Abstract
New ideas about the relative importance of the autonomic nervous system (and especially its sympathetic arm) in long-term blood pressure regulation are emerging. It is well known that mean arterial blood pressure is normally regulated in a fairly narrow range at rest and that blood pressure is also able to rise and fall ‘appropriately’ to meet the demands of various forms of mental, emotional and physical stress. By contrast, blood pressure varies widely when the autonomic nervous system is absent or when key mechanisms that govern it are destroyed. However, 24 h mean arterial pressure is still surprisingly normal under these conditions. Thus, the dominant idea has been that the kidney is the main long-term regulator of blood pressure and the autonomic nervous system is important in short-term regulation. However, this ‘renocentric’ scheme can be challenged by observations in humans showing that there is a high degree of individual variability in elements of the autonomic nervous system. Along these lines, the level of sympathetic outflow, the adrenergic responsiveness of blood vessels and individual haemodynamic patterns appear to exist in a complex, but appropriate, balance in normotension. Furthermore, evidence from animals and humans has now clearly shown that the sympathetic nervous system can play an important role in longer term blood pressure regulation in both normotension and hypertension. Finally, humans with high baseline sympathetic traffic might be at increased risk for hypertension if the ‘balance’ among factors deteriorates or is lost. In this context, the goal of this review is to encourage a comprehensive rethinking of the complexities related to long-term blood pressure regulation in humans and promote finer appreciation of physiological relationships among the autonomic nervous system, vascular function, ageing, metabolism and blood pressure.
The basis of this review article is the 2007 Michael de Burgh Daly Memorial Lecture given at the Life Sciences Meeting which was held in Glasgow, UK. As the title implies, the focus of the lecture was on the underappreciated role of the sympathetic nervous system in human blood pressure regulation. A main goal was to compare and contrast the role of the sympathetic nervous system with the currently dominant ‘renocentric’ view of blood pressure regulation. The aim of the presentation was to point out some of the limitations of the renocentric view of blood pressure regulation, and give some physiological examples of how the sympathetic nervous system might play a more prominent role in blood pressure regulation than is generally thought. Finally, ideas were explored about how the sympathetic nervous system, ageing and metabolism might operate in a synergistic negative way to promote weight gain and vasoconstriction with the resulting emergence of the hypertensive phenotype. These collective ideas shape the arguments that may stimulate a re-evaluation of the renocentric view of blood pressure regulation in humans and pose important questions about the role that increased baseline sympathetic activity might have in several linked pathophysiological conditions.
Who was Michael de Burgh Daly?
Professor de Burgh Daly (1922–2002) was a physiologist interested in how chemoreceptor and baroreceptor stimulation altered sympathetic outflow and governed the cardiovascular responses to a variety of stimuli, especially hypoxia and apnoea. His father (Ivan de Burgh Daly) was also a noted physiologist, and the two had the pleasure of collaborating on several projects (Daly & Daly, 1959a,b). Details of Professor de Burgh Daly’s life and an extensive obituary written by his collaborator Jennifer Angel James were published in Physiology News in 2003. This obituary provides an outstanding synopsis of Professor de Burgh Daly’s life, training and the history of his academic career. While there are many elements of Michael de Burgh Daly’s career and contributions that could be highlighted, of absolute fundamental importance was his work on chemoreflexes and sympathetic control during apnoea in diving animals. The monograph of the Physiological Society 46, Peripheral Arterial Chemoreceptors and Respiratory-Cardiovascular Integration, published in 1997, summarizes the main ideas of his work. Importantly, these ideas have continued translational utility related to medical conditions such as sleep apnoea, which is marked by chemoreceptor-mediated increases in sympathetic outflow and has emerged as a major risk factor for the development of hypertension in humans. Since sleep apnoea is a vast and still underappreciated medical condition with potentially devastating pathophysiological complications, including hypertension, it is likely that Professor de Burgh Daly’s ideas will be highly influential well into the future.
In this context, it should be noted that hypertension, a condition that can be diagnosed with a simple blood pressure cuff, and is a common disease with a long asymptomatic period. Even mild elevations in blood pressure are associated with an increased risk of cardiovascular disease, stroke and other significant medical problems (Kannel, 2000). With these thoughts as background, we will first summarize the evidence for the renocentric view of blood pressure regulation and discuss where these ideas came from.
The renocentric view of blood pressure regulation
There are multiple lines of evidence to suggest that the kidneys play the major role in blood pressure regulation in both humans and animals. From our perspective, several observations stand out in support of the renocentric view of blood pressure regulation.
