Abstract
Mutations in the DFNA5 gene are known to cause autosomal dominant non-syndromic hearing loss (ADNSHL). To date, five DFNA5 mutations have been reported, all of which were different in the genomic level. In this study, we ascertained a Korean family with autosomal dominant, progressive and sensorineural hearing loss and performed linkage analysis that revealed linkage to the DFNA5 locus on chromosome 7. Sequence analysis of DFNA5 identified a 3-bp deletion in intron 7 (c.991-15_991-13del) as the cause of hearing loss in this family. As the same mutation had been reported in a large Chinese family segregating DFNA5 hearing loss, we compared their DFNA5 mutation-linked haplotype with that of the Korean family. We found a conserved haplotype, suggesting that the 3-bp deletion is derived from a single origin in these families. Our observation raises the possibility that this mutation may be a common cause of autosomal dominant progressive hearing loss in East Asians.
Keywords: DFNA5, founder effect, hearing loss, mutation, non-syndromic
INTRODUCTION
Hereditary hearing loss is a clinically and genetically heterogeneous disorder. Autosomal dominant non-syndromic hearing loss (ADNSHL) occurs in approximately 20% of cases and usually shows a postlingual onset and progression.1 To date, approximately 59 loci (DFNA) for ADNSHL have been mapped and 22 of the causative genes have been identified.2
DFNA5 was mapped to chromosome 7p153,4 and the causative gene DFNA5 was identified in a large Dutch family.5 Since then, five different mutations have been identified in DFNA5.5–9 Except for one truncating mutation in exon 5, all mutations were located in intron 7 and 8, which leads to skipping of exon 8, a frameshift, and premature truncation of the protein. Several lines of evidence support the hypothesis that this DFNA5-associated hearing is caused by a gain-of-function mutation through a unique mechanism of skipping a specific exon.7 The age of onset of DFNA5 hearing loss varies from zero to 50 years, but all reported cases are non-syndromic, sensorineural and progressive.
In this study, we mapped ADNSHL to chromosome 7p15 in a Korean family and identified a deletion of three nucleotides in the polypyrimidine tract of intron 7 (c.991-15_991-13del, previously described as IVS7-17delCTT) in DFNA5. The same mutation has previously been reported in a Chinese family showing ADNSHL.9 We identified a shared DFNA5-linked haplotype that suggests a common ancestral founder for the mutation in the two families.
MATERIALS AND METHODS
Subjects and phenotype analysis
A Korean family segregating autosomal dominant hearing impairment was recruited from the Department of Otorhinolaryngology, Head and Neck Surgery, Ajou University, Suwon, Korea (Figure 1a). In all, 14 family members, including 7 affected and 7 normal individuals, participated in this study. After a physical and otoscopic examination, pure tone audiometry (PTA) was performed in a sound-treated room and the average of the thresholds at 0.5, 1, 2, 4 and 8 kHz was calculated. Vestibular function was also tested. All participants provided written informed consent according to the protocol approved by the ethics committee of Ajou University Hospital before the study.
Figure 1.
