Abstract
Objective
Smoking is highly intractable and the genetic influences on cessation are unclear. Identifying the genetic factors affecting smoking cessation could elucidate the nature of tobacco dependence, enhance risk assessment, and support treatment algorithm development. This study tests whether variants in the nicotinic receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) predict age of smoking cessation and relapse to smoking after a quit attempt.
Method
In a community-based, cross-sectional study (N=5,216) and a randomized comparative effectiveness smoking cessation trial (N=1,073), we used survival analyses and logistic regression to model relations between smoking cessation (self-reported quit age in a community study and point-prevalence abstinence at end-of-treatment in a clinical trial) and three common haplotypes in the CHRNA5-CHRNA3-CHRNB4 region defined by rs16969968 and rs680244.
Results
The genetic variants in the CHRNA5-CHRNA3-CHRNB4 region that predict nicotine dependence also predict a later age of smoking cessation in a community-based sample (X2=8.46, df=2, p=0.015). In the smoking cessation trial, these variants predict abstinence at end-of-treatment in individuals receiving placebo medication, but not amongst individuals receiving active medication. Genetic variants interact with treatment in affecting cessation success (X2=8.97, df=2, p=0.011).
Conclusions
Smokers with the high risk genetic variants have a three-fold increased likelihood of responding to pharmacologic cessation treatments, compared to smokers with the low risk genetic variants. The high-risk variants increase the risk of cessation failure, and this increased risk can be ameliorated by cessation pharmacotherapy. By identifying a high-risk genetic group with heightened response to smoking cessation pharmacotherapy, this work may support the development of personalized cessation treatments.
INTRODUCTION
Tobacco smoking is a serious public health problem. Unfortunately, smoking is a quintessential dependence disorder, evidenced by a characteristic withdrawal syndrome and heavy, uncontrolled use (1). Cardinal manifestations of uncontrolled use are both persistent use and an inability to quit successfully in a cessation attempt (1-3). Nicotine dependence is associated with both reduced likelihood of quitting over time (4) and a rapid return to smoking following a quit attempt (3, 5-7).Therefore, identification of the factors that contribute to either sustained smoking or more rapid smoking relapse should help elucidate the causal basis of tobacco dependence, permit more accurate prediction of dependence and relapse risk, and support more effective application of treatment.
A recent series of meta-analyses based on tens of thousands of subjects of European descent confirms the association of chromosome region 15q25.1with smoking heaviness, defined by cigarettes per day (8-11), with the most robust associations being reported for rs16969968 and rs1051730, two highly correlated variants (p<5.57*10−72)(10). In the CHRNA5-A3-B4 region, at least two independent signals have been identified (9, 12). The first signal tagged by rs16969968, a variant that results in an amino acid change in the α5 nicotinic cholinergic receptor (CHRNA5), alters nicotinic receptor conductance in vitro (13, 14). A second, distinct signal tagged by rs680244 is associated with variability in CHRNA5 mRNA levels(15). Resequencing of the CHRNA5-CHRNA3-CHRNB4 locus in subjects of European ancestry identified three common haplotypes in the region spanning CHRNA5 and the 3′ end of CHRNA3 (12), which can be defined by rs16969968 and rs680244 (15).
The CHRNA5-CHRNA3-CHRNB4 variants have been less consistently associated with cessation outcomes than with smoking heaviness measures. Five studies show an association between the CHRNA5-CHRNA3-CHRNB4 region and successful smoking cessation (16-20). All five found that the same genetic risk variants that contribute to smoking heaviness and nicotine dependence also predicted smoking cessation. Other studies, however, fail to confirm this association (21-23). A genome-wide association of three treatment cohorts did not identify any nicotinic receptor genes as predictors of prospectively-measured smoking cessation (23). One large genome-wide association meta-analysis that strongly supported the association between 15q25.1 and smoking heaviness failed to find an association with current versus former smoking as a measure of smoking cessation at a genome wide level of significance (10).
A logical case can be made for there being a relation between the CHRNA5-CHRNA3-CHRNB4 variants and smoking cessation. These variants are consistently related to measures of smoking heaviness and nicotine dependence (12, 16), and there is copious evidence that measures of nicotine dependence predict cessation likelihood (4, 5, 24). These findings encourage an examination of the involvement of the CHRNA5-CHRNA3-CHRNB4 variants with cessation, and further suggest that this relation will be mediated by dependence. If evidence for mediation is not found, and yet the variants are related to cessation, it would suggest that the variants influence cessation via routes that are independent to their influence on dependence. Moreover, previous research suggests that the relation between the CHRNA5-CHRNA3-CHRNB4 variants and cessation should be examined with regard to pharmacotherapy. There is mounting evidence that pharmacotherapies work by mitigating the risks for cessation failure that are related to severity of nicotine dependence (25, 26). This suggests that the connection between the variants and cessation may be strongest amongst individuals who do not receive pharmacotherapy for smoking cessation.
