Abstract
In social hierarchies, dominant individuals experience reproductive and health benefits, but the costs of social dominance remain a topic of debate. Prevailing hypotheses predict that higher-ranking males experience higher testosterone and glucocorticoid (stress hormone) levels than lower-ranking males when hierarchies are unstable but not otherwise. In this long-term study of rank-related stress in a natural population of savannah baboons (Papio cynocephalus), high-ranking males had higher testosterone and lower glucocorticoid levels than other males, regardless of hierarchy stability. The singular exception was the highest-ranking (alpha) males, who exhibited both high testosterone and high glucocorticoid levels. In particular, alpha males exhibited much higher stress hormone levels than second-ranking (beta) males, suggesting that being at the very top may be more costly than previously thought.
In many animal societies, a high dominance rank is beneficial (1, 2). High-ranking primates, for example, tend to experience higher reproductive success and/or greater offspring quality as measured by survival, growth rates, and accelerated maturation (3–8). Social rank also influences health (9). However, attaining and maintaining high dominance rank may entail substantial energetic costs, especially for males, if high-ranking individuals are involved in more agonistic and sexual activities (10).
Currently, no consensus exists about the rank-associated stress physiology of individuals in stratified mammal societies, with various studies producing apparently contradictory findings. In some studies, it is subordinate animals, and in others it is dominants, that exhibit greater stress levels (11, 12). These differences may arise from species-level variations in social and mating systems or from variability in methodology and housing (11, 12). Differences within social and mating systems, or even within species, may also occur as a function of hierarchy stability (13). For example, in a pioneering investigation, Sapolsky (13, 14), studying wild olive baboons, determined a male dominance hierarchy during each of seven annual three-month research periods. During research periods when the hierarchy was stable, high social ranks were associated with lower levels of glucocorticoids, but this advantage was lost during a research period when the hierarchy was unstable (when a high proportion of agonistic interactions involved ‘reversals’, i.e. a subordinate winning over a dominant male) (13, 14). Two other investigations of multi-male primate societies defined unstable periods as those in which rank changes occurred for males that were in the alpha position (semi-captive mandrills) (15), or in either the alpha and/or beta position (wild chacma baboons) (16). Both studies found an interaction between dominance and stability although the relationship between rank and fGC within periods was significant in only one of the two studies, perhaps because of differences in sample size. In contrast to those three studies, high-ranking chimpanzee males had higher glucocorticoid levels than did low-ranking ones during a period of stability (identified by no rank changes among adult males) (10).
Exposure to stressors activates a chain of endocrine reactions, including secretion of glucocorticoids by the adrenals, which mobilizes the energy necessary to adapt to the stressor (17). Short-term secretion is beneficial, but long-term exposure to high levels can lead to suppressed immune function (15, 18). Glucocorticoids can also suppress testosterone (9, 17), which is the major steroid contributing to sperm production, muscle mass, male secondary sexual characteristics, and sexual and aggressive behaviors (19–21). However, under some conditions, including mating in seasonally breeding species or in high-ranking individuals, the reproductive system can be insensitive to the action of glucocorticoids (21, 22). An animal may then exhibit elevated levels of both glucocorticoids and testosterone (21, 22). The relationship between social rank and testosterone, like that for glucocorticoids, may depend on hierarchy stability. During stable periods, testosterone levels are often independent of rank whereas during times of instability, high-ranking males may exhibit higher concentrations of testosterone (13), in agreement with the ‘challenge’ hypothesis, which originally proposed that testosterone concentrations rise according to anticipated needs (21).
We tested the predominant hypothesis that high-ranking males experience higher testosterone and glucocorticoid levels than other males when hierarchies are unstable but not during stability. To do so, we evaluated the relationship between male rank and physiological measures of stress and reproductive function in five social groups of wild savannah baboons in Amboseli, Kenya, over a nine-year period. Our dataset included physiological, behavioral, and ecological data for 125 adult males (23). We used General Linear Mixed Models (GLMM) to predict fecal glucocorticoids (fGC) and testosterone (fT), using a monthly average value for each hormone for each male sampled in a given month (2432 monthly values derived from 4543 hormone samples). Predictor variables included, for each month, individual dominance ranks, whether the dominance hierarchy was stable or not, and an interaction between rank and stability (23). A month was considered stable if males occupying the top three rank positions were the same as in the previous and following months (23). Because hormone levels are often influenced by age and environment (24), we also included these variables as fixed factors in the GLMM. Identity and social group were included as random factors (23). Variables that were not significant for either hormone were deleted from the final models (23).