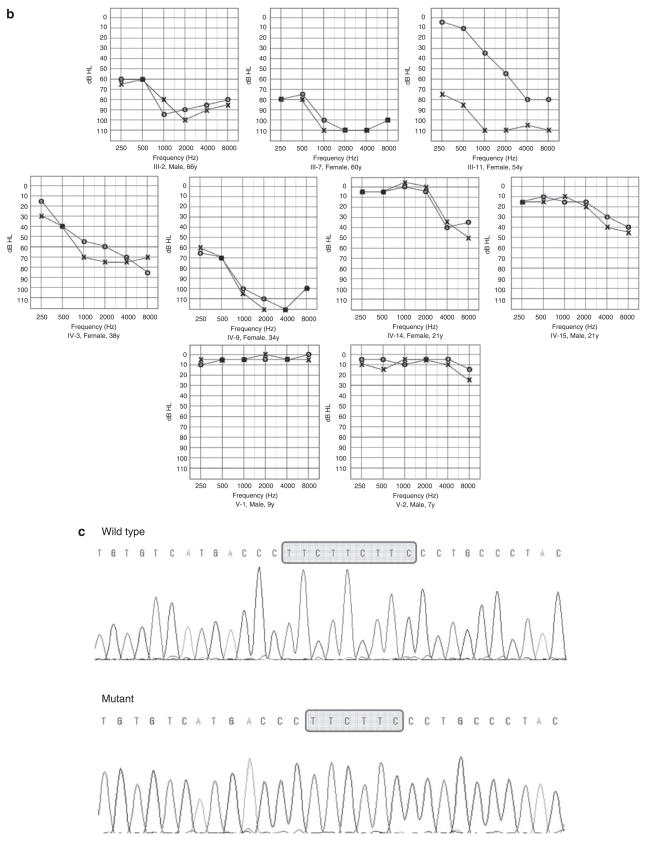
(a) Pedigree and haplotypes of the Korean family with autosomal dominant non-syndromic hearing loss (ADNSHL). The DFNA5-linked haplotype is enclosed in the box. Markers were selected according to their physical location on the human genome map (National Center for Biotechnology Information: www.ncbi.nlm.nih.gov). (b) Pure tone audiograms. The horizontal axis shows pure tone stimulus frequency (Hz); the vertical axis gives hearing threshold (dB HL). This cross-sectional analysis is consistent with a late onset, progressive hearing loss that initially affects high frequencies and deteriorates over time to involve all frequencies. (c) Nucleotide sequence of subcloned wild-type and mutant allele PCR products.
Genotype analysis
Genomic DNA was extracted from peripheral blood using a FlexiGene DNA extraction kit (Qiagen, Hilden, Germany). Samples were genotyped for markers flanking known 41 DFNA loci, using marker information provided by the Hereditary Hearing Loss Homepage (http://webh01.ua.ac.be/hhh/).
All 10 exons and flanking intronic sequences of DFNA5 were amplified by polymerase chain reaction (PCR) for sequence analysis. The amplified DNA fragments were purified using exonuclease I (USB Corporation, Cleveland, OH, USA) and shrimp alkaline phosphatase (USB Corporation) and sequenced using ABI PRISM Big Dye Terminator Cycle Sequencing Kit (V3.1) and an ABI PRISM 3130XL DNA analyzer (Applied Biosystems, Foster City, CA, USA). The data were analyzed using ABI Sequencing Analysis (v.5.0) and Lasergene–SeqMan software. When an identified sequence variant segregated with the phenotype within the family, the presence of the variant was evaluated in 70 unrelated Korean subjects with normal hearing. The PCR product with a 3-bp deletion (c.991-15_991-13del) was subcloned into pCR2.1-TOPO and sequenced to confirm the presence of the 3-bp deletion in the mutated allele.
Haplotype analysis
Intragenic single-nucleotide polymorphisms (SNPs) of DFNA5 were analyzed using PCR amplification and DNA sequencing, and four microsatellite markers flanking the DFNA5 gene were genotyped (Table 1, Supplementary 1). The Chinese samples were kindly provided by Dr Xiangyin Kong.9
Table 1.
DFNA5-linked microsatellite and SNP genotypes among Korean and Chinese families
| Nucleotide variations | SNP/STR marker | Distance to c.991-15_991-13del (bp) | Linked allelea (bp)
|
||
|---|---|---|---|---|---|
| Korean | Chinese | Allele frequency | |||
| D7S2525 | 745 871 | 185 | 185 | ND | |
| D7S1791 | 396 627 | 175 | 157 | ND | |
| c.1200A>G | rs17149912 | 3573 | A | G | 0.744 |
| c.1184-101 G>A | rs2240005 | 3456 | G | G | 0.733 |
| c.1184-133 T>C | rs2074142 | 3424 | T | T | 0.756 |
| c.1184-753 G>C | rs4719777 | 2804 | C | C | 0.689 |
| c.991-15_991-13 | c.991-15_991-13del | 0 | del | del | ND |
| c.991-27 A>G | rs2269812 | 13 | A | A | 0.773 |
| c.991-67 G>A | rs57866118 | 53 | G | G | ND |
| c.862+1393G>A | rs2240006 | 2441 | G | G | 0.79 |
| c.862+813insA | rs59938883 | 3022 | — | — | ND |
| c.862+644 G>T | rs17149943 | 3190 | G | G | 0.854 |
| c.862+87insC | rs35529766 | 3747 | — | — | ND |
| c.619G>A | rs12540919 | 10 942 | G | G | 0.811 |
| c.489 G>A | rs754555 | 12 744 | A | A | 0.522 |
| c.424C>A | rs754554 | 12 809 | A | A | 0.511 |
| c.399-15 G>A | rs754553 | 12 843 | A | A | 0.522 |
| c.212-30 C>T | rs2521768 | 38 394 | T | T | 0.791 |
| c.-19-36 C>T | rs10235527 | 43 440 | T | C | 0.096 |
| GATA137A12 | 1 607 773 | 220 | 212 | ND | |
| D7S2458 | 2 654 733 | 131 | 131 | ND | |
Abbreviations: ND, not determined; SNP, single-nucleotide polymorphism; STR, short tandem repeat.