Using data from a community-based study, the Atherosclerosis Risk in Communities study (ARIC(27)), and a University of Wisconsin Transdisciplinary Tobacco Use Research Center (UW-TTURC) smoking cessation clinical trial, we extend the research on the CHRNA5-CHRNA3-CHRNB4 region and smoking cessation success. This research was predicated on hypotheses about the relation among CHRNA5-CHRNA3-CHRNB4 variants, tobacco dependence, and smoking cessation. The two types of studies differ in study duration, amount of experimental contact and monitoring, and type of participants. However, complementary hypothesis were developed for these two types of research designs. In the case of the community trial, the assumption was that dependence would be manifest in cessation occurring at a later age (28-30). In the case of the clinical trial, the assumption was that both heightened genetic risk and dependence would be demonstrated as failed abstinence at end of treatment. Therefore, quitting difficulty was assessed in terms of both relatively long latency to quit in a community sample and failed abstinence at end of treatment in a treatment trial. Analyses addressed three major questions: 1) Does the natural history of smoking cessation vary by genetic variants in the chromosome 15q25 region? 2) Do these same variants predict cessation in a treatment trial? 3) Does the relation between the genetic variants and cessation depend upon treatment status? The results are relevant to understanding the nature of risk posed by the targeted variants for cessation, and whether the variants have pharmacogenetic implications in treatment assignment or application.
METHODS
ATHEROSCLEROSIS RISK IN COMMUNITIES (ARIC) STUDY
We used the ARIC study to examine the genetic association with age of cessation in a non-treatment setting. ARIC is a prospective epidemiologic study conducted in four U.S. communities to investigate atherosclerosis. In 1987, each ARIC field center recruited a cohort sample aged 45-64 from a defined population in their community, and almost 16,000 subjects participated. Self-reported age of cessation was assessed with the question “How old were you when you stopped smoking?” Genotyping was performed at the Broad Institute of MIT and Harvard on the Affymetrix 6.0 chip. Genetic and phenotype data are available on 12,771 subjects; data were obtained from the NCBI database of Genotypes and Phenotypes (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gap).
UNIVERSITY OF WISCONSIN TRANSDISCIPLINARY TOBACCO USE RESEARCH CENTER (UW-TTURC)
We used the UW-TTURC randomized, placebo-controlled smoking cessation trial (31) to examine the genetic association with time to relapse after quitting in a treatment trial setting. This sample has not been previously examined for the genetic risk on smoking cessation. The University of Wisconsin-Madison IRB approved this trial, and all subjects provided written informed consent.
Participants were eligible to participate if they were 18 years of age or older, smoked 10 or more cigarettes per day, and were motivated to quit smoking. Prior to randomization, participants completed baseline assessments of demographics, smoking history (including cigarettes smoked per day), and tobacco dependence including the Fagerström Test for Nicotine Dependence (FTND) (32). Participants provided a breath sample for alveolar carbon monoxide (CO) analysis to verify their smoking status and estimate their smoking heaviness.
Participants (N=1,073) were randomly assigned to one of six conditions: placebo (n = 132), nicotine patch (n = 187); nicotine lozenge (n = 183); bupropion SR (n = 188); nicotine patch and nicotine lozenge (n = 193); or bupropion and nicotine lozenge (n = 190). All participants received six brief (10 minute) individual counseling sessions.
Biochemically confirmed 7-day point prevalence abstinence was assessed at end-of-treatment (8 weeks post-quit). All of participants′ self-reports of abstinence during study visits were confirmed by an expired CO level of less than 10 parts per million. Relapse was defined as any smoking on 7 consecutive days after the target quit date. Time of relapse was determined via timeline follow-up assessment (33, 34)and was available for 1015 subjects.
Genotyping of the UW-TTURC sample was performed by the Center for Inherited Disease Research at Johns Hopkins University using the Illumina Omni2.5 microarray (www.illumina.com). Data cleaning was led by the GENEVA Coordinating Center at the University of Washington.
ANALYSIS
CHRNA5-CHRNA3-CHRNB4 haplotypes were analyzed. It is advantageous to model the genetic architecture by rational selection of haplotypes that maximizes information about the common variation at this locus and reflects potential underlying biological mechanisms. Three common haplotypes in the region spanning CHRNA5 and the 3′ end of CHRNA3 (12, 15) are defined by rs16969968 and rs680244. The risk allele of rs16969968 primarily occurs on the low mRNA expression allele of rs680244. Together, these variants identify three risk levels involving CHRNA5 representing two distinct mechanisms for nicotine dependence. We used these three haplotypes as our standard of analysis. In the ARIC dataset, these two variants were not genotyped, so two highly correlated SNPs were used as proxies to estimate haplotypes: rs951266 (r2 with rs16969968 is 0.97 in CEU 1000 Genomes Project) and rs6495306 (r2 with rs680244 is 1 in CEU 1000 Genomes Project; http://www.1000genomes.org/).
