Abstract
BACKGROUND
Women without stress urinary incontinence undergoing vaginal surgery for pelvicorgan prolapse are at risk for postoperative urinary incontinence. A midurethral sling may be placed at the time of prolapse repair to reduce this risk.
METHODS
We performed a multicenter trial involving women without symptoms of stress incontinence and with anterior prolapse (of stage 2 or higher on a Pelvic Organ Prolapse Quantification system examination) who were planning to undergo vaginal prolapse surgery. Women were randomly assigned to receive either a midurethral sling or sham incisions during surgery. One primary end point was urinary incontinence or treatment for this condition at 3 months. The second primary end point was the presence of incontinence at 12 months, allowing for subsequent treatment for incontinence.
RESULTS
Of the 337 women who underwent randomization, 327 (97%) completed follow-up at 1 year. At 3 months, the rate of urinary incontinence (or treatment) was 23.6% in the sling group and 49.4% in the sham group (P<0.001). At 12 months, urinary incontinence (allowing for subsequent treatment of incontinence) was present in 27.3% and 43.0% of patients in the sling and sham groups, respectively (P = 0.002). The number needed to treat with a sling to prevent one case of urinary incontinence at 12 months was 6.3. The rate of bladder perforation was higher in the sling group than in the sham group (6.7% vs. 0%), as were rates of urinary tract infection (31.0% vs. 18.3%), major bleeding complications (3.1% vs. 0%), and incomplete bladder emptying 6 weeks after surgery (3.7% vs. 0%) (P≤0.05 for all comparisons).
CONCLUSIONS
A prophylactic midurethral sling inserted during vaginal prolapse surgery resulted in a lower rate of urinary incontinence at 3 and 12 months but higher rates of adverse events. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health; OPUS ClinicalTrials.gov number, NCT00460434.)
One in five women will undergo surgery for pelvic-organ prolapse in her lifetime,1 and urinary incontinence commonly occurs with pelvic-organ prolapse. In previously continent women with pelvic-organ prolapse, urinary incontinence develops in approximately a quarter of them after prolapse repair; this phenomenon is referred to as occult, latent, de novo, iatrogenic, or potential stress urinary incontinence.2
In 2006, the Colpopexy and Urinary Reduction Efforts (CARE) trial2 showed that adding a bladder-neck suspension at the time of abdominal prolapse surgery in women without preoperative stress incontinence significantly reduced the risk of postoperative stress urinary incontinence (23.8%, vs. 44.1% in the control group). Recently, prolapse repairs have been increasingly performed transvaginally, and midurethral slings have largely replaced bladder-neck suspensions.3 Extrapolating from the CARE trial data, many surgeons prophylactically insert a concomitant midurethral sling in all continent women undergoing vaginal prolapse surgery4; others perform this additional procedure selectively, in women who have urinary stress incontinence on preoperative cough testing.5 An alternative strategy is to treat only those women in whom bothersome urinary incontinence develops postoperatively.4 The relative benefits and risks of these strategies are unclear.
We designed the Outcomes Following Vaginal Prolapse Repair and Midurethral Sling (OPUS) trial6 to determine whether, among women without stress incontinence who undergo vaginal prolapse surgery, the prevalence of urinary incontinence or treatment at 3 months and the prevalence of urinary incontinence (regardless of interval treatment) at 12 months differed with and without a concomitant midurethral sling. We also evaluated the role of preoperative prolapse-reduction stress testing and included an assessment of outcomes among women who declined to undergo randomization (patient-preference cohort).
METHODS
STUDY DESIGN
We conducted this multicenter, randomized, single-blind, sham-controlled, surgical-intervention trial at seven clinical sites. The methods have been published previously.6 The study protocol was approved by the institutional review board at each site and is available with the full text of this article at NEJM.org. The last author vouches for the accuracy and completeness of the reported data and for the fidelity of the study to the protocol. Participants provided written informed consent. Enrollment began in May 2007, and follow-up was completed in January 2011.
PATIENT POPULATION
Eligible participants were women planning to undergo vaginal prolapse surgery who reported the symptom of feeling or seeing a vaginal bulge but reported no symptoms of stress incontinence, which was defined as a positive response to any of the three questions regarding stress incontinence on the Pelvic Floor Distress Inventory.6,7 On pelvic examination, the anterior vaginal-wall prolapse had to be within 1 cm of the hymenal ring with straining. Women were excluded if they had undergone previous sling placement, were receiving treatment for stress urinary incontinence, had contraindications for a midurethral sling (prior urethral surgery or pelvic irradiation), were planning pregnancy in the first year after surgery, or had a history of two or more hospitalizations for medical illnesses in the previous year.
