Abstract
Quetiapine extended-release (quetiapine-XR) has been studied in patients with schizophrenia, bipolar mania, bipolar depression, major depressive disorder (MDD), and generalized anxiety disorder (GAD). The purpose of this study was to compare the tolerability and sensitivity of quetiapine-XR among these psychiatric conditions. The discontinuation due to adverse events (DAEs) and reported somnolence in randomized, double-blind, placebo-controlled studies of quetiapine-XR in these psychiatric conditions were examined. The absolute risk reduction or increase and the number needed to treat to benefit (NNTB) or harm (NNTH) for DAEs and reported somnolence of quetiapine-XR ≥300 mg/d relative to placebo were estimated. Data from one study in schizophrenia (n=465), one in mania (n=316), one in bipolar depression (n=280), two in refractory MDD (n=624), two in MDD (n=669) and three in GAD (n=1109) were available. The risk for DAEs of quetiapine-XR relative to placebo was significantly increased in bipolar depression (NNTH=9), refractory MDD (NNTH=8), MDD (NNTH=9), and GAD (NNTH=5), but not in schizophrenia and mania. The risk for reported somnolence of quetiapine-XR relative to placebo was significantly increased in schizophrenia (600 mg/d NNTH=15 and 800 mg/d NNTH=11), mania (NNTH=8), bipolar depression (NNTH=4), refractory MDD (NNTH=5), MDD (NNTH=5) and GAD (NNTH=5). These results suggest that patients with GAD had the poorest tolerability during treatment with quetiapine-XR, but they had a similar sensitivity as those with bipolar depression and MDD. Patients with schizophrenia or mania had a higher tolerability and a lower sensitivity than those with bipolar depression, MDD, or GAD.
Keywords: Atypical antipsychotic, bipolar disorder, generalized anxiety disorder, major depressive disorder, schizophrenia
Introduction
Schizophrenia, mood disorders, and anxiety disorders have been considered to be nosologically and aetiologically different disorders. Their differences have been supported by their phenomenological presentations, clinical courses, and neurobiological findings (De Luca et al. 2006; McDonald et al. 2004; Muir et al. 2001; Murray et al. 2004; Sun et al. 2009; Takahashi et al. 2009). However, the distinctions among these disorders have also been challenged by overlapping psychopathology, neuropsychology, and neurobiology (Altindag et al. 2006; Buckley et al. 2009; Ciapparelli et al. 2005; Craddock et al. 2005; Grant et al. 2005; Maier et al. 2005; Medved et al. 2001; Milak et al. 2007; Moffitt et al. 2007; Rice et al. 2004; Schretlen et al. 2007; Van Snellenberg & de Candia, 2009).
In our previous studies, we found that during acute treatment with haloperidol and newer atypical antipsychotics, patients with schizophrenia, bipolar mania, or bipolar depression had differential risks for the discontinuation due to adverse events (DAEs), reported somnolence and sedation, and extrapyramidal side-effects (Gao et al. 2008a, b). Patients with schizophrenia had a better tolerability and a lower sensitivity than those with bipolar disorder, especially when compared to those with bipolar depression. More recently, when comparing the tolerability and sensitivity of atypical antipsychotics in patients with bipolar depression, major depressive disorder (MDD), or generalized anxiety disorder (GAD), we found that patients with GAD had the highest risk for DAEs, but had a similar risk as those with MDD or bipolar depression for reported somnolence (Gao et al. in press).
Currently, with the exception of clozapine, all atypical antipsychotics have been approved by the Food and Drug Administration of the United States (US FDA) for the acute treatment of schizophrenia, bipolar mania and some have been approved for bipolar depression, bipolar maintenance, or adjunctive treatment to antidepressants for treatment-refractory MDD (Gajwani et al. 2006; Fleurence et al. 2009; Gao et al. 2005, in press; Ketter et al. 2006; Lenderts & Kalali, 2009). In addition, atypical antipsychotics have also increasingly been used off-label for refractory anxiety disorders (Gao et al. 2006, 2009; Pae et al. 2008). It remains unclear whether patients with MDD or an anxiety disorder have a similar tolerability and/or sensitivity to atypical antipsychotics compared to those with schizophrenia or bipolar mania. Such information will help clinicians to use these drugs more properly. In addition, the relationship between tolerability and sensitivity in each psychiatric condition may shed light on how patients with each disorder perceive and manage unwanted side-effects.
The quetiapine extended-release (XR) formulation produces a smoother pharmacokinetic profile and a delayed onset of sedation as compared to the quetiapine immediate-release (IR) formulation (Datto et al. 2009a; Figueroa et al. 2009; Mamo et al. 2008). Its efficacy and safety have been investigated in the acute treatment of patients with schizophrenia (Kahn et al. 2007), bipolar mania (Datto et al. 2009c), bipolar depression (Datto et al. 2009b), MDD (Cutler et al. 2009; El-Khalili et al. 2008a; Weisler et al. 2009), refractory MDD (Bauer et al. 2009; El-Khalili et al. 2008b), and GAD (Chouinard et al. 2008; Joyce et al. 2008; Merideth et al. 2008), but quetiapine-XR monotherapy has only been approved by the US FDA for schizophrenia and bipolar disorder, and as adjunctive therapy to antidepressants for treatment-resistant MDD. On the other hand, this form of marketing-oriented research approach with quetiapine-XR provides a unique dataset to indirectly compare the tolerability and sensitivity of patients with these different psychiatric conditions. Therefore, this review was undertaken to use the data on quetiapine-XR to compare the risks for DAEs and reported somnolence relative to placebo in the acute treatment of schizophrenia, bipolar mania, bipolar depression, MDD, refractory MDD, and GAD. The intent of such a comparison is 2-fold. First, is to provide evidence for clinicians as to whether any differences in tolerability and sensitivity profiles exist with quetiapine-XR across different psychiatric disorders. If so, clinicians should choose the most appropriate dose based in part upon diagnosis when using multi-functional drugs like quetiapine, either for the approved indication or off-label use. The second purpose is to provide evidence for researchers who are interested in studying multi-functional drugs in different psychiatric conditions. If patients with different psychiatric conditions have different tolerabilities and sensitivities to quetiapine-XR, researches may need to select an optimal dose of a multiple-functional drug for a specific psychiatric disorder to minimize the rate of DAEs.
