Abstract
Zebrafish are becoming increasingly popular models for examining the mechanisms of and treatments for neurological diseases. The available methods and technology to examine disease processes in vivo are increasing, however, detailed observations of subcellular structures and processes are complex in whole organisms. To address this need, we developed a primary motor neuron (MN) culture technique for utilization with zebrafish neurological disease models. Our protocol enables the culturing of cells from embryos older than 24 hours post-fertilization, at points after MN axonal development and outgrowth begins, which enables MN axons to develop in vivo in the context of the normal endogenous cues of the model organism, while also providing the accessibility of an in vitro system. When utilized with the increasing number of genetically modified or transgenic models of neurological diseases, this approach provides a novel tool for the examination of cellular and subcellular disease mechanisms, and offers a new platform for therapeutic discoveries in zebrafish.
Keywords: zebrafish, motor neuron, ex vivo culture, primary culture
1. Introduction
Our understanding of the cellular and molecular processes underlying human diseases relies heavily on information obtained from in vitro and in vivo models. In recent years, zebrafish (Danio rerio) have gained popularity as a model organism among scientists for the examination of cellular processes and for drug discovery (Chakraborty et al., 2009; Lieschke and Currie, 2007; Rinkwitz et al., 2011). Zebrafish are vertebrate organisms that develop quickly and produce large numbers of embryos that are optically transparent. Furthermore, zebrafish are easily amenable to genetic manipulation, and through forward genetic screens, transient genetic manipulation and stable transgenesis, the current applications of zebrafish models extend across a full biological disease spectrum.
While originally admired for its potential as a developmental biology tool, zebrafish are more recently being examined for their application to the study of neurodegenerative diseases (Bandmann and Burton, 2010; Kabashi et al., 2010; Rinkwitz et al., 2011; Sager et al., 2010). The neuromuscular system in zebrafish has been well characterized, and techniques exist to examine motor axon development and characterize neuromuscular junction formation at multiple levels in vivo (Beattie, 2000; Saint-Amant et al., 2008). Because embryos are optically transparent, the combination of fluorescent dye injection and live imaging has provided a valuable resource for understanding early motor neuron (MN) axon guidance, and immunohistochemistry (IHC) and staining techniques provide additional methods for observation of the neuromuscular system in developing zebrafish. What is lacking, however, is an approach that enables detailed observations of subcellular structures in zebrafish neurological disease models.
At this point, little emphasis has been placed on in vitro strategies based on zebrafish models (Lieschke and Currie, 2007). A small number of immortalized embryonic zebrafish cell lines are available, however characterization of these lines is minimal and they do not represent the tissues of interest for neuroscientists (Chen et al., 2002; Driever and Rangini, 1993; He et al., 2006). Primary culture techniques from zebrafish offer an alternative approach for the examination of neuronal populations. Primary neuron culture protocols from developing zebrafish ranging from blastula stage embryos to 19 hours post-fertilization (hpf) have been reported (Andersen, 2001; Ghosh et al., 1997; Liu and Westerfield, 1992), however, these cultures are derived prior to the normal course of MN axon pathfinding and neuromuscular development. In zebrafish, primary MN axons exit the spinal cord beginning around 18 hpf, whereas secondary MN axonal pathfinding occurs between 26-34 hpf (Beattie, 2000; Eisen, 1991; Eisen et al., 1986; Myers et al., 1986). Brain explants cultures (Hendricks and Jesuthasan, 2007) and primary cell culture techniques for muscle fibers (Dowling et al., 2009; Nixon et al., 2005; Saint-Amant et al., 2008) are possible at later developmental stages from zebrafish embryos; therefore, we have developed and optimized a method for dissociating zebrafish cells at developmental stages after axonal outgrowth has begun and maintaining primary zebrafish MNs in culture.