STUDY INTERVENTION AND RANDOMIZATION
At the time of vaginal prolapse surgery, women were randomly assigned to receive either a retropubic midurethral sling (Gynecare TVT, Ethicon) (the sling group) or two 1-cm suprapubic, superficial sham incisions, which mimicked the sling incisions (the sham group). Ethicon, the maker of the Gynecare TVT midurethral sling, was not involved in the study and did not provide funding or slings. Randomization, with the use of a permuted block design, was stratified according to surgeon and type of prolapse surgery (colpocleisis, apical suspension, or anterior repair). Women who declined to undergo randomization were offered the opportunity to participate in a patient-preference cohort in which the decision for a sling was left up to the patient and her surgeon.
STUDY MEASURES
Baseline assessments included the collection of demographic and general health data, examination for prolapse,8 a measurement of postvoiding residual volume, and a preoperative prolapse-reduction stress test (at a bladder volume of 300 ml, with the prolapsed organ repositioned inside the vagina with the use of one or two large swabs); the test was considered negative if the woman had no leakage with coughing or straining in either the supine or standing position. We also collected scores on the Medical Outcomes Study 36-Item Short-Form Health Survey (with normalized values having a mean of 50 and a standard deviation of 10 and higher scores indicating better health status),9 the Pelvic Floor Distress Inventory (with scores on the Urinary Distress Inventory subscale and prolapse subscale ranging from 0 to 300, scores on the colorectal–anal subscale ranging from 0 to 400, and higher scores indicating more symptoms),7 the Pelvic Floor Impact Questionnaire (with scores ranging from 0 to 400 and higher scores indicating a greater negative effect on quality of life),7 the Incontinence Severity Index (with scores ranging from 1 to 12 and higher scores indicating more severe incontinence),10 the Pelvic Organ Prolapse/Urinary Incontinence Sexual Functioning Questionnaire Short Form (with scores ranging from 0 to 48 and higher scores indicating better sexual function),11 and a visual analogue pain scale adapted for suprapubic pain (with scores ranging from 0 to 10 and higher scores indicating more pain).12 All surveys were administered by centralized telephone interviewers. Participants in the randomized cohort, interviewers, and coordinators were unaware of study-group assignments, and operative notes and surgical-consent forms did not reveal the study group.
Follow-up at 3, 6, and 12 months consisted of a medical history taking, administration of the surveys noted above, and an assessment of prolapse severity.8 A cough stress test, urinalysis, and measurement of postvoiding residual volume were performed at 3 and 12 months.
STUDY OUTCOMES
The two primary study end points6 (Table 1 in the Supplementary Appendix, available at NEJM.org) were urinary incontinence (stress, urge, or mixed) at 3 months, defined as a positive cough stress test, bothersome incontinence symptoms, or treatment for urinary incontinence, and urinary incontinence (stress, urge, or mixed) at 12 months, regardless of whether interim treatment for incontinence had been provided.
Secondary outcomes included measures of incontinence and quality of life (as noted above). Serious adverse events, expected complications (common complications attributable to slings), and unexpected nonserious adverse events were systematically ascertained. An independent data and safety monitoring board convened quarterly to review study conduct, adverse events, and interim analyses.
STATISTICAL ANALYSIS
For the randomized cohort, we calculated that 150 participants per group would provide the study with 80% power to detect a 15% between-group difference in the primary 3-month end point on the basis of a two-sample test of proportions, with a two-sided significance level of 5%. Given that the primary 12-month end point addresses a distinct hypothesis, we did not adjust for multiple comparisons and we estimated that the study would have 80% power to identify a 15% between-group difference. The first interim analysis was completed and reviewed by the data and safety monitoring board; however, a second planned interim analysis was not performed because enrollment ended earlier than anticipated. Thus, the nominal significance level was 0.0469 for the primary 3-month end point. A significance level of 5% was used for all other hypothesis testing.
The primary analyses for the randomized trial compared the proportion of participants who had urinary incontinence or had received treatment for it at 3 months and who had urinary incontinence at 12 months between the sling and the sham groups with the use of conditional logistic regression, stratified according to surgeon, with covariates for treatment and planned surgical repair, providing odds ratios for the treatment effect. To estimate the corresponding relative risks, we used a generalized linear model that was based on the binomial distribution with a log link, adjusting for planned surgical repair and including surgeon as a random effect.13 The analysis was based on an intention-to-treat approach, with participants who withdrew from the study or were missing components of the composite end point considered to have had treatment failures. Sensitivity analyses were performed to assess the effect of missing data by imputing treatment success in these cases instead of treatment failure and analyzing complete cases. Baseline characteristics, changes in quality-of-life measures, and adverse events were compared between the randomized groups with the use of Fisher’s exact test or the chi-square test for discrete variables and with the use of Student’s t-test or the Wilcoxon signed-ranktest for continuous variables. A similar approach was used to look for differences between the randomized and patient-preference cohorts as a measure of participation bias. An additional planned secondary analysis was a subgroup analysis that was based on the results of the preoperative prolapse-reduction stress test. To assess effect modification by this variable, we incorporated an interaction term in the conditional logistic model.14 All analyses were performed with the use of SAS software, version 9.2 (SAS Institute).