We used PLINK(35) to estimate haplotype probabilities for each individual. We assumed that pairs of haplotypes in an individual are “additive”. The posterior probability for the haplotypes was 1 for over 99% of the haplotypes. Dummy coding for different haplotypes was used.
We used a standard series of Cox proportional hazard models to analyze age of smoking cessation in the ARIC study, and we used logistic regression to analyze biochemically confirmed end-of-treatment (EOT) point-prevalence abstinence in the UW-TTURC study. In the UW-TTURC study, days to relapse constituted a secondary outcome which was analyzed with Cox proportional hazard models. The primary predictor variables of interest were haplotypes and a haplotype-by-treatment interaction in the smoking cessation study. Covariates included gender, age (in quartiles), cigarettes per day (in 4 levels: <10, 11-20, 21-30, >31), and treatment (placebo versus active treatment in the UW-TTURC study).
RESULTS
ATHEROSCLEROSIS RISK IN COMMUNITIES (ARIC) STUDY
Subjects were of European descent, identified as smokers (defined as smoking 400 cigarettes lifetime), and had genotype data (N=5,216). Demographic descriptions are given in Table S1. These subjects smoked on average 23.6 cigarettes per day (CPD) (standard deviation=13.6) when smoking. Haplotype counts and frequencies in the ARIC sample were: GC (24.4%), GT (42.4%), and AC (33.2%), and they were labeled as H1, H2, and H3, respectively.
We found a robust association between CHRNA5-CHRNA3-CHRNB4 haplotypes and smoking heaviness defined by CPD, consistent with previous findings. These three haplotypes are strongly associated with CPD (Wald=56.4, df=2, omnibus-p=5.8×10−13 for the overall haplotype effect) (see Table S2).
CHRNA5-CHRNA3-CHRNB4 haplotypes were also associated with age of self-reported smoking cessation in this community-based sample (Wald=8.64, df=2, omnibus-p=0.015). Compared to H1, the high-risk haplotype H3 was associated with a later quit age (RH=0.92, 95%CI=0.86-0.98, df=1, p=0.0088) (Table 1). The median age of smoking cessation was 57 years for those with haplotype H3, and 55 years for those with haplotypes H2 and H1 (Wald=8.46, df=2, omnibus p=0.015 for the overall haplotype effect). The strength of this association was more modest than the association with heaviness of smoking. To further understand this genetic association, we added CPD as a covariate into the model. Smoking a higher number of cigarettes per day was strongly associated with a later quit age (RH=0.63, 95%CI=0.61-0.65, p<1.0×10−8), and the haplotypes were no longer associated with age of cessation (Wald=1.72, df=2, omnibus-p=0.42 for the overall haplotype effect). Analyses were repeated with smoking duration (measured from self-reported age of onset of smoking to age of smoking cessation) as the outcome variable, and similar results were seen.
Table 1.
Haplotypic Risk for Age of Smoking Cessation in ARIC sample (N=5,216)
| Age of Smoking Cessation |
|||
|---|---|---|---|
| Predictors | Relative Hazard |
95% C.I. | P |
| Haplotypes (rs16969968-rs680244**) | (a) | ||
| H1- GC | reference | ||
| H2- GT | 0.98 | (0.92, 1.05) | 0.59 |
| H3- AC | 0.92 | (0.86, 0.98) | 0.0088 |
All models were adjusted for age (quartiles) and gender. ARIC = Atherosclerosis Risk in Communities Study.
In the ARIC sample, rs951266 was used as a proxy for rs16969968 (r2 = 0.97 in CEU in 1000 Genomes database); rs6495306 was used as a proxy for rs680244 (r2=1 in CEU in 1000 Genomes database).
Wald=8.46, df=2, omnibus p=0.015 for the overall haplotype effect.
UNIVERSITY OF WISCONSIN TRANSDISCIPLINARY TOBACCO USE RESEARCH CENTER (UW-TTURC)
Subjects of European ancestry with genotype data and relapse data were analyzed (N=1,073). Table S1 shows demographics. Haplotype counts and frequencies in the UW-TTURC sample were: GC (20.8%), GT (43.7%), and AC (35.5%), and they were labeled again as H1, H2, and H3, respectively. In this treatment seeking sample, CPD was associated with the three haplotypes after adjusting for age and gender (Wald=7.15, df=2, omnibus-p=0.028 for the overall haplotype effect), but this effect was modest in this heavy smoking cohort (Table S2).