Here, we describe our novel method for the ex vivo examination of zebrafish MNs from embryos and larvae. This protocol is based on previous zebrafish culture protocols (Andersen, 2001; Beattie, 2000; Dowling et al., 2009; Eisen, 1991; Eisen et al., 1986; Ghosh et al., 1997; Hendricks and Jesuthasan, 2007; Liu and Westerfield, 1992; Myers et al., 1986; Nixon et al., 2005; Saint-Amant et al., 2008) and protocols in our laboratory deriving primary MN cultures from embryonic rats (Lunn et al., 2009; Vincent et al., 2004a; Vincent et al., 2004b). The current technique enables zebrafish MNs to initially develop in vivo, taking advantage of the endogenous developmental cues of the model organism. Furthermore, we demonstrate the versatility of this protocol for application to embryonic and larval zebrafish of different ages, and present alternative approaches for enhancing the purity of specific cell types. Finally, we characterize the cell types present within the mixed ex vivo cultures derived from dissociated zebrafish, and demonstrate the potential of this cell culture approach for detailed examination of subcellular structures. This technique provides a novel tool that can increase our understanding of the pathogenesis of neurodegenerative disorders and promote mechanism-based drug development for neurologic disorders.
2. Materials and Methods
2.1. Zebrafish
All zebrafish were maintained in compliance with University of Michigan’s Institutional Animal Care and Use Committee (UCUCA) standards and utilized per approved protocols. To facilitate the visualization of MNs and subcellular organelles, transgenic zebrafish lines were utilized that express GFP in tissue-specific or organelle-specific manners. Embryos used for the current studies were collected after timed mating of an adult AB strain zebrafish crossed with a homozygous transgenic zebrafish expressing GFP in MNs (HB9:mGFP) (Flanagan-Steet et al., 2005), or with a homozygous transgenic zebrafish expressing mitochondrially-targeted GFP (MLS-EGFP) (Kim et al., 2008). Embryos were incubated at 28°C in 0.5X E2 media (Westerfield, 2007) until processed for primary cell culture or fixed overnight in 4% paraformaldehyde for whole-mount confocal imaging.
2.2. Primary cell culture from dissociated embryos
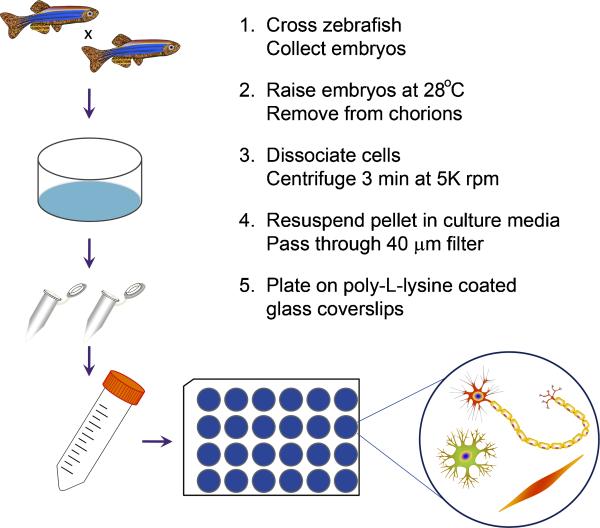
The goal of the current study was to develop a simple protocol to establish primary MN cultures from zebrafish embryos at a point after MN axons have developed. To prepare the zebrafish embryos for dissociation and primary cell culture, embryos at 24, 48 or 96 hpf were manually dechorionated with fine forceps and approximately 30 embryos per 1.5 ml microcentrifuge tube were rinsed in sterile 0.5X E2 medium. Unless otherwise noted, ex vivo cultures were prepared from whole embryos; however, some cultures were prepared from only the zebrafish body and tail in order to examine the potential to enhance spinal MN density. Dissection was performed using fine forceps to remove the anterior portion of the embryos prior to dissociation. To dissociate the embryos, culture media containing collagenase type II (1 mg/ml; Worthington, Lakewood, NJ) was added and embryos were incubated with gentle rotation and periodic trituration at room temperature, with time intervals between trituration and for dissociation dependent on the age of the zebrafish. For 24 hpf embryos, zebrafish were triturated every 10 min for approximately 30 min. For 48 hpf embryos, dissociation proceeded for approximately 45 min to 1 h, with trituration occurring in 15 min intervals. For 96 hpf larvae, zebrafish were incubated for approximately 1.5-2 h, with trituration every 30 min. Culture media consisted of CO2-independent media (Gibco BRL, Invitrogen, Carlsbad, CA) supplemented with 1X penicillin/streptomycin/neomycin (PSN; Gibco BRL). Zebrafish are dissociated until only small pieces are visible, but not so long that cells begin to dissolve. Once cell dissociation was sufficient, the cells were centrifuged for 3 min at 5000 rpm, the supernatant was removed and the pellet was resuspended in 1 ml culture media. The solution was passed through a 40 μm filter, after which the dissociated cells were plated on poly-L-lysine-coated glass coverslips at a density of 40 μl / coverslip. For some experiments, dissociated cells underwent fluorescence activated cell sorting (FACS) using a BD FACS AriaTM II instrument at the University of Michigan Flow Cytometry Core (http://www.med.umich.edu/flowcytometry/) prior to plating to enhance spinal MN purity. After allowing 1-2 h for the cells to adhere to the coverslips, additional culture media was added for maintenance of the cultures, after which cultures underwent a 50% media change daily. Cultures were incubated at 37°C in 5% CO2. See Figure 1 for an overview of the protocol.
