Abstract
Purpose
Epidermal growth factor receptor variant 3 (EGFRvIII) has been detected in several cancers where tumors expressing this truncated growth factor receptor demonstrate more aggressive behavior. The molecular mechanisms that contribute to EGFRvIII-mediated tumor progression that are amenable to targeted therapy are incompletely understood. The present study aimed to better define the role of src family kinases in EGFRvIII mediated cell motility and tumor growth of head and neck squamous cell carcinomas (HNSCC).
Experimental Design
HNSCC models expressing EGFRvIII were treated with dasatinib, a pharmacologic inhibitor of src family kinases (SFKs).
Results
SFK inhibition significantly decreased cell proliferation, migration and invasion of EGFRvIII-expressing HNSCC cells. Administration of dasatinib to mice bearing EGFRvIII-expressing HNSCC xenografts resulted in a significant reduction of tumor volume compared with controls. Immunoprecipitation with anti- c-Src, Lyn, Fyn and Yes antibodies followed by immunoblotting for phosphorylation of the SFK activation site (Y416) demonstrated specific activation of Lyn kinase in EGFRvIII expressing HNSCC cell lines and human HNSCC tumor specimens. Selective inhibition of Lyn using siRNA decreased cell migration and invasion of EGFRvIII- expressing HNSCC compared to vector-control cells.
Conclusions
These findings demonstrate that Lyn mediates tumor progression of EGFRvIII-expressing HNSCC where strategies to inhibit SFK may represent an effective therapeutic strategy.
Keywords: EGFRvIII, lyn, dasatinib, head and neck cancer, src
Introduction
Epidermal growth factor receptor variant 3 (EGFRvIII) is the most common EGFR alteration in many cancers including head and neck squamous cell carcinoma (HNSCC). EGFRvIII lacks exons 2–7, is constitutively active and is absent in normal tissue (1, 2). EGFRvIII expression has been shown to contribute to increased cell survival, proliferation, motility, invasiveness and treatment resistance (3–5). EGFRvIII has been most extensively characterized in glioma where src family kinases (SFKs) have been implicated in the EGFRvIII oncogenic phenotype. Abrogation of SFK signaling in an in vivo EGFRvIII positive glioma xenograft model significantly reduces EGFRvIII mediated tumorigenesis (6). Further studies in glioma found the src family kinases Fyn and c-Src to be key mediators in EGFRvIII signaling (7).
SFKs have been implicated in many normal cellular functions such as cell adhesion, migration, proliferation, survival, angiogenesis and differentiation where deregulation of these pathways contributes to tumorigenesis, tumor progression and metastasis of cancers expressing wild-type EGFR (8). SFKs are rarely mutated in cancer (8) and are activated in response to stimulation of several cellular factors including PDGFR, EGFR, IGF-1R, GPCRs, cytokine receptors, integrins, and cell adhesion complexes (9). Activated c-Src is common in colorectal and breast cancers and elevated levels of c-Src protein have been reported in several cancers including colon, breast, lung, endometrial, ovarian, pancreatic and HNSCC (8). c-Src has been reported to be activated in HNSCC compared to levels in normal mucosa where pSFK expression correlates with invasiveness and lymph node metastasis (10). Aberrant c-Src activation has been shown to contribute to HNSCC progression and metastasis (11, 12). SFK blockade inhibited proliferation in several tumor models including breast cancer, HNSCC, prostate cancer and glioma (11, 13–15). Treatment of cancer cell lines with a SFK inhibitor or siRNA directed against c-Src abrogated tumor cell invasion and migration (12, 14, 15). In HNSCC, c-Src, Lyn, Fyn and Yes are expressed at detectable levels in cell lines and tumors (16). Given the paucity of EGFRvIII cancer cell models and the difficulty of detecting EGFRvIII in human tumors, few studies have elucidated the role of SFK in cancers characterized by EGFRvIII expression.
The role of SFK in EGFRvIII-expressing HNSCC has not been explored, however, studies in wtEGFR only HNSCC have found that SFK can mediate proliferation, invasion and migration through various pathways (12). Glioma expressing-EGFRvIII (as compared to wtEGFR) preferentially signals through the Akt/PI3K and MAPK pathways (17, 18) and we have shown previously that inhibition of the PI3K/Akt pathway reduces cell proliferation but has no effect on cell motility or invasion in EGFRvIII expressing HNSCC (19). In wtEGFR-expressing HNSCC SFK inhibition reduced cell motility and invasion by regulating downstream cell adhesion molecules such as FAK (12). SFK is part of the focal adhesion complex which functions to link integrins to the cytoskeleton. In this complex SFK is involved in FAK activation (at tyrosines 576/577 and 861) and with other proteins, SFK promotes cell motility by turnover of the focal adhesion. Reduced cell motility is observed through SFK inactivation by c-Src tyrosine kinase (20). FAK also contains an autophosphorylation site (tyrosine 397) and when autophosphorylated creates a binding site for SFK via the SH2 domain of SFK which activates SFK by displacing the inhibitory phosphorylation at Y527 (21).
EGFRvIII is expressed in 17–42% of HNSCC, always in conjunction with wild type EGFR (wtEGFR) (22–24). HNSCC cells expressing EGFRvIII have been shown to be resistant to apoptosis by cisplatin in vitro and cetuximab tumor inhibition in vivo (22). A phase III clinical trial with the anti-EGFR monoclonal antibody cetuximab combined with radiation prolonged overall survival but did not alter the incidence of metastasis (25). We have shown previously that EGFRvIII expressing HNSCC cells are resistant to cetuximab-mediated inhibition of cell motility and invasion (19). A recent report of a phase II trial of cetuximab in combination with docetaxel in recurrent or metastatic HNSCC found that EGFRvIII expression was associated with reduced progression free survival (24). In EGFRvIII-expressing glioma, genetic and chemical inhibition of SFKs in several xenograft models have shown decreased tumor growth and metastasis compared to controls (6, 7). The role of SFK in EGFRvIII-expressing HNSCC has not been defined. We undertook the present study to determine the contribution of SFK in EGFRvIII-expressing HNSCC, where SFK targeting could represent an alternative therapeutic strategy in the setting of EGFRvIII-mediated cetuximab resistance. Identification of alternative therapeutic targets in the setting of EGFRvIII may improve treatment responses.
Materials and Methods
Cell lines, reagents and cell culture
Cal33 (site of origin: tongue) and UMSCC1 (SCC1) (site of origin: oral cavity) cells were a kind gift from Dr. Gerard Milano (Centre Antoine-Lacassagne, Nice, France) and Dr. Thomas E. Carey (University of Michigan, Michigan, USA), respectively. FaDu (site of origin: pharynx) and 293T cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). All cells were maintained in Dulbecco's modified Eagle's medium (Mediatech Inc., Herndon, VA, USA) with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA). UMSCC1 cells were supplemented with 0.4 ug/ml hydrocortisone (Invitrogen) and FaDu cells were supplemented with 1% NEAA (Invitrogen). Cells were incubated at 37°C in the presence of 5% CO2. All cell lines were genotyped by STR profiling using the AmpFℓSTR® Profiler PCR Amplification Kit (Applied Biosystems).
EGFRvIII-transfected HNSCC cells (Cal33vIII) and vector control-transfected HNSCC cells (Cal33control) have been previously described (19). EGFRvIII was subcloned into the pMSCV Neo plasmid (Clontech, Mountain View, CA). EGFRvIII plasmid DNAs were a kind gift from Dr Frank Furnari (Ludwig Institute for Cancer Research, La Jolla, CA). UMSCC1 and FaDu cells were infected with vector alone (MSCV) or EGFRvIII vector (vIII). Briefly, 293T cells were plated at 80–90% confluency in a 10 cm dish and reverse transfected using lipofectamine 2000 (Invitrogen), manufacturer’s plasmids and parent vector or EGFRvIII plasmid overnight. Fresh media was placed on the cells after 16 hours and virus produced over 48 hours. Target cells were plated in 10 cm dishes at 25% confluency 16 hours prior to treatment to allow cells to adhere. Viral supernatant was collected, centrifuged, filtered, supplemented with polybrene and placed on target cells for 72 hours. Viral supernatant was replaced with complete media for 24 hours and cells were selected with 0.5 mg/ml G418 (Invitrogen) for 72 hours. The resulting population of cells were maintained under selection pressure and tested for EGFRvIII expression via RT-PCR as described previously (19, 22). Briefly, total RNA was isolated from HNSCC cell lines using the RNeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. Total RNA (0.5ug) was reverse transcribed and amplified using SuperScript One-Step RT–PCR with Platinum Taq (Invitrogen). For EGFRvIII (primers previously described (22) and GAPDH the following primers and conditions were used: GAPDH forward 5’-TGGAATTTGCCATGGGTG-3’ and reverse 5’-GTGAAGGTCGGAGTCAAC-3’; Reverse transcription was performed for 30 minutes at 50°C followed by 2 minutes at 94°C. PCR amplification was performed for 40 cycles of: 94°C one minute, 67°C one minute and 72°C one minute followed by a final amplification at 72°C for 5 minutes. To detect only wtEGFR, primers were designed in the exon 2–7 region of EGFR. Primers were as follows: Forward 5’-ACAAGCTCACGCAGTTGGGCA-3’; Reverse 5’-GGCAGACCAGGCAGTCGCTC-3’; conditions were as above with denaturation at 62°C.