In this trial, 47.3% of participants were abstinent at end-of-treatment (8 weeks post-quit). In a simple logistic regression model, abstinence is predicted by treatment assignment (placebo versus active treatment) after adjusting for age and gender. Having any pharmacologic treatment, compared to the placebo group, increased abstinence by over 85% (OR=1.87, 95% CI=1.42-2.45, df=1, p=8.1×10−6). Smoking fewer cigarettes per day was the strongest predictor of abstinence (OR=0.67, 95% CI=0.60-0.75, p =3.3×10−11).
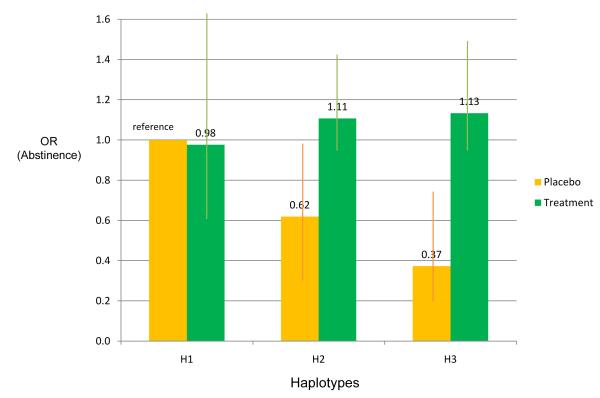
Haplotypes did not predict abstinence in the entire sample including both placebo and treatment groups. However, the association of haplotypes with abstinence depended upon treatment condition. In the placebo group, haplotype H3, which is associated with heavy smoking, predicts failed abstinence in comparison to haplotype H1 (OR=0.37, 95% CI=0.19-0.78, p=0.0081 for H3 compared to H1); overall haplotypes were associated with abstinence (X2=7.02, df=2, omnibus-p=0.030 for the overall haplotype effect in the placebo group). However, haplotype status was not associated with abstinence amongst individuals receiving active pharmacotherapy (X2=1.45, df=2, omnibus-p=0.48 for the overall haplotype effect). This is reflected by a significant interaction between treatment (placebo versus active treatment) and haplotypes (X2=8.97, df=2, omnibus-p=0.011 for the interaction) (Table 2; Figure 1).
Table 2.
Interaction of Haplotypic Risk and Treatment Effect on Abstinence at End-of-Treatment in the Wisconsin TTURC Cessation Trial (N=1,073)
| Abstinence at End of Treatment |
|||
|---|---|---|---|
| Predictors | Odds Ratio |
95% C.I. | P |
| Haplotypes (rs16969968-rs680244*) | |||
| H1- GC | reference | ||
| H2- GT | 0.62 | (0.33, 1.17) | 0.14 |
| H3- AC | 0.37 | (0.19, 0.75) | 0.0057 |
| Treatment Status | |||
| Placebo | reference | ||
| Active Treatment | 0.98 | (0.57, 1.69) | 0.93 |
| Interaction of Haplotypes and Intervention | (a) | ||
| H1* Active Treatment | reference | ||
| H2* Active Treatment | 1.83 | (0.93, 3.63) | 0.083 |
| H3* Active Treatment | 3.12 | (1.48, 6.55) | 0.0027 |
All models were adjusted for age (quartiles) and gender. TTURC = Transdisciplinary Tobacco Use Research Center
chi squared=8.97, df=2, omnibus p=0.011 for the overall interaction effect.
Figure 1. Interaction of Haplotypic Risk and Treatment Effect on Abstinence at End-of-Treatment.
The interaction of haplotypes and treatment on abstinence is significant (X2=8.97, df=2, p=0.011).
Abstinence is defined at end of 8 Week treatment. Odds ratios are adjusted for age and gender.
Haplotypes based on 2 SNPs (rs16969968, rs680244): H1=GC(20.8%), H2=GT(43.7%), H3=AC(35.5%).
N=1073 (132 in the placebo group; 941 in the treatment group).
To further understand the interplay between the haplotypes and treatment, we added cigarettes per day (CPD) and the interaction between CPD and treatment to determine if the relationship between the haplotype status and abstinence depended upon smoking heaviness (as a measure of dependence). Heavier smoking was associated with less abstinence (OR=0.67, 95% CI=0.60-0.75, p=2.8×10−11), but this relationship did not differ by treatment status (X2=0.98, df=1, omnibus-p=0.32 for the interaction). The interaction between the haplotypes and treatment remained significant (X2=8.61, df=2, omnibus-p=0.013 for the interaction; Table S3), after adjusting for CPD.
