Abstract
Background. To understand and model the impact of pneumococcal conjugate vaccines at the population level, we need to know the transmission dynamics of individual pneumococcal serotypes. We estimated serotype-specific clearance and acquisition rates of nasopharyngeal colonization among Kenyan children.
Methods. Children aged 3–59 months who were identified as carriers in a cross-sectional survey were followed-up approximately 1, 2, 4, 8, 16, and 32 days later and monthly thereafter until culture of 2 consecutive swabs yielded an alternative serotype or no pneumococcus. Serotype-specific clearance rates were estimated by exponential regression of interval-censored carriage durations. Duration was estimated as the reciprocal of the clearance rate, and acquisition rates were estimated on the basis of prevalence and duration, assuming an equilibrium state.
Results. Of 2840 children sampled between October 2006 and December 2008, 1868 were carriers. The clearance rate was 0.032 episodes/day (95% confidence interval [CI], .030–.034), for a carriage duration of 31.3 days, and the rate varied by serotype (P < .0005). Carriage durations for the 28 serotypes with ≥10 carriers ranged from 6.7 to 50 days. Clearance rates increased with year of age, adjusted for serotype (hazard ratio, 1.21; 95% CI, 1.15–1.27). The acquisition rate was 0.061 episodes/day (95% CI, .055–.067), which did not vary with age. Serotype-specific acquisition rates varied from 0.0002 to 0.0022 episodes/day. Serotype-specific acquisition rates correlated with prevalence (r = 0.91; P < .00005) and with acquisition rates measured in a separate study involving 1404 newborns in Kilifi (r = 0.87; P < .00005).
Conclusions. The large sample size and short swabbing intervals provide a precise description of the prevalence, duration, and acquisition of carriage of 28 pneumococcal serotypes. In Kilifi, young children experience approximately 8 episodes of carriage per year. The declining prevalence with age is attributable to increasing clearance rates.
Pneumococcal conjugate vaccines (PCVs) were highly efficacious against invasive pneumococcal disease (IPD) in randomized controlled trials in developed and developing countries [1–4] and were highly effective when introduced into the national vaccination programs of developed countries [5, 6]. On this evidence, the GAVI Alliance has pledged funding support for PCVs in developing countries, and several of these countries have recently introduced 10- or 13-valent PCV. The programmatic effectiveness of PCV against IPD has been confirmed by large-scale longitudinal surveillance systems that are not generally available in developing countries [5, 6]. In the United States, vaccination of young children has led to a reduction of IPD in older children, neonates, adults, and elderly individuals [5, 7, 8]. Studies of PCV consistently show a reduction in carriage of vaccine-serotypes among vaccinees, which provides a plausible explanation for indirect protection [9–12]. A corollary of this effect is an increase in the carriage of nonvaccine serotypes, and in some settings this has led to an increase in IPD both among children and elderly individuals [6, 13]. The unpredictability of this effect and the lack of monitoring systems for IPD in developing countries have raised the question of whether studies of pneumococcal carriage can help understand or predict indirect vaccine effects in different epidemiological settings [14].
For the purpose of modeling, this problem can be divided into 2 components. The first component characterizes the epidemiology of transmission in a stable prevaccine population and explores the impact of vaccine introduction by using estimates of vaccine efficacy against nasopharyngeal colonization that are derived from field studies of PCV. This type of model also requires epidemiologic data on carriage prevalence [15, 16]; the force of infection, defined as the rate of acquisition among uncolonized individuals [17]; and competition between serotypes for the nasopharyngeal niche [18–20]. The second component overlays the risk of disease associated with a colonization episode on top of the transmission model to predict the population rate of IPD. Because the risk of IPD is thought to be most closely associated with acquisition of colonization rather than with duration, the second component uses the “attack rate” (calculated as the rate of pneumococcal acquisition divided by the rate of IPD) [16, 21, 22] to produce credible estimates of disease incidence.
With the exception of prevalence, few data exist on the dynamics of pneumococcal carriage to support these models. Furthermore, what little can be derived is confined to highly prevalent types, which are generally those included in vaccine formulations [19, 23]. In this study, we describe the prevalence, duration, and rates of acquisition of pneumococcal carriage for a wide range of serotypes, including those that are most likely to predominate in the postvaccine era, in a representative sample of African children prior to PCV introduction.
MATERIALS AND METHODS
Study Population
The study was conducted among children aged 3–59 months who were residents of the Kilifi Health and Demographic Surveillance System (KHDSS) [24]. This is a longitudinal surveillance of 250 000 people living in a well-defined geographic area around Kilifi District Hospital. At the midpoint of the present study (23 January 2008), the KHDSS had a population of 42 345 individuals aged 3–59 months.
Study Design
This was a longitudinal study of individuals with prevalent nasopharyngeal carriage who were followed until the index episode of carriage was terminated. The carrier population was defined by a cross-sectional survey of 3570 children selected at random from the KHDSS population register, of whom consent to participate was received for 2840 [25].
Carrier status was defined by a baseline nasopharyngeal swab that yielded pneumococci on culture, and children who were found to be carriers were swabbed again on approximately days 1, 2, 4, 8, 16, and 32 after the baseline swab and monthly thereafter until the episode of carriage was terminated. We defined termination as the observation of 2 consecutive swabs in which the original serotype was not detected on culture. Clearance was subcategorized as immune clearance, if the first of these 2 swabs was negative for any pneumococcal growth, or as competitive displacement, if the first of these 2 swabs was positive for an alternative serotype of pneumococcus. For any one serotype, recruitment of carriers into the longitudinal study ceased when we had identified 50 episodes of carriage of that serotype, although data for additional carriers beyond these 50 were included in prevalence estimates.