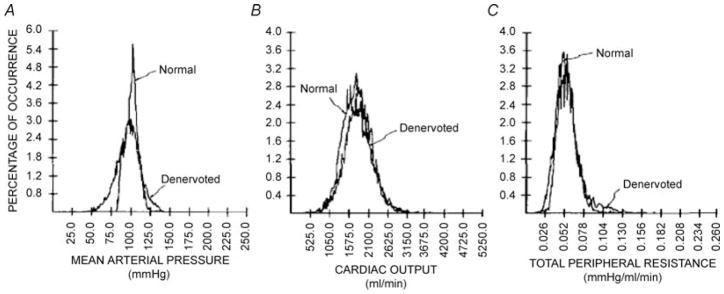
First, when animals are baro-denervated there is no change in mean arterial pressure over 24 h (Fig. 1; Cowley et al. 1973). However, blood pressure does become more variable. Additionally, the mean values and distributions of both cardiac output and total peripheral resistance remain essentially unchanged after baro-denervation. One obvious interpretation of these data is that the baroreflexes and their autonomic effector systems serve to regulate short-term changes in arterial pressure but have little influence over the average value of blood pressure over longer periods of time.
Figure 1. Distribution of mean arterial pressure (A), cardiac output (B) and total peripheral resistance (C) in a conscious dog before and after baro-denervation.
Twenty-four hour mean arterial pressure was normal but more variable after baro-denervation. By contrast, both the average values and distribution for cardiac output and total peripheral resistance were normal after baro-denervation. (From Cowley et al. 1973, with permission.)
Second, when kidneys from inbred strains of rats that predictably develop hypertension are transplanted into rats that do not develop hypertension and vice versa, rats with ‘hypertensive’ kidneys develop hypertension, and genetically hypertensive rats with normotensive kidneys are protected from hypertension (Rettig et al. 1990). While there are a number of nuances to transplantation experiments, the simplified summary above reflects the broad-based results from a number of studies, and again is seen as powerful evidence for the predominant role that the kidneys play in long-term blood pressure regulation.
Third, when normotensive animals are subjected to salt loading this usually suppresses the renin–angiotensin system and there is little rise in arterial pressure. However, addition of modest amounts of exogenous renin or angiotensin in the presence of a high-salt diet generates the hypertensive phenotype in the animals (Romero & Feckelhoff, 1999).
Fourth, in humans, blood pressure tends to rise with age. When this rise is compared in different cultures it is linearly related to daily sodium intake and renal sodium excretion (Meneton et al. 2005). Thus, human populations that consume very low-sodium diets have minimal age-related increases in blood pressure. By contrast, societies that consume a lot of dietary salt show yearly average increases in blood pressure of almost 1 mmHg per year after age 30.
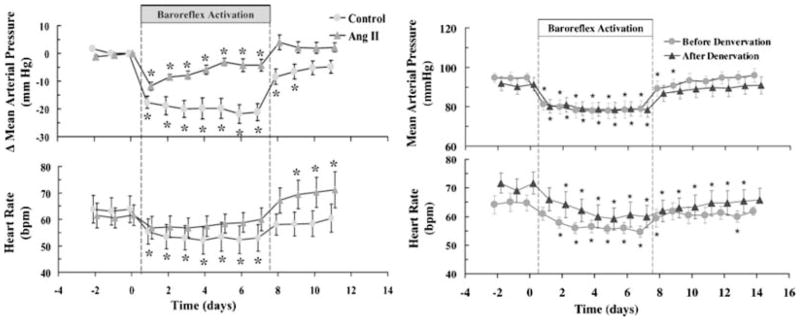
Fifth, in an angiotensin model of hypertension, chronic electrical stimulation of the carotid sinus, which normally evokes reflex-mediated reduction in sympathetic outflow, has only a modest impact on long-term blood pressure (Fig. 2; Lohmeier et al. 2005). This contrasts with the more pronounced effects of chronic electrical stimulation of the carotid sinus nerve on blood pressure in normotensive animals.
Figure 2. Effects of electrical stimulation on Ang II hypertension.
Left panel shows the effects of baroreflex activation on blood pressure in normal animals and animals administered angiotensin II to evoke hypertension. Electrical stimulation of baroreflexes caused sustained reductions in blood pressure in the control animals but the effects in the animals administered angiotensin II waned over time. Right panel shows effects of chronic electrical stimulation of the carotid sinus (baroreflex activation) on animals before and after renal denervation. The sustained reduction in blood pressure was unaffected by renal denervation. (From Lohmeier et al. 2005, 2007b, with permission.)
Thus, the general idea is that a reduction in blood pressure activates renal mechanisms that tend to conserve sodium and expand blood volume over time and the opposite occurs when blood pressure is elevated. In this context, chronic increases in blood pressure and hypertension occur when sodium-retaining and volume-expanding mechanisms are activated even though blood pressure is adequate or ‘normal’.