RESULTS
STUDY POPULATION
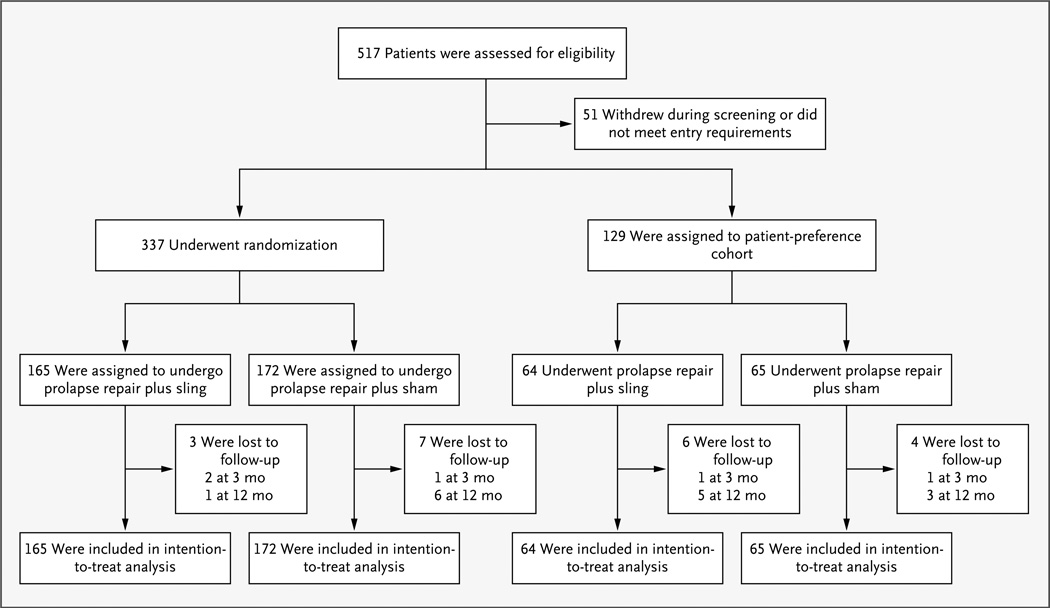
Between May 2007 and October 2009, a total of 517 women presenting to the clinical sites for prolapse repair were screened for eligibility, and 337 underwent randomization (Fig. 1). Two participants in the sling group did not receive a sling; all participants in the sham group received sham incisions. Of those who underwent randomization, 327 (97%) completed follow-up at 1 year; 306 (91%) underwent evaluation for the primary outcome at 3 months, and 287 (85%) at 12 months. The baseline demographic and clinical characteristics were similar between the two groups (Table 1, and Table 2 in the Supplementary Appendix).
Figure 1. Enrollment, Randomization, and Analysis.
Of 517 patients who were assessed for eligibility, 337 were randomly assigned to receive a midurethral sling or sham incisions, 129 decided to participate in a patient-preference cohort, and 51 withdrew or did not meet eligibility requirements. Data from the women in the randomized and patient-preference cohorts were included in the intention-to-treat analyses.
Table 1.
Demographic and Clinical Characteristics at Baseline, According to Study Group in the Randomized-Trial Cohort.*
| Characteristic | Sling (N = 165) |
Sham (N = 172) |
|---|---|---|
| Age — yr | 63.4 ± 10.8 | 62.2 ± 10.2 |
| Race or ethnic group — no. (%)† | ||
| White | 143 (87) | 143 (83) |
| Black | 10 (6) | 14 (8) |
| Asian | 3 (2) | 2 (1) |
| Hispanic | 21 (13) | 27 (16) |
| Other | 9 (5) | 13 (8) |
| Body-mass index‡ | 27.8 ± 4.9 | 28.1 ± 5.5 |
| POP-Q stage — no. (%)§ | ||
| 2 | 45 (27) | 48 (28) |
| 3 | 107 (65) | 106 (62) |
| 4 | 13 (8) | 18 (10) |
| Annual income <$30,000 — no./total no. (%) | 31/64 (48) | 27/65 (42) |
| Married — no./total no. (%) | 121/163 (74) | 101/161 (63) |
| Completed high school or less — no./total no. (%) | 61/163 (37) | 71/161 (44) |
| Positive cough stress test — no. (%) | 54 (33) | 57 (33) |
| Anterior vaginal-prolapse repair — no./total no. (%)¶ | ||
| Anterior repair only | 20/164 (12) | 17/172 (10) |
| Apical suspension only | 32/164 (20) | 42/172 (24) |
| Both anterior repair and apical suspension | 101/164 (62) | 100/172 (58) |
| Colpocleisis | 11/164 (7) | 13/172 (8) |
| Posterior vaginal-prolapse repair — no. (%) | 74 (45) | 80 (47) |
| Previous hysterectomy — no. (%) | 62 (38) | 66 (38) |
| Concomitant hysterectomy — no. (%) | 82 (50) | 83 (48) |
Plus–minus values are means ±SD. P>0.05 for all comparisons. An expanded list of baseline characteristics is provided in Table 2 in the Supplementary Appendix.