Laboratory Assay
The study followed World Health Organization guidelines for nasopharyngeal studies of Streptococcus pneumoniae [26]. Nasopharyngeal specimens were sampled using Dacron-tipped flexible wire swabs passed via the anterior nares to the posterior nasopharynx. The swab tip was immersed in skim-milk tryptone glucose glycerol (STGG) transport medium, separated from its handle with wire cutters, and transported at ambient temperature to the laboratory, where they were cultured directly. Internal quality control of STGG was conducted to ensure sterility and the ability to support pneumococcal growth. STGG samples were vortexed for 20 seconds, and a 10-µL specimen was inoculated onto a blood agar plate containing 2.5 μg/mL gentamicin and incubated overnight at 37°C in 5% CO2. Pneumococci were identified by α-hemolysis, optochin sensitivity, and presence of capsule. We serotyped 1 colony per plate, using the Quellung reaction and polyclonal rabbit antisera (Statens Seruminstitut, Copenhagen, Denmark). Antisera to differentiate serotype 6C from 6A were not available at the time of this study.
Analysis
The analyses were performed using Stata v11.2 (StataCorp, College Station, TX). The total rate of clearance was estimated by fitting an exponential function to the interval-censored carriage durations, in which the intervals were defined by the date on which the last swab positive for the prevalent type was collected and the date on which the first swab with a negative or other result was collected. We excluded children with <3 swabs from the analysis. Children who did not clear the prevalent serotype were right censored at the date on which the last swab positive for that serotype was collected. Multivariable exponential regression models were undertaken that included age in 10 strata, sex, and serotype. With the assumption of a constant rate of loss, the average duration of carriage was estimated as the reciprocal of the rate of clearance.
The analysis of clearance combined both immune clearance and competitive displacement. To calculate the rate of immune clearance alone, we right censored displaced episodes at the date on which the last swab positive for the prevalent serotype was collected.
Acquisition rates were estimated by using the relationship between prevalence, incidence, and duration in a steady-state population [27]. With constant prevalence, the population rates of acquisition and of clearance must be equal. By assuming, for simplicity, that acquisition occurs only among uncolonized children, the individual rate of clearance, b, among the proportion of the population who are carriers, p, is equal to the individual incidence of acquisition, a, among the proportion who are noncarriers [1 – p], which can be expressed as follows: log a = log [p/(1 – p)] + log b. To validate these indirect estimates of acquisition, we compared them against rates measured directly in a separate study of children in Kilifi, reported elsewhere [28]. Uncolonized newborn infants were studied to determine the force of infection. Infants were swabbed weekly for 13 weeks or until they were colonized with S. pneumoniae. Serotype-specific acquisition hazard rates (HRs) were estimated using survival analysis.
The Kenya Medical Research Institute/National Ethical Review Committee and The Oxford Tropical Research Ethics Committee approved the study, and written informed consent was obtained for all participants.
RESULTS
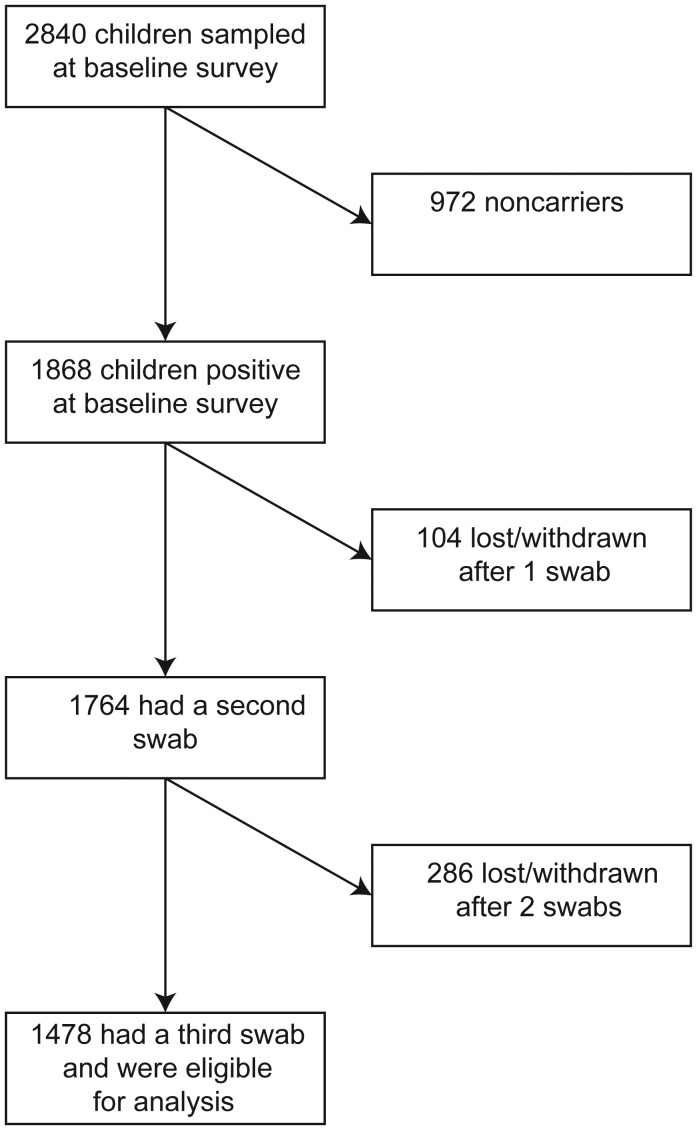
In total, 9466 swabs were collected throughout the study, yielding 7331 pneumococcal isolates. The baseline survey took place between 23 October 2006 and 2 December 2008; 2840 children were sampled [25]. The baseline survey identified 1868 carriers, but only 1478 were eligible for analysis (Figure 1). Of these, 444 carriage episodes were terminated by clearance, 613 were terminated by displacement, and 421 persisted either until the participant withdrew or until the end of the study period (Table 1). The last swab was collected on 17 February 2009. The median time between the 2 swabs used for interval censoring was 6 days. We calculated clearance and acquisition rates for the 28 serotypes that had ≥10 episodes of carriage, which represented 96% of all carried types.