Figure 1. Primary culture protocol from zebrafish embryos.
Embryos are collected following timed mating of adult zebrafish and raised to the desired developmental timepoint. Embryos are then dechorionated, if necessary, and incubated in culture media containing 1 mg/ml collagenase type 2 with periodic trituration until dissociation is sufficient. Samples are centrifuged to pellet the dissociated cells, which are subsequently resuspended in fresh culture media. Prior to plating, resuspended cells are passed through a 40 μm filter to remove large debris. After cells adhere to poly-L-lysine-coated glass coverslips, additional culture media is supplemented and cultures are incubated until the desired timepoint for further processing.
2.3. IHC
Primary cultures grown on glass coverslips were fixed at various time points between 24 and 72 h by incubating in 4% PFA for 5 min followed by washing and storage in 1X PBS at 4°C. IHC was performed as previously described (Dowling et al., 2009; Lunn et al., 2009; Vincent et al., 2004a; Vincent et al., 2004b). Briefly, coverslips were permeabilized for 5 min in 0.1% Triton in 1X PBS, and blocked for 2 h at room temperature in 5% normal goat serum (NGS) / 0.1% Triton in 1X PBS. The coverslips were incubated overnight at 4°C in the following primary antibodies: for muscle, F59 (1:100; Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA); for glia, GFAP (1:100; Cell Signaling); for neurons, Islet-1/2 (1:100, DSHB), SV2 (1:200, DSHB) and neurofilament (NF; 1:1000, Abcam, Cambridge, MA). Coverslips were then washed and incubated with AlexaFluor-594-conjugated anti-mouse secondary antibody (1:250, Molecular Probes, Invitrogen, Eugene, OR) for 2 h at room temperature. Following subsequent PBS washes, coverslips were mounted onto glass slides using ProLong-Gold antifade reagent with DAPI (Molecular Probes).
2.4. Imaging and analysis
Live imaging of whole embryos was performed using a Nikon AZ100 microscope. Imaging and analysis of IHC results and fixed whole embryos was performed using an Olympus FluoView 500 confocal microscope. MN density was determined by calculating the percentage of GFP-positive MNs among the total number of DAPI-positive cells over at least 10 independent image fields per condition. All results are representative of at least 3 independent experiments.
3. Results
3.1. Primary cell culture from dissociated embryos
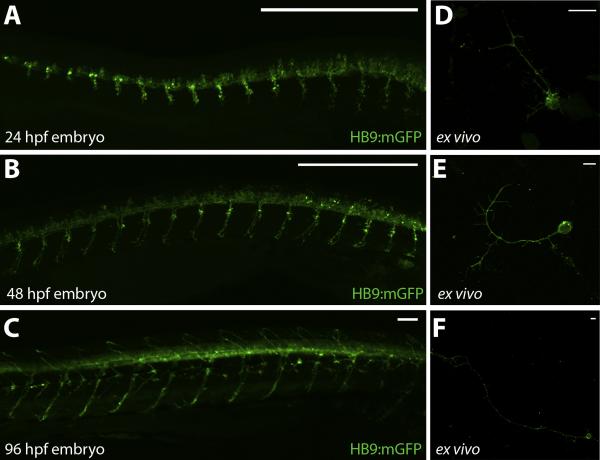
Ex vivo preparations from HB9:mGFP transgenic zebrafish embryos provide a simple approach for culturing and examining zebrafish MNs. This protocol enables the dissociation of MNs from zebrafish embryos at multiple different ages after MN axonal development has begun. Imaging of HB9:mGFP embryos at 24, 48 and 96 hpf demonstrate the extent of MN axonal development at these timepoints (Fig. 2 A-C). Representative images of GFP expression in dissociated cell preparations from 24, 48 and 96 hpf HB9:mGFP embryos are shown after 48 h in culture (Fig. 2 D-F). These results demonstrate the versatility of the culture technique for embryos of different ages, and further represent the ability to image MN axonal integrity in ex vivo cultures.