In addition, we found similar results when modeling the secondary cessation outcome (time to relapse post-quit over 60 days). There was a consistent interaction effect between the haplotypes and treatment status (H3 compared to H1: RH=0.54, 95% CI=0.33-0.89, p=0.015; H2 compared to H1: RH=0.60, 95% CI=0.37-0.95, p=0.031; omnibus p-value=0.040 for the overall haplotype effect) (Table S4). Figure S1 illustrates the risk for relapse by haplotypes in the entire sample, and stratified by treatment status. Haplotypes were associated with risk of relapse in the placebo group, but not in the active treatment group. Figure S3 shows the risk for relapse by treatment status, stratified by the three haplotype groups. Active treatment was strongly associated with a decreased risk of relapse in individuals with the H3 haplotype (RH=0.48, 95%CI=0.36-0.64, p=9.7×10−7) and H2 haplotype (RH=0.48, 95%CI=0.37-0.62, p=2.7×10−8). Active treatment had no significant effect in those with the H1 haplotype (RH=0.83, 95%CI=0.56-1.24, p= 0.36).
Furthermore, there was no significant difference in haplotypic risks on abstinence or relapse between different active treatment groups (Bupropion only, NRT only, and Combined) (Table S5), and no association between haplotypes and abstinence or relapse in any specific medication groups (Figures S2, S4). Additional analyses using single SNPs showed consistent results (Tables S6, S7).
DISCUSSION
This study reveals an interaction between the genetic variants in CHRNA5-CHRNA3-CHRNB4and smoking cessation pharmacotherapy in relation to smoking cessation. Smokers with the high-risk genetic variants have a three-fold increased likelihood of responding to pharmacologic cessation treatments, compared to smokers with the low-risk genetic variants.
In addition to the interaction effect, this study showed that the genetic variants in the chromosome 15q25 region that predict heavy smoking and nicotine dependence also predict a later age of smoking cessation in a large community-based sample. Those with the high-risk genetic variants quit later than those at low genetic risk, manifested as a two-year delay in median quit age. Recruitment for the ARIC sample began in the late 1980′s when the population utilization of nicotine replacement therapy was quite low (36, 37), so most ARIC subjects quit smoking without any pharmacologic treatment, thus representing a “natural history” of smoking cessation. The observed association between haplotype status and quitting latency was no longer significant once smoking heaviness was taken into account. This suggests that the targeted risk haplotypes confer heightened risk of heavy smoking and that this, in turn, constitutes an obstacle to successful quitting.
The large smoking cessation trial offers a distinct, complementary test of the association between haplotype status and cessation. In this study, the genetic associations with smoking cessation are manifested in the placebo group, which is consistent with the results obtained in the ARIC “natural history” sample. In contrast, these genetic variants do not predict abstinence across active treatment conditions, and this reduced genetic effect in the context of active pharmacological treatments suggests that cessation treatments differ in effectiveness across the haplotypes and mitigate the genetic risks for cessation difficulty. Pharmacological cessation treatment significantly increased the likelihood of abstinence in individuals with the high-risk haplotype H3, but exerted little effect in individuals with the low-risk haplotype H1.
These findings may explain discrepancies in prior studies of these genetic variants and smoking cessation. Some previous studies indicate that the chromosome 15q25 region is associated with smoking cessation (16-20), whereas other studies do not (21-23). Given our findings, we believe that genetic risk varies by pharmacologic intervention and weaker (or even no) genetic effect will be seen in pharmacologic trials if an interaction effect with treatment (active treatment versus placebo) is not included. We believe that the effect of this genetic locus will be seen most clearly in placebo arms, or in a sample where pharmacotherapy use is rare. For example, Sarginson et al reported very little association between CHRNA5-CHRNA3-CHRNB4 variants and cessation during the pharmacologic treatment phase of the trial, but the association increased at the 1-year follow-up after a maintenance phase with no medication. Our present findings suggest that a genetic risk by environmental interaction might account for such inconsistency because the targeted genetic effects are most strongly expressed in environments that provide little support for cessation (e.g., no effective pharmacotherapy). None of the previous studies has reported this interaction, which could potentially explain the apparently inconsistent results in this area. Such interactions between genetic variants and treatment can serve as the basis for personalized medicine. The TAG consortium examined a different cessation phenotype (former vs. current smoker) and reported a modest association with CHRNA5-CHRNA3-CHRNB4 (p <1*10−4) (10).