Figure 1.
Flow of participants through the study.
Table 1.
Number of Swab Observations Involved in Defining Each Episode of Carriage, by Outcome of the Episode
| Variable | Episode Terminated by Immune Clearance | Episode Terminated by Competitive Displacement | Episode Not Terminated |
|---|---|---|---|
| Swabs defining the episode, no. | |||
| 3 | 124 | 165 | 148 |
| 4 | 62 | 81 | 85 |
| 5 | 67 | 75 | 58 |
| 6 | 66 | 69 | 49 |
| 7 | 59 | 94 | 30 |
| 8 | 34 | 75 | 26 |
| 9 | 14 | 30 | 11 |
| 10 | 6 | 13 | 4 |
| 11 | 6 | 5 | 4 |
| 12 | 4 | 3 | 2 |
| 13 | 0 | 2 | 2 |
| 14 | 0 | 0 | 1 |
| 15 | 1 | 0 | 0 |
| 16 | 0 | 0 | 0 |
| 17 | 1 | 1 | 1 |
| Overall | 444 | 613 | 421 |
| Swabs/child, no., mean | 5.3 | 5.5 | 4.9 |
| Follow-up duration, days | |||
| Mean | 34 | 44 | 33 |
| Maximum | 333 | 297 | 486 |
Data are no. of study subjects, unless otherwise indicated. A total of 7818 swabs from 1478 children who had at least 3 swabs are included in the analysis. In addition, 1362 children were sampled in the baseline survey, and 286 were sampled a second time but did not enter the longitudinal analysis. Among children whose episode was not observed to the end by the study definition, 153 had a final swab that was negative for the original serotype.
Rates of Clearance
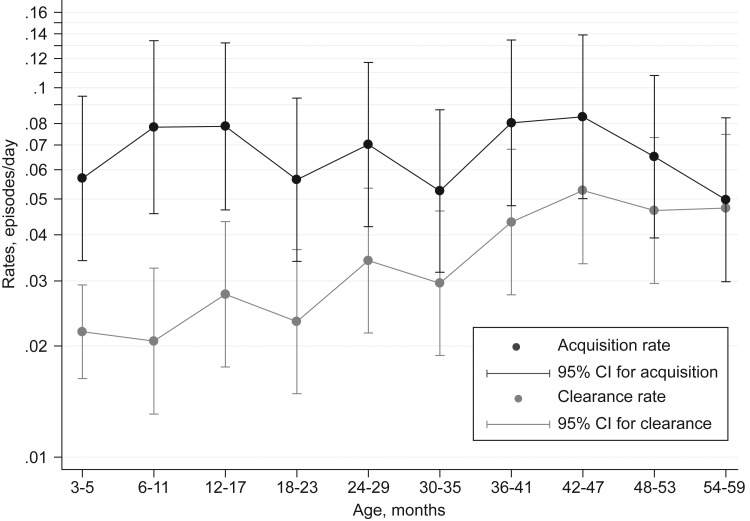
The overall rate of clearance for the colonizing pneumococci identified at the cross-sectional survey was 0.032 episodes/day (95% confidence interval [CI], .030–.034), giving a carriage duration estimate of 31.3 days (95% CI, 29.7–33.6 days) and a clearance half-life of 22 days. The clearance rate did not vary by sex but increased progressively with age (HR, 1.25 per year; 95% CI, 1.20–1.31) (Figure 2 and Supplementary Table 1). Older children are colonized less frequently by the most commonly observed serotypes [25]; however, the association between clearance rate and age was little altered by adjustment for serotype (HR, 1.21 per year; 95% CI, 1.15–1.27), suggesting that the age-related change in clearance reflects changes in duration within serotype and not only a changing serotype composition with age.
Figure 2.
Rates of clearance and acquisition of pneumococcal colonization, by age. Abbreviation: CI, confidence interval.