Hints that the sympathetic nervous plays an underappreciated role in blood pressure regulation
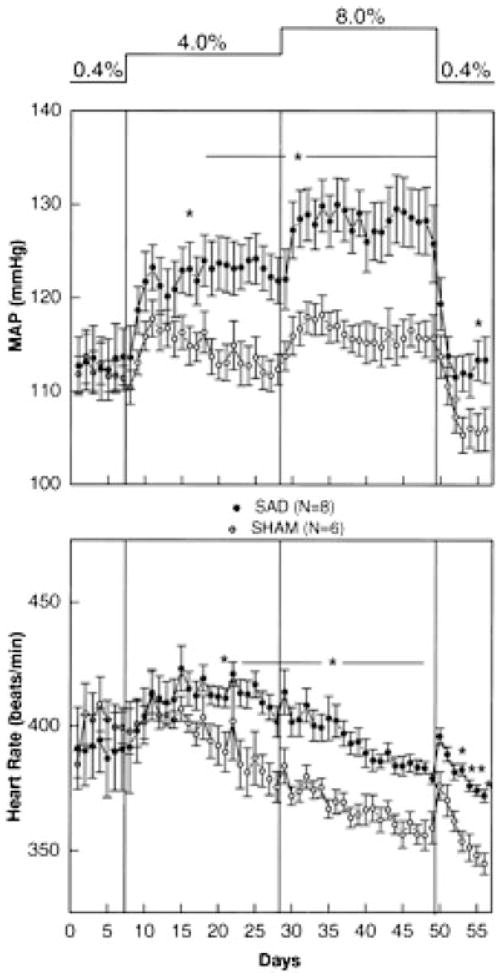
The observations summarized above are consistent with the renocentric view of blood pressure regulation and especially the long-term factors that contribute to hypertension. However, it should also be noted that normotensive animals fed a high-salt and even a very high-salt diet are relatively resistant to marked elevations in blood pressure, but when animals fed similar diets are baro-denervated the rise in blood pressure with increased dietary salt is much more dramatic (Fig. 3; Osborn & Hornfeldt, 1998). Additionally, chronic baroreflex stimulation in normotensive animals does cause marked and sustained reductions in blood pressure, and there is little evidence that this reduction in blood pressure activates renal mechanisms that tend to conserve sodium, increase blood volume and restore blood pressure to normal values (Lohmeier et al. 2004, 2005). Therefore, as convincing as the arguments are that indicate the kidney is the dominant factor in blood pressure regulation, there is clearly evidence that the sympathetic nervous system and arterial baroreflexes play an important role.
Figure 3. Blood pressure responses to sodium loading in sham-operated and sinoaortic denervated (SAD) animals.
Both 4 and 8% saline drinking water had only modest effects on mean arterial pressure in the sham-operated (SHAM) animals. By contrast, sodium loading after sinoaortic denervation led to marked increases in mean arterial pressure. (From Osborn & Hornfeldt, 1998, with permission.)
There are other important observations suggesting that the sympathetic nervous system plays an important role in long-term blood pressure regulation. For example, when large numbers of young normotensive humans are exposed to standardized stressors (e.g. cold pressor testing, mental stress or handgripping) the rise in blood pressure associated with these stressors can be highly variable. This acute rise in blood pressure is mediated by the sympathoexcitation in response to the stressors. More importantly, normotensive individuals who show the most marked rise in arterial pressure in response to sympathoexcitatory stress are at much higher risk for the future development of hypertension than their counterparts who can be described as ‘non-responders’ (Matthews et al. 2004). This observation again suggests that the role of the sympathetic nervous system in long-term blood pressure regulation and the risk for hypertension is underappreciated. This then leads to a subsequent question: how does baseline sympathetic activity in humans relate to blood pressure?
Baseline sympathetic activity and blood pressure in humans
Since the late 1960s it has been possible to record multi-unit efferent sympathetic traffic to skeletal muscle and skin from peripheral nerves in humans. The technical aspects of how this is done and the history of the technique have been summarized recently (Vallbo et al. 2004; Wallin & Charkoudian, 2007). A key point is that from the earliest recordings it became clear that baseline muscle sympathetic nerve activity (MSNA) can vary widely in normotensive humans, as much as five- to 10-fold. In practical terms, this means that some young normotensive subjects display only five or 10 sympathetic bursts per minute while others (also normotensive) have 40 or 50 bursts per minute. It is also important to note that these values for burst frequency are stable in a given individual over time but tend to increase with age (Fagius & Wallin, 1993). Additionally, there is strong evidence to suggest that the baseline level of muscle sympathetic nerve activity is correlated with whole-body noradrenaline spillover, and both renal and cardiac noradrenaline spillover (Wallin et al. 1992, 1996). This suggests that individuals with high baseline sympathetic activity directed at their skeletal muscle vascular beds also have high sympathetic activity directed at the heart and kidneys. Finally, manoeuvres that cause acute increases in MSNA are associated with marked vasoconstriction (Seals, 1989).