The results of this study should be interpreted in the context of several limitations. First, there was relatively little power to compare the magnitude of the targeted genetic effects among different active treatment conditions. The genetic risk for cessation was similar across the different pharmacotherapy conditions, indicating that multiple cessation interventions have the potential to be effective with such high-risk individuals. It is unclear if pharmacotherapy mitigates particular biological processes that lead to cessation failure, or if the genetic effect is more likely expressed at high overall levels of quitting difficulty. Second, the placebo group in the cessation trial is fairly small, which adds to the importance of future replication. However, reported associations between these genetic variants and cessation in multiple samples suggest a valid relation between these variants and cessation in some environmental contexts. Third, the smoking reports in the ARIC sample were not supported by biochemical confirmation. However, such confirmation was obtained in the UW-TTURC trial, and research shows that self-report is a valid indicant of current smoking, especially when there are no strong incentives to deceive (38). In addition, this work only studies one genetic locus and it is clear that multiple genes contribute to smoking cessation success. Future research should explore whether greater accuracy in predicting treatment response can be attained by the addition of other genetic variants implicated in differential treatment effects (39). Finally, this study only included subjects of European descent while the frequency and effects of these genetic variants differ by ancestry.
These results suggest that the biological effects of these haplotypes affect both smoking heaviness and a decreased ability to quit and that the interaction suggests that pharmacologic treatments are more effective for individuals who are biologically predisposed to have difficulty quitting. However, it is unclear whether the haplotypes are linked to both outcomes via the same mechanisms. In the ARIC sample, the effects of the targeted haplotypes on smoking heaviness mediated the influence of the haplotypes on cessation. Such a meditational role was not found in the UW-TTURC trial, indicating that more research is needed to clarify the causal paths from the haplotypes to cessation success. However, regardless of the specific causal paths that are ultimately determined, if the CHRNA5-CHRNA3-CHRNB4 haplotypes are indeed meaningfully related to both heaviness of smoking and cessation, it underscores their important role in the development and expression of nicotine dependence.
While acknowledging the limitations of our study, we note that this work complements and builds upon previous research on treatment and genetic effects on smoking cessation. Using diverse methods, this work underscores the relation between the targeted haplotypes and smoking cessation, shows a significant interaction between these haplotypes and treatment on cessation success, and reveals that cessation treatment effectiveness is modulated by the haplotypes.
In summary, our findings strengthen the case for the development and rigorous testing of treatments that target patients with different genetic risk profiles based on the chromosome 15q25 region that includes CHRNA5-CHRNA3-CHRNB4. Those with the high/intermediate-risk haplotypes appear more biologically predisposed to have difficulty quitting without treatment, but this risk may be ameliorated by effective pharmacological treatment. Further research that identifies genes related to responsiveness to treatment for nicotine addiction may lead to treatment algorithms that further the promise of personalized medicine(40).
Supplementary Material
ACKNOWLEDGMENTS
The Wisconsin State Laboratory of Hygiene provided considerable technical assistance in this research effort. Glaxo Wellcome provided bupropion at no cost in the UW-TTURC clinical trial. The authors thank John Budde and Nick McKenna for their technical assistance with Open Array platform genotyping, Louis Fox, Joseph Mullaney, and Thomas Przybeck for their assistance in preparing and analyzing the data, and Sherri Fisher for her assistance in project coordination and editing/preparing the manuscript.
FUNDING SUPPORT This research was supported by NIH grants P01 CA089392 (LJB), P50 CA84724 (TBB), and K05CA139871 (TBB) from the National Cancer Institute, P50 DA19706 (TBB), R01 DA026911 (NLS), K02 DA021237 (LJB), and K08 DA030398 (LSC) from the National Institute on Drug Abuse, U01 HG004422 (LJB) from the National Human Genome Research Institute, and sub-award KL2 RR024994 (LSC) from the National Center for Research Resources. Genotyping services for the UW-TTURC sample were provided by the Center for Inherited Disease Research (CIDR). Funding support for CIDR was provided by NIH grant U01HG004438and NIH contract HHSN268200782096C to The Johns Hopkins University. Assistance with genotype cleaning was provided by the Gene Environment Association Studies (GENEVA) Coordinating Center (U01 HG004446). The Atherosclerosis Risk in Communities Study (ARIC) is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, N01-HC-55022, R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. The ARIC datasets used for the analyses described in this manuscript were obtained from dbGaP at http://www.ncbi.nlm.nih.gov/sites/entrez?db=gapthrough dbGaP accession number phs000090.v1.p1.
Footnotes
DISCLOSURES Dr. Bierut, Dr. Goate, and Dr. Wang are listed as inventors on issued U.S. patent 8,080,371, “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. Dr. Chen, Dr. Baker, Dr. Piper, Dr. Breslau, Dr. Cannon, Dr. Doheny, Dr. Gogarten, Dr. Johnson, Dr. Saccone and Dr. Weiss declare no potential conflict of interest.