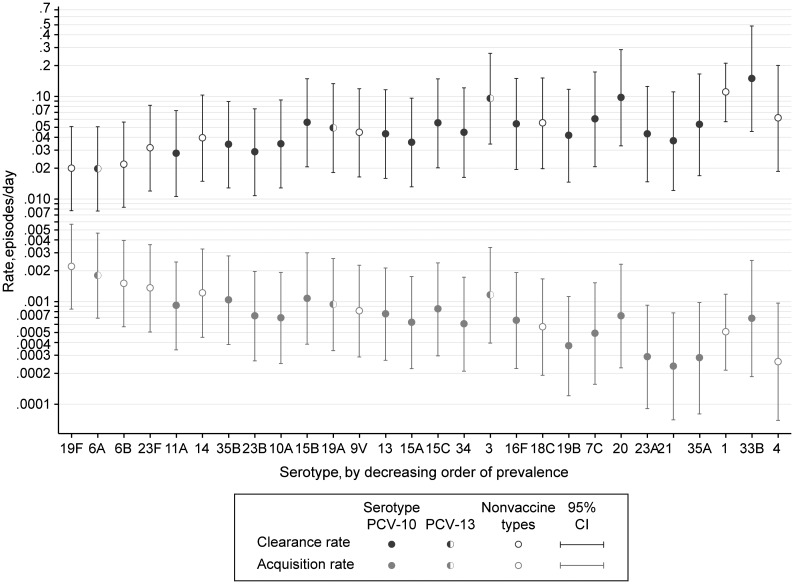
The rate of clearance varied significantly by serotype (P < .0005). Among the 28 most common serotypes, the clearance rate varied almost 8-fold, from 0.0197 episodes/day for serotype 6A to 0.149 episodes/day for serotype 33B (Table 2 and Figure 4), representing a range in carriage durations of 6.7 to 50 days (Table 2). Serotypes can be categorized by vaccine formulation as 7-valent serotypes (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F), 10-valent serotypes (7-valent serotypes plus serotypes 1, 5, and 7F), 13-valent serotypes (10-valent serotypes plus serotypes 3, 6A, and 19A), and all others. The mean duration of carriage in these 4 groups was 38.1, 7.7, 39.8, and 24.4 days, respectively.
Table 2.
Pneumococcal Acquisition, Carriage Duration, and Clearance, by Serotype
| Serotype | Carriers, No.a | Clearance Rate, Episodes/Day (95% CI) | Carriage Duration, Days, Mean (95% CI) | Acquisition Rate, Episodes/Day (95% CI) |
|---|---|---|---|---|
| 19F | 283 | 0.0198 (.0077–.0509) | 50.5 (19.7–130) | 0.0022 (.0008–.0057) |
| 6A | 237 | 0.0197 (.0076–.0506) | 50.8 (19.7–131) | 0.0018 (.0007–.0047) |
| 6B | 184 | 0.0216 (.0083–.0563) | 46.3 (17.8–120) | 0.0015 (.0006–.0039) |
| 23F | 117 | 0.0313 (.0120–.0821) | 31.9 (12.2–83.5) | 0.0013 (.0005–.0036) |
| 11A | 90 | 0.0278 (.0106–.0728) | 36.0 (13.7–94.4) | 0.0009 (.0003–.0024) |
| 14 | 85 | 0.0392 (.0149–.1032) | 25.5 (9.7–67.2) | 0.0012 (.0004–.0033) |
| 35B | 84 | 0.0339 (.0128–.0894) | 29.5 (11.2–77.9) | 0.0010 (.0004–.0028) |
| 23B | 70 | 0.0286 (.0108–.0757) | 35.0 (13.2–92.8) | 0.0007 (.0003–.0020) |
| 10A | 56 | 0.0344 (.0128–.0924) | 29.1 (10.8–77.9) | 0.0007 (.0002–.0019) |
| 15B | 54 | 0.0554 (.0206–.1489) | 18.1 (6.7–48.6) | 0.0011 (.0004–.0030) |
| 19A | 53 | 0.0492 (.0182–.1332) | 20.3 (7.5–55.1) | 0.0009 (.0003–.0026) |
| 9V | 51 | 0.0442 (.0164–.1191) | 22.6 (8.4–60.9) | 0.0008 (.0003–.0023) |
| 13 | 49 | 0.0430 (.0159–.1164) | 23.3 (8.6–63.0) | 0.0008 (.0003–.0021) |
| 15A | 49 | 0.0356 (.0132–.0962) | 28.1 (10.4–76.0) | 0.0006 (.0002–.0018) |
| 15C | 43 | 0.0547 (.0201–.1484) | 18.3 (6.7–49.6) | 0.0008 (.0003–.0024) |
| 34 | 38 | 0.0444 (.0163–.1213) | 22.5 (8.2–61.5) | 0.0006 (.0002–.0017) |
| 3 | 34 | 0.0951 (.0344–.2635) | 10.5 (3.8–29.1) | 0.0012 (.0004–.0034) |
| 16F | 34 | 0.0539 (.0194–.1500) | 18.6 (6.7–51.5) | 0.0007 (.0002–.0019) |
| 18C | 29 | 0.0547 (.0198–.1513) | 18.3 (6.6–50.6) | 0.0006 (.0002–.0017) |
| 19B | 25 | 0.0414 (.0146–.1174) | 24.2 (8.5–68.4) | 0.0004 (.0001–.0011) |
| 7C | 23 | 0.0598 (.0207–.1732) | 16.7 (5.8–48.4) | 0.0005 (.0002–.0015) |
| 20 | 21 | 0.0971 (.0330–.2859) | 10.3 (3.5–30.3) | 0.0007 (.0002–.0023) |
| 23A | 19 | 0.0429 (.0147–.1251) | 23.3 (8.0–67.9) | 0.0003 (.0001–.0009) |
| 21 | 18 | 0.0366 (.0121–.1108) | 27.3 (9.0–82.6) | 0.0002 (.0001–.0008) |
| 35A | 15 | 0.0529 (.0169–.1657) | 18.9 (6.0–59.3) | 0.0003 (.0001–.0010) |
| 1 | 13 | 0.1094 (.0567–.2109) | 9.1 (4.7–17.6) | 0.0005 (.0002–.0012) |
| 33B | 13 | 0.1488 (.0455–.4860) | 6.7 (2.1–22.0) | 0.0007 (.0002–.0025) |
| 4 | 12 | 0.0611 (.0186–.2005) | 16.4 (5.0–53.7) | 0.0003 (.0001–.0010) |
Abbreviation: CI, confidence interval.
a Data exclude 69 individuals who were colonized with serotypes that were observed too infrequently to permit estimation of clearance and acquisition rates [25].