Methods
English-language literature published and cited in Medline from January 1966 to December 2009 was initially searched with the terms: schizophrenia, mania, bipolar depression, bipolar disorder, MDD, or GAD, atypical antipsychotic, brand and generic names of aripiprazole, olanzapine, quetiapine, quetiapine-XR, risperidone, or ziprasidone, randomized, and placebo-controlled trial. The initial search was supplemented by manual examination of cross-references. Only quetiapine-XR was found being investigated in the treatment of schizophrenia, bipolar disorder, MDD, and GAD. Therefore, the original placebo-controlled trials of quetiapine-XR in the acute treatment of schizophrenia, mania, bipolar depression, MDD, and GAD were further examined. Studies designed for schizoaffective disorder, prodromal psychosis, or maintenance treatments were excluded for further examination. In addition, randomized, double-blind, placebo-controlled, monotherapy studies of quetiapine-XR in the five psychiatric conditions presented at major scientific meetings were also reviewed.
After reviewing all relevant articles, we found that adverse events were reported differently in different studies of quetiapine-XR treatment; however, the incidences of DAEs, an overall indicator of tolerability and reported somnolence, and an indicator of sensitivity were reported in all studies. Thus comparing the incidences of DAEs and reported somnolence in the five psychiatric conditions could shed light on the differential tolerability and sensitivity of various populations to quetiapine-XR. Since the detailed comparisons of the tolerability and sensitivity between patients with bipolar depression, MDD, and GAD to atypical antipsychotics including quetiapine-XR have been conducted (Gao et al. in press) and a dose lower than quetiapine-XR 300 mg/d in schizophrenia, mania, bipolar depression was never studied, the focus of this study is solely to compare the tolerability and sensitivity of five psychiatric conditions at higher doses of quetiapine-XR (≥300 mg/d).
Number needed to treat (NNT) is defined as the number of patients one would expect to treat with T to have one more success (or one less failure) than if the same number were treated with C (Kraemer & Kupfer, 2006). `T' refers to treatment and `C' refers to control. Therefore, according to the outcome of success or failure relative to control, the NNT can be estimated as number needed to treat to benefit (NNTB) or harm (NNTH). Mathematically, NNTB=1/absolute risk reduction (ARR) and NNTH=1/absoluate risk increase (ARI). These measures are believed to provide more clinically relevant information than relative risk reduction or odds ratios (Jaeschke et al. 1995) and have been advocated for use in systematic reviews (Guyatt et al. 2002; McQuay & Moore, 1997). In this review, the assumption was that quetiapine-XR would cause a greater occurrence of DAEs and reported somnolence than placebo. The NNT was calculated as 1/(control event rate – experimental event rate). Therefore a negative value, presented with a NNTH and an ARI, was indicative of a higher risk for DAEs or somnolence with quetiapine-XR than with placebo. On the other hand, a positive value, presented with a NNTB and an ARR, was indicative of a lower risk for DAEs or somnolence with quetiapine-XR than with placebo. For more than one clinical trials of a similar study design, the values of outcome measures were recalculated based on a pooled sample.
Significance testing between quetiapine-XR and placebo were presented with confidence interval (95% CI=mean±1.96 standard error). The rationale for the use of CI, instead of p value, is that the CI not only provides a more quantifiable comparison, but also helps to interpret the result, especially when there is no statistical significance (Altman 2005; Montori et al. 2004). For quetiapine-XR and placebo comparisons, a statistical significance was claimed when a CI did not include 0. For comparisons among the five psychiatric conditions, statistical significance was claimed when there was no overlap between their 95% CIs. Such conservative interpretation might miss statistical significance (Belia et al. 2005; Cumming & Finch 2005; Payton et al. 2003), but the NNTB or NNTH and its CI overlap can help clinicians determine the degree of clinical significance. Forest plots were created with risk differences between quetiapine-XR ≥300 mg/d and placebo.
Results
One randomized, double-blind, placebo-controlled, monotherapy in schizophrenia (Kahn et al. 2007), one in bipolar mania (Datto et al. 2009c), one in bipolar depression (Datto et al. 2009b), two in MDD (Cutler et al. 2009; Weisler et al. 2009), two in refractory MDD (Bauer et al. 2009; El-Khalili et al. 2008b), and three in GAD (Chouinard et al. 2008; Joyce et al. 2008; Merideth et al. 2008) were identified.
DAEs in schizophrenia, mania, bipolar depression, MDD and GAD
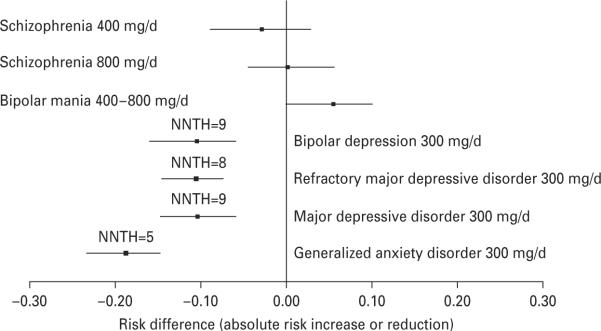
In patients with schizophrenia, there was no significant difference between quetiapine-XR 400 mg/d, 600 mg/d, or 800 mg/d and placebo in DAEs (Table 1). Similarly, in patients with acute mania, quetiapine-XR 400–800 mg/d was as well tolerated as placebo (Table 1) in terms of DAEs. However, in bipolar depression, there was a significantly higher risk for DAEs with quetiapine-XR 300 mg/d than placebo, with a mean ARI of 10.7% (95% CI −17.2 to −5.0) and a NNTH of 9 (95% CI −20 to −6), respectively (Table 1).
Table 1.