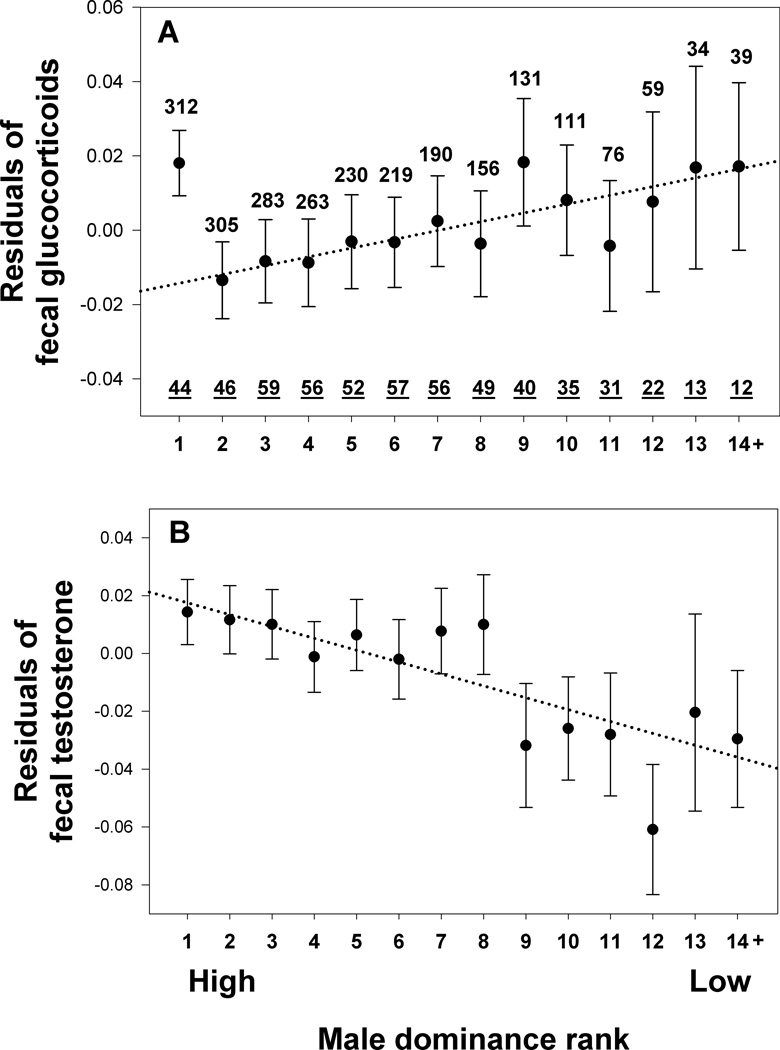
Overall, fGC concentrations increased with declining rank, with the striking exception of alpha males, who exhibited higher levels of these stress hormones than predicted from the linear pattern across ranks (Table 1 rows 1 & 2; see also Fig. 1A). The distinctiveness of alpha males is highlighted by comparing alpha to other relatively high-ranking males and to low-ranking males. Alpha males had higher fGC levels than males ranked 2–8 (F1,1881=4.367, p=0.037) but similar levels to males ranked 9–14 (F1,333=0.403, p=0.526). In contrast to fGC levels, fT levels were simply a linear function of rank; higher ranking males exhibited higher fT levels (Table 1; Fig. 1B).
Table 1.