Figure 4.
Rates of clearance and acquisition of pneumococcal colonization by serotype. Point estimates for serotypes contained in the 10-valent pneumococcal conjugate vaccine (PCV-10) are shown with open circles; those contained in the 13-valent vaccine (PCV-13) but not in the 10-valent vaccine are shown with half circles. Abbreviation: CI, confidence interval.
The log rates for serotype-specific clearance were inversely correlated with the log odds of prevalence (r = −0.80; P < .00005) (Supplementary Figure 1).
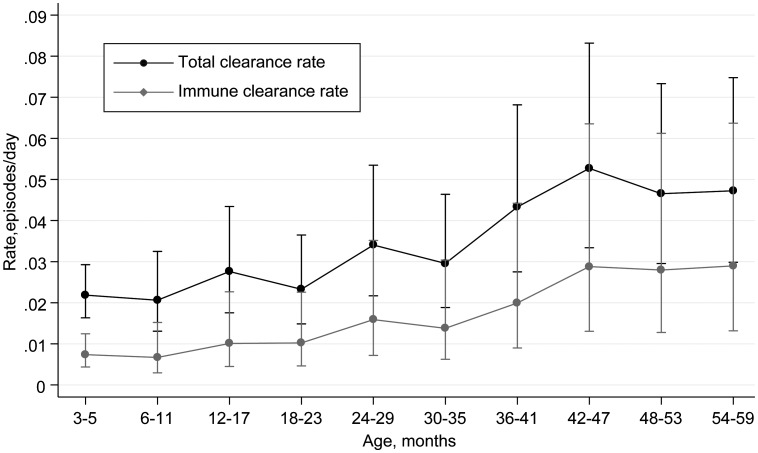
The rate of immune clearance was substantially lower than the total clearance rate, at 0.0148 episodes/day (95% CI, 0.0135–0.0163); the average carriage duration, in the absence of competition, was 68 days. However, the immune clearance rate was similarly associated with age (HR, 1.40 per year; 95% CI, 1.30–1.51), and the increase in total clearance rates with age closely reflects the pattern of immune clearance rates with age (Figure 3 and Supplementary Table 2).
Figure 3.
Rates of clearance of pneumococcal colonization by age, for immune and total clearance. Whiskers denote 95% confidence intervals.
Rates of Acquisition
The estimated rate of acquisition of pneumococcal colonization was 0.061 episodes/day (95% CI, .055–.067), suggesting that each child experiences 1 new acquisition on average every 16.4 days.
Acquisition rates did not vary systematically with age (Figure 2) but did vary by serotype, from 0.0002 episodes/day for serotype 21 to 0.0022 episodes/day for serotype 19F. These represent 1 new acquisition of serotype 19F every 15 child-months and 1 new acquisition of serotype 21 every 14 child-years. The sum of all serotype-specific acquisition rates, 0.064 episodes/day, was very close to the acquisition rate estimate for all pneumococci treated as a single strain. When the serotypes were ranked in order of decreasing prevalence, the serotype-specific acquisition rates declined, and the serotype-specific clearance rates increased in approximately equal magnitudes (Figure 4). By serotype, the acquisition rates were highly correlated with prevalence (r = 0.91; P < .00005); and the log rates of acquisition and clearance were negatively correlated (r = −0.38; P = .046) (Supplementary Figure 1).
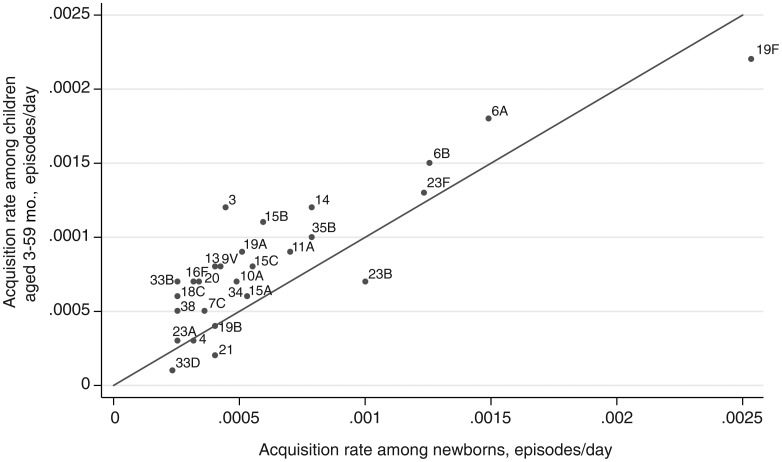
We compared the serotype-specific rates of acquisition derived in this study with serotype-specific rates of acquisition measured directly in a population of 1404 newborns in the same setting in Kilifi (Figure 5) [28]. Among the most frequent 28 serotypes in the newborn study, acquisition rates were highly correlated (r = 0.87; P < .00005). For the 28 most common serotypes, the serotype-specific acquisition rates in this study were on average 1.47 times those observed for newborns.
Figure 5.
Scatterplot and line of equality for acquisition rates estimated in 2 different studies in Kilifi. The illustration shows the scatter of acquisition rates of the most frequently observed 28 serotypes directly measured in newborns (age, 0–3 months) against the acquisition rates among children aged 3–59 months calculated in the present study. r = 0.78; P < .00005.