BCR-Abl/Src inhibitor dasatinib (Das) was a kind gift from Bristol-Myers Squibb (New York, NY, USA).
Immunoblotting
Cell lines and tumor pieces were lysed in detergent containing 1% NP-40, 0.1mM phenylmethylsulfonyl fluoride, 1mg/ml leupeptin and 1mg/ml aprotinin, and protein levels were determined using the Bio-Rad protein assay method (Bio-Rad Laboratories, Hercules, CA, USA). Total protein (40µg) were separated on 8%SDS–page gel and transferred to nitrocellulose membranes using the semidry transfer machine (Bio-Rad Laboratories). Membranes were blocked with Odyssey blocking buffer (Li-Cor Biosciences; Lincoln, NE), probed with primary and subsequently secondary antibodies and visualized using Odyssey Infrared Imaging System (Li-Cor Biosciences) according to manufacturer’s instructions. Quantification of western blots was performed using the Li-Cor Odyssey system to record the near infrared signal (NIR) according to the manufacturer’s instructions. Primary antibodies used for blotting included β-actin, phospho-Akt (Ser473), Akt, phospho-Src (Y416), , Lyn, Fyn, c-Src, and Yes from Cell Signaling Technology (also used for immunoprecipitations), Beverly, MA, Src B-12 (Santa Cruz Biotechnology; Santa Cruz, CA), and EGFR (BD Transduction Laboratories; San Jose, CA), anti-phospho-Src Y416 clone 9A6 (Millipore, Temecula, CA). Secondary antibodies used for blotting included goat anti rabbit IRDye 680 or goat anti mouse IRDye 800CW (Li Cor Biosciences).
Proliferation assays
To assay proliferation 5000 cells per well were plated in triplicate in a black-walled 96 well plate and allowed to adhere overnight. Following adhesion cells were treated with DMSO (control) or 100nM dasatinib for 72 hours and subsequently assayed with CellTiter-Glo Luminescent Cell Viability Assay (Promega; Madison WI) according to manufacturer’s instructions. Breifly, 100 ul Cell Titer Glo reagent was added to each well and the plate was rocked gently for 2 minutes and the luminescent signal allowed to stabilize for 10 minutes before the plate was read on a Victor3V 1420 multilabel counter with Wallac 1420 software (Perkin Elmer; Waltham, MA). Values were normalized to DMSO vector control cells and plotted in GraphPad Prism (version 4.03; GraphPad Prism Software, Inc.; La Jolla, CA).
Matrigel invasion assay and cell migration assay
Cell invasion was evaluated in vitro using Matrigel-coated semi-permeable modified Boyden inserts with a pore size of 8µm (Becton Dickinson/Biocoat, Bedford, MA, USA). Cell migration was evaluated in vitro using semi-permeable modified Boyden inserts with a pore size of 8µm (Becton Dickinson/Biocoat). For both assays, cells were plated in duplicate at a density of 1.3 × 104 cells per well in serum-free media in the insert. At the same time, cells were plated in 24-well plates to serve as loading and cell viability controls. Both the insert and the holding well were subjected to the same medium composition (DMEM with 7ng/ml EGF (Sigma-Aldrich Corp. St Louis, MO, USA) with the exception of serum. The insert contained no serum, whereas the lower well contained 10% FBS that served as a chemoattractant. After 24h of treatment at 37°C in a 5% CO2 incubator, the cells in the insert were removed by wiping gently with a cotton swab. Cells on the reverse side of the insert were fixed and stained with Hema 3 (Fisher Scientific, Hampton, NH, USA) according to the manufacturer's instructions. Cells plated in 24-well plates were subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays and the cell numbers across the groups were normalized. The number of invading or migrating cells was adjusted accordingly.
In vivo studies
Nu/nu athymic nude mice (Harlan Sprague-Dawley, Indianapolis, IN, USA) were injected subcutaneously with 5×105 cells per flank suspended in 100 µl serum free media with Cal33control and Cal33vIII in opposing flanks. Tumor volumes were measured in two dimensions with vernier calipers and calculated using the formula: (length × width2) × 0.52. At the end of the study, mice were killed by cervical dislocation under anesthesia; the tumors surgically excised and snap frozen in dry ice. Tumors were allowed to develop and 10 days after inoculation tumors were measured and stratified randomization performed dividing the mice into 2 groups of 8 mice per group. Mice were treated with either 80mM citric acid in PBS by oral gavage, or, dasatinib (50mg/kg daily) by oral gavage. All studies involving animal use and care were in strict compliance with institutional guidelines established by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Immunoprecipitation
HNSCC cells were plated at 50% confluency in a 10cm dish for 48 hours and harvested as indicated in immunoblotting. For immunoprecipitation cells were serum starved for 24 hours following cell adhesion. 1 mg of whole cell lysate from cell lines or 200 ng from patient specimens, 2–4ug antibody and 40 ul Protein G beads (Millipore, Temecula, CA) were combined and allowed to rotate overnight. Beads were pelleted the following day by centrifugation and washed 3 times with fresh lysis buffer. Beads were resuspended in 1× western blot loading dye and boiled for 8 min before loading onto a 10% SDS-page gel. Lyn, Fyn, c-Src, and Yes antibodies were used (Cell Signaling Technology, Beverly, MA) for immunoprecipitations and subsequent immunoblotting.
Patient tissues
Tumors were obtained from HNSCC patients with tumors in the oral cavity, oropharynx, hypopharyx, or larynx who provided written informed consent. Tissues were collected under the auspices of a tissue bank protocol approved by the University of Pittsburgh Institutional Review Board. Tissues were evaluated by a pathologist to confirm >70% tumor composition and fresh frozen for further studies. RNA was isolated and assayed for the presence of EGFRvIII by RT-PCR as previously described (19). EGFRvIII bands were excised from the agarose gel, purified using the QIAquick gel extraction kit according to manufacturer’s protocol (Qiagen, Valencia, CA) and sent for standard Sanger sequencing at the University of Pittsburgh Genomics and Proteomics Core Laboratories.
siRNA transfections
The siRNA sequences targeting Lyn human mRNA (sense: AAUGGUGGAAAGCAAAGUCCCUU, antisense: GGGACUUUGCUUUCCACCAUUUU; Sigma-Aldrich, St. Louis, MO, USA) were transfected into HNSCC cells for target silencing. The nontargeting siRNA (D-001210-01, sense 5′-UAGCGACUAAACACAUCAAUU-3′ and antisense 5-UUGAUGUGUUUAGUCGCUAUU-3′; Thermo Scientific Dharmacon) was used as a control. The siRNA or transfections were performed using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. HNSCC cells were transfected with 800pmol of siRNA or nontargeting control siRNA per 10cm dish. The transfection medium was replaced with complete media after 4–6h of transfection and cells incubated for 48 hours before plating for invasion and migration assays or harvested for western blotting.
Statistical analysis
For migration and invasion studies, the statistical significance of differences in the number of invading or migrating cells was assessed using Wilcoxon–Mann–Whitney exact test. Statistical analysis and graphs were created in GraphPad Prism (version 4.03; GraphPad Prism Software, Inc.; La Jolla, CA).