Effect of hierarchy stability and social dominance on hormone concentrations.
| Dependant variable: log fGC | |||||
|---|---|---|---|---|---|
| Variables* | b | Numerator df |
Denominator df |
F | Sig. |
| Intercept | 1 | 429.665 | 4758.378 | <0.001 | |
| Dominance rank | 0.0043 | 1 | 1578.129 | 5.390 | 0.020 |
| Alpha status | 0.0437† | 1 | 2334.854 | 9.134 | 0.003 |
| Hierarchy stability | −0.0182‡ | 1 | 2351.302 | 5.666 | 0.017 |
| Season | −0.0239§ | 1 | 2324.398 | 9.100 | 0.003 |
| Temperature | −0.0062∥ | 1 | 2337.664 | 0.393 | 0.531 |
| Age | 0.0034 | 1 | 522.355 | 2.153 | 0.143 |
| Dependant variable: log fT | |||||
|---|---|---|---|---|---|
| Variables* | b | Numerator df |
Denominator df |
F | Sig. |
| Intercept | 1 | 625.525 | 3891.482 | <0.001 | |
| Dominance rank | −0.0124 | 1 | 2374.909 | 29.102 | <0.001 |
| Alpha status | −0.0117† | 1 | 2360.698 | 0.473 | 0.492 |
| Hierarchy stability | 0.0146‡ | 1 | 2301.445 | 2.776 | 0.096 |
| Season | 0.0705§ | 1 | 2290.120 | 60.702 | <0.001 |
| Temperature | 0.0266∥ | 1 | 2294.284 | 5.481 | 0.019 |
| Age | −0.0249 | 1 | 1816.997 | 59.963 | <0.001 |
Statistically significant results appear in bold.
Factors that were not significant for either hormone were dropped. These included the interaction between rank and hierarchy stability (a fixed factor) and social group (a random factor). For the four categorical variables (Alpha Status, Hierarchy stability, Season and Temperature), a positive value of b indicates that the hormone concentration was higher
for the alpha male,
when the hierarchy was stable,
during the wet season,
in cooler months. The variable ‘Dominance Rank’ captures the linear component of the functional relationship between rank and the hormones; the binary variable ‘Alpha Status’ captures the non-linear component.
Fig. 1.
Relationship between male dominance rank and glucocorticoids (A) or testosterone (B) concentrations. The y axis represents the residuals of log-transformed hormone concentration obtained from a GLMM including age, environmental factors and hierarchy stability as fixed factors, male identity as a random factor (23). Each value represents the mean ± SE across male monthly averages. The dotted lines represent the regression lines determined using all the monthly male hormone values. N = number of monthly averages, N = number of males. Sample sizes in A and B are the same. Note that this visualization is not a substitute for the full statistical model results which are presented in table 1.
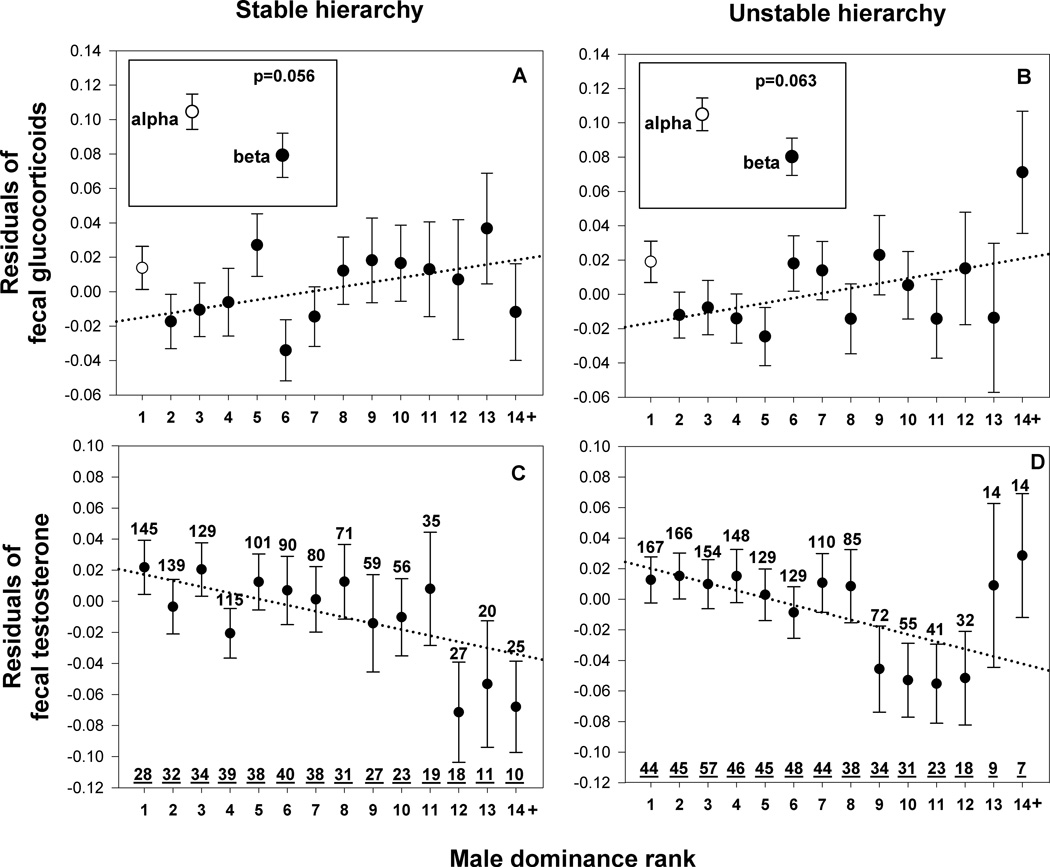