Race or ethnic group was self-reported, and more than one category may have been selected.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Stages in the Pelvic Organ Prolapse Quantification (POP-Q) system are ordinal categories based on the lowest point of prolapse, with 1 indicating that the lowest point of prolapse is more than 1 cm above the hymen, 2 that it is within 1 cm above or below the hymen, 3 that it is more than 1 cm below the hymen but protrudes no more than 2 cm less than the total vaginal length, and 4 that there is complete vaginal eversion.8
Anterior repairs included paravaginal repairs, colporrhaphy, and mesh augmentation. Apical suspensions included uterosacral-ligament suspension, sacrospinous-ligament suspension, McCall culdoplasty, iliococcygeal repair, purse-string repair of enterocele, and use of an apical-suspension kit.
The patient-preference cohort had 129 participants, with 64 receiving slings (Fig. 1). As compared with participants in the randomized trial, women in the patient-preference cohort were more likely to be white (95.4% vs. 84.9%, P = 0.02) and to have received only an apical-prolapse suspension (34.9% vs. 22%, P = 0.01); other baseline demographic and clinical characteristics did not differ significantly between the two groups (Table 3 in the Supplementary Appendix).
RANDOMIZED-TRIAL COHORT
Three-Month Outcomes
Three months after surgery, the rate of urinary incontinence or treatment for it was 23.6% in the sling group and 49.4% in the sham group (adjusted odds ratio, 0.31; 95% confidence interval [CI], 0.19 to 0.50; P<0.001) (Table 2). The corresponding unadjusted relative risk was 0.48 (95% CI, 0.35 to 0.65), and the adjusted relative risk was 0.49 (95% CI, 0.35 to 0.69).13 Women who were randomly assigned to receive a sling had lower rates of a positive cough stress test and bothersome symptoms of urinary incontinence, but the rates of postoperative treatment of incontinence did not differ significantly between the groups (Table 2). The number needed to treat with a sling to prevent one case of urinary incontinence or treatment of incontinence at 3 months was 3.9 (95% CI, 2.8 to 6.5).
Table 2.
End Points at 3 and 12 Months, According to Study Group in the Randomized-Trial Cohort.
| End Point | Sling (N = 165) |
Sham (N = 172) |
Difference, Sling vs. Sham (95% Cl)* |
Adjusted Odds Ratio (95% Cl) |
P Value† |
|---|---|---|---|---|---|
| percentage points | |||||
| At 3 mo | |||||
| Urinary incontinence or treatment — no. (%)‡ | 39 (23.6) | 85 (49.4) | −25.8 (−36.1 to −15.5)§ | 0.31 (0.19 to 0.50) | <0.001 |
| Positive cough stress test — no./total no. (%)¶ | 10/158 (6.3) | 54/157 (34.4) | −28.1 (−37.0 to −19.2) | <0.001 | |
| Symptoms of incontinence — no./total no. (%)‖ | 15/160 (9.4) | 41/165 (24.8) | −15.5 (−23.7 to −7.3) | <0.001 | |
| Treatment for incontinence — no./total no. (%)** | 11/164 (6.7) | 13/172 (7.6) | −0.9 (−6.45 to 4.7) | 0.76 | |
| At 12 mo | |||||
| Urinary incontinence — no. (%)†† | 45 (27.3) | 74 (43.0) | −15.7 (−26 to −5.5)‡ | 0.48 (0.30 to 0.77) | <0.01 |
| Positive cough stress test — no./total no. (%) | 5/143 (3.5) | 31/151 (20.5) | −17.0 (−24.5 to −9.5) | <0.001 | |
| Symptoms of incontinence — no./total no. (%) | 18/158 (11.4) | 30/160 (18.8) | −7.4 (−15.2 to 0.5) | 0.07 | |
Percentage-point differences may vary slightly from percents reported, because of rounding.