Results
EGFRvIII is expressed in engineered HNSCC cell lines
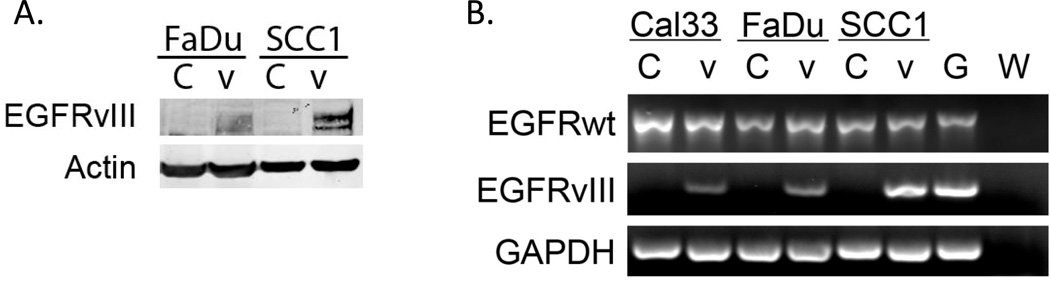
It has been previously reported that EGFRvIII is present in 17–42% of HNSCC (22–24). EGFRvIII is generally lost in vitro for unknown reasons, consequently EGFRvIII mechanistic studies must be performed on exogenously transfected model systems (26). We previously reported engineering the Cal33 HNSCC cell line to stably express EGFRvIII (19). For the present study we also stably expressed EGFRvIII constructs in 2 additional HNSCC cell lines, FaDu and UMSCC1. Differential transfection and infection efficiencies exist between cell lines and can affect the level of expression of exogenously introduced constructs. EGFRvIII expression in these cells was confirmed by immunoblotting whole cell lysates with an EGFRvIII antibody and by RT-PCR of isolated RNA (Figure 1).
Figure 1. EGFRvIII expression in transfected HNSCC cell lines.
EGFRvIII was stably introduced into the HNSCC cell lines FaDu and UMSCC1. A) Cells expressing the vector alone (labeled ‘C’) or EGFRvIII (labeled ‘V’) were probed for EGFRvIII using the 4–5H antibody to demonstrate protein expression of EGFRvIII. B) HNSCC cells expressing vector alone (labeled ‘C’) or EGFRvIII (labeled ‘V’) were assayed for EGFRvIII expression by RT-PCR as described in materials and methods. The glioma cell line U87MG EGFRvIII was used as a positive control (labeled ‘G’). A water control lane is included to ensure no contamination of reagents (labeled ‘W’), which contains reagents added with water in place of transcript. The experiment was repeated 3 times with similar results.
Src family kinases mediate proliferation, invasion and migration in EGFRvIII-expressing HNSCC cells
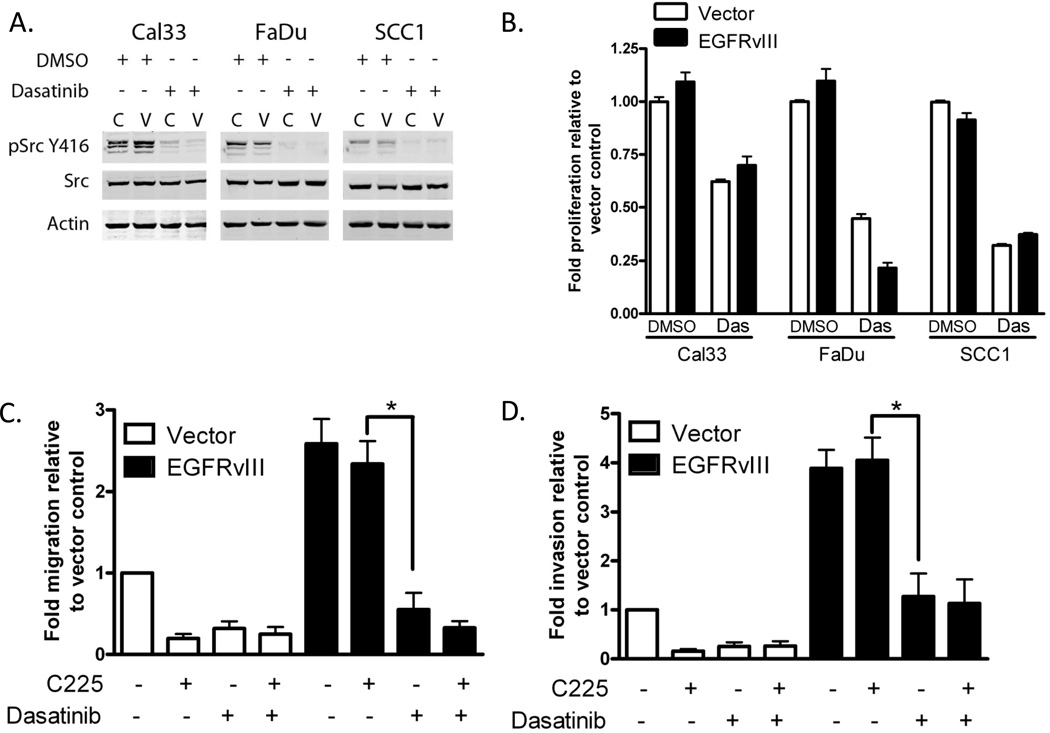
We previously reported that expression of EGFRvIII enhances HNSCC cell motility and invasion in vitro and tumor growth in vivo (22, 27). Previous reports show that EGFRvIII does not always increase proliferation in vitro but consistently augments tumor growth in vivo (28, 29). Additionally, in EGFRvIII-expressing HNSCC cells cetuximab treatment did not abrogate cell motility or invasion as it did in control cells (19). EGFRvIII has been most studied in glioma where SFKs have increased phosphorylation downstream of EGFRvIII compared to parental cell lines and mediate EGFRvIII-induced cell motility and tumor growth (7). We assessed SFK activation in EGFRvIII-expressing HNSCC by immunoblotting for phosphorylation at the activation site (Y416). All three EGFRvIII-expressing cell lines and vector control cells demonstrated phosphorylation at Y416, which was abrogated by treatment with the SFK inhibitor dasatinib (Figure 2A). Basal phosphorylation levels of SFKs varied among the HNSCC cell lines used. To assess the role of SFKs in EGFRvIII-expressing HNSCC cell proliferation, cells were treated with dasatinib and assayed for cell proliferation as indicated in materials and methods (Figure 2B). We found that dasatinib significantly inhibited cell proliferation in EGFRvIII expressing and vector control cells compared to vehicle treated cells (p<0.0001). These results indicate that SFK are activated in vector control and EGFRvIII-expressing HNSCC where treatment with the SFK inhibitor dasatinib abrogates proliferation.
Figure 2. SFK inhibition decreases proliferation, migration and invasion in EGFRvIII expressing HNSCC.
(A) Dasatinib inhibits phosphorylation of SFKs at tyrosine 416 in HNSCC expressing EGFRvIII. EGFRvIII (‘V’) expressing clones and vector control (‘C’) were cultured for 24 hours in the presence of DMSO or 100nM dasatinib and immunoblotted for SFK activation via phosphorylation at Y416. (B) Dasatinib inhibits cell proliferation in vector control (open bars) and EGFRvIII (closed bars) HNSCC cells. Cell proliferation was assayed in each cell line following 72 hours of treatment with DMSO or 100nM dasatinib. The experiment was repeated 4 times and the Mann-Whitney test was used to determine statistically significant differences between each cell line’s DMSO and dasatinib treatments; p<0.0001 for all comparisons of DMSO to treatment. (C) Dasatinib reduces EGFRvIII-mediated cell migration. UMSCC1 vector control (open bars) and EGFRvIII (closed bars) expressing cells were assayed for cell motility in the presence of DMSO or 100nM dasatinib (Das). The experiment was repeated 4 times and assessed for significance by the Mann-Whitney test: p=0.0006. (D) Dasatinib reduces EGFRvIII-mediated cell invasion. UMSCC1 vector control (open bars) and EGFRvIII (closed bars) expressing cells were assayed for cell invasion in the presence of DMSO or 100nM dasatinib (Das). The experiment was repeated 4 times and assessed for significance by the Mann-Whitney test: p=0.0009.