Hierarchy stability in a given group did not affect fT concentrations among males (F1,2301=2.776, p=0.096) but was a significant predictor of their fGC levels (F1,2351=5.666, p=0.017; Table 1); fGC concentrations were higher when the hierarchy was unstable. However, despite the overall elevation of fGC during instability, no interaction was found between dominance rank and hierarchy stability for either fGC or fT (fGC: rank*stability: F1,2338=0.034, p=0.853; fT: rank*stability: F1,2293=0.109, p=0.741). In other words, hierarchy stability did not influence the relationship between dominance rank and hormone levels (as illustrated in Fig.2).
Fig. 2.
Relationship between male dominance rank and glucocorticoid (A, B) and testosterone (C, D) concentrations in stable and in unstable hierarchies, illustrating the similar relationships with dominance rank in both stable and unstable conditions (identified statistically as the absence of a significant interaction between dominance rank and stability; see table 1 and text). Separate GLMM models were created for each condition (stable and unstable) and each hormone. In each case, values on the Y axis represent residuals of log-transformed hormone concentrations from the respective GLMM model, which included age and environmental factors as fixed factors, and male identity as a random factor (23). Each plotted value represents the mean ± SE across male monthly averages. The dotted lines represent the regression lines determined using all the monthly male hormone values. N = number of monthly averages, N = number of males. Sample sizes in A and C and in B and D are the same. Inserts represent the alpha vs. beta comparison using the reduced dataset that included only alpha and beta males. Note that this visualization is not a substitute for the full statistical model results which are presented in table 1.
The relationship between alpha and beta males is of special interest, both conceptually and empirically. Males of these ranks achieve most of the matings and father most of the offspring. Alpha and beta males in our data set were strikingly different in their fGC levels, as determined in a reduced model comparing only alpha and beta males (F1,595=8.741, p=0.003). This result was reinforced by (1) a comparison of hormone levels within individuals who had occupied the alpha and beta position in different months (paired t-test: t37=2.179, p=0.036), and (2) a comparison of hormone levels across alpha and beta males in the same group within months (paired t-test: t220=2.191, p=0.029). In contrast, there was no difference in fT levels between alpha and beta males (F1,597=0.221, p=0.638). Furthermore, the relationship between the hormone profiles of alpha and beta males was similar whether the hierarchy was stable or unstable (Stable hierarchy, fGC: F1,174=3.694, p=0.056, see insert in Fig. 2A; Unstable hierarchy, fGC: F1,324=3.493, p=0.063, see insert in Fig. 2B; Stable hierarchy, fT: F1,277=0.075, p=0.785; Unstable hierarchy, fT: F1,299=0.773, p=0.380).