P values for primary end points were calculated from a conditional logistic-regression model, stratified according to surgeon, and adjusted for type of prolapse-repair procedure (colpocleisis, apical suspension, anterior repair, or both apical suspension and anterior repair). P values for components of the primary end points were calculated by means of chi-square (unadjusted) tests.
The end point at 3 months was a positive cough stress test, report of bothersome symptoms, or additional treatment for urinary incontinence. Some patients had overlapping criteria, and those for whom end-point data were missing or who discontinued the study were regarded as having incontinence, per protocol.
Between-group differences for the composite end points were compared with the use of the conditional logistic model.
A positive cough stress test was defined as leakage of urine with coughing or straining in either the supine or standing position with the bladder filled through a urethral catheter to 300 ml.
Symptoms of urinary incontinence were defined as symptoms that were at least moderately bothersome to the participant (as measured by a response of “moderately” or “quite a bit” to any of the four items on the Pelvic Floor Distress Inventory regarding leakage).
Treatment for urinary incontinence was defined as the need for treatment for any urinary incontinence, including surgery, medication, pessary for incontinence, supervised pelvic-muscle exercises, timed voiding and fluid management, periurethral injection, botulinum toxin injection, neuromodulation, or other treatment for incontinence.
The end point at 12 months was only a positive cough stress test or report of bothersome symptoms. Some patients had overlapping criteria, and those for whom end-point data were missing or who discontinued the study were regarded as having incontinence, per protocol.
Twelve-Month Outcomes
Twelve months after surgery, participants who were randomly assigned to receive a sling had a lower rate of urinary incontinence than those in the sham group (27.3% vs. 43.0%; adjusted odds ratio, 0.48; 95% CI, 0.30 to 0.77; P = 0.002), allowing for subsequent treatment for urinary incontinence (Table 2). The corresponding unadjusted relative risk was 0.63 (95% CI, 0.47 to 0.85), and the adjusted relative risk was 0.64 (95% CI, 0.47 to 0.85).13 At 12 months, the number needed to treat with a sling to prevent one case of urinary incontinence, allowing for subsequent treatment for incontinence, was 6.3 (95% CI, 3.9 to 18).
During follow-up, 12 women (7.3%) in the sling group underwent subsequent treatment for urinary incontinence, including 1 (0.6%) who underwent surgery for incontinence. In the sham group, 19 women (11.0%) underwent treatment for incontinence, including 8 (4.7%) who underwent surgery for incontinence. Four women who were randomly assigned to receive a sling (2.4%) underwent surgery for voiding dysfunction in the first year. At both 3 and 12 months, sensitivity analyses assessing the effect of missing data did not alter our conclusions regarding efficacy.
CHANGES IN HEALTH STATUS AND SYMPTOMS
The changes from baseline in urinary symptoms as measured on the Pelvic Floor Distress Inventory, including the Urinary Distress Inventory stress subscale, and the Incontinence Severity Index at 3 months were greater in the sling group than in the sham group (indicating a greater reduction in symptoms) (Table 3). At 12 months, only the changes from baseline for the stress urinary incontinence subscale and Incontinence Severity Index were greater in the sling group than in the sham group. There were no significant between-group differences at 3 and 12 months in changes in generic health (Table 3), other pelvic-floor symptoms, effect on quality of life, sexual function, or pain (Table 4 in the Supplementary Appendix).
Table 3.