DISCUSSION
This is the largest single study of nasopharyngeal carriage dynamics conducted in a developing country and includes almost 10 000 swabs. In a setting where the carriage prevalence is 66% [25], the study shows that a child acquires a new colonizing strain once every 16 days and that the average duration of carriage is 32 days. By estimating the relatively lower rates of immune clearance, the study illustrates the significant role of competitive displacement in pneumococcal ecology. It also shows that the declining prevalence of carriage with age is explained by a reduction in clearance rates rather than by an increase in acquisition rates and that the relative rate of acquisition of different serotypes is most cogently explained by the relative prevalence of those types in the nasopharynges of this subpopulation.
Although we did not detect the onset of carriage, we were able to obtain a valid estimate of carriage duration by using the reciprocal of the clearance rates from an exponential regression model. Participants who remained colonized at the end of the study contributed valid risk time to these clearance rate estimates. Normally, in a longitudinal carriage study of intermittent swabbing there is uncertainty about the timing of acquisition and uncertainty about the timing of clearance. The approach we have adopted eliminates one of these sources of uncertainty. We also optimized the estimate of clearance by using the data set as a whole to inform the clearance function, rather than by imposing an arbitrary rule (eg, the midpoint). Finally, compared with the standard sampling schedule of fortnightly or monthly swabs, the intense sampling in the first 2 weeks of the study increased the precision of our estimates of short-duration episodes. Studies of smaller size, wider sampling intervals, and midpoint censoring will obtain an estimated carriage duration that is biased upward. Not surprisingly, therefore, the estimate of carriage duration in this study, 32 days, is shorter than that estimated in several other studies. For example, in longitudinal studies of Swedish and British children <5 years old, the mean carriage durations were 43 and 51 days [29, 30], and among Gambian children aged 1–4 years, the mean duration was 84 days [31].
Comparisons of carriage duration across populations are, however, of limited usefulness given geographical variations in serotype prevalence and the 8-fold variation in serotype-specific clearance rates seen in this data set. Nonetheless, even within serotype-specific comparisons, our estimates of duration are shorter than those observed in other developing countries. For example, the average carriage duration of serotypes 6A and 6B (46–51 days) was less than that of serotype 6A in the Gambia (77 days) or for serogroup 6 in Papua New Guinea (63 days) [31, 32].
Consistent with other studies [30, 31], we observed an increase in the rate of clearance with age. The stability of acquisition rates with age indicates that the progressive decline in carriage prevalence between the ages of 3 and 59 months is attributable to the progressive decline in the carriage duration. Although the pattern of serotypes carried varies significantly with age among children [25], the increased rate of clearance with age is not attributable to confounding by changing serotype patterns, suggesting that it is an inherent characteristic of the host. When competitive displacement was excluded from our analyses of clearance, the residual rate of immune clearance closely shadowed the change in total clearance rates by age, further supporting the role of host immune maturation [33, 34].
We used an established epidemiological relationship between acquisition, duration, and prevalence to estimate acquisition rates [27]. The estimate obtained, 0.064 episodes/day, is consistent with an estimate from Papua New Guinea (≥0.046 episodes/day) [32] but substantially higher than the estimate among children aged <3 years in England (0.012 episodes/day), where the prevalence and risk of exposure are much lower [29]. The method used assumes equilibrium between acquisition and clearance around a constant prevalence. For all pneumococcal carriage, there is wide variation in prevalence by season [25, 35], but performing the baseline prevalence survey over a period of 23 months likely mitigated the impact of season in our study.
The fact that the serotype-specific estimates of acquisition in the present study are similar to those measured directly in a separate study of newborns in the same area provides a reassuring validation of the epidemiological model. The sum of all serotype-specific acquisition rates in the present study also approximates the summary estimate of acquisition for pneumococcal carriage as a whole, further validating the approach. The study was designed specifically to observe carriage episodes of short duration, but in most of these short-duration episodes termination was by displacement rather than by immune clearance. This raises 2 significant issues for interpretation: the role competition in the nasopharynx and the need to quantify multiple-serotype carriage.
Acquisition of carriage can occur in an uncolonized individual or in an individual already colonized with another type. Similarly, an episode of carriage can be terminated by clearance to the uncolonized state or by colonization with any other serotype. For pneumococci as a whole, our estimate of the immune clearance rate is lower than the total clearance rate, which confirms the important role of intraspecies competition in the determination of prevalence. It is likely that acquisition rates among uncolonized individuals are also higher than those among individuals who are already colonized. A significant limitation of the present study, therefore, is that it does not account for this competition. We have specified a separate model that includes parameters for transitions between colonization with each of the most common 27 serotypes and for the capacity of each serotype to resist displacement, and we have resolved these through maximum likelihood on the present data [36]. The transition rates in that model, expressed as acquisition among uncolonized individuals and clearance to no colonization, are highly correlated with the present results [36].
A further constraint of the present design is its assumption that a child is only colonized with a single serotype of pneumococcus. Studies of multiple colonization have suggested that 10%–38% of children can be colonized with >1 strain simultaneously [37, 38]. Given the size of the present study and the lack of sensitive methods to determine multiple-serotype carriage at the outset, this simplifying assumption was unavoidable. In categorizing data, we may therefore have defined a carriage episode as terminated (by the presence of another serotype) when the original serotype was still present in low numbers. This would lead us to underestimate the duration of carriage and to overestimate the rates of acquisition. At the beginning of the observed episodes, we may have detected a serotype from a stable minority population that was rapidly “replaced” in subsequent swabs by our detection of the dominant serotype. Such observations would substantially exaggerate the observed rate of clearance and the calculated rate of acquisition.