Risk estimate for discontinuation due to adverse events of quetiapine-XR ≥300 mg/d relative to placebo in the acute treatment of schizophrenia, mania, bipolar depression, MDD, and GAD
| No. of patients |
|||||||
|---|---|---|---|---|---|---|---|
| Duration | ARIor ARR(%) | NNTB or NNTH | |||||
| Diagnosis | Trial | Treatment arm | (wk) | Total | DAEs | Mean (95 % CI) | Mean (95 % CI) |
| Schizophrenia | Kahn et al. (2007) | Quetiapine-XR 400 mg/d | 6 | 113 | 6 | −2.8 (−8.8 to 2.7) | −36 (37 to −11) |
| Quetiapine-XR 600 mg/d | 113 | 3 | −0.1 (−0.5 to 4.9) | −889 (21 to −19) | |||
| Quetiapine-XR 800 mg/d | 121 | 3 | 0.1 (−4.5 to 5.0) | 1586 (20 to −21) | |||
| Placebo | 118 | 3 | |||||
| Mania | Datto et al. (2009c) | Quetiapine-XR 400–800 mg/d | 3 | 155 | 4 | 4.9 (−0.1 to 10.2) | 21 (10 to −934) |
| Placebo | 161 | 12 | |||||
| Bipolar depression | Datto et al. (2009b) | Quetiapine-XR 300 mg/d | 8 | 140 | 17 | −10.7 (−17.2 to −5.0) | −9 (−20 to −6) |
| Placebo | 140 | 2 | |||||
| MDD | Cutler et al. (2009) | Quetiapine-XR 300 mg/d | 6 | 331 | 57 | −11.3 (−16.2 to −6.5) | −9 (−15 to −6) |
| Weisler et al. (2009) | Placebo | 338 | 20 | ||||
| Refractory MDD | El-Khalili et al. (2008b) | Quetiapine-XR 300 mg/d | 6 | 315 | 46 | −12.3 (−16.8 to −8.2) | −8 (−12 to −6) |
| Bauer et al. (2009) | Placebo | 309 | 7 | ||||
| GAD | Chouinard et al. (2008) | Quetiapine-XR 300 mg/d | 8 | 444 | 110 | − 19.1 (−23.5 to −14.8) | −5 (−7 to −4) |
| Joyce et al. (2008) | Placebo | 665 | 38 | ||||
| Merideth et al. (2008) | |||||||
DAEs, Discontinuation due to adverse events; ARI, absolute risk increase; ARR, absolute risk reduction; NNTB, number needed to treat to benefit; NNTH, number needed to treat to harm; CI, confidence interval; MDD, major depressive disorder; GAD, generalized anxiety disorder.
In patients with MDD, there was a significantly higher risk for DAEs with quetiapine-XR 300 mg/d than with placebo (Table 1). The mean ARI and NNTH were 11.3% (95% CI −16.2 to −6.5) and 9 (95% CI −15 to −6). Similarly, in patients with refractory MDD, there was also a significantly higher risk for DAEs with quetiapine 300 mg/d than with placebo. The mean ARI and NNTH were 12.3% (95% CI −16.8 to −8.2) and 9 (95% CI −12 to −6), respectively.
In patients with GAD, there was a significantly higher risk for DAEs with quetiapine-XR 300 mg/d than with placebo (Table 1). The mean ARI and NNTH were 19.1% (95% CI −23.5 to −14.8) and 5 (95% CI −7 to −4), respectively.
As illustrated in Fig. 1, there were no significant differences in the risk for DAEs of quetiapine-XR 400 mg/d or 800 mg/d relative to its placebo in schizophrenia and that of quetiapine-XR 400– 800 mg/d relative to its placebo in mania. There were also no significant differences in the risk for DAEs of quetiapine-XR 300 mg/d relative to its placebo in the patients with bipolar depression, MDD, refractory MDD, or GAD because their 95% CIs of ARI overlapped (Fig. 1). However, the 95% CIs of ARI of quetiapine-XR 300 mg/d relative to its placebo in bipolar depression, MDD, refractory MDD, and GAD did not overlap with that of quetiapine-XR 800 mg/d in schizophrenia and that of quetiapine-XR 400– 800 mg/d in mania. Moreover, patients with mania exhibited a trend for better tolerability to quetiapine-XR than to placebo. In contrast, patients with bipolar depression, MDD, or GAD had a better tolerability to placebo than to quetiapine-XR. In addition, the 95% CI of ARI of quetiapine-XR 300 mg/d relative to its placebo in GAD did not overlap with that of quetiapine-XR 400 mg/d relative to placebo in schizophrenia and only marginally overlapped with those of quetiapine-XR 300 mg/d relative to its placebo in bipolar depression, MDD, and refractory MDD.
Fig. 1.
Risk for discontinuation due to adverse events relative to placebo during quetiapine-XR ≥300 mg/d treatment of schizophrenia, bipolar mania, bipolar depression, major depressive disorder, and generalized anxiety disorder. NNTH, Number needed to treat to harm.
Reported somnolence in schizophrenia, mania, bipolar depression, MDD and GAD
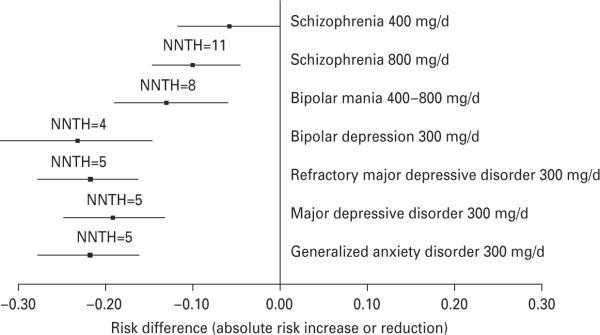
In patients with schizophrenia, there were no significant differences between quetiapine-XR 400 mg/d and its placebo in the risk for reported somnolence. However, there was a significantly higher risk for reported somnolence with quetiapine-XR 600 mg/d or 800 mg/d relative to its placebo (Table 2). The mean NNTH were 15 (95% CI −112 to −8) for quetiapine-XR 600 mg/d and 11 (95% CI −35 to −7) for quetiapine-XR 800 mg/d, respectively.
Table 2.