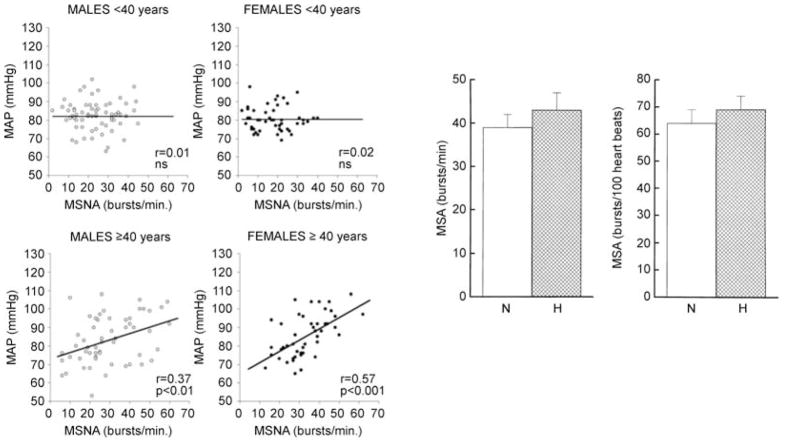
Along similar lines, while there is no (Sundlöf & Wallin, 1978; Kienbaum et al. 2001; Charkoudian et al. 2005, 2006a,b) or minimal relationship (Weyer et al. 2000) between baseline MSNA and blood pressure in humans under 40 years old, there are modest relationships between MSNA and blood pressure in humans over 40 years old (Fig. 4; Narkiewicz et al. 2005). Additionally, when groups of individuals with mild hypertension are compared with normotensive individuals, the hypertensive cohorts show (at most) modest increases in baseline muscle sympathetic nerve activity, and there is substantial overlap in the distributions of MSNA between the groups (Yamada et al. 1989; Gudbjörnsdóttir et al. 1996; Anderson et al. 1998).
Figure 4. MSNA and blood pressure.
Left panel shows the relationship between muscle sympathetic nerve activity (MSNA) and mean arterial pressure in males and females below and above 40 years of age. In the younger cohorts there was no relationship between MSNA and mean arterial pressure. By contrast, in those over 40 years of age, increases in MSNA showed some influence on mean arterial pressure. Right panel shows MSNA expressed either as bursts per minute or bursts per 100 heartbeats in middle-aged, normotensive and hypertensive subjects. In these cohorts, there are no dramatic differences in MSNA. (Left panel from Narkiewicz et al. 2005; right panel from Gudbjörnsdóttir et al. 1996, with permission.)
Thus, the lack of a clear and dramatic association between baseline sympathetic outflow and chronic blood pressure levels would appear to support the argument that the sympathetic nervous system does not play a major role in baseline blood pressure regulation. Additionally, these data raise the third major question about the sympathetic nervous system and blood pressure regulation: why is hypertension not ‘obligatory’ in individuals with high levels of baseline MSNA?
Why high baseline sympathetic activity is not synonymous with high blood pressure
A possible explanation for the apparent lack of relationship between baseline MSNA and blood pressure is that there is a reciprocal relationship between sympathetic activity and counterbalancing vasodilating pathways. In this context, Skarphedinsson et al. (1997) demonstrated a linear relationship between plasma nitrates (a marker of whole body NO tone) and MSNA in normotensive humans such that individuals with higher resting MSNA tended to have higher levels of circulating plasma nitrates. These data suggested that the tonic level of nitric oxide-mediated vasodilator tone was higher in subjects with high MSNA and that this high vasodilator tone might limit the blood pressure-raising effects of high sympathetic traffic.
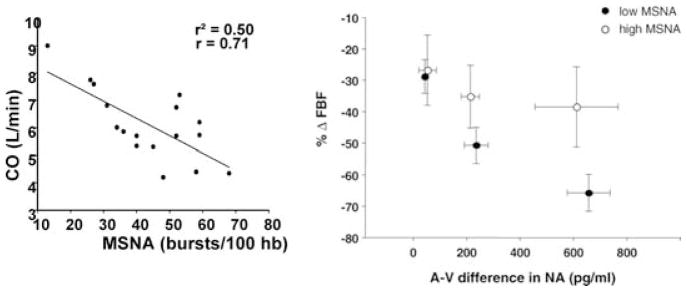
In an effort to explore the specific interactions among resting MSNA, systemic haemodynamics and resting NO tone, we sought to make detailed haemodynamic measurements in normotensive subjects to better understand why high levels of MSNA could be observed in normotensive subjects. We also sought to test the overall hypothesis that a complex set of relationships exist among factors which tend to raise and factors which tend to lower blood pressure (Fig. 5; Charkoudian et al. 2005, 2006a,b). First, we demonstrated that there was a wide range of resting cardiac output values observed in normotensive subjects and a reciprocal relationship between the baseline level of MSNA and cardiac output such that individuals with high baseline MSNA had low cardiac outputs and vice versa. Initially, we were surprised by the extent of inter-individual variability in the cardiac output measurements, but subsequent review of earlier literature suggested that a similar range of values had been obtained in large numbers of subjects using invasive techniques during the 1960s by Drs Stevo Julius and James Conway at the University of Michigan (Julius & Conway, 1968). Second, in a follow-up to our initial observations we wanted to evaluate whether vascular α-adrenergic sensitivity (responsiveness) varied depending on the level of baseline sympathetic activity. The hypothesis was that individuals with high baseline MSNA would be relatively insensitive to the vasoconstricting actions of noradrenaline. To test this hypothesis, we infused tyramine, a substance which causes endogenous noradrenaline release, into the brachial artery and measured the associated arteriovenous noradrenaline differences as well as the forearm vasoconstrictor responses to the tyramine. We noted an inverse relationship between baseline MSNA and forearm vasoconstriction to a given level of noradrenaline during three doses of tyramine infusion. Together, the cardiac output–MSNA relationships and the adrenergic sensitivity–MSNA relationships explain in part why hypertension is not ‘obligatory’ in individuals with high MSNA.