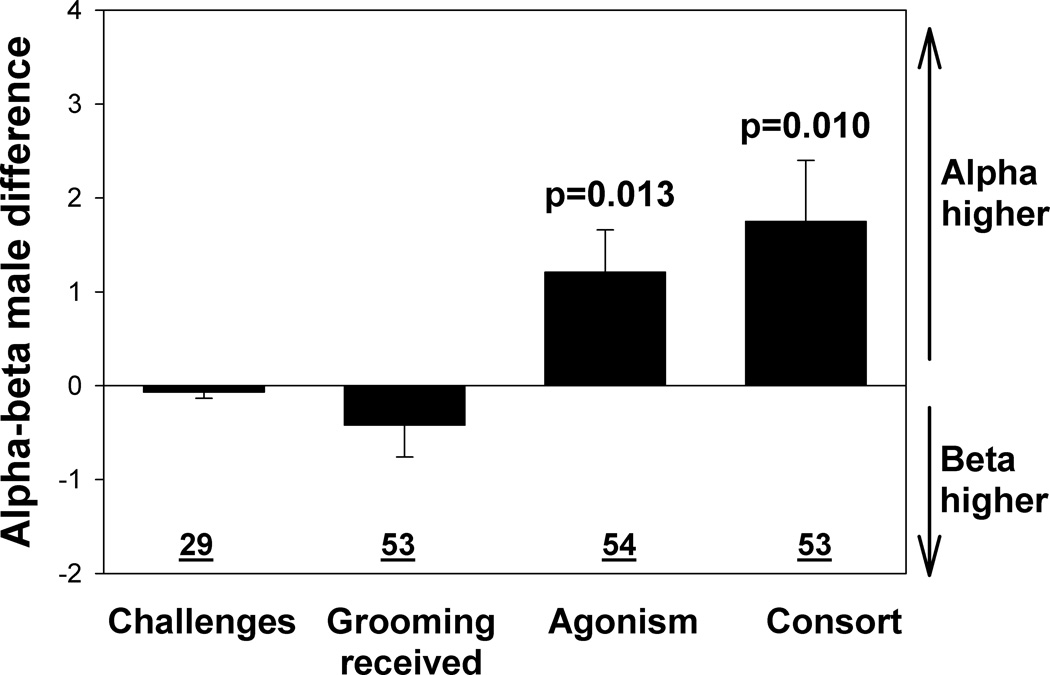
These physiological results led us to ask what energetic or psychological mechanisms might contribute to the observed hormone differences between alpha and beta males. We were able to examine several major potential factors. First, Sapolsky found that high glucocorticoids were predicted by a male experiencing a high proportion of dominance interactions that involved reversals from males close in rank below him (14). However, alpha and beta males in our study received similar rates of such challenges (Wilcoxon Signed ranks test: Z=−0.868, p=0.385, N=29 alpha-beta pairs, Fig. 3) (23). Differences in stress levels might also be accounted for by differences in access to coping outlets such as received grooming; even if two individuals were exposed to similar stressors, the one that received more grooming might then have lower levels of stress hormones (25). However, alpha and beta males received similar rates of grooming from adult females (the class that performs most of the grooming in baboon groups) (Wilcoxon Signed ranks test: Z=−1.207, p=0.227, N=53 alpha-beta pairs, Fig. 3) (23). In contrast, alpha males differed from beta males in two energetically costly activities, maintenance of dominance rank through agonistic encounters (10, 11) and mate guarding of fertile females (‘consortships’ in primates) (26, 27). Alpha males experienced a 17% higher rate of agonistic encounters and spent 29% more time in sexual consortships than did beta males (Wilcoxon Signed ranks test: Z=−2.461, p=0.014, N=54 male pairs, for agonistic encounters and Z= −2.572, p=0.010, N=53 male pairs for consort time, Fig 3) (23). Mating and agonistic behaviors are also generally positively associated with higher levels of testosterone, thus one would predict higher fT levels in alpha than beta males. We did not find such a difference (model results above and residual visualization in Fig. 1B), possibly because of the inhibitory effect of glucocorticoids on testosterone secretion (9).
Fig. 3.
Differences between alpha and beta males in the proportion of challenges received, rate of grooming received, number of agonistic encounters and consort time. Each bar represents the mean ± SE of the difference between the alpha and beta male within a group-month (alpha value minus beta value). N = number of alpha-beta pairs. For each analysis, an alpha-beta pair is represented by a single value that represents the overall difference over time for that pair. Any pair is included if data were available for the pair for at least three months and if at least one member of the pair had a non-zero value during that time (23).