Figure 2. HB9:mGFP embryo and primary culture imaging.
A-C. MN development in HB9:mGFP embryos at 24, 48 and 96 hpf. D-F. Primary cultures from 24, 48 and 96 hpf dissociated HB9:mGFP embryos after 48 h in culture. Zebrafish and cellular images are acquired using an Olympus BX-51 confocal microscope at 10X or 60X magnification, respectively. Scale bar equals 50 μm (A-C) or 20 μm (D-F).
3.2. Cellular analysis of primary cultures
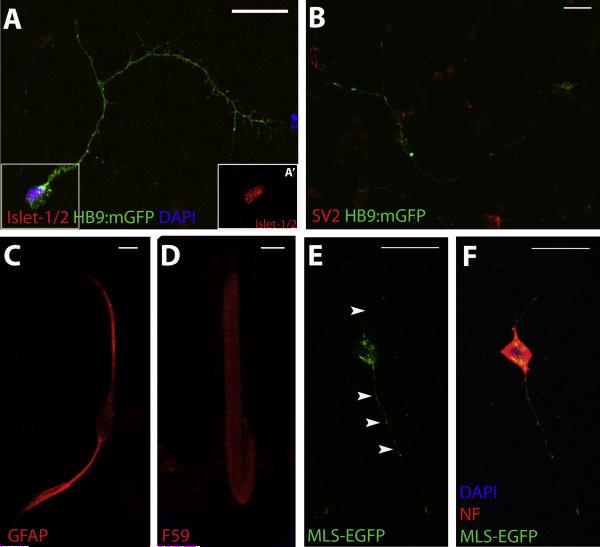
Primary cultures enable MN axons to develop in vivo while still providing a readily accessible system for detailed cellular analysis. While HB9:mGFP transgenic zebrafish embryos enable the visualization of cultured MNs without additional IHC processing, IHC with various cellular markers validate this protocol for the detailed examination of MNs and demonstrate some potential applications for the technique. Islet-1 expression in GFP-positive cells validates that MNs can be identified using GFP fluorescence when cultures are obtained from HB9:mGFP transgenic zebrafish embryos (Flanagan-Steet et al., 2005), and as shown in Figure 4A, nuclear expression of Islet-1 (see inset, Fig. 4A’) is evident in GFP-positive neurons. Furthermore, general neuronal labeling with SV2 (Fig. 4B) demonstrates that GFP expression is restricted to the MNs, as also evidenced in studies characterizing the transgenic HB9:mGFP zebrafish line (Flanagan-Steet et al., 2005).
Figure 4. Characterization of primary cultures.
A-D. IHC for various neuronal and non-neuronal markers demonstrate the presence of MNs (Islet-1/2; A, A’(inset of boxed region in A)), synaptic vesicles (SV2; B), glia (GFAP; C) and muscle fibers (F59; D) in primary cultures from 48 hpf HB9:mGFP embryos. E-F. Utilization of MLS-EGFP transgenic zebrafish for primary cultures enables visualization of mitochondria in cell bodies and axons (arrowheads; E) of neurons, as demonstrated by IHC for a neuronal marker (NF; F). GFP expression in transgenic zebrafish lines in MNs is under control of the HB9 promoter (HB9:mGFP; A-D) or in mitochondria using a mitochondrial-localization signal (MLS-EGFP; E-F). Images are acquired using an Olympus BX-51 confocal microscope at 60X magnification. Scale bar equals 20 μm.