We previously reported that EGFRvIII increases cell motility in vitro in HNSCC where EGFRvIII cells are resistant to cetuximab-mediated inhibition of cell motility (19). To determine if inhibition of SFK could inhibit cell motility, vector control and EGFRvIII-expressing HNSCC cells (SCC1) were treated with dasatinib and/or cetuximab followed by assessment of migration and invasion. We found that dasatinib was effective at inhibiting migration (p=0.0006, Figure 2C) and invasion (p=0.0009, Figure 2D) in EGFRvIII expressing cells as well as vector control cells. Specifically, dasatnib reduced cell migration in EGFRvIII expressing cells by 72% while there was no significant inhibition of cell migration with cetuximab treatment. Combined treatment with cetuximab and dasatinib did not significantly enhance the inhibition of cell migration over dasatinib alone in either vector control or EGFRvIII expressing HNSCC cells. Dasatinib reduced invasion in EGFRvIII expressing cells by 58% but cetuximab treatment failed to significantly inhibit invasion in EGFRvIII expressing cells as it did in vector control HNSCC cells. Addition of cetuximab to the dasatinib treatment did not have any additional inhibitory effect on cell invasion over dasatinib alone. These results were confirmed in a second HNSCC cell line, FaDu (Supplemental Figure 1). The Cal33 cell line did not migrate or invade in our in vitro model systems.
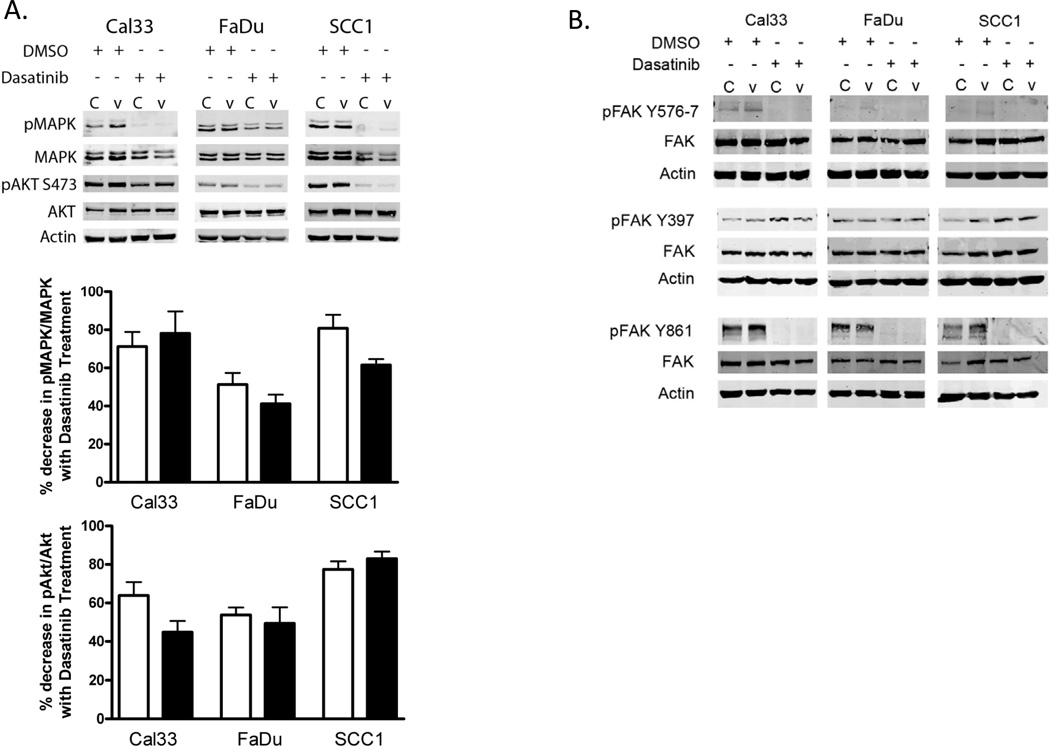
Dasatinib is known to inhibit several other kinases in addition to SFKs (30) and EGFRvIII has been shown to preferentially signal through several pathways including Akt/PI3K and MAPK in glioma (17, 18). To verify that dasatinib abrogated downstream signaling pathways involved in cell proliferation and motility we performed immunoblots of dasatinib-treated EGFRvIII expressing HNSCC cells to evaluate phosphorylation of the active sites of MAPK, Akt and FAK. EGFRvIII HNSCC cell lines treated with dasatinib demonstrated reduced phosphorylation of Akt and MAPK (Figure 3A). EGFRvIII-expressing HNSCC cells exposed to dasatinib also demonstrated reduced FAK phosphorylation at tyrosine 576/577 and tyrosine 861. Both of these phosphorylation sites are phosphorylated by active SFK. As anticipated, phosphorylation of FAK at the autophosphorylation site tyrosine 397 was unchanged following dasatinib treatment of EGFRvIII-expressing HNSCC cells. This site, when autophosphorylated creates a binding site for SFK via the SH2 domain of SFK which activates SFK by displacing the inhibitory phosphorylation at Y527 (21) (Figure 3B). Phosphorylation of Akt, MAPK and FAK was significantly reduced by dasatinib in HNSCC cells expressing EGFRvIII as well as vector-control cells (STAT3 was also evaluated, Supplemental Figure 2). It appears that biochemically dasatinib is equally effective in EGFRvIII and control HNSCC cells.
Figure 3. Dasatinib inhibits signaling pathways for proliferation and cell motility.
EGFRvIII (‘V’) expressing and vector control (‘C’) HNSCC cells were cultured for 24 hours in the presence of DMSO or 100nM dasatinib and immunoblotted for: (A) MAPK and AKT activation via phosphorylation or (B) FAK activation via phosphorylation at the SFK activating sites Y576/7 and Y861 and the autophosphorylation site Y397. The experiment was repeated 3 times with similar results. Near infrared signal quantification is provided as percent decreased signal with dasatinib treatment (n=3), open bars are vector control HNSCC, closed bars are EGFRvIII expressing HNSCC.
Dasatinib inhibits tumor growth of EGFRvIII-expressing HNSCC xenografts
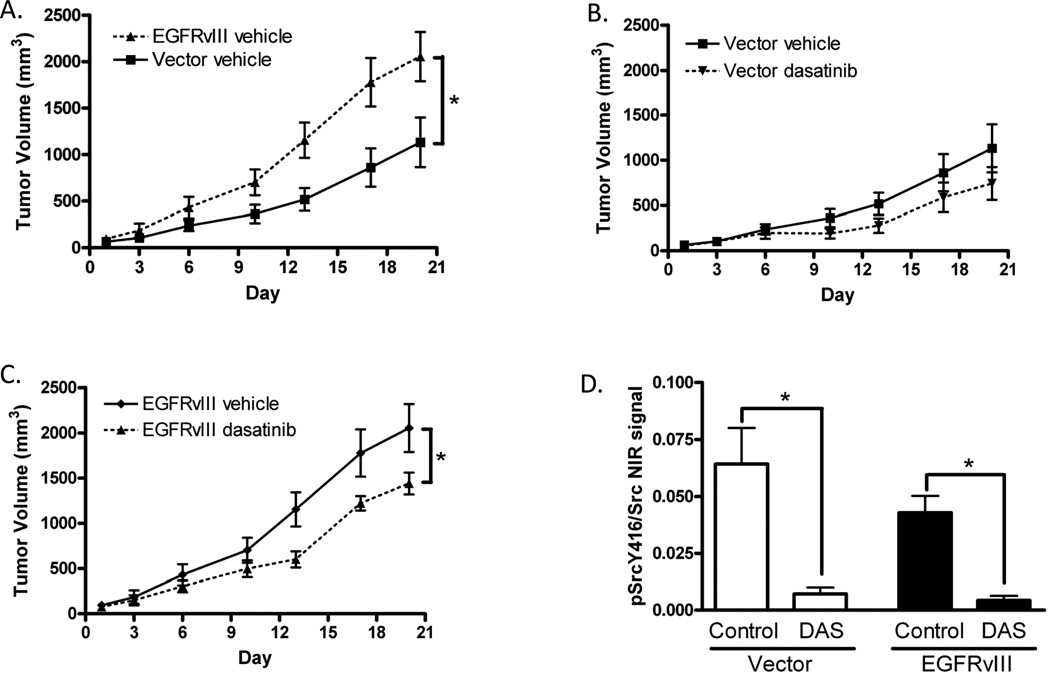
We previously reported that EGFRvIII-expressing HNSCC xenografts grow more rapidly than controls and are relatively insensitive to cetuximab, the only FDA approved molecular targeted therapy for HNSCC (22). Since dasatinib was effective at abrogating proliferation, migration, invasion and signaling in EGFRvIII-expressing cells, we hypothesized that inhibition of SFK would demonstrate antitumor effects in a non-metastatic subcutaneous model of EGFRvIII-expressing xenografts in vivo. To test this hypothesis, we innoculated nude mice with vector control or EGFRvIII-expressing HNSCC cells (Cal33) and initiated treatment when tumors were palpable and of equal volume. Mice were treated with vehicle control or dasatinib to assess the effects of SFK inhibition on tumor volume. EGFRvIII-expressing HNSCC xenografts demonstrated increased tumor volumes over HNSCC xenografts derived from vector-transfected control cells (p=0.019 on day 20, Figure 4A). In HNSCC xenografts derived from vector-transfected control cells only expressing wtEGFR, SFK inhibition failed to significantly reduce tumor volume compared to vehicle control treatment (p=0.19 on day 20, Figure 4B). However, in EGFRvIII-expressing xenografts, SFK inhibition significantly reduced tumor volume (p=0.047 on day 20 Figure 4C, p=0.02 on day 13 Supplemental Figure 3A), indicating that SFK may represent a plausible therapeutic target in HNSCC expressing EGFRvIII. These results were also validated in a second HNSCC xenograft model (FaDu, Supplemental Figure 3B,C). When cetuximab was combined with dasatinib, EGFRvIII expressing tumor volumes were lower than with dasatinib alone (Supplemental Figure 3D). Dasatinib did not significantly increase the efficacy of cetuximab in xenografts derived from vector control cells (data not shown). To verify that SFKs were durably inhibited by dasatinib treatment we immunoblotted lysates prepared from control and treated xenografts and probed for phosphorylated SFK. We found that phosphorylated SFK at the activation site was significantly decreased in dasatinib treated xenografts expressing EGFRvIII and vector control (p=0.0003, Figure 4D). We also found no difference in the phosphorylation of SFKs at tyrosine 416 between vector control and EGFRvIII expressing xenografts (Figure 4D).