Failure to account for competition and multiple serotype colonization is likely to affect our estimates of less competitive (and therefore less common) serotypes disproportionately. Indeed, the more common the serotype in this study, the closer the correlation was with directly observed acquisition rates (Figure 5), and the correlation for the commonest 28 serotypes was high (r = 0.87).
By using quantitative molecular methods, several studies have identified a gradient in pneumococcal carriage density and an association between invasive disease and high nasopharyngeal load [39, 40]. Colonization density may also affect the probability of transmission between individuals. We did not measure colonization density, but the results do reflect the sensitivity of nasopharyngeal sampling, which is likely to be affected by colonization load [41].
For the 28 most common serotypes, clearance rates were inversely correlated with prevalence, and the increase in pneumococcal clearance rates with age closely reflected a decreased prevalence with age. These observations suggest that prevalence both of pneumococci and of individual serotypes is strongly determined by the rate of clearance in the nasopharynx. Serotype-specific acquisition rates were very strongly correlated with prevalence, and again the most plausible direction for this association is that acquisition is a function of exposure and that exposure is a direct function of the population prevalence. The finding that colonization prevalence, but not acquisition rate, declines with age is consistent with the fact that the acquisition experience of any one child, regardless of age, is determined by the mean prevalence in the population, which does not vary. The pattern of associations between clearance and acquisition rates with prevalence—by age and by serotype—suggest that carriage prevalence is maintained by the host clearance rate and that the acquisition rate is determined by the opportunity for exposure expressed as population prevalence.
We have reported a large carriage study that used an epidemiologically efficient design to obtain serotype-specific rates of acquisition and clearance and serotype-specific prevalence in a well-defined population of Kenyan children. With the exception of intraspecies competition, this study has provided a credible description of the major forces driving transmission and prevalence of the commonest 28 serotypes: acquisition is strongly determined by prevalence, and prevalence in turn is determined by the clearance rate. Immune maturation with age increases clearance rates and reduces prevalence.
The serotype-specific rates also provide baseline estimates to investigate the likely indirect effects of multivalent pneumococcal conjugate vaccines. First, comparison of serotype-specific acquisition rates with matched rates of invasive pneumococcal disease could provide a valid estimate of pneumococcal “attack rates” [21, 22]. This highlights the circulating serotypes that have the greatest potential to cause serotype replacement disease. The prevalence and the rate of acquisition can be used to estimate the force of infection required for population modeling of transmission, which, when combined with the attack rates, creates a population model of disease. Given that the 10-valent pneumococcal conjugate vaccine has recently been introduced into the study setting, these data provide a baseline against which to test the usefulness of carriage observations in predicting and understanding the impact of vaccine on the prevalence and acquisition of nonvaccine serotypes and the risk of serotype replacement disease.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the participating families of the Kilifi District, for taking part in the study, and the field staff, for their tremendous work.
This article is published with the permission of the Director of the Kenya Medical Research Institute.
Financial support. A. S. is funded by a research fellowship from the Wellcome Trust (grant 081835). Work by M. L. was funded in part by US National Institutes of Health (grant R01 AI04893). The KEMRI-Wellcome Trust Research Programme is supported by core funding from the Wellcome Trust (grant 092654/Z/10/A).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente vaccine study center group. Pediatr Infect Dis J. 2000;19:187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Cutts FT, Zaman SMA, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 3.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien KL, Moulton LH, Reid R, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003;362:355–61. doi: 10.1016/S0140-6736(03)14022-6. [DOI] [PubMed] [Google Scholar]
- 5.Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2005;54:893–7. [PubMed] [Google Scholar]
- 6.Miller E, Andrews N, Waight P, Slack M, George R. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–8. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 7.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043–51. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 8.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295:1668–74. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 9.Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180:1171–6. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 10.Obaro SK, Adegbola RA, Banya WA, Greenwood BM. Carriage of pneumococci after pneumococcal vaccination. Lancet. 1996;348:271–2. doi: 10.1016/s0140-6736(05)65585-7. [DOI] [PubMed] [Google Scholar]
- 11.Dagan R, Givon-Lavi N, Zamir O, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis. 2002;185:927–36. doi: 10.1086/339525. [DOI] [PubMed] [Google Scholar]
- 12.Cheung Y-B, Zaman SMA, Nsekpong ED, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian children who participated in a 9-valent pneumococcal conjugate vaccine trial and in their younger siblings. Pediatr Infect Dis J. 2009;28:990–5. doi: 10.1097/INF.0b013e3181a78185. [DOI] [PubMed] [Google Scholar]