Risk estimate for reported somnolence of quetiapine-XR ≥300 mg/d relative to placebo in the acute treatment of schizophrenia, mania, bipolar depression, MDD, and GAD
| No. of patients |
|||||||
|---|---|---|---|---|---|---|---|
| Duration | Reported | ARI or ARR(%) | NNTB or NNTH | ||||
| Diagnosis | Trial | Treatment arm | (wk) | Total | somnolence | Mean (95% CI) | Mean (95% CI) |
| Schizophrenia | Kahn et al. (2007) | Quetiapine-XR 400 mg/d | 6 | 113 | 8 | −5.4 (−11.8 to 0.1) | −19 (927 to −8) |
| Quetiapine-XR 600 mg/d | 113 | 10 | −6.5 (−12.8 to −0.9) | −15 (−112 to −8) | |||
| Quetiapine-XR 800 mg/d | 121 | 14 | −8.7 (−15.1 to −2.8) | −11 (−35 to −7) | |||
| Placebo | 118 | 2 | |||||
| Mania | Datto et al. (2009 c) | Quetiapine-XR 400–800 mg/d | 3 | 151 | 25 | −12.2 (−19.3 to −5.5) | −8 (−18 to −5) |
| Placebo | 160 | 7 | |||||
| Bipolar depression | Datto et al. (2009 b) | Quetiapine-XR 300 mg/d | 8 | 137 | 40 | −23.5 (−32.0 to −14.5) | −4 (−7 to −3) |
| Placebo | 140 | 8 | |||||
| MDD | Cutler et al. (2009), | Quetiapine-XR 300 mg/d | 6 | 331 | 93 | −19.2 (−24.9 to −13.5) | −5 (−7 to −4) |
| Weisler et al. (2009) | Placebo | 338 | 30 | ||||
| Refractory MDD | El-Khalili et al. (2008b) | Quetiapine-XR 300 mg/d | 6 | 315 | 80 | −22.1 (−27.4 to −16.9) | −5 (−6 to −4) |
| Bauer et al. (2009) | Placebo | 309 | 10 | ||||
| GAD | Chouinard et al. (2008) | Quetiapine-XR 300 mg/d | 8 | 444 | 145 | −22.4 (−27.4 to −17.5) | −5 (−6 to −4) |
| Joyce et al. (2008) | Placebo | 665 | 68 | ||||
| Merideth et al. (2008) | |||||||
ARI, absolute risk increase; ARR, absolute risk reduction; NNTB, number needed to treat to benefit; NNTH, number needed to treat to harm; CI, confidence interval; MDD, major depressive disorder; GAD, generalized anxiety disorder.
In patients with mania, there was a significantly higher risk for reported somnolence with quetiapine-XR 400–800 mg/d relative to its placebo (Table 2). The mean ARI and NNTH were 12.2% (95% CI −19.3 to −5.5) and 8 (95% CI −18 to −5), respectively. Similarly, in patients with bipolar depression, there was also a significantly higher risk for reported somnolence with quetiapine-XR 300 mg/d relative to its placebo (Table 2). The mean ARI and NNTH were 23.5% (95% CI −32.0 to −14.5) and 4 (95% CI −7 to −3), respectively.
In patients with MDD, quetiapine-XR 300 mg/d had a significantly higher risk for reported somnolence relative to its placebo (Table 2). In patients with refractory MDD, the mean ARI and NNTH were 22.1% (95% CI −27.4 to −16.9) and 5 (95% CI −6 to −4), respectively. In patients with non-refractory MDD, the mean ARI and NNTH were 19.2% (95% CI −24.9 to −13.5) and 5 (95% CI −7 to −4), respectively.
Similarly, in patients with GAD, quetiapine-XR 300 mg/d had a significantly higher risk for reported somnolence than its placebo (Table 2). The mean ARI and NNTH were 22.4% (95% CI −27.4 to −17.5) and 5 (95% CI −6 to −4), respectively.
As illustrated in Fig. 2, there was no significant difference in the risk for reported somnolence between quetiapine-XR 400 mg/d or 800 mg/d relative to its placebo in schizophrenia and that of quetiapine-XR 400–800 mg/d relative to its placebo in mania. There were also no significant differences in the risk for reported somnolence of quetiapine-XR 300 mg/d relative to its placebo in bipolar depression, MDD, refractory MDD, and GAD because their 95% CIs of ARI overlapped (Fig. 2). However, the 95% CIs of ARI of quetiapine-XR 300 mg/d relative to its placebo in bipolar depression, MDD refractory MDD, and GAD did not overlap with that of quetiapine-XR 400 mg/d relative to its placebo in schizophrenia. Moreover, the 95% CIs of ARI of quetiapine-XR 300 mg/d relative to its placebo in refractory MDD and GAD did not overlap with that of quetiapine-XR 800 mg/d relative to its placebo in schizophrenia.
Fig. 2.
Risk for reported somnolence relative to placebo during quetiapine-XR ≥300 mg/d treatment of schizophrenia, bipolar mania, bipolar depression, major depressive disorder, and generalized anxiety disorder. NNTH, Number needed to treat to harm.
Discussion
To our knowledge, this review represents the first comparisons of the ARI and NNTH for DAEs and reported somnolence with quetiapine-XR in the acute treatment of schizophrenia, mania, bipolar depression, MDD and GAD, which provides an estimate for clinicians of how many patients need to be treated for one to discontinue the treatment due to intolerable adverse events, and one to report somnolence relative to placebo in these five psychiatric conditions. In addition, this study expands our previous findings that patients with schizophrenia, mania and bipolar depression had differential risks for DAEs, reported somnolence, and extrapyramidal side-effects during treatment with haloperidol and atypical antipsychotics (Gao et al. 2008a, b).
One important finding is that patients with schizophrenia and bipolar mania had the highest tolerability to quetiapine-XR treatment among all psychiatric disorders studied so far. This is supported by the finding that of insignificant difference between quetiapine-XR ≥400 mg/d and placebo in the risk for DAEs in patients with schizophrenia or bipolar mania, whereas significant differences emerged between quetiapine XR 300 mg/d and placebo in the risk for DAEs in patients with bipolar depression, MDD, and GAD. The higher tolerability in patients with schizophrenia and bipolar mania were further supported by (1) the majority of 95% CIs for the ARI at quetiapine-XR dosage of 300 mg/d in bipolar depression, MDD and GAD did not overlap with those of schizophrenia or mania (Table 1 and Fig. 1); (2) the doses of quetiapine-XR in schizophrenia and bipolar mania were higher than those used in the treatment of bipolar depression, MDD, and GAD. A higher tolerability to olanzapine and quetiapine-IR was also observed among patients with schizophrenia and bipolar mania compared to patients with bipolar depression (Gao et al. 2008a), suggesting that the different tolerabilities in the different psychiatric conditions are more likely due to the nature of illness of each individual psychiatric disorder.