References
- 1.Breslau N, Johnson EO, Hiripi E, Kessler R. Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Archives of General Psychiatry. 2001;58(9):810–6. doi: 10.1001/archpsyc.58.9.810. [see comment] [DOI] [PubMed] [Google Scholar]
- 2.Hughes JR, Baker T, Breslau N, Covey L, Shiffman S. Applicability of DSM criteria to nicotine dependence. Addiction. 2011;106(5):894–5. doi: 10.1111/j.1360-0443.2010.03281.x. discussion 895-7. [DOI] [PubMed] [Google Scholar]
- 3.West R. In: Bock G, Goode J, editors. Defining and assessming nicotine dependence in humans; Understanding nicotine and tobacco addiction Novartis Foundation Symposium, No 275; Chichester, UK: Wiley; 2005. pp. 36–58. [Google Scholar]
- 4.Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. Am J Public Health. 2000;90(7):1122–7. doi: 10.2105/ajph.90.7.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, Colby S, Conti D, Giovino GA, Hatsukami D, Hyland A, Krishnan-Sarin S, Niaura R, Perkins KA, Toll BA. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9(Suppl 4):S555–70. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendricks PS, Prochaska JJ, Humfleet GL, Hall SM. Evaluating the validities of different DSM-IV-based conceptual constructs of tobacco dependence. Addiction. 2008;103(7):1215–23. doi: 10.1111/j.1360-0443.2008.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend. 1994;34(3):211–6. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 8.Liu JZ, Tozzi F, Waterworth DM, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42(5):436–40. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, Kong X, Landi MT, Ma JZ, Short SE, Stephens SH, Stevens VL, Sun L, Wang Y, Wenzlaff AS, Aggen SH, Breslau N, Broderick P, Chatterjee N, Chen J, Heath AC, Heliovaara M, Hoft NR, Hunter DJ, Jensen MK, Martin NG, Montgomery GW, Niu T, Payne TJ, Peltonen L, Pergadia ML, Rice JP, Sherva R, Spitz MR, Sun J, Wang JC, Weiss RB, Wheeler W, Witt SH, Yang BZ, Caporaso NE, Ehringer MA, Eisen T, Gapstur SM, Gelernter J, Houlston R, Kaprio J, Kendler KS, Kraft P, Leppert MF, Li MD, Madden PA, Nothen MM, Pillai S, Rietschel M, Rujescu D, Schwartz A, Amos CI, Bierut LJ. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6(8) doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TAG Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441–7. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, Sulem P, Rafnar T, Esko T, Walter S, Gieger C, Rawal R, Mangino M, Prokopenko I, Magi R, Keskitalo K, Gudjonsdottir IH, Gretarsdottir S, Stefansson H, Thompson JR, Aulchenko YS, Nelis M, Aben KK, den Heijer M, Dirksen A, Ashraf H, Soranzo N, Valdes AM, Steves C, Uitterlinden AG, Hofman A, Tonjes A, Kovacs P, Hottenga JJ, Willemsen G, Vogelzangs N, Doring A, Dahmen N, Nitz B, Pergadia ML, Saez B, De Diego V, Lezcano V, Garcia-Prats MD, Ripatti S, Perola M, Kettunen J, Hartikainen AL, Pouta A, Laitinen J, Isohanni M, Huei-Yi S, Allen M, Krestyaninova M, Hall AS, Jones GT, van Rij AM, Mueller T, Dieplinger B, Haltmayer M, Jonsson S, Matthiasson SE, Oskarsson H, Tyrfingsson T, Kiemeney LA, Mayordomo JI, Lindholt JS, Pedersen JH, Franklin WA, Wolf H, Montgomery GW, Heath AC, Martin NG, Madden PA, Giegling I, Rujescu D, Jarvelin MR, Salomaa V, Stumvoll M, Spector TD, Wichmann HE, Metspalu A, Samani NJ, Penninx BW, Oostra BA, Boomsma DI, Tiemeier H, van Duijn CM, Kaprio J, Gulcher JR, McCarthy MI, Peltonen L, Thorsteinsdottir U, Stefansson K. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42(5):448–53. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, Singh NA, Baird L, Coon H, McMahon WM, Piper ME, Fiore MC, Scholand MB, Connett JE, Kanner RE, Gahring LC, Rogers SW, Hoidal JR, Leppert MF. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4(7):e1000125. doi: 10.1371/journal.pgen.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J, Jr., Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165(9):1163–71. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol Pharmacol. 2011;79(1):119–25. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, Budde JP, Duan W, Fox L, Grucza RA, Kern J, Mayo K, Reyes O, Rice J, Saccone SF, Spiegel N, Steinbach JH, Stitzel JA, Anderson MW, You M, Stevens VL, Bierut LJ, Goate AM. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker TB, Weiss RB, Bolt D, von Niederhausern A, Fiore MC, Dunn DM, Piper ME, Matsunami N, Smith SS, Coon H, McMahon WM, Scholand MB, Singh N, Hoidal JR, Kim SY, Leppert MF, Cannon DS. Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11(7):785–96. doi: 10.1093/ntr/ntp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, Smith GD, Frayling TM, Hattersley AT. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18(15):2922–7. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munafo MR, Johnstone EC, Walther D, Uhl GR, Murphy MF, Aveyard P. CHRNA3 rs1051730 Genotype and Short-Term Smoking Cessation. Nicotine Tob Res. 2011;13(10):982–8. doi: 10.1093/ntr/ntr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, Murphy GM., Jr. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(3):275–84. doi: 10.1002/ajmg.b.31155. [DOI] [PubMed] [Google Scholar]