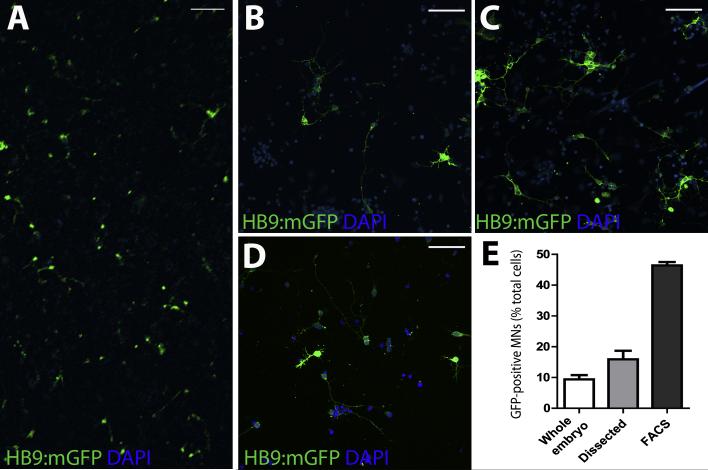
Because cells are obtained from dissociated whole embryos, the resulting cultures contain a heterogeneous population of cell types. As demonstrated in Figure 3A, GFP-expressing MNs represent only a portion of the cultured cells. IHC for glial (Fig. 4C) and muscle cell (Fig. 4D) markers demonstrate the presence of both cell types within the mixed cultures and support the extension of this protocol to other cell types of interest if desired. It is possible, however, to enhance the ratio of particular cell types in the cultures using dissection techniques or FACS. For example, to enhance the spinal MN density in our ex vivo cultures using dissection techniques, we prepared cultures from the trunk and tail portion of zebrafish embryos (Fig. 3C). Alternatively, cultures were prepared from dissociated embryos that underwent FACS prior to plating (Fig. 3D). Quantification of the percentage of GFP-positive cells in these cultures compared to those from whole embryos (Fig. 3B) demonstrate that it is possible to significantly increase the purity of MNs (Fig. 3E). Overall, these results confirm that it is possible to enhance the proportion of a cell type of interest in zebrafish ex vivo cultures.
Figure 3. MN density in primary cultures.
A. Representative low magnification imaging of ex vivo cultures demonstrates typical MN density. B-D. Approaches to enhance the MN density typically seen in ex vivo cultures from whole embryos (B) include dissection of embryos prior to dissociation (C) and FACS (D). E. Quantification of the percentage of GFP-positive MNs in ex vivo cultures prepared from whole embryos, dissected embryos or following FACS. Images are acquired using an Olympus BX-51 confocal microscope at 10X (A) or 20X (B-D). Scale bar equals 100 μm (A-D).
Potential applications of this technique also include the detailed examination of subcellular structures including organelles. Primary cultures from MLS-EGFP transgenic zebrafish embryos which express mitochondrially-targeted GFP demonstrate fluorescence in mitochondria that can be resolved with confocal microscopy (Fig. 4E-F). Overall, these data demonstrate that in the heterogeneous population of cells obtained from dissociating whole embryos, the combination of available transgenic zebrafish lines and IHC techniques allow a simple approach for the examination of detailed MN biology.
4. Discussion
A small number of protocols exist for the ex vivo culturing of embryonic cells from various zebrafish tissues at early developmental stages; however, no reports of a system for culturing MNs after axonal outgrowth has begun are noted to our knowledge. In the current manuscript, we describe a novel protocol for deriving cultures from embryonic and larval zebrafish (Fig. 1). MN axons first exit the spinal cord after 18 hpf; therefore, we dissociated and cultured cells from zebrafish at 24, 48, and 96 hpf (Fig. 2). These time points enable the MNs to develop in vivo, following endogenous pathfinding cues within the embryo, receive cues from supporting cells within the spinal cord and musculature and form neuromuscular contacts. While we acknowledge that dissociation of cells from zebrafish embryos and larvae may alter the integrity of MN axons that have already developed, we maintain that subsequent axonal outgrowth occurring in vitro reflects developmental cues received by spinal MNs prior to dissociation. Neurites are observed on some cells within 1 h of plating (data not shown), and the increased axonal length observed in preparations from embryos at 24, 48 and 96 hpf (Fig. 2) reflects the various developmental stages and potential of these MNs. This culture approach provides a novel tool to study MNs from zebrafish models of neurological diseases.
Along with MNs, several additional cell types are also present within the ex vivo cultures. Using IHC, we have identified neurons, glia and myofibers within our preparations (Fig. 4), and other cells are also present. The presence of these cell types increase the versatility of this technique beyond that of just studies based on neurological disease models. Furthermore, the heterogeneous population in the cultures recapitulates a cellular environment that resembles one normally present in vivo. As demonstrated in Figure 3, however, adaptations to the protocol can increase the proportion of MNs if desired. Enhanced myofiber and MN density is possible by preparing cultures from zebrafish embryo trunks and tails, and MN purity can be significantly enhanced using FACS. The current results reflect cultures with approximately 50% MNs following FACS (Fig. 3D-E); however, studies are currently underway to optimize FACS parameters and investigate additional approaches to achieve more pure primary zebrafish MN cultures. Overall, the primary culture technique presented here provides an adaptable resource that can be easily modified to highlight and study specific cell types present in the cultures.