Another important finding is that patients with GAD had the lowest tolerability to quetiapine-XR treatment as manifested by the smallest mean NNTH of 5 for quetiapine 300 mg/d relative to its placebo. The 95% CIs of ARI of quetiapine 300 mg/d in GAD overlapped with those of quetiapine-XR 300 mg/d in bipolar depression, MDD, and refractory MDD, suggesting that they are not statistically significant, but the clinical significance of the tolerability between patients with GAD and those with bipolar depression or MDD cannot be ignored. On the other hand, patients with bipolar depression, MDD, or refractory MDD had comparable tolerability to quetiapine-XR as reflected by a similar mean NNTH of 8–9 at a quetiapine-XR 300 mg/d dose in these three psychiatric conditions (Table 1, Fig. 1).
In terms of sensitivity, with the exception of quetiapine-XR 400 mg/d in patients with schizophrenia, all other patients studied were highly sensitive to quetiapine-XR relative to placebo. However, patients with bipolar depression, MDD and GAD had a higher sensitivity to quetiapine-XR than those with schizophrenia and bipolar mania as reflected by the fact that the 95% CIs of ARI reported for somnolence in those conditions either did not overlap or marginally overlapped with that in schizophrenia or mania. A higher risk for reported somnolence in patients with bipolar depression than those with bipolar mania or schizophrenia was also observed with the treatment of olanzapine and quetiapine-IR (Gao et al. 2008a). In contrast to the difference in tolerability between patients with bipolar depression or MDD and GAD, the sensitivity in these three psychiatric conditions was quite similar (Table 2, Fig. 2).
The reasons for the different tolerabilities and sensitivities to quetiapine-XR treatments in different psychiatric conditions are unclear. However, some disease-related or unrelated mechanisms might be involved. For example, previous exposure to anti-psychotics in patients with schizophrenia might cause `desensitization' to quetiapine-XR. It is also possible that underlying psychopathology in schizophrenia could decrease the sensitivity and increase the tolerability to antipsychotics including quetiapine-XR. For patients with acute mania, it has been speculated that an increase in dopamine release or an enhanced post-synaptic dopamine activity is associated with manic symptoms (Anand et al. 2002; McTavish et al. 2001; Silverstone, 1985). By blocking dopamine activity with quetiapine-XR, patients who are in an increased wakefully manic state may easily experience somnolence. Meanwhile, the increased dopamine activity may overcome somnolence and keep patients in the study.
Increased release of dopamine in the mesolimbic area is believed to play an important role in the genesis of anxiety disorders including GAD (Gao et al. 2006). Similar to patients in a manic state, the dopamine blockade may account for the higher risk of reported somnolence in patients with GAD. However, it is difficult to determine the magnitude of the effect of dopamine blockade on the tolerability and sensitivity in patients with GAD. Lower tolerability in patients with anxiety has also been reported with other medication treatments (Fava et al. 2008; Feske et al. 2000). Cognitive distortion of the severity of side-effects in patients with GAD was proposed as a cause for premature discontinuation from a study (Wurthmann et al. 1997). Similar sensitivity to quetiapine-XR among the patients with bipolar depression, MDD, and GAD, but a lower tolerability in patients with GAD, suggests that the perception and interpretation of the adverse events in patients with GAD may be different from those with bipolar depression and MDD.
In contrast to the potential blockade of possible high dopamine activity in patients with mania and GAD, the lower tolerability to quetiapine-XR in bipolar depression and MDD may be due to a decreased dopamine function. This assumption is supported by findings that pramipexole, a dopamine D2/3 receptor agonist and bupropion, a dopamine reuptake inhibitor, may improve depressive symptoms in bipolar depression (Goldberg et al. 2004; Zarate et al. 2004) and MDD (Dhillon et al. 2008; Stahl et al. 2003). A further blockade of dopamine activity with quetiapine-XR may increase somnolence and lower the tolerability to the adverse events. However, D2 receptor blockade is unlikely to be the only possible cause. Quetiapine works on multiple neurotransmitter systems and multiple receptors including histamine1 receptors that can cause somnolence and sedation (Arnt & Skarsfeldt 1998).
Quetiapine-XR at fixed doses of 50 mg/d, 150 mg/d, and 300 mg/d has been studied in the treatment of MDD and GAD. Dose-dependent tolerability and sensitivity of patients with these two psychiatric conditions to quetiapine-XR were observed (Gao et al. in press). In most cases, higher doses of quetiapine-XR were more likely to cause DAEs and somnolence/sedation relative to placebo in these two conditions. However, the true cause for the differential tolerabilities and sensitivities to quetiapine-XR and other psychotropic medications among the different psychiatric conditions remains to be determined. The results of this review and our previous reviews (Gao et al. 2008a, b; in press) suggest that from the safety and tolerability point of view, the doses of antipsychotics should be justified according to individual disorders and different phases of each individual disorder such as mania vs. depression of bipolar I disorder.
As approved by the US FDA, quetiapine-XR monotherapy can be used as a first-line agent for the acute and long-term treatment of schizophrenia, bipolar mania, and bipolar depression. Although adjunctive quetiapine-XR has been approved for treating episodes of MDD that are inadequately responsive to an anti-depressant, its use should be carefully justified due to the scarcity of long-term safety data in this population. In the acute treatment of MDD, quetiapine-XR monotherapy was superior to placebo and as effective as duloxetine 60 mg/d (Cutler et al. 2009; Weisler et al. 2009), but it is potentially associated with the side-effects of somnolence, EPS, and metabolic dysregulation that may relegate its use to a second- or third-line agent after failure of a traditional antidepressant. Similarly, although quetiapine-XR monotherapy was superior to placebo and as effective as paroxetine 20 mg/d and escitalopram 10 mg/d in the acute treatment of GAD (Gao et al. 2009), its use as monotherapy might be considered after an inadequate response or treatment failure with approved agents. On the other hand, in patients with comorbid anxiety disorders, the anxiolytic and hypnotic effects of quetiapine-XR, in addition to its antidepressant and mood-stabilizing properties, may allow for minimization of poly-pharmacy when used earlier in the treatment course. In all instances, consideration should be given to balancing the risks and benefits in this heterogeneous and complex group of patients.
