Abstract
S-adenosyl-L-methionine (SAM) activated the virus-associated RNA polymerase of cytoplasmic polyhedrosis virus in vitro. Synthesis of single-stranded viral RNA (mRNA) proceeded depending on the presence of SAM.
A methyl residue of SAM was incorporated into an RNA molecule. A ribose moiety of adenylic acid in the 5′-terminal region of the nascent RNA was methylated in the very early stage of the transcription. The dependence of the viral transcription on the presence of SAM and the methylation of terminal nucleotide suggests that the transcription of CPV is a “methylation-coupled” reaction.
Full text
PDF





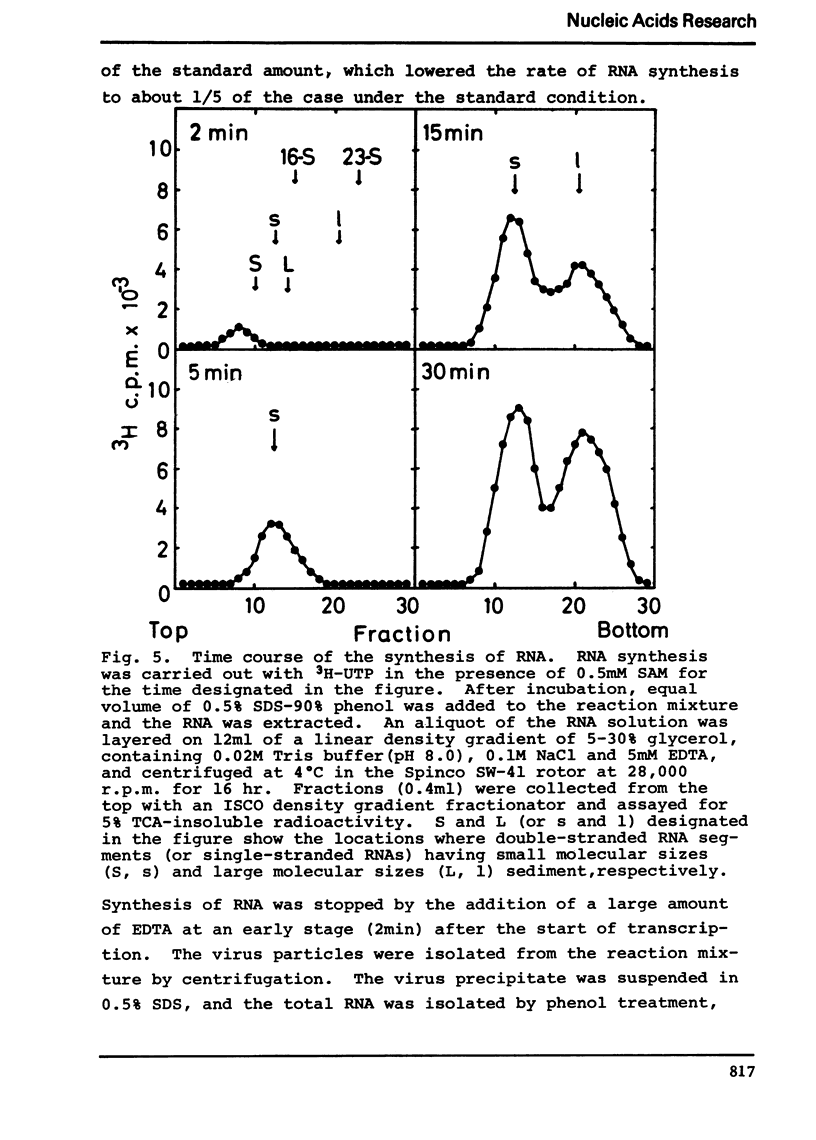




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOM A. D., ROBINS R. K. THE DIRECT PREPARATION OF 2'-O-METHYLADENOSINE FROM ADENOSINE. J Am Chem Soc. 1965 Mar 5;87:1145–1146. doi: 10.1021/ja01083a045. [DOI] [PubMed] [Google Scholar]
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Barchas J. Inhibition of transmethylations of biogenic amines by S-adenosylhomocysteine. Enhancement of transmethylation by adenosylhomocysteinase. J Biol Chem. 1971 May 25;246(10):3175–3181. [PubMed] [Google Scholar]
- Furuichi Y., Miura K. I. Identity of the 3'-terminal sequences in ten genome segments of silkworm cytoplasmic polyhedrosis virus. Virology. 1973 Oct;55(2):418–425. doi: 10.1016/0042-6822(73)90183-9. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Miura K. The 3'-termini of the genome RNA segments of silkworm cytoplasmic polyhedrosis virus. J Mol Biol. 1972 Mar 14;64(3):619–632. doi: 10.1016/0022-2836(72)90087-3. [DOI] [PubMed] [Google Scholar]
- Gantt R. R., Stromberg K. J., Montes de Oca F. Specific RNA methylase associated with avian myeloblastosis virus. Nature. 1971 Nov 5;234(5323):35–37. doi: 10.1038/234035a0. [DOI] [PubMed] [Google Scholar]
- HALL R. H. ON THE 2'-O-METHYLRIBONUCLEOSIDE CONTENT OF RIBONUCLEIC ACIDS. Biochemistry. 1964 Jul;3:876–880. doi: 10.1021/bi00895a001. [DOI] [PubMed] [Google Scholar]
- Huismans H., Verwoerd D. W. Control of transcription during the expression of the bluetongue virus genome. Virology. 1973 Mar;52(1):81–88. doi: 10.1016/0042-6822(73)90400-5. [DOI] [PubMed] [Google Scholar]