Figure 4. Dasatinib inhibits tumor growth in EGFRvIII expressing cells in vivo.
Mice were inoculated with Cal33 cells expressing vector alone (Vector) or EGFRvIII constructs and treated daily with vehicle control (Control) (n=8) or dasatinib (n=8) as described in materials and methods. (A) Tumor volumes of Cal33 vector and EGFRvIII expressing cells under control treatment plotted by day (Day 1 = treatment initiation). Final tumor volumes (Day 20) were analyzed for statistical significance using the Mann-Whitney test; p=0.019. (B) Tumor volumes of Cal33 vector expressing cells treated with vehicle control or dasatinib and plotted by day. Final tumor volumes (Day 20) were analyzed for statistical significance using the Mann-Whitney test; p=0.19. (C) Tumor volumes of Cal33 EGFRvIII-expressing cells treated with vehicle control or dasatinib and plotted by day. Final tumor volumes (Day 20) were analyzed for statistical significance using the Mann-Whitney test; p=0.047. (D) Quantification of the near infrared signal ratio is provided for immunoblot of xenografts probed for SFK phosphorylation at Y416, and total SFK. Mann-Whitney test, p=0.0003 for untreated compared to treated in both vector control (open bars) and EGFRvIII (closed bars) expressing xenografts, there were no significant differences between vector control and EGFRvIII expressing xenografts.
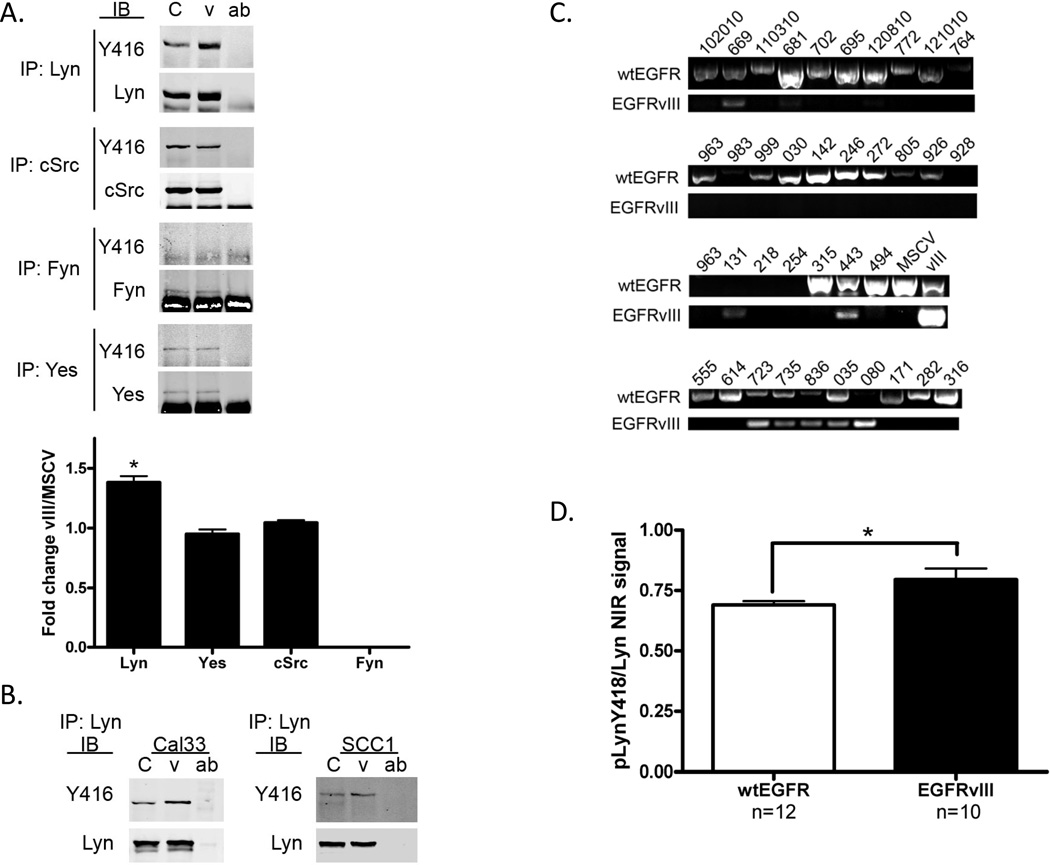
Lyn kinase mediates migration and invasion in EGFRvIII-expressing HNSCC
Assessment of SFK phosphorylation at the active site (Y416) or pharmacologic inhibition of SFK cannot distinguish the precise role of individual SFKs. Reports in glioma-expressing EGFRvIII indicate that Fyn and c-Src are key effectors of EGFRvIII signaling (7). We therefore sought to determine which SFKs were activated in EGFRvIII-expressing HNSCC. Previous studies in HNSCC expressing wtEGFR indicated that Lyn, Fyn, c-Src and Yes are all expressed in HNSCC (16). We therefore immunoprecipitated Lyn, Fyn, c-Src or Yes followed by immunoblotting with SFK Y416 and total SFK protein antibodies. We found that Lyn was the only SFK demonstrating increased phosphorylation in EGFRvIII-expressing HNSCC cells (p=0.019, Figure 5A). Fyn, while expressed, was not phosphorylated and c-Src and Yes were not differentially phosphorylated in HNSCC cells expressing EGFRvIII or wtEGFR. Increased phosphorylation of Lyn in EGFRvIII-expressing HNSCC cells was also detected in two other head and neck cell lines expressing EGFRvIII and 3 separate clones of the Cal33 EGFRvIII expressing cell line (Figure 5B, SCC1 p=0.03, Cal33 p=0.01; Supplemental Figures 4 and 5).
Figure 5. Increased Lyn phosphorylation in EGFRvIII-expressing HNSCC compared to wtEGFR only HNSCC.