Limitations
This review is limited by the computer search parameters, which included only English-language publications and primarily focused on randomized, placebo-controlled, acute trials, and only used DAEs and reported somnolence for comparison. Although placebo was used as a direct comparator among the different psychiatric conditions, it could still be confounded by the original study designs, including the inclusion and exclusion criteria (in-patient vs. outpatient); sample sizes; study durations; medication dosages; or concomitant use of other psychotropic agents. More importantly, these data may not generalize to routine clinical settings since all these studies were carried out in relatively `pure' populations of patients with each disorder. Therefore, quetiapine-XR as monotherapy or adjunctive therapy in patients with schizophrenia, mania, bipolar depression, MDD, or GAD should be carefully justified. In addition, since there were no data of lower doses of quetiapine-XR in schizophrenia and bipolar disorder available, the tolerability and sensitivity to quetiapine-XR at lower doses in these two conditions could not be compared to the tolerability and sensitivity to quetiapine-XR at lower doses in MDD and GAD. On the other hand, the doses used in schizophrenia and bipolar mania were higher than the dose of quetiapine-XR 300 mg/d in bipolar depression, MDD, and GAD. Since there were no significant differences between placebo and quetiapine-XR at higher doses in schizophrenia and bipolar mania in the risk for DAEs, it is unlikely that a lower dose, such as quetiapine-XR 300 mg/d, in these two conditions would have a significant difference between placebo and quetiapine-XR. This assumption is supported by one of our previous analyses that showed within a certain dose range, the tolerability and sensitivity to antipsychotics is inversely related to dose (Gao et al. in press).
Conclusions
Among the patients with schizophrenia, mania, bipolar depression, refractory MDD, non-refractory MDD, and GAD, patients with GAD had the poorest tolerability to quetiapine-XR 300 mg/d although they had a similar sensitivity as those with bipolar depression or MDD. Patients with schizophrenia or mania had the highest tolerability and the lowest sensitivity among all patients across five psychiatric conditions.
Acknowledgements
Support was received for this manuscript in part by NIMH P20 MH-66054 (J.R.C). Z. Wang was a visiting research fellow in the Department of Psychiatry, Mood Disorders Program at the University Hospitals Case Medical Center/Case Western Reserve University School of Medicine.
Statement of Interest Dr Calabrese has received grant support and honoraria from Abbott, AstraZeneca, Bristol–Myers Squibb, GlaxoSmithKline, Lilly, Pfizer, and Janssen. Dr Gao has received grant support from AstraZeneca and NARSAD, is on an advisory board of Schering-Plough and a speaker's bureau of Pfizer. Dr Kemp has acted as a consultant to Bristol-Myers Squibb and has served on a speaker's bureau for AstraZeneca and Pfizer. Dr Kemp has received grant support from NIH grant 1KL2RR024990.
References
- Anand A, Verhoeff P, Seneca N, Zoghbi SS, et al. Brain SPECT imaging of amphetamine-induced dopamine release in euthymic bipolar disorder patients. American Journal of Psychiatry. 2002;157:1108–1114. doi: 10.1176/appi.ajp.157.7.1108. [DOI] [PubMed] [Google Scholar]
- Altindag A, Yanik M, Nebioglu M. The comorbidity of anxiety disorders in bipolar I patients: prevalence and clinical correlates. Israel Journal of Psychiatry and Related Sciences. 2006;43:10–15. [PubMed] [Google Scholar]
- Altman DG. Why we need confidence intervals. World Journal of Surgery. 2005;29:554–556. doi: 10.1007/s00268-005-7911-0. [DOI] [PubMed] [Google Scholar]
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pretorius HW, Constant EL, Earley WR, et al. Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. Journal of Clinical Psychiatry. 2009;70:540–549. doi: 10.4088/jcp.08m04629. [DOI] [PubMed] [Google Scholar]
- Belia S, Fidler F, Williams J, Cumming G. Researchers misunderstand confidence intervals and standard error bars. Psychological Methods. 2005;10:389–396. doi: 10.1037/1082-989X.10.4.389. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophrenia Bulletin. 2009;35:383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard G, Bobes J, Ahokas A, Eggens I, et al. Once-daily extended release quetiapine fumarate (quetiapine XR) monotherapy in generalized anxiety disorder (GAD): A placebo-controlled study with active-comparator Paroxetine. European Psychiatry. 2008;23(Suppl. 2):S211. [Google Scholar]
- Ciapparelli A, Paggini R, Marazziti D, Carmassi C, et al. Researcher misunderstood confidence intervals and standard error bars. Psychological Methods. 2005;10:389–396. doi: 10.1037/1082-989X.10.4.389. [DOI] [PubMed] [Google Scholar]
- Craddock N, O′Donovan MC, Owen MJ. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. Journal of Medical Genetics. 2005;42:193–204. doi: 10.1136/jmg.2005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming G, Finch S. Confidence Inference by eye: confidence intervals and how to read pictures of data. American Psychologist. 2005;60:170–180. doi: 10.1037/0003-066X.60.2.170. [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Montgomery SA, Feifel D, Lazarus A, et al. Extended release quetiapine fumarate monotherapy in major depressive disorder: a placebo- and duloxetine-controlled study. Journal of Clinical Psychiatry. 2009;70:526–539. doi: 10.4088/jcp.08m04592. [DOI] [PubMed] [Google Scholar]
- Datto C, Berggren L, Patel JB, Eriksson H. Self-reported sedation profile of immediate-release quetiapine fumarate compared with extended-release quetiapine fumarate during dose initiation: a randomized, double-blind, crossover study in healthy adult subjects. Clinical Therapeutics. 2009a;31:492–502. doi: 10.1016/j.clinthera.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Datto C, Minkwitz M, Nordenhem A, Walker C, et al. Effctiveness of the new extended-release formulation of quetiapine as monotherapy for the treatment of acute bipolar depression. European Psychiatry. 2009b;24(Suppl. 1):S574. doi: 10.1016/j.jad.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Datto C, Nordenhem A, Minkwitz M, Dettore B, et al. Effectiveness of extended-release formulation of quetiapine as monotherapy for the treatment of acute bipolar mania. European Psychiatry. 2009c;24(Suppl. 1):S573. [Google Scholar]