Changes in Health Status and Lower Urinary Tract Symptoms from Baseline, According to Study Group in the Randomized-Trial Cohort.*
| Variable | Sling Group (N = 165) |
Sham Group (N = 172) |
Difference, Sling vs. Sham (95% Cl)† |
P Value |
|---|---|---|---|---|
| SF-36 mental component summary‡ | ||||
| At 3 mo | ||||
| No. of patients | 156 | 154 | ||
| Score change | 1.5 ± 9.25 | 0.6 ± 8.44 | 0.8 (−1.1 to 2.8) | 0.40 |
| At 12 mo | ||||
| No. of patients | 154 | 152 | ||
| Score change | 1.9 ± 8.40 | 2.0 ± 8.94 | −0.1 (−2.1 to 1.9) | 0.92 |
| SF-36 physical component summary‡ | ||||
| At 3 mo | ||||
| No. of patients | 156 | 154 | ||
| Score change | 2.1 ± 8.21 | 1.5 ± 7.52 | 0.6 (−1.2 to 2.4) | 0.50 |
| At 12 mo | ||||
| No. of patients | 154 | 152 | ||
| Score change | 3.1 ± 9.23 | 2.3 ± 7.51 | 0.8 (−1.1 to 2.7) | 0.42 |
| PFDI UDI§ | ||||
| At 3 mo | ||||
| No. of patients | 160 | 155 | ||
| Score change | −44.9 ± 48.24 | −34.3 ± 44.92 | −10.6 (−20.9 to −0.2) | 0.04 |
| At 12 mo | ||||
| No. of patients | 157 | 152 | ||
| Score change | −43.1 ± 44.25 | −39.3 ± 40.93 | −3.7 (−13.3 to 5.8) | 0.44 |
| UDI obstructive symptom subscale¶ | ||||
| At 3 mo | ||||
| No. of patients | 160 | 158 | ||
| Score change | −27.3 ± 22.69 | −28.0 ± 20.81 | 0.7 (−4.1 to 5.6) | 0.76 |
| At 12 mo | ||||
| No. of patients | 157 | 154 | ||
| Score change | −26.4 ± 22.69 | −27.1 ± 21.39 | 0.7 (−4.2 to 5.6) | 0.77 |
| UDI irritative symptom subscale¶ | ||||
| At 3 mo | ||||
| No. of patients | 160 | 158 | ||
| Score change | −12.5 ± 18.83 | −10.5 ± 17.06 | −2.0 (−6.0 to 2.0) | 0.33 |
| At 12 mo | ||||
| No. of patients | 157 | 154 | ||
| Score change | −10.9 ± 17.15 | −11.6 ± 15.87 | 0.6 (−3.0 to 4.3) | 0.73 |
| UDI stress subscale¶ | ||||
| At 3 mo | ||||
| No. of patients | 160 | 155 | ||
| Score change | −5.1 ± 16.29 | 4.2 ± 20.42 | −9.4 (−13.5 to −5.3) | <0.001 |
| At 12 mo | ||||
| No. of patients | 157 | 152 | ||
| Score change | −5.7 ± 14.62 | −0.5 ± 16.89 | −5.3 (−8.8 to −1.7) | 0.004 |
| Incontinence Severity Index‖ | ||||
| At 3 mo | ||||
| No. of patients | 157 | 156 | ||
| Score change | −0.9 ± 3.01 | 0.6 ± 3.26 | −1.5 (−2.2 to −0.8) | <0.001 |
| At 12 mo | ||||
| No. of patients | 154 | 152 | ||
| Score change | −0.9 ± 2.70 | 0.1 ± 2.70 | −1.0 (−1.6 to −0.4) | <0.001 |
Plus–minus values are means ±SD.
Percentage-point differences may vary slightly from percents reported, because of rounding.
Scores on the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) have normalized values with a mean of 50 and a standard deviation of 10, with higher scores indicating better health status.
Scores on the Pelvic Floor Distress Inventory (PFDI) Urinary Distress Inventory (UDI) subscale range from 0 to 300, with higher scores indicating more symptoms.
Scores on the UDI subscales range from 0 to 100, with higher scores indicating more symptoms.
Scores on the Incontinence Severity Index range from 1 to 12, with higher scores indicating greater severity.
ADVERSE EVENTS
The rates of serious or unexpected adverse events did not differ significantly between groups at the end of the trial (Table 4, and Tables 7 and 8 in the Supplementary Appendix). The mean operative time was 11.4 minutes longer and the mean estimated blood loss 24 ml higher in the sling group than in the sham group (P = 0.05 and P = 0.03, respectively). Rates of bladder perforation, urinary tract infection, major bleeding complications, and incomplete bladder emptying in the first 6 weeks after surgery were all higher in the sling group than in the sham group (Table 4). There were no mesh erosions resulting from sling placement. Bladder perforations due to sling placement were all managed during surgery without long-term consequences.
Table 4.
Adverse Events during the First Year after Surgery, According to Study Group in the Randomized-Trial Cohort.*
| Event | Sling (N = 165) |
Sham (N = 172) |
P Value |
|---|---|---|---|
| Serious adverse events — no. of patients (%) | 28 (17.0) | 20 (11.6) | 0.16 |
| Unexpected adverse events — no. of patients (%) | 14 (8.5) | 14 (8.1) | 1.0 |
| Ureteral injury | 6 (3.6) | 3 (1.7) | 0.33 |
| Other | 8 (4.8) | 11 (6.4) | 0.64 |
| Expected adverse events — no. of patients/total no. (%) | |||
| Bladder perforation during sling placement | 11/164 (6.7) | 0/172 | <0.01 |
| Mesh erosion or exposure | 0/160 (0) | 0/171 | NA |
| Urinary tract infection† | 49/158 (31.0) | 30/164 (18.3) | 0.008 |
| Major bleeding or vascular complication | 5/164 (3.0) | 0/172 | 0.03 |
| Incomplete bladder emptying‡ | |||
| At hospital discharge | 69/162 (42.6) | 51/170 (30.0) | 0.02 |
| At 2 wk | 9/163 (5.5) | 1/169 (0.6) | 0.01 |
| At 6 wk | 6/162 (3.7) | 0/170 | 0.01 |
| Urethrolysis for voiding dysfunction | 4/165 (2.4) | 0/172 | 0.06 |
A serious adverse event was defined as any untoward medical occurrence (whether or not it was plausibly related to the index surgery) that resulted in death, was life threatening, required inpatient hospitalization, resulted in persistent or serious disability or incapacity, resulted in a congenital anomaly or birth defect, or constituted a medically important condition. An unexpected adverse event was defined as any other untoward event that did not qualify as an expected adverse event. Expected adverse events were defined as common side effects attributable to the placement of a sling. Any expected or unexpected adverse event that qualified as a serious adverse event was counted as such. Details of serious and unexpected adverse events are reported in Tables 7 and 8 in the Supplementary Appendix, respectively. A patient may have had more than one adverse event.