Modification of culture conditions for the protocol provides additional versatility for extension of this technique to different disease models and cell types. During the optimization of this protocol, different culture media types were tested (data not shown) which all demonstrated successful growth and maintenance of the cultures. The cultures presented here are grown in CO2-independent media, however, we were also able to culture cells in Dulbecco’s Modified Eagle Medium supplemented with fetal bovine serum and PSN, as well as modified Neurobasal media utilized for our rodent primary MN cultures (Lunn et al., 2009; Vincent et al., 2004a; Vincent et al., 2004b). Furthermore, an additional advantage of zebrafish ex vivo cultures is their ability to grow at temperatures ranging from room temperature to 37°C. Optimal zebrafish development occurs at 28.5°C and studies are underway to compare the effects of culture temperatures on the stabilization of various cell types. Our results, however, demonstrate successful growth of MNs at 37°C, and other protocols demonstrate growth at room temperature as well (Andersen, 2001; Saint-Amant et al., 2008). Overall, the foundation of the protocol enables flexibility depending on the cell type of interest and experimental demands.
Many transgenic zebrafish lines that express GFP and other fluorescent reporters in a cell- or organelle-specific manner are available that further enhance the potential of this ex vivo culture technique. Because our group is interested in MN degeneration, the current results are based on experiments utilizing HB9:mGFP transgenic zebrafish, which express GFP in MNs under control of the HB9 promoter element (Flanagan-Steet et al., 2005). Additional transgenic zebrafish lines with tissue-specific GFP expression are also reported, including another MN-specific line, a glial-specific line and muscle-specific reporter lines (Bernardos and Raymond, 2006; Higashijima et al., 1997; Paquet et al., 2009; Park et al., 2000). Using FACS prior to plating in combination with these transgenic zebrafish lines with tissue-specific GFP expression can achieve cultures featuring various desired labeled populations of cells. Organelle-specific GFP expression is also available in transgenic zebrafish lines such as the MLS-EGFP line, where GFP is localized to mitochondria (Kim et al., 2008). As shown in Figure 4E-F, mitochondria in MNs are possible to resolve in our ex vivo cultures, which would be difficult in whole developing organisms as the GFP is expressed in mitochondria of all cells. Ex vivo cultures utilizing the MLS-EGFP zebrafish line, for example, may provide a novel tool to assess altered axonal mitochondrial transport, a proposed mechanism in the pathogenesis of neurodegeneration (Chevalier-Larsen and Holzbaur, 2006; Duffy et al., 2011). Overall, the combination of such transgenic lines with IHC represents the potential to examine specific structures or cell types in detail, and offers a system to examine the effects of exogenous cues on cellular morphology and subcellular organelle distribution, for example.
With the ability to examine detailed cellular features using primary cultures, the protocol described here adds an impressive tool to the many advantages of zebrafish as model organism for neurological disease and many other human diseases. Genetic manipulation of zebrafish to model specific diseases and altering the expression of genes of interest in specific subsets of cells in zebrafish, in combination with this technique, offers an exciting approach to learn more about disease mechanisms and provides the potential to scrutinize subcellular events. Furthermore, the application of exogenous factors to cultured primary cells will provide a new platform for analyzing and developing novel therapies for neurological diseases. Overall, this technique provides a novel approach for the detailed examination of MN biology and therapeutic discovery in zebrafish. Primary culture from zebrafish embryos and larvae has the potential to provide tremendous insight into disease mechanisms and treatments for multiple human diseases.
Highlights.