Total length of STR marker is indicated as allele. The allele frequency is given for each SNP and was based on the Hapmap. When two families had in the different alleles (for example, rs17149912), the allele frequency in the Korean family is represented.
RESULTS
We ascertained a five-generation Korean family segregating NSHL. Among 14 members examined for clinical features, seven (two males and five females) were affected. The pattern of inheritance was consistent with autosomal dominant or X-linked inheritance (Figure 1a), although the similar severity of hearing loss observed in females and males of similar ages is more suggestive of autosomal inheritance. The hearing loss initially affects high frequencies in the second decade of life, and progresses with increasing age to a down-sloping audiometric configuration (Figure 1b). None of the patients had vestibular symptoms or other clinical abnormalities.
As autosomal dominant inheritance seemed most likely, we analyzed markers linked to known DFNA loci and identified linkage to DFNA5 on chromosome 7p15. We genotyped additional markers to confirm linkage and fine-map the genetic interval. Haplotype analysis revealed a 19-cM critical interval between D7S2557 and D7S1808, in which the DFNA5 locus resides. All 10 exons and flanking intronic sequences of DFNA5 were screened by direct sequencing of genomic PCR amplicons. A 3-bp deletion (c.991-15_991-13del) was identified in intron 7. All affected family members were heterozygous for this mutation. We confirmed this mutation by sequencing subcloned PCR products (Figure 1c). The 3-bp deletion co-segregated with DFNA5 hearing loss in the family and was not found among 70 control Korean samples. Two sons (V-1, 9 years; V-2, 7 years) of an affected member (IV-3) had normal hearing and were heterozygous for the 3-bp deletion (Figure 1b).
As this mutation has previously been reported for a Chinese DFNA5 family,9 we compared the DFNA5-linked haplotypes of flanking microsatellite markers and intragenic SNPs between the Korean and Chinese DFNA5 alleles (Table 1 and Supplementary 1). As shown in Table 1, comparison of the linked haplotypes showed identical alleles within the DFNA5 gene, but different alleles flanking the gene.
DISCUSSION
There are five different reported DFNA5 mutations identified in European and Asian families segregating DFNA5 hearing loss. The clinical features of all the DFNA5 families are similar and all the mutations cause exon 8 skipping at the mRNA level.7 This is the first report of a specific DFNA5 mutation identified in more than one population.
A founder effect has been described for the GJB2 mutations 35delG in Caucasians and 235delC in East Asians, as well as for the SLC26A4 mutation H723R in Asians.10–12 In this study we show that a founder effect may also exist for the 3-bp deletion mutation of DFNA5 gene among East Asians. The small region of the shared mutation-linked haplotype indicates that a common founder would have been very ancient. However, the most closely linked SNPs are less informative than the more distant microsatellite markers and we cannot rule out the possibility that the mutational event occurred twice on different ancestor chromosomes.
Genetic screening for the 3-bp deletion in East Asian ADNSHL patients is needed to assess its prevalence among this population. These studies will further define the genetic epidemiology of deafness and facilitate genetic testing for hearing loss and deafness in East Asians.