- De Luca V, Likhodi O, Van Tol HH, Kennedy JL, et al. Regulation of alpha7-nicotinic receptor subunit and alpha7-like gene expression in the prefrontal cortex of patients with bipolar disorder and schizophrenia. Acta Psychiatrica Scandinavica. 2006;114:211–215. doi: 10.1111/j.1600-0447.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- Dhillon S, Yang LP, Curran MP. Spotlight on bupropion in major depressive disorder. CNS Drugs. 2008;22:613–617. doi: 10.2165/00023210-200822070-00006. [DOI] [PubMed] [Google Scholar]
- El-Khalili N, Banov M, Bortnick B, Adson D, et al. Efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in major depressive disorder (MDD): a randomized, placebo-controlled clinical trial (Study 003). 63rd Annual Society of Biological Psychiatry; Washington DC, USA. 2008a. [DOI] [PubMed] [Google Scholar]
- El-Khalili N, Joyce M, Atkinson S, Buynak R, et al. Adjunctive extended-release quetiapine fumarate (quetiapine XR) in patients with major depressive disorder and inadequate antidepressant response. 161st Annual Meeting of the American Psychiatric Association; Washington, DC, USA. 2008b. [Google Scholar]
- Fava M, Rush AJ, Alpert JE, Balasubramani GK, et al. Difference in treatment outcome in outpatients with anxious vs. nonanxious depression: a STAR*D report. American Journal of Psychiatry. 2008;165:342–351. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- Feske U, Frank E, Mallinger AG, Houck PR, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. American Journal of Psychiatry. 2000;157:956–962. doi: 10.1176/appi.ajp.157.6.956. [DOI] [PubMed] [Google Scholar]
- Figueroa C, Brecher M, Hamer-Maansson JE, Winter H. Pharmacokinetic profiles of extended release quetiapine fumarate compared with quetiapine immediate release. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33:199–204. doi: 10.1016/j.pnpbp.2008.09.026. [DOI] [PubMed] [Google Scholar]
- Fleurence R, Williamson R, Jing Y, Kim E, et al. A systematic review of augmentation strategies for patients with major depressive disorder. Psychopharmacology Bulletin. 2009;42:57–90. [PubMed] [Google Scholar]
- Gajwani P, Kemp DE, Muzina DJ, Xia G, et al. Acute treatment of mania: an update on new medications. Current Psychiatry Reports. 2006;8:504–509. doi: 10.1007/s11920-006-0058-3. [DOI] [PubMed] [Google Scholar]
- Gao K, Gajwani P, Elhaj O, Calabrese JR. Typical and atypical antipsychotics in bipolar depression. Journal of Clinical Psychiatry. 2005;66:1376–1385. doi: 10.4088/jcp.v66n1106. [DOI] [PubMed] [Google Scholar]
- Gao K, Ganocy SJ, Gajwani P, Muzina DJ, et al. A review of sensitivity and tolerability of antipsychotics in patients with bipolar disorder or schizophrenia: focus on somnolence. Journal of Clinical Psychiatry. 2008a;69:302–309. doi: 10.4088/jcp.v69n0217. [DOI] [PubMed] [Google Scholar]
- Gao K, Kemp DE, Ganocy SJ, Gajwani P, et al. Antipsychotic-induced extrapyramidal side effects in bipolar disorder and schizophrenia: a systematic review. Journal of Clinical Psychopharmacology. 2008b;28:203–209. doi: 10.1097/JCP.0b013e318166c4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Kemp DE, Fein E, Wang ZW, et al. Number needed to treat to harm for discontinuation due to adverse events in the treatment in bipolar depression, major depressive disorder, and generalized anxiety disorder with atypical antipsychotics. Journal of Clinical Psychiatry. doi: 10.4088/JCP.09r05535gre. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Muzina D, Gajwani P, Calabrese JR. Efficacy of typical and atypical antipsychotics for primary and comorbid anxiety symptoms or disorders: a review. Journal of Clinical Psychiatry. 2006;67:1327–1340. doi: 10.4088/jcp.v67n0902. [DOI] [PubMed] [Google Scholar]
- Gao K, Sheehan DV, Calabrese JR. Atypical antipsychotics in primary generalized anxiety disorder or comorbid with mood disorders. Expert Review of Neurotherapeutics. 2009;9:1147–1158. doi: 10.1586/ern.09.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JF, Burdick KE, Endick CJ. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. American Journal of Psychiatry. 2004;161:564–566. doi: 10.1176/appi.ajp.161.3.564. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Hasin DS, Dawson DA, et al. Prevalence, correlates, and comorbidity of bipolar I disorder and axis I and II disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2005;66:1205–1215. doi: 10.4088/jcp.v66n1001. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Cook D, Devereaux PJ, Meade M, et al. Therapy. In: Guyatt G, Rennie D, editors. Users Guides: Essentials of Evidence-based Clinical Practice. American Medical Association; Chicago: 2002. pp. 55–79. [Google Scholar]
- Jaeschke R, Guyatt G, Shannon H, Walter S, et al. Basic statistics for clinicians: 3. Assessing the effects of treatment: measures of association. Canadian Medical Association Journal. 1995;152:351–357. [PMC free article] [PubMed] [Google Scholar]
- Joyce M, Khan A, Atkinson S, Eggens I, et al. Efficacy and safety of extended release quetiapine fumarate (quetiapine XR) monotherapy in patients with generalized anxiety disorder (GAD). 161st Annual Meeting of the American Psychiatric Association; Washington, DC, USA. 2008. [Google Scholar]
- Kahn RS, Schulz SC, Palazov VD, Reyes EB, et al. Efficacy and tolerability of once-daily extended release quetiapine fumarate in acute schizophrenia: a randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry. 2007;68:832–842. doi: 10.4088/jcp.v68n0603. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Nasrallah HA, Fagiolini A. Mood stabilizers and atypical antipsychotics: bimodal treatments for bipolar disorder. Psychopharmacology Bulletin. 2006;39:120–146. [PubMed] [Google Scholar]
- Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biological Psychiatry. 2006;59:990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Lenderts S, Kalali A. Treatment of depression: an update on antidepressant monotherapy and combination therapy. Psychiatry (Edgmont) 2009;6:15–17. [PMC free article] [PubMed] [Google Scholar]
- Maier W, Höfgen B, Zobel A, Rietschel M. Genetic models of schizophrenia and bipolar disorder: overlapping inheritance or discrete genotypes? European Archives of Psychiatry and Clinical Neuroscience. 2005;255:159–166. doi: 10.1007/s00406-005-0583-9. [DOI] [PubMed] [Google Scholar]
- Mamo DC, Uchida H, Vitcu I, Barsoum P, et al. Quetiapine extended-release vs. immediate-release formulation: a positron emission tomography study. Journal of Clinical Psychiatry. 2008;69:81–86. doi: 10.4088/jcp.v69n0111. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Archives of General Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- McQuay HJ, Moore AR. Using numerical results from systematic reviews in clinical practice. Annals of Internal Medicine. 1997;126:712–720. doi: 10.7326/0003-4819-126-9-199705010-00007. [DOI] [PubMed] [Google Scholar]
- McTavish SF, McPherson MH, Harmer CJ, Clark L, et al. Antidopaminergic effects of dietary tyromine depletion in healthy subjects and patients with manic illness. British Journal of Psychiatry. 2001;179:356–360. doi: 10.1192/bjp.179.4.356. [DOI] [PubMed] [Google Scholar]
- Medved V, Petrovic R, Isgum V, Szirovicza L, et al. Similarities in the pattern of regional brain dysfunction in negative schizophrenia and unipolar depression: a single photon emission-computed tomography and auditory evoked potentials study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2001;25:993–1009. doi: 10.1016/s0278-5846(01)00172-5. [DOI] [PubMed] [Google Scholar]
- Merideth C, Cutler A, Neijber A, She F, et al. Efficacy and tolerability of extended release quetiapine fumarate (Quetiapine XR) monotherapy in the treatment of GAD. 21st European College of Neuropsychopharmacology Congress; Barcelona, Spain. 2008. [Google Scholar]
- Milak MS, Aniskin DB, Eisenberg DP, Prikhojan A, et al. The negative syndrome as a dimension: factor analyses of PANSS in major depressive disorder and organic brain disease compared with negative syndrome structures found in the schizophrenia literature. Cognitive and Behavioral Neurology. 2007;20:113–120. doi: 10.1097/WNN.0b013e3180653c35. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, Kim-Cohen J, et al. Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Archives of General Psychiatry. 2007;64:651–660. doi: 10.1001/archpsyc.64.6.651. [DOI] [PubMed] [Google Scholar]
- Montori VM, Kleinbart J, Newman TB, Keitz S, et al. Tips for learners of evidence-based medicine: 2. Measures of precision (confidence intervals) Canadian Medical Association Journal. 2004;171:611–615. doi: 10.1503/cmaj.1031667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir WJ, Thomson ML, McKeon P, Mynett-Johnson L, et al. Markers close to the dopamine D5 receptor gene (DRD5) show significant association with schizophrenia but not bipolar disorder. American Journal of Medical Genetics. 2001;105:152–158. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1163>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Murray RM, Sham P, Van Os J, Zanelli J, et al. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophrenia Research. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Pae CU, Serretti A, Patkar AA, Masand PS. Aripiprazole in the treatment of depressive and anxiety disorders: a review of current evidence. CNS Drugs. 2008;22:367–388. doi: 10.2165/00023210-200822050-00002. [DOI] [PubMed] [Google Scholar]
- Payton ME, Greenstone MH, Schenker N. Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance. Journal of Insect Science. 2003;3:34. doi: 10.1093/jis/3.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, van den Bree MB, Thapar A. A population-based study of anxiety as a precursor for depression in childhood and adolescence. BMC Psychiatry. 2004;4:43. doi: 10.1186/1471-244X-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen DJ, Cascella NG, Meyer SM, Kingery LR, et al. Neuropsychological functioning in bipolar disorder and schizophrenia. Biological Psychiatry. 2007;62:179–186. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone T. Dopamine in manic depressive illness. A pharmacological synthesis. Journal of Affective Disorders. 1985;8:225–231. doi: 10.1016/0165-0327(85)90020-5. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Zhang L, Damatarca C, Grady M. Brain circuits determine destiny in depression: a novel approach to the psychopharmacology of wakefulness, fatigue, and executive dysfunction in major depressive disorder. Journal of Clinical Psychiatry. 2003;64(Suppl. 14):6–17. [PubMed] [Google Scholar]
- Sun J, Maller JJ, Daskalakis ZJ, Furtado CC, et al. Morphology of the corpus callosum in treatment-resistant schizophrenia and major depression. Acta Psychiatrica Scandinavica. 2009;120:265–273. doi: 10.1111/j.1600-0447.2009.01389.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Soulsby B, Kawasaki Y, et al. An MRI study of the superior temporal subregions in first-episode patients with various psychotic disorders. Schizophrenia Research. 2009;113:158–166. doi: 10.1016/j.schres.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Van Snellenberg JX, de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Archives of General Psychiatry. 2009;66:748–755. doi: 10.1001/archgenpsychiatry.2009.64. [DOI] [PubMed] [Google Scholar]
- Weisler R, Joyce M, McGill L, Lazarus A, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectrums. 2009;14:299–313. doi: 10.1017/s1092852900020307. [DOI] [PubMed] [Google Scholar]
- Wurthmann C, Klieser E, Lehmann E. Side effects of low dose neuroleptics and their impact on clinical outcome in generalized anxiety disorder. Progress in Neuropsychopharmacology and Biological Psychiatry. 1997;21:601–609. doi: 10.1016/s0278-5846(97)00035-3. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Payne JL, Singh J, Quiroz JA, et al. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biological Psychiatry. 2004;56:54–60. doi: 10.1016/j.biopsych.2004.03.013. [DOI] [PubMed] [Google Scholar]