A urinary tract infection was diagnosed either by a positive urinalysis in clinic, resulting in treatment with antibiotics, or because patients reported symptoms, resulting in treatment with antibiotics.
A urethral catheter was left in place until day 1 after surgery. A successful voiding trial was defined as a postvoiding residual volume of less than 150 ml. Women with poor bladder emptying received treatment according to the clinical care standards at that site until voiding was successful.
PREOPERATIVE PROLAPSE-REDUCTION STRESS TEST
Overall, 33.5% of women (111 of the 331 patients who completed a baseline prolapse-reduction stress test) had a positive prolapse-reduction stress test before surgery. Among these women, 29.6% in the sling group, as compared with 71.9% in the sham group, had urinary incontinence at 3 months (adjusted odds ratio, 0.13; 95% CI, 0.05 to 0.34) (Table 5 in the Supplementary Appendix). Women with a positive prolapse-reduction stress test before surgery appeared to receive more benefit from a sling at 3 months than did those with a negative test (P = 0.06 for interaction), although this difference was not significant at 12 months (P = 0.16 for interaction).
PATIENT-PREFERENCE COHORT
Women in the patient-preference cohort who had a positive prolapse-reduction stress test were more likely to undergo a sling procedure at the time of prolapse repair than those who had a negative test (57.8% vs. 22.2%, P<0.001). Otherwise, there were no significant differences in baseline demographic or clinical characteristics between the study groups in the patient-preference cohort. Consistent with the randomized cohort, the rate of urinary incontinence or treatment for incontinence at 3 months was lower among women who received a sling than among those who did not (adjusted odds ratio, 0.31; 95% CI, 0.11 to 0.86). At 12 months, rates of urinary incontinence in the two groups were similar to those in the randomized trial, but differences were not significant (Table 6 in the Supplementary Appendix).
DISCUSSION
In our randomized trial of sling placement versus sham placement in women without preoperative symptoms of stress incontinence who were planning to undergo vaginal surgery for apical or anterior prolapse, the odds of urinary incontinence or treatment for urinary incontinence 3 months after surgery among the women in the sling group were substantively reduced, as compared with those in the sham group. The beneficial effect of the sling on urinary incontinence remained significant at 12 months. A benefit was observed regardless of the results of preoperative prolapse-reduction stress testing; there was modest evidence (P = 0.06) to suggest that at 3 months, patients with a positive prolapse-reduction stress test before surgery may have received more benefit than those with a negative test, but this was not apparent at 12 months.
A recent survey study of 132 women who underwent vaginal prolapse surgery and had a negative prolapse-reduction stress test before surgery showed that 42% had postoperative urinary incontinence, as assessed by subjective criteria, which was similar to the 38% rate in our study.15 Moreover, approximately one third of participants who responded to the survey were moderately or greatly bothered by their symptoms, and 5% underwent surgery for these symptoms. These findings highlight the potential role for effective preventive strategies.
Our results support earlier findings of the CARE trial,2 in which the addition of a Burch colposuspension at the time of abdominal prolapse surgery reduced the incidence of postoperative stress urinary incontinence. A smaller randomized trial involving women undergoing vaginal prolapse repair, which was limited to women with occult stress incontinence, showed a 4% rate of incontinence, as assessed by subjective criteria, at 2 years among women who received a sling, as compared with a rate of 36% among women in the control group.5 The absence of blinding in that trial may in part explain the greater observed difference between groups. When the end point includes a subjective component, it is particularly important that participants and outcome assessors be unaware of the study-group assignments.
From the perspective of the individual patient, benefits must be balanced against the higher rates of clinically relevant adverse effects and the need for additional surgery. In our trial, nearly 5% of the women in the sham group underwent a sling procedure in the first 12 months after prolapse-repair surgery. In contrast, surgery to remove the sling was required in only 2.4% of women in the sling group.