Figure 5. MSNA, cardiac output and adrenergic sensitivity.
Left panel shows the relationship between muscle sympathetic nerve activity (in bursts per 100 heart beats) and cardiac output (CO) in normotensive subjects. Individuals with high levels of MSNA tended to have lower levels of cardiac output. Right pane shows the forearm vasoconstrictor responses (ΔFBF; forearm blood flow) to the release of noradrenaline (expressed as arteriovenous difference in NA) evoked by brachial artery administration of tyramine in subjects with low and high levels of MSNA. Subjects with high levels of MSNA showed blunted vasoconstrictor responses to a given level of noradrenaline. These observations help to explain how normotension is maintained in subjects with high levels of MSNA. (Figure from Charkoudian et al. 2005, 2006b, with permission.)
In our next study, we sought to follow up on the studies of Skarphedinsson et al. (1997) to evaluate the relationship between resting MSNA and systemic NO ‘tone’. We measured MSNA and cardiac output in 18 normotensive male subjects and then performed systemic infusions of the nitric oxide synthase inhibitor NG-monomethyl-L-arginine (L-NMMA). In response to systemic L-NMMA infusions, individuals with high baseline MSNA showed a greater rise in blood pressure than individuals with low levels of baseline MSNA. However, these differences in pressor responses appeared to be due to differences in cardiac output between groups. The changes in total peripheral resistance with L-NMMA were not different between individuals with low and high nerve activity. This series of studies provided several mechanistic integrative explanations for the lack of obvious relationship between baseline MSNA and blood pressure in normotensive humans. They also provide important clues and raise additional questions about high levels of baseline MSNA and the development of hypertension in some subjects.
Hypertension and MSNA
As noted above, when large numbers of subjects with essential hypertension are studied, a range of MSNA values are observed. This range of values shows significant overlap with the range of values seen in normotensive subjects, and some studies on relatively large groups of subjects report no obvious increases in baseline MSNA in mildly hypertensive subjects. Others report a modest increase in MSNA in hypertension (Yamada et al. 1989; Gudbjörnsdóttir et al. 1996; Anderson et al. 1998; Weyer et al. 2000; Narkiewicz et al. 2005). The key points are: first, if there is a rise in baseline MSNA seen in cohorts of hypertensive subjects it is typically modest; and second, since the distributions of MSNA overlap so much between cohorts of normotensive subjects and those with essential hypertension, there are many hypertensive subjects with lower values for MSNA than seen in normotensive subjects. Again, at face value this evidence suggests that high levels of MSNA do not cause hypertension.
When are increased levels of sympathetic nerve activity important in blood pressure regulation?
Sympathetic activity in general and muscle sympathetic nerve activity specifically rise with both ageing and obesity. Ageing and obesity are also associated with increased levels of blood pressure (Sundlöf & Wallin, 1978; Ng et al. 1993; Seals & Bell 2004). By age 60 or 70 years, healthy older subjects have MSNA values that are on average about twofold greater than those seen in younger subjects. Obese, younger humans also have high levels of MSNA and these levels fall with significant weight loss (Alvarez et al. 2002; Trombetta et al. 2003). There is also evidence that sympathetic ‘support’ of blood pressure is greater in both older subjects and obese humans (Jones et al. 2001; Shibao et al. 2007). For example (Fig. 6), when either older subjects or obese subjects (especially obese, hypertensive subjects) are given ganglionic blockers to eliminate autonomic outflow, they experience a fall in blood pressure that is greater than that seen in lean, young subjects during ganglionic blockade. These observations have profound implications for Western society, which is both rapidly ageing and getting fatter. In this context, the epidemiological stage has been set for an epidemic of high blood pressure that has a strong sympathetically mediated pump. How might this happen?
Figure 6. Effects of ganglionic blockade on the fall in systolic blood pressure (ΔSBP) in lean subjects, obese subjects and obese subjects with hypertension (HTA).