Primary neuron cultures from 24-96 hours post-fertilizaiton zebrafish embryos
Cultures are obtained from embryos after in vivo motor neuron axon outgrowth begins
Mixed cultures include neurons, muscle fibers, glia and many other cell types
Provides a novel tool to examine cellular processes in zebrafish disease models
Acknowledgements
The authors would like to thank Dr. John Kuwada and Dr. Seok-Yong Choi for providing the HB9:mGFP and MLS-EGFP transgenic zebrafish, respectively. The authors would like to thank Dr. Stephen Lentz at the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center (funded by NIH5P60 DK20572 from the National Institute of Diabetes & Digestive & Kidney Diseases) for microscopy assistance. The authors would also like to thank the University of Michigan Flow Cytometry Core Facility (http://www.med.umich.edu/flowcytometry/) for assistance with FACS. The authors would also like to thank Ms. Judith Boldt for excellent secretarial support during the preparation of this manuscript. This work is supported by the A. Alfred Taubman Medical Research Institute and the Program for Neurology Research and Discovery. Support for SAS is provided by the National Institutes of Health (T32 NS007222-28).
Abbreviations
- DSHB
Developmental Studies Hybridoma Bank
- FACS
fluorescence activated cell sorting
- hpf
hours post-fertilization
- IHC
immunohistochemistry
- MLS
mitochondria-localization signal
- MN
motor neuron
- NF
neurofilament
- NGS
normal goat serum
- PSN
penicillin/streptomycin/neomycin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SS. Preparation of dissociated zebrafish spinal neuron cultures. Methods Cell Sci. 2001;23:205–9. doi: 10.1023/a:1016349232389. [DOI] [PubMed] [Google Scholar]
- Asakawa K, Kawakami K. Targeted gene expression by the Gal4-UAS system in zebrafish. Dev Growth Differ. 2008;50:391–9. doi: 10.1111/j.1440-169X.2008.01044.x. [DOI] [PubMed] [Google Scholar]
- Bandmann O, Burton EA. Genetic zebrafish models of neurodegenerative diseases. Neurobiol Dis. 2010;40:58–65. doi: 10.1016/j.nbd.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Beattie CE. Control of motor axon guidance in the zebrafish embryo. Brain Res Bull. 2000;53:489–500. doi: 10.1016/s0361-9230(00)00382-8. [DOI] [PubMed] [Google Scholar]
- Bernardos RL, Raymond PA. GFAP transgenic zebrafish. Gene Expr Patterns. 2006;6:1007–13. doi: 10.1016/j.modgep.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Chakraborty C, Hsu CH, Wen ZH, Lin CS, Agoramoorthy G. Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab. 2009;10:116–24. doi: 10.2174/138920009787522197. [DOI] [PubMed] [Google Scholar]
- Chen W, Burgess S, Golling G, Amsterdam A, Hopkins N. High-throughput selection of retrovirus producer cell lines leads to markedly improved efficiency of germ line-transmissible insertions in zebra fish. J Virol. 2002;76:2192–8. doi: 10.1128/jvi.76.5.2192-2198.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochimica et biophysica acta. 2006;1762:1094–108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Dowling JJ, Vreede AP, Low SE, Gibbs EM, Kuwada JY, Bonnemann CG, Feldman EL. Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet. 2009;5:e1000372. doi: 10.1371/journal.pgen.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W, Rangini Z. Characterization of a cell line derived from zebrafish (Brachydanio rerio) embryos. In Vitro Cell Dev Biol Anim. 1993;29A:749–54. doi: 10.1007/BF02631432. [DOI] [PubMed] [Google Scholar]
- Duffy LM, Chapman AL, Shaw PJ, Grierson AJ. The role of mitochondria in the pathogenesis of Amyotrophic Lateral Sclerosis. Neuropathol Appl Neurobiol. 2011;37:336–52. doi: 10.1111/j.1365-2990.2011.01166.x. [DOI] [PubMed] [Google Scholar]
- Eisen JS. Motoneuronal development in the embryonic zebrafish. Development (Cambridge, England) 1991;(Suppl 2):141–7. [PubMed] [Google Scholar]
- Eisen JS, Myers PZ, Westerfield M. Pathway selection by growth cones of identified motoneurones in live zebra fish embryos. Nature. 1986;320:269–71. doi: 10.1038/320269a0. [DOI] [PubMed] [Google Scholar]
- Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development (Cambridge, England) 2005;132:4471–81. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- Ghosh C, Liu Y, Ma C, Collodi P. Cell cultures derived from early zebrafish embryos differentiate in vitro into neurons and astrocytes. Cytotechnology. 1997;23:221–30. doi: 10.1023/A:1007915618413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Salas-Vidal E, Rueb S, Krens SF, Meijer AH, Snaar-Jagalska BE, Spaink HP. Genetic and transcriptome characterization of model zebrafish cell lines. Zebrafish. 2006;3:441–53. doi: 10.1089/zeb.2006.3.441. [DOI] [PubMed] [Google Scholar]