(A) FaDu vector control or EGFRvIII cells were serum starved for 24 hours and harvested for immunoprecipitation (IP) with an SFK antibody as noted. Following IP proteins were immunoblotted for phosphorylation at Y416 and subsequently for total protein. Near infrared signal was recorded. pSFK was normalized to total SFK and graphed as a fold increase in EGFRvIII expressing cells compared to vector only cells. Mann-Whitney test p=0.019. (B) Increased phosphorylation of Lyn Y416 was confirmed in Cal33 and UMSCC1 vector and EGFRvIII expressing cells as noted in panel A (p=0.01 and 0.03 respectively). (C) Representative sample of RT-PCR of HNSCC patient specimens for EGFRvIII positivity. EGFRvIII bands were excised and subsequently sequenced. FaDu MSCV (MSCV) was used as a wtEGFR only control, FaDu vIII (vIII) was used as a EGFRvIII positive control. (D) HNSCC tumor lysates positive (n=10) or negative (n=12) for EGFRvIII were immunoprecipitated with a Lyn specific antibody, immunoblotted for phosphorylation at Y416 and subsequently for total Lyn protein. Near infrared signal was recorded and taken as a ratio of phosphoprotein to total protein and plotted. Mann-Whitney test p=0.035.
To confirm that increased phosphorylation of Lyn in EGFRvIII-expressing HNSCC was indeed a clinically relevant phenomena we screened a cohort of 52 patient HNSCC tumors for EGFRvIII expression via RT-PCR followed by sequencing. We found that 12/52 (23%) tumors expressed detectable levels of EGFRvIII (Figure 5C). From this cohort we chose tumors based on tissue availability to perform Lyn immunoprecipitation. Due to the high level of consensus in the active site sequence of SFKs there is no pLyn antibody of sufficient specificity for immunohistochemical analysis. 22 HNSCC patient specimens, 10 tumors harboring EGFRvIII and 12 tumors without EGFRvIII expression had sufficient tissue for immunoprecipitation. Immunoblotting with pSFK Y416 and total Lyn protein showed a modest but significant increase in phosphorylation of Lyn in patient tumors with EGFRvIII expression compared to wtEGFR only tumors (p=0.035, Figure 5D) demonstrating that phosphorylation of Lyn is increased in EGFRvIII-expressing human HNSCC.
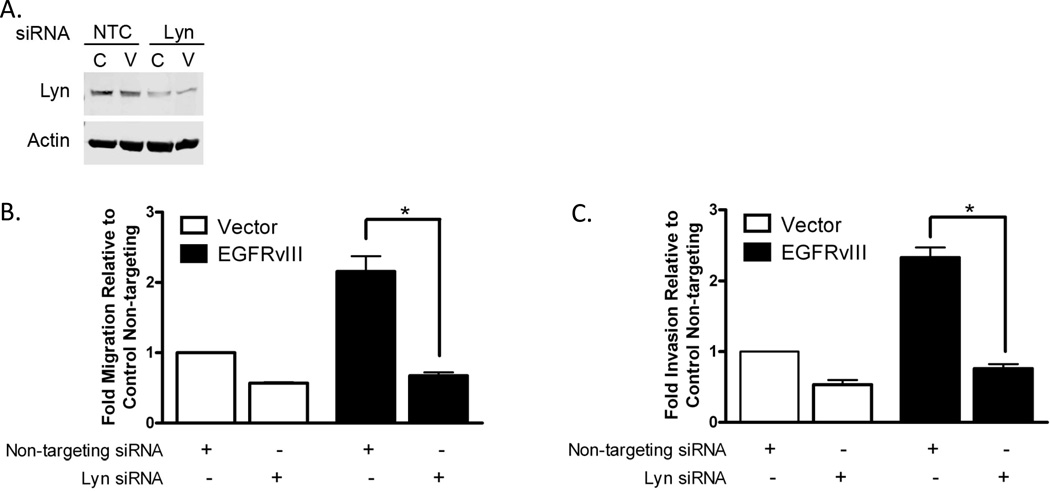
We next examined the effects of Lyn knockdown on the EGFRvIII phenotype of cell motility to evaluate the hypothesis that phosphorylation of Lyn is a key signaling intermediate for EGFRvIII-mediated invasion and migration. We treated SCC1 HNSCC cells for 48 hours with Lyn siRNA (Figure 6A, siRNA specificity Supplemental Figure 6) and subjected them to migration and invasion assays in vitro. We found that Lyn siRNA significantly reduced invasion and migration in EGFRvIII expressing cells (p=0.014 for both invasion and migration) and vector control cells (p=0.014, Figure 6B, C). These results were confirmed in a second HNSCC cell line (FaDu, Supplemental Figure 7). In SCC1 EGFRvIII expressing cells the percent inhibition in migration (68.5%) and invasion (67.2%) was significantly greater than the percent inhibition of vector control expressing cells (migration 43.2%, invasion 46.7%) (p=0.029 for both) indicating that Lyn is a likely intermediate in EGFRvIII-mediated cell motility.
Figure 6. Knockdown of Lyn inhibits EGFRvIII-mediated HNSCC migration and invasion.
(A) UMSCC1 vector and EGFRvIII expressing cells were treated with Lyn siRNA for 48 hours and immunoblotted to confirm Lyn knockdown. (B) UMSCC1 vector control (open bars) and EGFRvIII expressing (closed bars) cells were assayed for cell motility in the presence of non-targeting siRNA or Lyn siRNA. The experiment was repeated 4 times and assessed for significance by the Mann-Whitney test p=0.014. (C) UMSCC1 vector control (open bars) and EGFRvIII expressing (closed bars) cells were assayed for cell invasion in the presence of non-targeting siRNA or Lyn siRNA. The experiment was repeated 4 times and assessed for significance by the Mann-Whitney test p=0.014.
Discussion
EGFRvIII is reportedly more tumorigenic than wtEGFR (1, 3, 18) despite the fact that there is no difference in the cytoplasmic signaling domain of wtEGFR and EGFRvIII. Differential activation of EGFRvIII signaling pathways compared to wtEGFR has been reported. Altered oncogenic phenotypes may be attributed to differential signaling kinetics due to EGFRvIII retention at the plasma membrane which results in low level constitutive signaling (31). EGFRvIII expression is found in 17–42% of HNSCC (22, 23) in addition to other tumor types including glioma, breast, lung (2, 32) and prostate cancers (33). EGFRvIII has been shown to contribute to increased oncogenicity through various signaling pathways (34) and a recent study indicates that HNSCC patients with EGFRvIII have reduced disease control rates and shortened progression free survival when treated with a cetuximab-containing regiment (24). The mechanisms through which EGFRvIII expression increases oncogenicity and confers cetuximab resistance are incompletely understood.
EGFRvIII in glioma has been shown in vitro and in human tumor samples to demonstrate constitutive activation of the PI3K/Akt pathway (17, 35). Blockade of this pathway has been shown to reduce the EGFRvIII enhanced oncogenic phenotype (17). We previously reported that in EGFRvIII-expressing HNSCC, inhibition of the PI3K/Akt pathway reduces cell proliferation but has no effect on cell motility or invasion (19). In the present study, Akt phosphorylation was detected in EGFRvIII-expressing HNSCC cells and was abrogated by SFK inhibition (Figure 3). There are conflicting reports on the role of MAPK signaling in EGFRvIII-expressing models where some studies report activation (18, 36) and others fail to suggest a role (29). In this study we found that MAPK phosphorylation in EGFRvIII-expressing HNSCC cells was significantly reduced by SFK inhibition (Figure 3).
