- 2.The Price of Excess: Identifying Waste in Healthcare Spending. Price Waterhourse Coopers’ Health Research Institute. 2009. < www.pwc.com/us/en/healthcare/publications/the-price-of-excess.jhtml> (Accessed June 13, 2012).
- 3.World Health Organization . The World Health Report – Health Systems Financing: The Path to Universal Coverage. Geneva: World Health Organization; 2010. < www.who/int/int/whr/2010/en/index.html> (Accessed June 13, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute for Clinical Evaluative Sciences . Payments to Ontario Physicians from Ministry of Health and Long-Term Care Sources 1992/93 to 2009/10 ICES Investigative Report. Toronto: Institute for Clinical Evaluative Sciences; 2012. < www.ices.on.ca/file/ICES_PhysiciansReport_2012.pdf> (Accessed June 30, 2012). [Google Scholar]
- 5.Canadian Institute for Health Information National Health Expenditure Trends 1975–2011. < https://secure.cihi.ca/free_products/nhex_trends_report_2011_en.pdf> (Accessed June 13, 2012).
- 6.Thoma A, Farrokhyar F, Bhandari M, Tandan V. Users’ guide to the surgical literature: How to assess a randomized controlled trial in surgery. Can J Surg. 2004;47:200–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Thoma A, Cornacchi SD, Lovrics PJ, Goldsmith CH. Users’ guide to the surgical literature: How to assess an article on health-related quality of life. Can J Surg. 2008;51:215–24. [PMC free article] [PubMed] [Google Scholar]
- 8.Thoma A, Sprague S, Tandan V. Users’ guide to the surgical literature: How to use an article on economic analysis. Can J Surg. 2001;44:347–54. [PMC free article] [PubMed] [Google Scholar]
- 9.Thoma A, Strumas N, Rockwell G, McKnight L. The use of cost-effectiveness analysis in plastic surgery clinical research. Clin Plastic Surg. 2008;35:285–96. doi: 10.1016/j.cps.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Thoma A, Ignacy TA, Li YK, Coroneos CJ. Reporting the level of evidence in the Canadian Journal of Plastic Surgery: Why is it important? Can J Plast Surg. 2012;20:12–6. doi: 10.1177/229255031202000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barua B, Rovere M, Skinner BJ. Waiting Your Turn: Wait Times for Health Care in Canada 2011 Report. < www.fraserinstitute.org/uploadedFiles/fraser-ca/Content/research-news/research/publications/waiting-your-turn-2011.pdf> (Accessed June 13, 2012).
- 12.Health Canada. First Minister’s Meeting on the Future of Healthcare 2004: A 10-Year Plan to Strengthen Health Care <www.hc-sc.gc.ca/hcs-sss/delivery-prestation/fptcollab/2004-fmm-rpm/index-eng.php> (Accessed June 16, 2012).
- 13.Canadian Institute for Health Information Wait Times in Canada – A summary 2012. < https://secure.cihi.ca/free_products/WaitTimesSummary2012_EN.pdf > (Accessed June 13, 2012).