- Lewandowski L. J., Kalmakoff J., Tanada Y. Characterization of a Ribonucleic Acid Polymerase Activity Associated with Purified Cytoplasmic Polyhedrosis Virus of the Silkworm Bombyx mori. J Virol. 1969 Dec;4(6):857–865. doi: 10.1128/jvi.4.6.857-865.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski L. J., Traynor B. L. Comparison of the structure and polypeptide composition of three double-stranded ribonucleic acid-containing viruses (diplornaviruses): cytoplasmic polyhedrosis virus, wound tumor virus, and reovirus. J Virol. 1972 Nov;10(5):1053–1070. doi: 10.1128/jvi.10.5.1053-1070.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- Loening U. E. Molecular weights of ribosomal RNA in relation to evolution. J Mol Biol. 1968 Dec;38(3):355–365. doi: 10.1016/0022-2836(68)90391-4. [DOI] [PubMed] [Google Scholar]
- Manor H., Goodman D., Stent G. S. RNA chain growth rates in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):1–29. doi: 10.1016/0022-2836(69)90329-5. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Miura K., Fujii I., Sakaki T., Fuke M., Kawase S. Double-stranded Ribonucleic Acid from Cytoplasmic Polyhedrosis Virus of the Silkworm. J Virol. 1968 Oct;2(10):1211–1222. doi: 10.1128/jvi.2.10.1211-1222.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. L., Hay A. J., Joklik W. K. 5'-terminal nucleotide sequence of reovirus mRNA synthesized in vitro. Nat New Biol. 1972 Jan 26;235(56):105–107. doi: 10.1038/newbio235105a0. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Shatkin A. J. Replication of reovirus. Adv Virus Res. 1969;14:63–87. doi: 10.1016/s0065-3527(08)60557-6. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotono K., Miura K. Single-stranded RNA synthesis in vitro by the RNA polymerase associated with cytoplasmic polyhedrosis virus containing double-stranded RNA. J Biochem. 1973 Jul;74(1):117–125. doi: 10.1093/oxfordjournals.jbchem.a130214. [DOI] [PubMed] [Google Scholar]
- Shimotono K., Miura K. Transcription of double-stranded RNA in cytoplasmic polyhedrosis virus in vtro. Virology. 1973 May;53(1):283–286. [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Prevec L., Graham A. F. Specificity in transcription of the reovirus genome. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1040–1046. doi: 10.1073/pnas.58.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A. Nuclear RNA metabolism. Annu Rev Biochem. 1973;42:329–354. doi: 10.1146/annurev.bi.42.070173.001553. [DOI] [PubMed] [Google Scholar]