Both STAT3 and SFKs have been implicated as key mediators in the EGFRvIII oncogenic phenotype in glioma. In glioma specimens there was a significant correlation between activated STAT3 levels and EGFRvIII (but not wtEGFR) (37). We have previously shown that STAT3 is required for EGFRvIII expressing HNSCC motility and invasion (19). Many human cancers have shown a role for SFK in constitutive STAT activation and in HNSCC, STAT3 is a SFK-dependent mediator of EGFR-stimulated growth in vitro (38), decreased apoptosis (39) and increased tumor growth in vivo (40). SFKs have been shown to contribute to invasion in EGFRvIII-expressing gliomas where genetic and molecular inhibition of SFKs reduced invasion (7). In HNSCC expressing only wtEGFR, it has been reported that SFK inhibition using dasatinib reduces cell motility and invasion by regulating downstream cell adhesion molecules such as FAK (12). In the present study, we found that dasatinib significantly decreases proliferation, invasion and cell motility of vector control and EGFRvIII expressing HNSCC cells (Figure 2). Total phosphorylated SFK levels at baseline do not appear to correlate with the biological effects of dasatinib. This has also been noted previously in HNSCC cells expressing only wtEGFR (12). We also detected reduced Akt and MAPK phosphorylation in addition to decreased FAK phosphorylation by dasatinib treatment (Figure 3). pFAK Y397, which is the FAK autophosphorylation site and is a docking site for SFKs appeared to be unchanged with SFK inhibition. Dasatinib treatment of HNSCC cells by Johnson et al. also showed no change in pFAK Y397 levels (12). This is not surprising as integrins and several RTKs activate this site (Y397) which can then recruit SFKs (21). While invasion and cell motility were significantly inhibited by SFK blockade, it appears that there are other mechanisms that contribute to EGFRvIII-expressing HNSCC-mediated invasion and cell motility as cell motility was not entirely abrogated in the presence of SFK inhibition. Persistent phosphorylation of FAK at Y397 may contribute to invasion and migration even in the setting of dasatinib treatment.
Dasatinib is known to inhibit SFKs as well as Abl, c-kit, PDGF-R and EphA2 (30, 41). Studies in HNSCC show that treatment with imatinib (an inhibitor of Abl, c-kit and PDGF-R) did not affect cell cycle progression or apoptosis (42, 43). EphA2 is inhibited by dasatinib but dasatinib inhibition of EphA2 activation levels did not correlate with the effects of dasatinib on cell cycle progression or apoptosis (12). It is therefore likely that not all of the effects observed from dasatinib treatment in HNSCC are due to SFK inhibition.
In glioma several studies have evaluated the effects of altered SFK activity on EGFRvIII-expressing tumors. Genetic disruption of c-Src using a dominant-negative approach in EGFRvIII-expressing glioma xenografts decreased tumor growth rates and significantly increased the efficacy of EGFRvIII-specific Ab treatment (6). Dasatinib has also demonstrated antitumor effects in glioma model systems. In an endogenously expressing EGFRvIII in vivo model dasatinib has been shown to inhibit growth and increase survival and apoptosis (7). SFK inhibition through dasatinib treatment of HNSCC xenografts has been primarily reported in the context of downstream signaling markers and the effects of dasatinib on tumor growth in vivo or metastasis are still being defined (44). We found that in vivo treatment with dasatinib had a more profound inhibitory effect on tumor volumes in EGFRvIII-expressing HNSCC xenografts compared to tumors derived from vector control cells expressing wtEGFR only (Figure 4). This is in agreement with a recent report that a wtEGFR HNSCC orthotopic xenograft model treated with dasatinib alone had no effect on tumor volume (45). The cause of the discrepancy between in vitro and in vivo efficacies in wtEGFR-expressing HNSCC is as yet unknown but may be related to differences in drug exposure (in vitro cells receive a single dose for 24–72 hrs while in vivo dosing occurs daily over weeks). These results indicate that tumor growth of EGFRvIII expressing cells is mediated, at least in part, by SFK signaling. In glioma, EGFRvIII did not appear to initiate differential expression of specific SFKs but rather preferentially activated Fyn and c-Src compared with Lyn, Yes, Hck, and Blk (7). Lyn has been implicated as a key signaling mediator in several solid cancers including prostate (46), glioma (47), and breast (48). Further experiments showed that while Fyn, c-Src and Yes were phosphorylated equally in EGFRvIII and vector control expressing cells, Lyn phosphorylation was increased in EGFRvIII cells and human HNSCC tumors expressing EGFRvIII. It is therefore plausible that the increased phosphorylation of Lyn in EGFRvIII expressing cells contributes to the greater efficacy of SFK inhibition to EGFRvIII-expressing HNSCC in vivo. Evaluation of patient HNSCC samples demonstrated increased phosphorylation of Lyn in EGFRvIII expressing HNSCC. Selective targeting of Lyn expression using siRNA inhibited the migration and invasion of EGFRvIII-expressing HNSCC cells, implicating a functional role of increased Lyn activation (Figure 6). To our knowledge, this is the first report of a specific role for Lyn in HNSCC. These results suggest that therapeutic agents that selectively target Lyn could be effective in this cancer (49). The Lyn/BCR-ABL inhibitor bafetinib is currently under investigation in phase II clinical trials for patients with prostate cancer, B-cell chronic lymphocytic leukemia, and brain tumors (50). To the best of our knowledge there are limited studies in HNSCC that have evaluated lymph node and distant metastasis. In a wtEGFR orthotopic mouse model (44) with dasatinib treatment no metastasis of any type was identified in control or treatment groups. Further investigation of SFK/Lyn inhibition in metastatic orthotopic mouse models is warranted.
The majority of reports on EGFRvIII in cancer conclude that EGFRvIII contributes to increased tumor growth and poor prognosis. In HNSCC, EGFRvIII expression has been associated with cetuximab resistance in preclinical models and now in a human HNSCC cohort (24). Therapeutic strategies that target SFK, specifically Lyn, may be effective alternative strategies in these patients.
Supplementary Material
Translational Relevance.
Head and neck squamous cell carcinoma (HNSCC) expressing the constitutively active altered EGFR variant three (EGFRvIII) protein is resistant to treatment with cetuximab in preclinical models and a recently reported clinical trial. EGFRvIII oncogenic signaling in HNSCC is incompletely understood and further elucidation of the pathways contributing to EGFRvIII mediated tumor progression may allow for identification of druggable targets to overcome cetuximab resistance. Here we present preclinical in vitro and in vivo evidence that Lyn is the primary src family kinase activated by EGFRvIII in HNSCC, which can be targeted with dasatinib with resulting anti-tumor effects.
Acknowledgments
Grant Support: P50CA097190, R01CA098372, U01CA84968, Bristol-Myers Squibb and the American Cancer Society (JRG), 1F31DE020223 (SW)
References
- 1.Batra SK, Castelino-Prabhu S, Wikstrand CJ, Zhu X, Humphrey PA, Friedman HS, et al. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ. 1995;6(10):1251–1259. [PubMed] [Google Scholar]
- 2.Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51(8):2164–2172. [PubMed] [Google Scholar]
- 3.Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56(21):5079–5086. [PubMed] [Google Scholar]
- 4.Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, et al. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62(12):3335–3339. [PubMed] [Google Scholar]
- 5.Mukherjee B, McEllin B, Camacho CV, Tomimatsu N, Sirasanagandala S, Nannepaga S, et al. EGFRvIII and DNA double-strand break repair: a molecular mechanism for radioresistance in glioblastoma. Cancer Res. 2009;69(10):4252–4259. doi: 10.1158/0008-5472.CAN-08-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johns TG, Perera RM, Vernes SC, Vitali AA, Cao DX, Cavenee WK, et al. The efficacy of epidermal growth factor receptor-specific antibodies against glioma xenografts is influenced by receptor levels, activation status, and heterodimerization. Clin Cancer Res. 2007;13(6):1911–1925. doi: 10.1158/1078-0432.CCR-06-1453. [DOI] [PubMed] [Google Scholar]
- 7.Lu KV, Zhu S, Cvrljevic A, Huang TT, Sarkaria S, Ahkavan D, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69(17):6889–6898. doi: 10.1158/0008-5472.CAN-09-0347. PMCID: 2770839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22(4):337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- 9.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6(3):209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Mandal M, Myers JN, Lippman SM, Johnson FM, Williams MD, Rayala S, et al. Epithelial to mesenchymal transition in head and neck squamous carcinoma: association of Src activation with E-cadherin down-regulation, vimentin expression, and aggressive tumor features. Cancer. 2008 doi: 10.1002/cncr.23410. [DOI] [PubMed] [Google Scholar]