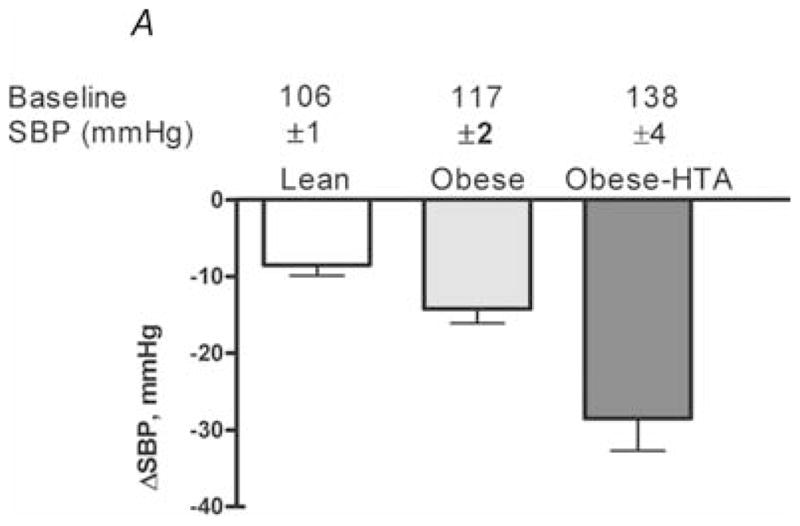
Obese subjects with hypertension also had elevated muscle sympathetic nerve activity. Note the evidence for increased autonomic support of blood pressure in obese hypertensive subjects. (Figure from Shibao et al. 2007, with permission.)
As noted above, sympathetic activity increases with both ageing and obesity. The rise in MSNA with ageing appears likely to be due to a combination of changes in central autonomic regulation, a stiffening of the baroreflexes and changes in body composition. Similar factors, along with sleep-disturbed breathing, probably contribute to the rise in MSNA with obesity (Narkiewicz et al. 1998). In general, there is a strong relationship between either total body fat or markers of visceral obesity and MSNA in humans (Alvarez et al. 2002; Seals & Bell, 2004). Obesity and ageing are also associated with so-called oxidative stress, which would probably limit either the bioavailability or the production of nitric oxide from the vascular endothelium. Ageing and (probably) obesity are also associated with changes in the vascular endothelium that favour production of vasoconstricting as opposed to vasodilating prostanoids (Taddei et al. 1997; Schrage et al. 2006). All of the changes noted above would favor the emergence of the hypertensive state and could explain the augmented sympathetic autonomic support of blood pressure in these populations. Additionally, this general scheme is supported by animal studies showing that obesity-associated hypertension can be treated by long-term baroreceptor activation via electrical stimulation of the carotid sinus nerve (Lohmeier et al. 2007a). There is also emerging anecdotal evidence that carotid sinus nerve stimulation might be a useful treatment in drug-resistant human hypertension (Fig. 7; Mohaupt et al. 2007).
Figure 7. Effects of chronic electrical stimulation in carotid sinus nerve in a human patient with resistant hypertension.
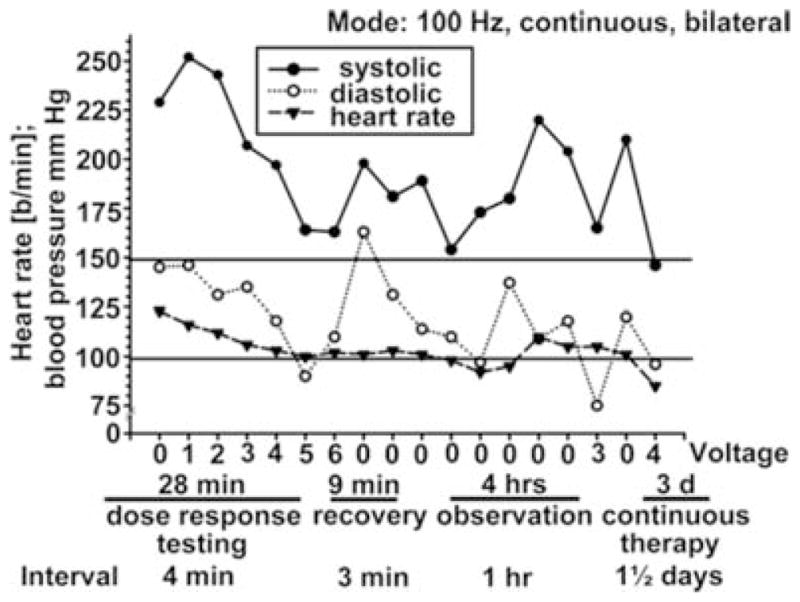
During 28 min of dose–response testing, blood pressure fell from ~225/110 to ~170/100 mmHg. The stimulator was turned off for 9 min and blood pressure rose. During 4 h of observation in the laboratory, blood pressure was lower during stimulation. With 3 days of treatment (far right), sustained reductions in blood pressure were seen in this patient who was resistant to multiple combinations to drug therapy. (From Mohaupt et al. 2007, with permission.)