- Hendricks M, Jesuthasan S. Electroporation-based methods for in vivo, whole mount and primary culture analysis of zebrafish brain development. Neural Dev. 2007;2:6. doi: 10.1186/1749-8104-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Okamoto H, Ueno N, Hotta Y, Eguchi G. High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Developmental biology. 1997;192:289–99. doi: 10.1006/dbio.1997.8779. [DOI] [PubMed] [Google Scholar]
- Kabashi E, Champagne N, Brustein E, Drapeau P. In the swim of things: recent insights to neurogenetic disorders from zebrafish. Trends Genet. 2010;26:373–81. doi: 10.1016/j.tig.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kang KH, Kim CH, Choi SY. Real-time imaging of mitochondria in transgenic zebrafish expressing mitochondrially targeted GFP. Biotechniques. 2008;45:331–4. doi: 10.2144/000112909. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–99. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–67. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Liu DW, Westerfield M. Clustering of muscle acetylcholine receptors requires motoneurons in live embryos, but not in cell culture. J Neurosci. 1992;12:1859–66. doi: 10.1523/JNEUROSCI.12-05-01859.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JS, Sakowski SA, Kim B, Rosenberg AA, Feldman EL. Vascular endothelial growth factor prevents G93A-SOD1-induced motor neuron degeneration. Developmental neurobiology. 2009;69:871–84. doi: 10.1002/dneu.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers PZ, Eisen JS, Westerfield M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J Neurosci. 1986;6:2278–89. doi: 10.1523/JNEUROSCI.06-08-02278.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–20. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Wegner J, Ferguson C, Mery PF, Hancock JF, Currie PD, Key B, Westerfield M, Parton RG. Zebrafish as a model for caveolin-associated muscle disease; caveolin-3 is required for myofibril organization and muscle cell patterning. Hum Mol Genet. 2005;14:1727–43. doi: 10.1093/hmg/ddi179. [DOI] [PubMed] [Google Scholar]
- Paquet D, Bhat R, Sydow A, Mandelkow EM, Berg S, Hellberg S, Falting J, Distel M, Koster RW, Schmid B, Haass C. A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J Clin Invest. 2009;119:1382–95. doi: 10.1172/JCI37537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HC, Kim CH, Bae YK, Yeo SY, Kim SH, Hong SK, Shin J, Yoo KW, Hibi M, Hirano T, Miki N, Chitnis AB, Huh TL. Analysis of upstream elements in the HuC promoter leads to the establishment of transgenic zebrafish with fluorescent neurons. Developmental biology. 2000;227:279–93. doi: 10.1006/dbio.2000.9898. [DOI] [PubMed] [Google Scholar]
- Rinkwitz S, Mourrain P, Becker TS. Zebrafish: An integrative system for neurogenomics and neurosciences. Prog Neurobiol. 2011;93:231–43. doi: 10.1016/j.pneurobio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Sager JJ, Bai Q, Burton EA. Transgenic zebrafish models of neurodegenerative diseases. Brain Struct Funct. 2010;214:285–302. doi: 10.1007/s00429-009-0237-1. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Sprague SM, Hirata H, Li Q, Cui WW, Zhou W, Poudou O, Hume RI, Kuwada JY. The zebrafish ennui behavioral mutation disrupts acetylcholine receptor localization and motor axon stability. Developmental neurobiology. 2008;68:45–61. doi: 10.1002/dneu.20569. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Feldman EL, Song DK, Jung V, Schild A, Zhang W, Imperiale MJ, Boulis NM. Adeno-associated viral-mediated insulin-like growth factor delivery protects motor neurons in vitro. Neuromolecular Medicine. 2004a;6:79–86. doi: 10.1385/NMM:6:2-3:079. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Mobley BC, Hiller A, Feldman EL. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol Dis. 2004b;16:407–16. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 5 ed The University of Oregon Press; Eugene, OR: 2007. [Google Scholar]
- Xu Q, Stemple D, Joubin K. Microinjection and cell transplantation in zebrafish embryos. Methods Mol Biol. 2008;461:513–20. doi: 10.1007/978-1-60327-483-8_35. [DOI] [PubMed] [Google Scholar]