Taken together, the findings reported in this study revealed that being at the very top of a social hierarchy may be more stressful than being immediately below because of physiological costs of life at the top. In the Amboseli baboons, these costs are probably largely energetic rather than psychological in origin. In fact, alpha males in Amboseli usually maintain their rank for a relatively short period (28), and they often fail to monopolize access to reproductive females to the extent predicted by a simple rank-based model of access. In contrast, beta males do slightly better than predicted (29). This failure of alpha males to reach their full reproductive potential may stem from the health costs associated with high levels of glucocorticoids and testosterone. Both hormones are costly as they both have immunosuppressive effects at high levels (15, 18) and reduce individual survival (30). Parasite richness, for example, has been shown to positively correlate with glucocorticoid and testosterone levels in chimpanzees (31), and parasite load was higher in high-ranking males in a study of the Amboseli baboons (32).
Although alpha males and the males of the lower part of the hierarchy experienced comparably high stress hormone levels in our study (Fig 1a), we suggest that the sources of stress for these two classes of males may be different. In particular, a major energetic source of stress for alpha males seems to be high levels of agonistic and mating activities, as proposed for chimpanzees (10). In contrast, males in the lower part of the hierarchy are likely to experience energetic costs associated with limited access to resources (such as food), a commonly recognized phenomenon for low-ranking individuals (9).
A final important insight from our study is that individual rank positions in animal (and possibly human) societies may have unique costs and benefits associated with them; these will then be obscured by the common practice of pooling data across many ranks to categorize individuals as simply ‘high’ vs. ‘low’ ranking. Use of full ordinal ranks will help elucidate the conditions under which rank categories are heterogeneous vs. homogenous and will thereby, provide new insights into the functioning of social hierarchies and their mechanisms.
Supplementary Material
Acknowledgments
Supported by NSF (IOB-0322781, BCS-0323596), NIH (R03 MH65294), NIA (P30 AG024361, R01-AG034513). Thanks to the Kenya Wildlife Services, Amboseli-Longido pastoralist communities, and Amboseli research team, particularly R. S. Mututua, S. Sayialel, J. K. Warutere, T. Wango, V. K. Oudu. Thanks to S. Mukherjee, G. Rodriguez and J. Tung for advice on analysis and to J. Beehner, N. Goldman, C. Markham, J. Silk, B. Singer for comments on previous drafts of this paper. All protocols were non-invasive and approved in Kenya (MOEST 13/001/C351 Vol. II) and the U.S.A. (Princeton University IACUC 1689). This article has been supported by NIA Grant P01AG031719.
Footnotes
Publisher's Disclaimer: This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reporduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Summary: Top-ranking males exhibit higher stress hormone levels than second-ranking males in a wild baboon society suggesting that being at the very top of a social hierarchy may be more costly than previously thought.
REFERENCES AND NOTES
- 1.Clutton-Brock TH, editor. Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems. Chicago and London: Univ. Chicago Press; 1988. [Google Scholar]
- 2.Ellis L. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol. Sociobiol. 1995;16:257–333. [Google Scholar]
- 3.Cowlishaw G, Dunbar RIM. Dominance rank and mating success in male primates. Anim. Behav. 1991;41:1045–1056. [Google Scholar]
- 4.Pusey A, Williams J, Goodall J. The influence of dominance rank on the reproductive success of female chimpanzees. Science. 1997;277:828–831. doi: 10.1126/science.277.5327.828. [DOI] [PubMed] [Google Scholar]
- 5.Dixson AF. Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes and Human Beings. New York: Oxford Univ. Press; 1998. [Google Scholar]
- 6.Cheney DL, et al. Reproduction and Fitness in Baboons: Behavioral, Ecological, and Life History Perspectives. In: Swedell L, Leigh SR, editors. Springer Series Developments in Primatology: Progress and Prospects. New York: Springer; 2006. pp. 147–176. [Google Scholar]