- 14.Lee JE, Fos PJ, Zuniga MA, Kastl PR, Sung JH. Assessing health-related quality of life in cataract patients: The relationship between utility and health-related quality of life. Qual Life Res. 2000;9:1127–35. doi: 10.1023/a:1016645523769. [DOI] [PubMed] [Google Scholar]
- 15.Busbee BG, Brown MM, Brown GC, Sharma S. Incremental cost-effectiveness of initial cataract surgery. Ophthalmology. 2002;109:606–12. doi: 10.1016/s0161-6420(01)00971-x. [DOI] [PubMed] [Google Scholar]
- 16.Lee JE, Fos PJ, Zuniga MA, Kastl PR, Sung JH. Health-related quality of life of cataract patients: Cross-cultural comparisons of utility and psychometric measures. Ophthalmic Epidemiol. 2003;10:177–91. doi: 10.1076/opep.10.3.177.15084. [DOI] [PubMed] [Google Scholar]
- 17.Thoma A, Sprague S, Veltri K, Duku E, Furlong W. A prospective study of patients undergoing breast reduction surgery: Health-related quality of life and clinical outcomes. Plast Reconstr Surg. 2007;120:13–26. doi: 10.1097/01.prs.0000263370.94191.90. [DOI] [PubMed] [Google Scholar]
- 18.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd edn. New York: Oxford University Press; 2005. [Google Scholar]
- 19.Gold MR, Seigel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 20.Thoma A, McKnight LL. Quality-adjusted life year as a surgical outcome: A primer for plastic surgeons. Plast Reconstr Surg. 2010;125:1279–87. doi: 10.1097/PRS.0b013e3181d0ae58. [DOI] [PubMed] [Google Scholar]
- 21.Ziolkowski N, Voineskos S, Ignacy T, Thoma A. Systematic Review of Economic Evaluations in Plastic Surgery. Presented at the 66th Annual Meeting of the Canadian Society of Plastic Surgeons; Toronto. June 5 to 9, 2012. [Google Scholar]
- 22.Brooks R. EuroQol: The current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 23.Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (HUI): Concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. < www.hqlo.com/content/1/1/54> (Accessed June 15, 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan RM, Anderson JP. A general health policy model: Update and applications. Health Serv Res. 1988;23:203–35. [PMC free article] [PubMed] [Google Scholar]
- 25.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473–81. [PMC free article] [PubMed] [Google Scholar]
- 26.Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analysis in orthopaedic surgery. J Bone Joint Surg (Am) 2005;87:1253–9. doi: 10.2106/JBJS.D.02152. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Health and Clinical Excellence Incorporating health economics in guidelines and assessing resource impact. National Institute for Health and Clinical Excellence. 2007. < www.nice.org.uk/niceMedia/pdf/GuidelinesManualChapter8.pdf> (Accessed July 3, 2012).
- 28.Cohen DJ, Breall JA, Ho KK, et al. Economics of elective coronary revascularization: Comparison of the costs and charges for conventional angioplasty, directional atherectomy, stenting and bypass surgery. J Am Coll Cardiol. 1993;22:1052–9. doi: 10.1016/0735-1097(93)90415-w. [DOI] [PubMed] [Google Scholar]
- 29.Taira DA, Seto TB, Siegrist R, Cosprove R, Berezin R, Cohen DJ. Comparison of analytic approaches for economic evaluation of new technologies alongside multicenter clinical trials. Am Heart J. 2003;145:452–8. doi: 10.1067/mhj.2003.3. [DOI] [PubMed] [Google Scholar]
- 30.Chung KC, Oda T, Saddawi-Konefka D, Shauver MJ. An economic analysis of hand transplantation in the United States. Plast Reconstr Surg. 2010;125:589–98. doi: 10.1097/PRS.0b013e3181c82eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thoma A, Wong VH, Sprague S, Duku E. A cost-utility analysis of open and endoscopic carpal tunnel release. Can J Plast Surg. 2006;14:15–20. doi: 10.1177/229255030601400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockwell GM, Thoma A. Should the donor radius be plated prophylactically after harvest of a radial osteocutaneous flap? A cost-effectiveness analysis. J Reconstr Microsurg. 2004;20:297–306. doi: 10.1055/s-2004-824887. [DOI] [PubMed] [Google Scholar]
- 33.Thoma A, Khuthaila D, Rockwell G, Veltri K. Cost-utility analysis comparing free and pedicled TRAM flap for breast reconstruction. Microsurgery. 2003;23:287–95. doi: 10.1002/micr.10138. [DOI] [PubMed] [Google Scholar]
- 34.Thoma A, Veltri K, Khuthaila D, Rockwell G, Duku E. Comparison of the deep inferior epigastric perforator (DIEP) and free transverse rectus abdominis myocutaneous (TRAM) flap in post-mastectomy reconstruction: A cost-effectiveness analysis. Plast Reconstr Surg. 2004;113:1650–61. doi: 10.1097/01.prs.0000117196.61020.fd. [DOI] [PubMed] [Google Scholar]
- 35.Thoma A, Haines T, Veltri K, Goldsmith CH, O’Brien BJ, Quartly C. A methodological guide to performing a cost-utility comparing surgical techniques. Can J Plast Surg. 2004;12:179–187. doi: 10.1177/229255030401200404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ofman JJ, Sullivan SD, Neumann PJ, et al. Examining the value and quality of health economic analyses: Implications of utilizing the QHES. J Managed Care Pharm. 2003;9:53–61. doi: 10.18553/jmcp.2003.9.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]