Acknowledgments
We are grateful to the family for their collaboration in this study. We also thank Dr Xiangyin Kong for providing Chinese samples. This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (R01-2008-000-10431-0), a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea, A080588 (UKK) and the National Institutes of Health intramural research fund Z01-DC-000060.
Footnotes
Supplementary Information accompanies the paper on Journal of Human Genetics website (http://www.nature.com/jhg)
References
- 1.Morton NE. Genetic epidemiology of hearing impairment. Ann NY Acad Sci. 1991;630:16–31. doi: 10.1111/j.1749-6632.1991.tb19572.x. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee A, Jalvi R, Pandey N, Rangasayee R, Anand A. A novel locus DFNA59 for autosomal dominant nonsyndromic hearing loss maps at chromosome 11p14.2-q12.3. Hum Genet. 2009;124:669–675. doi: 10.1007/s00439-008-0596-3. [DOI] [PubMed] [Google Scholar]
- 3.van Camp G, Coucke P, Balemans W, van Velzen D, van de Bilt C, van Laer L, et al. Localization of a gene for non-syndromic hearing loss (DFNA5) to chromosome 7p15. Hum Mol Genet. 1995;4:2159–2163. doi: 10.1093/hmg/4.11.2159. [DOI] [PubMed] [Google Scholar]
- 4.Van Laer L, Van Camp G, van Zuijlen D, Green ED, Verstreken M, Schatteman I, et al. Refined mapping of a gene for autosomal dominant progressive sensorineural hearing loss (DFNA5) to a 2-cM region, and exclusion of a candidate gene that is expressed in the cochlea. Eur J Hum Genet. 1997;5:397–405. [PubMed] [Google Scholar]
- 5.Van Laer L, Huizing EH, Verstreken M, van Zuijlen D, Wauters JG, Bossuyt PJ, et al. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat Genet. 1998;20:194–197. doi: 10.1038/2503. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff AM, Luijendijk MW, Huygen PL, van Duijnhoven G, De Leenheer EM, Oudesluijs GG, et al. A novel mutation identified in the DFNA5 gene in a Dutch family: a clinical and genetic evaluation. Audiol Neurootol. 2004;9:34–46. doi: 10.1159/000074185. [DOI] [PubMed] [Google Scholar]
- 7.Cheng J, Han DY, Dai P, Sun HJ, Tao R, Sun Q, et al. A novel DFNA5 mutation, IVS8+4 A4G, in the splice donor site of intron 8 causes late-onset non-syndromic hearing loss in a Chinese family. Clin Genet. 2007;72:471–477. doi: 10.1111/j.1399-0004.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Laer L, Meyer NC, Malekpour M, Riazalhosseini Y, Moghannibashi M, Kahrizi K, et al. A novel DFNA5 mutation does not cause hearing loss in an Iranian family. J Hum Genet. 2007;52:549–552. doi: 10.1007/s10038-007-0137-2. [DOI] [PubMed] [Google Scholar]
- 9.Yu C, Meng X, Zhang S, Zhao G, Hu L, Kong X. A 3-nucleotide deletion in the polypyrimidine tract of intron 7 of the DFNA5 gene causes nonsyndromic hearing impairment in a Chinese family. Genomics. 2003;82:575–579. doi: 10.1016/s0888-7543(03)00175-7. [DOI] [PubMed] [Google Scholar]
- 10.Park HJ, Shaukat S, Liu XZ, Hahn SH, Naz S, Ghosh M, et al. Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. J Med Genet. 2003;40:242–248. doi: 10.1136/jmg.40.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Laer L, Coucke P, Mueller RF, Caethoven G, Flothmann K, Prasad SD, et al. A common founder for the 35delG GJB2 gene mutation in connexin 26 hearing impairment. J Med Genet. 2001;38:515–518. doi: 10.1136/jmg.38.8.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan D, Park HJ, Ouyang XM, Pandya A, Doi K, Erdenetungalag R, et al. Evidence of a founder effect for the 235delC mutation of GJB2 (connexin 26) in East Asians. Hum Genet. 2003;114:44–50. doi: 10.1007/s00439-003-1018-1. [DOI] [PubMed] [Google Scholar]