Findings in the patient-preference cohort were remarkably consistent with those in the randomized cohort, suggesting that nonparticipation bias probably had a minimal effect on the study findings. Not surprisingly, once women chose to participate in the patient-preference cohort, those with a positive preoperative stress test were more likely to undergo surgery to receive a sling, suggesting that knowledge of the results of the stress test may have influenced the decisions of participants and their surgeons.
The limitations of our study should be considered. It is possible that the incidence of postoperative incontinence may differ according to the type of anterior repair or apical suspension, but our study was not powered to assess these subgroups. Participant knowledge of the study intervention may have had an effect on the subjective outcomes; however, our strategies for masking should have lessened this risk. Some women may have been unwilling to undergo another surgery within the first year, even if they had symptoms. Finally, our findings should not be extrapolated beyond 12 months, although the benefits of sling placement with concomitant prolapse surgery for the treatment of incontinence have been shown to be durable beyond 1 year.16
Adding a midurethral sling at the time of vaginal-prolapse surgery in women without preoperative symptoms of stress urinary incontinence reduces the likelihood of urinary incontinence at 3 and 12 months after surgery but increases the likelihood of adverse events. Counseling of women who are planning to undergo vaginal-prolapse surgery should include attention to both the benefits and the risks of sling placement.
Supplementary Material
Acknowledgments
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (2U01 HD41249, 2U10 HD41250, 2U10 HD41261, 2U10 HD41267, 1U10 HD54136, 1U10 HD54214, 1U10 HD54215, and 1U10 HD54241) and the National Institutes of Health Office of Research on Women’s Health.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116:1096–1100. doi: 10.1097/AOG.0b013e3181f73729. [DOI] [PubMed] [Google Scholar]
- 2.Brubaker L, Cundiff GW, Fine P, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354:1557–1566. doi: 10.1056/NEJMoa054208. [DOI] [PubMed] [Google Scholar]
- 3.Richter HE, Albo ME, Zyczynski HM, et al. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066–2076. doi: 10.1056/NEJMoa0912658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatton B. Is there any evidence to advocate SUI prevention in continent women undergoing prolapse repair? An overview. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:235–245. doi: 10.1007/s00192-008-0734-4. [DOI] [PubMed] [Google Scholar]
- 5.Meschia M, Pifarotti P, Spennacchio M, Buonaguidi A, Gattei U, Somigliana E. A randomized comparison of tension-free vaginal tape and endopelvic fascia plication in women with genital prolapse and occult stress urinary incontinence. Am J Obstet Gynecol. 2004;190:609–613. doi: 10.1016/j.ajog.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Wei J, Nygaard I, Richter H, et al. Outcomes following vaginal Prolapse repair and mid Urethral Sling (OPUS) Trial — design and methods. Clin Trials. 2009;6:162–171. doi: 10.1177/1740774509102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185:1388–1395. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 8.Bump RC, Mattiasson A, Bø K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 9.Ziebland S. The Short Form 36 Health Status Questionnaire: clues from the Oxford region’s normative data about its usefulness in measuring health gain in population surveys. J Epidemiol Community Health. 1995;49:102–105. doi: 10.1136/jech.49.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47:497–499. doi: 10.1136/jech.47.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers RG, Kammerer-Doak D, Villarreal A, Coates K, Qualls C. A new instrument to measure sexual function in women with urinary incontinence or pelvic organ prolapse. Am J Obstet Gynecol. 2001;184:552–558. doi: 10.1067/mob.2001.111100. [DOI] [PubMed] [Google Scholar]
- 12.Hartanto VH, DiPiazza D, Ankem MK, Baccarini C, Lobby NJ. Comparison of recovery from postoperative pain utilizing two sling techniques. Can J Urol. 2003;10:1759–1763. [PubMed] [Google Scholar]
- 13.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 14.Selvin S. Statistical analysis of epidemiological data. New York: Oxford University Press; 1996. [Google Scholar]
- 15.Al-Mandeel H, Ross S, Robert M, Milne J. Incidence of stress urinary incontinence following vaginal repair of pelvic organ prolapse in objectively continent women. Neurourol Urodyn. 2011;30:390–394. doi: 10.1002/nau.20947. [DOI] [PubMed] [Google Scholar]
- 16.Schraffordt Koops SE, Bisseling TM, van Brummen HJ, Heintz AP, Vervest HA. Result of the tension-free vaginal tape in patients with concomitant prolapse surgery: a 2-year follow-up study: an analysis from the Netherlands TVT database. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:437–442. doi: 10.1007/s00192-006-0170-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.