- 11.Koppikar P, Choi SH, Egloff AM, Cai Q, Suzuki S, Freilino M, et al. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14(13):4284–4291. doi: 10.1158/1078-0432.CCR-07-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11(19 Pt 1):6924–6932. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi K, Kugimiya T, Miyazaki T. Substance P receptor in U373 MG human astrocytoma cells activates mitogen-activated protein kinases ERK1/2 through Src. Brain Tumor Pathol. 2005;22(1):1–8. doi: 10.1007/s10014-005-0178-1. [DOI] [PubMed] [Google Scholar]
- 14.Chang YM, Bai L, Liu S, Yang JC, Kung HJ, Evans CP. Src family kinase oncogenic potential and pathways in prostate cancer as revealed by AZD0530. Oncogene. 2008;27(49):6365–6375. doi: 10.1038/onc.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jallal H, Valentino ML, Chen G, Boschelli F, Ali S, Rabbani SA. A Src/Abl kinase inhibitor, SKI-606, blocks breast cancer invasion, growth, and metastasis in vitro and in vivo. Cancer Res. 2007;67(4):1580–1588. doi: 10.1158/0008-5472.CAN-06-2027. [DOI] [PubMed] [Google Scholar]
- 16.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278(34):31574–31583. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 17.Moscatello DK, Holgado-Madruga M, Emlet DR, Montgomery RB, Wong AJ. Constitutive activation of phosphatidylinositol 3-kinase by a naturally occurring mutant epidermal growth factor receptor. J Biol Chem. 1998;273(1):200–206. doi: 10.1074/jbc.273.1.200. [DOI] [PubMed] [Google Scholar]
- 18.Moscatello DK, Montgomery RB, Sundareshan P, McDanel H, Wong MY, Wong AJ. Transformational and altered signal transduction by a naturally occurring mutant EGF receptor. Oncogene. 1996;13(1):85–96. [PubMed] [Google Scholar]
- 19.Wheeler SE, Suzuki S, Thomas SM, Sen M, Leeman-Neill RJ, Chiosea SI, et al. Epidermal growth factor receptor variant III mediates head and neck cancer cell invasion via STAT3 activation. Oncogene. 29(37):5135–5145. doi: 10.1038/onc.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rengifo-Cam W, Konishi A, Morishita N, Matsuoka H, Yamori T, Nada S, et al. Csk defines the ability of integrin-mediated cell adhesion and migration in human colon cancer cells: implication for a potential role in cancer metastasis. Oncogene. 2004;23(1):289–297. doi: 10.1038/sj.onc.1207041. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28(1–2):35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- 22.Sok JC, Coppelli FM, Thomas SM, Lango MN, Xi S, Hunt JL, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12(17):5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 23.Chau NG, Perez-Ordonez B, Zhang K, Pham NA, Ho J, Zhang T, et al. The association between EGFR variant III, HPV, p16, c-MET, EGFR gene copy number and response to EGFR inhibitors in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck Oncol. 3:11. doi: 10.1186/1758-3284-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tinhofer I, Klinghammer K, Weichert W, Knodler M, Stenzinger A, Gauler T, et al. Expression of Amphiregulin and EGFRvIII Affect Outcome of Patients with Squamous Cell Carcinoma of the Head and Neck Receiving Cetuximab-Docetaxel Treatment. Clin Cancer Res. 2011;17(15):5197–5204. doi: 10.1158/1078-0432.CCR-10-3338. [DOI] [PubMed] [Google Scholar]
- 25.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 26.Bigner SH, Humphrey PA, Wong AJ, Vogelstein B, Mark J, Friedman HS, et al. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res. 1990;50(24):8017–8022. [PubMed] [Google Scholar]
- 27.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91(16):7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luwor RB, Zhu HJ, Walker F, Vitali AA, Perera RM, Burgess AW, et al. The tumor-specific de2-7 epidermal growth factor receptor (EGFR) promotes cells survival and heterodimerizes with the wild-type EGFR. Oncogene. 2004;23(36):6095–6104. doi: 10.1038/sj.onc.1207870. [DOI] [PubMed] [Google Scholar]
- 30.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47(27):6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 31.Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD, et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272(5):2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 32.Wikstrand CJ, Hale LP, Batra SK, Hill ML, Humphrey PA, Kurpad SN, et al. Monoclonal antibodies against EGFRvIII are tumor specific and react with breast and lung carcinomas and malignant gliomas. Cancer Res. 1995;55(14):3140–3148. [PubMed] [Google Scholar]
- 33.Olapade-Olaopa EO, Moscatello DK, MacKay EH, Horsburgh T, Sandhu DP, Terry TR, et al. Evidence for the differential expression of a variant EGF receptor protein in human prostate cancer. Br J Cancer. 2000;82(1):186–194. doi: 10.1054/bjoc.1999.0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal. 2009;2(87):re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- 35.Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, et al. Analysis of the phosphatidylinositol 3'-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63(11):2742–2746. [PubMed] [Google Scholar]
- 36.Montgomery RB, Moscatello DK, Wong AJ, Cooper JA, Stahl WL. Differential modulation of mitogen-activated protein (MAP) kinase/extracellular signal-related kinase kinase and MAP kinase activities by a mutant epidermal growth factor receptor. J Biol Chem. 1995;270(51):30562–30566. doi: 10.1074/jbc.270.51.30562. [DOI] [PubMed] [Google Scholar]
- 37.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 38.Rubin Grandis J, Drenning SD, Chakraborty A, Zhou M, Zeng Q, Pitt A, et al. Requirement of Stat3 but not Stat1 for EGFR-mediated cell growth in vitro. J Clin Invest. 1998;102(7):1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, et al. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A. 2000;97(8):4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kijima T, Niwa H, Steinman RA, Drenning SD, Gooding WE, Wentzel AL, et al. STAT3 activation abrogates growth factor dependence and contributes to head and neck squamous cell carcinoma tumor growth in vivo. Cell Growth Differ. 2002;13(8):355–362. [PubMed] [Google Scholar]
- 41.Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, et al. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67(5):2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 42.Johnson FM, Saigal B, Donato NJ. Induction of heparin-binding EGF-like growth factor and activation of EGF receptor in imatinib mesylate-treated squamous carcinoma cells. Journal of cellular physiology. 2005;205(2):218–227. doi: 10.1002/jcp.20383. [DOI] [PubMed] [Google Scholar]
- 43.Johnson FM, Yang P, Newman RA, Donato NJ. Cyclooxygenase-2 induction and prostaglandin E2 accumulation in squamous cell carcinoma as a consequence of epidermal growth factor receptor activation by imatinib mesylate. Journal of experimental therapeutics & oncology. 2004;4(4):317–325. [PubMed] [Google Scholar]
- 44.Sen B, Saigal B, Parikh N, Gallick G, Johnson FM. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res. 2009;69(5):1958–1965. doi: 10.1158/0008-5472.CAN-08-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sen B, Peng S, Saigal B, Williams MD, Johnson FM. Distinct interactions between c-Src and c-Met in mediating resistance to c-Src inhibition in head and neck cancer. Clin Cancer Res. 2011;17(3):514–524. doi: 10.1158/1078-0432.CCR-10-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai H, Smith DA, Memarzadeh S, Lowell CA, Cooper JA, Witte ON. Differential transformation capacity of Src family kinases during the initiation of prostate cancer. Proc Natl Acad Sci U S A. 2011;108(16):6579–6584. doi: 10.1073/pnas.1103904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding Q, Stewart J, Jr., Olman MA, Klobe MR, Gladson CL. The pattern of enhancement of Src kinase activity on platelet-derived growth factor stimulation of glioblastoma cells is affected by the integrin engaged. J Biol Chem. 2003;278(41):39882–39891. doi: 10.1074/jbc.M304685200. [DOI] [PubMed] [Google Scholar]
- 48.Choi YL, Bocanegra M, Kwon MJ, Shin YK, Nam SJ, Yang JH, et al. LYN is a mediator of epithelial-mesenchymal transition and a target of dasatinib in breast cancer. Cancer Res. 2010;70(6):2296–2306. doi: 10.1158/0008-5472.CAN-09-3141. PMCID: 2869247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimura S, Naito H, Segawa H, Kuroda J, Yuasa T, Sato K, et al. NS-187, a potent and selective dual Bcr-Abl/Lyn tyrosine kinase inhibitor, is a novel agent for imatinib-resistant leukemia. Blood. 2005;106(12):3948–3954. doi: 10.1182/blood-2005-06-2209. [DOI] [PubMed] [Google Scholar]
- 50.Santos FP, Kantarjian H, Cortes J, Quintas-Cardama A. Bafetinib, a dual Bcr-Abl/Lyn tyrosine kinase inhibitor for the potential treatment of leukemia. Curr Opin Investig Drugs. 2010;11(12):1450–1465. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.