- 7.Johnson SE. Reproduction and Fitness in Baboons: Behavioral, Ecological, and Life History Perspectives. In: Swedell L, Leigh SR, editors. Springer Series Developments in Primatology: Progress and Prospects. New York: Springer; 2006. pp. 177–198. [Google Scholar]
- 8.Rodriguez-Llanes JM, Verbeke G, Finlayson C. Reproductive benefits of high social status in male macaques (Macaca) Anim. Behav. 2009;78:643–648. [Google Scholar]
- 9.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 10.Muller MN, Wrangham RW. Dominance, cortisol and stress in wild chimpanzees. Behav. Ecol. Sociobiol. 2004;55:332–340. [Google Scholar]
- 11.Creel S. Social dominance and stress hormones. Trends Ecol. Evol. 2001;16:491–497. [Google Scholar]
- 12.Abbott DH, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 13.Sapolsky RM. Endocrine aspects of social instability in the olive baboon (Papio anubis) Am. J. Primatol. 1983;5:365–379. doi: 10.1002/ajp.1350050406. [DOI] [PubMed] [Google Scholar]
- 14.Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992;17:701–702. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- 15.Setchell JM, Smith T, Wickings EJ, Knapp LA. Stress, social behaviour, and secondary sexual traits in a male primate. Horm. Behav. 2010;58:720–728. doi: 10.1016/j.yhbeh.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM, Whitten PL. Correlates of stress in free-ranging male chacma baboons, Papio hamadryas ursinus. Anim. Behav. 2005;70:703–713. [Google Scholar]
- 17.Nelson RJ. An Introduction to Behavioral Endocrinology. ed. 3. Sunderland, MA: Sinauer Associates; 2005. chap. 11. [Google Scholar]
- 18.Roberts ML, Buchanan KL, Evans MR. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 2004;68:227–239. [Google Scholar]
- 19.Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The "Challenge Hypothesis": theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 1990;136:829–846. [Google Scholar]
- 20.Michael RP, Zumpe D. A review of hormonal factors influencing the sexual and aggressive behavior of macaques. Am. J. Primatol. 1993;30:213–241. doi: 10.1002/ajp.1350300306. [DOI] [PubMed] [Google Scholar]
- 21.Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: when and how. J. Neuroendocrinol. 2003;15:711–724. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- 22.Bercovitch FB, Ziegler TE. Current topics in primate socioendocrinology. Annu. Rev. Anthropol. 2002;31:45–67. [Google Scholar]
- 23.Information on methods is available on Science Online
- 24.Gesquiere LR, Onyango PO, Alberts SC, Altmann J J. Endocrinology of year-round reproduction in a highly seasonal habitat: environmental variability in testosterone and glucocorticoids in baboon males. Amer. J. Phys. Anthropol. 2010 doi: 10.1002/ajpa.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheney DL, Seyfarth RM. Stress and coping mechanisms in female primates. Adv. Stud. Behav. 2009;39:1–44. [Google Scholar]
- 26.Rasmussen KLR. Changes in the activity budgets of yellow baboons (Papio cynocephalus) during sexual consortships. Behav. Ecol. Sociobiol. 1985;17:161–170. [Google Scholar]
- 27.Alberts SC, Altmann J, Wilson ML. Mate guarding constrains foraging activity of male baboons. Anim. Behav. 1996;51:1269–1277. [Google Scholar]
- 28.Alberts SC, Watts HE, Altmann J. Queuing and queue-jumping: long-term patterns of reproductive skew among male savannah baboons. Anim. Behav. 2003;65:821–840. [Google Scholar]
- 29.Alberts SC, Buchan JC, Altmann J. Sexual selection in wild baboons: from mating opportunities to paternity success. Anim. Behav. 2006;72:1177–1196. [Google Scholar]
- 30.Mills SC, et al. Testosterone-mediated effects on fitness-related phenotypic traits and fitness. Am. Nat. 2009;173:475–487. doi: 10.1086/597222. [DOI] [PubMed] [Google Scholar]
- 31.Muehlenbein MP. Intestinal parasite infections and fecal steroid levels in wild chimpanzees. Amer. J. Phys. Anthropol. 2006;130:546–550. doi: 10.1002/ajpa.20391. [DOI] [PubMed] [Google Scholar]
- 32.Hausfater G, Watson DF. Social and reproductive correlates of parasite ova emissions by baboons. Nature. 1976;262:688–689. doi: 10.1038/262688a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.