- 20.King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, Kaprio J, Lerman C, Park PW. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology. 2012;37(3):641–50. doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breitling LP, Twardella D, Hoffmann MM, Witt SH, Treutlein J, Brenner H. Prospective association of dopamine-related polymorphisms with smoking cessation in general care. Pharmacogenomics. 2010;11(4):527–36. doi: 10.2217/pgs.10.1. [DOI] [PubMed] [Google Scholar]
- 22.Conti DV, Lee W, Li D, Liu J, Van Den Berg D, Thomas PD, Bergen AW, Swan GE, Tyndale RF, Benowitz NL, Lerman C. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol Genet. 2008;17(18):2834–48. doi: 10.1093/hmg/ddn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, David SP, Niaura R, Lerman C. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65(6):683–93. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piper ME, McCarthy DE, Baker TB. Assessing tobacco dependence: a guide to measure evaluation and selection. Nicotine Tob Res. 2006;8(3):339–51. doi: 10.1080/14622200600672765. [DOI] [PubMed] [Google Scholar]
- 25.Loh WY, Piper ME, Schlam TR, Fiore MC, Smith SS, Jorenby DE, Cook JW, Bolt DM, Baker TB. Should all smokers use combination smoking cessation pharmacotherapy? Using novel analytic methods to detect differential treatment effects over 8 weeks of pharmacotherapy. Nicotine & Tobacco Research. 2011 doi: 10.1093/ntr/ntr147. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stapleton JA, Sutherland G. Treating heavy smokers in primary care with the nicotine nasal spray: randomized placebo-controlled trial. Addiction. 2011;106(4):824–32. doi: 10.1111/j.1360-0443.2010.03274.x. [DOI] [PubMed] [Google Scholar]
- 27.Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, Tell GS. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279(2):119–24. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- 28.Croghan IT, Hurt RD, Ebbert JO, Croghan GA, Polk OD, Stella PJ, Novotny PJ, Sloan J, Loprinzi CL. Racial differences in smoking abstinence rates in a multicenter, randomized, open-label trial in the United States. Z Gesundh Wiss. 2010;18(1):59–68. doi: 10.1007/s10389-009-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frosch ZA, Dierker LC, Rose JS, Waldinger RJ. Smoking trajectories, health, and mortality across the adult lifespan. Addict Behav. 2009;34(8):701–4. doi: 10.1016/j.addbeh.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy DT, Romano E, Mumford E. The relationship of smoking cessation to sociodemographic characteristics, smoking intensity, and tobacco control policies. Nicotine Tob Res. 2005;7(3):387–96. doi: 10.1080/14622200500125443. [DOI] [PubMed] [Google Scholar]
- 31.Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–62. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 33.Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, Baker TB. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine Tob Res. 2007;9(9):947–54. doi: 10.1080/14622200701540820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobell LC, Sobell MB. In: Timeline Follow-Back: A technique for assessing self-reported alcohol consumption, in Measuring alcohol consumption: Psychosocial and biological methods. Litten RZ, Allen J, editors. Human Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- 35.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alberg AJ, Patnaik JL, May JW, Hoffman SC, Gitchelle J, Comstock GW, Helzlsouer KJ. Nicotine replacement therapy use among a cohort of smokers. J Addict Dis. 2005;24(1):101–13. doi: 10.1300/J069v24n01_09. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention C Use of FDA-approveed pharmacologic treatments for tobacco dependence--United States, 1984-1998. morbidity and Mortality Weekly Report. 2000;49(29):665–668. [PubMed] [Google Scholar]
- 38.Verification SSoB Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 39.Berrettini WH, Wileyto EP, Epstein L, Restine S, Hawk L, Shields P, Niaura R, Lerman C. Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biol Psychiatry. 2007;61(1):111–8. doi: 10.1016/j.biopsych.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Rutter JL. Symbiotic relationship of pharmacogenetics and drugs of abuse. AAPS J. 2006;8(1):E174–84. doi: 10.1208/aapsj080121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.