High levels of baseline sympathetic outflow may be a risk factor for weight gain in a high-calorie world
The ideas outlined above suggest a causal link between changes in body composition, sympathetic outflow, vascular function and blood pressure. Is it also possible that high levels of baseline MNSA might have a long-term influence on weight gain or body composition? There is some evidence to suggest that resting metabolic rate might be higher in individuals with higher levels of baseline cardiac output (Julius & Conway, 1968) who probably had low baseline MSNA (Charkoudian et al. 2005). Additionally, lean, older subjects with relatively high levels of baseline MSNA have lower resting metabolic rates than their younger counterparts (who have lower baseline MSNA). Older individuals also demonstrate less β-adrenergic support of resting metabolism than younger subjects (Bell et al. 2001; Seals & Bell, 2004). These observations suggest a potential inverse relationship between baseline sympathetic activity and resting metabolic rate in human subjects. Additionally, there is an acute increase in oxygen consumption associated with the consumption of a meal (i.e. the thermic effect of food; TEF). In older subjects TEF is lower than in younger individuals (Jones et al. 2004); perhaps TEF is also lower in younger subjects with high levels of baseline MSNA (Jones et al. 2004).
If the relationships between β-adrenergic support of resting metabolism and TEF seen in older subjects and obese subjects are causally linked to their high levels of sympathetic outflow, perhaps younger subjects with high levels of MSNA are at increased risk for weight gain. Additionally, if such a scheme did operate, then a self-reinforcing cycle of high MSNA and weight gain and ultimately hypertension might occur and make things even worse. Whether or not there is a relationship between baseline MSNA and metabolism in young subjects and to what extent it has a major or minor role in the phenomena of weight gain and the associated hypertension remains to be determined, but a number of laboratories throughout the world are considering this problem.
Some unanswered questions
Several fundamental questions remain unanswered. What is the origin of, and/or the reason for, the inter-individual variability in baseline sympathetic outflow in young, lean, healthy subjects? Is it due to fundamental differences in central sympathetic outflow? Perhaps some people just have ‘big’ hearts and large stroke volumes and ‘need’ less sympathetic support for their blood pressure to be normal. Is it due to alterations in the baroreflex function so that, at a given level of distension, signals from the mechanosensitive areas of the carotid arteries and aortic arch are somehow less effective in suppressing sympathetic outflow in some subjects? Is it due to differences at the level of the adrenergic receptors? Are there subtle genetic, genomic or proteomic differences in α-adrenergic receptors that generate more vasoconstriction in some subjects than in others, and perhaps the varying levels of sympathetic outflow are simply a compensatory response to these changes in the periphery? Are there differences in vasodilator systems which contribute to the variability? While there are speculative answers to all of these questions and many ideas about how they might interact, definitive information on any of these topics is currently not available and represents a ripe field for study on a critical topic that appears to resist a simple reductionist explanation.
Summary
In summary, the renocentric view of blood pressure regulation has predominated for the last 30–40 years. In this invited review, we have shown that the sympathetic nervous system and the arterial baroreflexes play an important role in long-term blood pressure regulation. We have also described the mechanisms that prevent high levels of baseline MSNA from causing increases in blood pressure in normotensive subjects. Finally, we have given specific examples of how and under what pathophysiological conditions high levels of MSNA clearly contribute to increases in blood pressure. We expect sympathetically mediated hypertension to be an important pathophysiological phenomenon as the developed world and the developing world gets both older and fatter.
Acknowledgments
The authors’ work was funded by NIH NS-32352, HL-83947 and Swedish Medical Research Council Grant 12170.
References
- Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1998;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- Angell-James J. Michael de Burgh Daly obituary. Physiology News. 2003;50:39–40. [Google Scholar]
- Bell C, Seals DR, Monroe MB, Day DS, Shapiro LF, Johnson DG, Jones PP. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab. 2001;86:4440–4444. doi: 10.1210/jcem.86.9.7855. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Barnes S, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Relationship between muscle sympathetic nerve activity and systemic hemodynamics during nitric oxide synthase inhibition in humans. Am J Physiol Heart Circ Physiol. 2006a;291:H1378–H1383. doi: 10.1152/ajpheart.00234.2006. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Wallin BG. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–321. doi: 10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006b;572:821–827. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley AW, Jr, Liard JF, Guyton AC. Role of the baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- de Burgh Daly I, de Burgh Daly M. The effects of stimulation of the carotid body chemoreceptors on the pulmonary vascular bed in the dog: the ‘vasosensory controlled perfused living animal’ preparation. J Physiol. 1959a;148:201–219. doi: 10.1113/jphysiol.1959.sp006282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Burgh Daly I, de Burgh Daly M. The effects of stimulation of the carotid sinus baroreceptors on the pulmonary vascular bed in the dog. J Physiol. 1959b;148:220–226. doi: 10.1113/jphysiol.1959.sp006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Burgh Daly M. Peripheral Arterial Chemoreceptors and Respiratory-Cardiovascular Integration. Oxford University Press; 1997. [Google Scholar]
- Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res. 1993;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- Gudbjörnsdóttir S, Lönnroth P, Sverrisdóttir YB, Wallin BG, Elam M. Sympathetic nerve activity and insulin in obese normotensive and hypertensive men. Hypertension. 1996;27:276–280. doi: 10.1161/01.hyp.27.2.276. [DOI] [PubMed] [Google Scholar]
- Jones PP, Shapiro LF, Keisling GA, Jordan J, Shannon JR, Quaife RA, Seals DR. Altered autonomic support of arterial blood pressure with age in healthy men. Circulation. 2001;104:2424–2429. doi: 10.1161/hc4501.099308. [DOI] [PubMed] [Google Scholar]
- Jones PP, Van Pelt RE, Johnson DG, Seals DR. Role of sympathetic neural activation in age- and habitual exercise-related differences in the thermic effect of food. J Clin Endocrinol Metab. 2004;89:5138–5144. doi: 10.1210/jc.2004-0101. [DOI] [PubMed] [Google Scholar]
- Julius S, Conway J. Hemodynamic studies in patients with borderline blood pressure elevation. Circulation. 1968;38:282–288. doi: 10.1161/01.cir.38.2.282. [DOI] [PubMed] [Google Scholar]
- Kannel WB. Fifty years of Framingham Study contributions to understanding hypertension. J Human Hypertens. 2000;14:83–90. doi: 10.1038/sj.jhh.1000949. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmeier TE, Dwyer TM, Hildebrandt DA, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension. 2005;46:1194–1200. doi: 10.1161/01.HYP.0000187011.44201.2e. [DOI] [PubMed] [Google Scholar]
- Lohmeier TE, Dwyer TM, Irwin ED, Rossing MA, Kieval RS. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension. 2007a;49:1307–1314. doi: 10.1161/HYPERTENSIONAHA.107.087874. [DOI] [PubMed] [Google Scholar]
- Lohmeier TE, Hildebrandt DA, Dwyer TM, Barrett AM, Irwin ED, Rossing MA, Kieval RS. Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure. Hypertension. 2007b;49:373–379. doi: 10.1161/01.HYP.0000253507.56499.bb. [DOI] [PubMed] [Google Scholar]
- Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA Study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. doi: 10.1152/physrev.00056.2003. [DOI] [PubMed] [Google Scholar]
- Mohaupt MG, Schmidli J, Luft FC. Management of uncrontrollable hypertension with a carotid sinus stimulation device. Hypertension. 2007;5:825–828. doi: 10.1161/HYPERTENSIONAHA.107.099416. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Phillips BG, Kato M, Hering D, Bieniaszewski L, Somers VK. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension. 2005;45:522–525. doi: 10.1161/01.HYP.0000160318.46725.46. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–776. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Hornfeldt BJ. Arterial baroreceptor denervation impairs long-term regulation of arterial pressure during dietary salt loading. Am J Physiol Heart Circ Physiol. 1998;275:H1558–H1566. doi: 10.1152/ajpheart.1998.275.5.H1558. [DOI] [PubMed] [Google Scholar]
- Rettig R, Folberth C, Stauss H, Kopf D, Waldherr R, Unger T. Role of the kidney in primary hypertension: a renal transplantation study in rats. Am J Physiol Renal Physiol. 1990;258:F606–F611. doi: 10.1152/ajprenal.1990.258.3.F606. [DOI] [PubMed] [Google Scholar]
- Romero JC, Feckelhoff JF. Role of angiotensin and oxidative stress in essential hypertension. Hypertension. 1999;34:943–949. doi: 10.1161/01.hyp.34.4.943. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol. 2006;579:227–236. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol. 1989;66:2472–2478. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- Seals DR, Bell C. Chronic sympathetic activation: consequences and cause of age-associated obesity? Diabetes. 2004;53:276–284. doi: 10.2337/diabetes.53.2.276. [DOI] [PubMed] [Google Scholar]
- Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- Skarphedinsson JO, Elam M, Jungersten L, Wallin BG. Sympathetic nerve traffic correlates with the release of nitric oxide in humans: implications for blood pressure control. J Physiol. 1997;501:671–675. doi: 10.1111/j.1469-7793.1997.671bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- Trombetta IC, Batalha LT, Rondon MU, Laterza MC, Kuniyoshi FH, Gowdak MM, Barretto AC, Halpern A, Villares SM, Negrão CE. Weight loss improves neurovascular and muscle metaboreflex control in obesity. Am J Physiol Heart Circ Physiol. 2003;285:H974–H982. doi: 10.1152/ajpheart.01090.2002. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth K-E, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol. 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Charkoudian N. Sympathetic neural control of integrated cardiovascular function: insights from measurement of human sympathetic nerve activity. Muscle Nerve. 2007;36:595–614. doi: 10.1002/mus.20831. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491:881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C, Pratley RE, Snitker S, Spraul M, Ravussin E, Tataranni PA. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. Hypertension. 2000;36:531–537. doi: 10.1161/01.hyp.36.4.531. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Miyajima E, Tochikubo O, Matsukawa T, Ishii M. Age-related changes in muscle sympathetic nerve activity in essential hypertension. Hypertension. 1989;13:870–877. doi: 10.1161/01.hyp.13.6.870. [DOI] [PubMed] [Google Scholar]