- 13.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784–92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 14.Weinberger D, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flasche S, Van Hoek AJ, Sheasby E, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8:e1001017. doi: 10.1371/journal.pmed.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberger DM, Harboe ZB, Flasche S, Scott JA, Lipsitch M. Prediction of serotypes causing invasive pneumococcal disease in unvaccinated and vaccinated populations. Epidemiology. 2011;22:199–207. doi: 10.1097/EDE.0b013e3182087634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Temime L, Guillemot D, Boelle PY. Short- and long-term effects of pneumococcal conjugate vaccination of children on penicillin resistance. Antimicrob Agents Chemother. 2004;48:2206–13. doi: 10.1128/AAC.48.6.2206-2213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipsitch M. Vaccination against colonizing bacteria with multiple serotypes. Proc Natl Acad Sci U S A. 1997;94:6571–6. doi: 10.1073/pnas.94.12.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melegaro A, Choi Y, Pebody R, Gay N. Pneumococcal carriage in United Kingdom families: estimating serotype-specific transmission parameters from longitudinal data. Am J Epidemiol. 2007;166:228–35. doi: 10.1093/aje/kwm076. [DOI] [PubMed] [Google Scholar]
- 20.Auranen K, Mehtala J, Tanskanen A, S Kaltoft M. Between-strain competition in acquisition and clearance of pneumococcal carriage—epidemiologic evidence from a longitudinal study of day-care children. Am J Epidemiol. 2010;171:169–76. doi: 10.1093/aje/kwp351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sleeman KL, Griffiths D, Shackley F, et al. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis. 2006;194:682–8. doi: 10.1086/505710. [DOI] [PubMed] [Google Scholar]
- 22.Yildirim I, Hanage WP, Lipsitch M, et al. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine. 2010;29:283–8. doi: 10.1016/j.vaccine.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auranen K, Arjay E, Leino T, Takala AK. Transmission of pneumococcal carriage in families: a latent Markov process model for binary longitudinal data. J Am Stat Assoc. 2000;95:1044–53. [Google Scholar]
- 24.Scott JA, Bauni E, Moisi J, et al. The Kilifi Health and Demographic Surveillance System (KHDSS) Int J Epidemiol. 2012;41:650–7. doi: 10.1093/ije/dys062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdullahi O, Karani A, Tigoi CC, et al. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One. 2012;7:e30787. doi: 10.1371/journal.pone.0030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien KL, Nohynek H. The WHO pneumococcal vaccine trials carriage working group. Report from a WHO working group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:133–40. doi: 10.1097/01.inf.0000048676.93549.d1. [DOI] [PubMed] [Google Scholar]
- 27.Freeman J, Hutchison GB. Prevalence, incidence and duration. Am J Epidemiol. 1980;112:707–23. doi: 10.1093/oxfordjournals.aje.a113043. [DOI] [PubMed] [Google Scholar]
- 28.Tigoi CC, Gatakaa H, Karani A, et al. Rates of acquisition of pneumococcal colonization and transmission probabilities, by serotype, among newborn infants in Kilifi District, Kenya. Clin Infect Dis. 2012;55:180–8. doi: 10.1093/cid/cis371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melegaro A, Gay NJ, Medley GF. Estimating the transmission parameters of pneumococcal carriage in households. Epidemiol Infect. 2004;132:433–41. doi: 10.1017/s0950268804001980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hogberg L, Geli P, Ringberg H, Melander E, Lipsitch M, Ekdahl K. Age and serogroup related differences in the observed duration of nasopharyngeal carriage of penicillin-resistant pneumococci. J Clin Microbiol. 2007;45:948–52. doi: 10.1128/JCM.01913-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill PC, Townend J, Antonio M, et al. Transmission of Streptococcus pneumoniae in rural Gambian villages: a longitudinal study. Clin Infect Dis. 2010;50:1468–76. doi: 10.1086/652443. [DOI] [PubMed] [Google Scholar]
- 32.Smith T, Lehmann D, Montgomery J, Gratten M, Riley ID, Alpers MP. Acquisition and invasiveness of different serotypes of Streptococcus pneumoniae in young children. Epidemiol Infect. 1993;111:27–39. doi: 10.1017/s0950268800056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bogaert D, Weinberger D, Thompson C, Lipsitch M, Malley R. Impaired innate and adaptive immunity to Streptococcus pneumoniae and its effect on colonization in an infant mouse model. Infect Immun. 2009;77:1613–22. doi: 10.1128/IAI.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci USA. 2005;102:4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdullahi O, Nyiro J, Lewa P, Slack M, Scott JAG. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr Infect Dis J. 2008;27:59–64. doi: 10.1097/INF.0b013e31814da70c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipsitch M, Abdullahi O, D'Amour A, et al. Rates of carriage acquisition and clearance and competitive ability for pneumococcal serotypes in Kilifi District, Kenya: application of a Markov transition model. Epidemiology. 2012;23:510–9. doi: 10.1097/EDE.0b013e31824f2f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montgomery JM, Lehmann D, Smith T, et al. Bacterial colonization of the upper respiratory tract and its association with acute lower respiratory tract infections in Highland children of Papua New Guinea. Rev Infect Dis. 1990;12(Suppl 8):S1006–16. doi: 10.1093/clinids/12.supplement_8.s1006. [DOI] [PubMed] [Google Scholar]
- 38.Smillie WG, Jewett OF. The relationship of immediate family contact to the transmission of type-specific pneumococci. Am J Hyg. 1940;32:79–88. [Google Scholar]
- 39.Vu HT, Yoshida LM, Suzuki M, et al. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr Infect Dis J. 2011;30:11–8. doi: 10.1097/INF.0b013e3181f111a2. [DOI] [PubMed] [Google Scholar]
- 40.Albrich WC, Madhi SA, Adrian PV, et al. Use of a rapid test of pneumococcal colonization density to diagnose pneumococcal pneumonia. Clin Infect Dis. 2012;54:601–9. doi: 10.1093/cid/cir859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdullahi O, Wanjiru E, Musyimi R, Glass N, Scott JAG. Validation of nasopharyngeal sampling and culture techniques for detection of Streptococcus pneumoniae in children in Kenya. J Clin Microbiol. 2007;45:3408–10. doi: 10.1128/JCM.01393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.