Abstract
Staphylococcus aureus necrotizing pneumonia is a life-threatening disease that is frequently preceded by influenza infection. The S. aureus toxin Panton–Valentine leukocidin (PVL) is most likely causative for necrotizing diseases, but the precise pathogenic mechanisms of PVL and a possible contribution of influenza virus remain to be elucidated. In this study, we present a model that explains how influenza virus and PVL act together to cause necrotizing pneumonia: an influenza infection activates the lung epithelium to produce chemoattractants for neutrophils. Upon superinfection with PVL-expressing S. aureus, the recruited neutrophils are rapidly killed by PVL, resulting in uncontrolled release of neutrophil proteases that damage the airway epithelium. The host counteracts this pathogen strategy by generating PVL-neutralizing antibodies and by neutralizing the released proteases via protease inhibitors present in the serum. These findings explain why necrotizing infections mainly develop in serum-free spaces (eg, pulmonary alveoli) and open options for new therapeutic approaches.
Bacterial pneumonia is a well-described complication of influenza [1, 2]. In recent years, infections caused by community-onset, methicillin-resistant Staphylococcus aureus (CA-MRSA) strains, which have widely spread, have emerged as contributors to morbidity and mortality in patients with influenza [3, 4]. Furthermore, there has also been considerable increase in the incidence of S. aureus infections in otherwise healthy and immunocompetent individuals, which, in the United States, is mainly caused by CA-MRSA strains [5]. In this respect, a rapidly progressive, hemorrhagic, and necrotizing pneumonia with severe leukopenia has been reported. This type of S. aureus pneumonia is often preceded by an influenza infection or influenzalike symptoms and seems to be a specific disease entity with a high lethality rate. Because of the necrotic histopathological appearance of the lung, the illness has been designated S. aureus necrotizing pneumonia [6]. However, the involved staphylococcal and possibly viral components have not been identified with certainty.
The staphylococcal factor that has been most closely associated with necrotizing infections is the Panton–Valentine leukocidin (PVL) [6, 7]. Panton–Valentine leukocidin is a bicomponent leukotoxin composed of the proteins LukS-PV and LukF-PV, which mainly target human neutrophils [8]. Clinical studies reported that almost exclusively PVL-positive S. aureus strains can complicate influenzalike illness in otherwise healthy young patients, with rapid progression to severe necrotizing pneumonia. By contrast, PVL-negative strains generally induce nonspecific S. aureus pneumonia, which is less fulminant and occurs in older adults (aged ≥60 years) [6, 7].
Besides the epidemiological evidence of PVL and influenzalike illness as causative agents, the pathogenic mechanisms leading to necrotizing pneumonia are largely undefined. For PVL, it is well known that low doses of toxin activate and rapidly kill human neutrophils. These effects are strongly species- and cell-specific because they are mainly restricted to neutrophils from humans and rabbits and could not be reproduced in other cell types, such as epithelial cells [8–10]. By contrast, many viral infections are known to activate epithelial cells by inducing a cytokine and chemokine response, which are important signals to promote neutrophil migration to the inflammatory site [11, 12]. In general, neutrophils are the first cells recruited during inflammation and form the earliest line of defense against invading microorganisms [13]. They are short-lived cells that destroy invading microorganisms through production of reactive oxygen intermediates, proteolytic enzymes, and defensins—compounds that are potentially harmful for the host as well [14]. In a recently published rabbit model of PVL-induced necrotizing pneumonia, it was demonstrated that the presence of neutrophils is the prerequisite to lung inflammation [15].
Taken together, there are many inflammatory and harmful factors, such as PVL and other staphylococcal components, influenza virus, and accumulation of neutrophils, that could play an interacting role in necrotizing pneumonia. The aim of this study is to characterize the specific factors and to understand the complex interplay between S. aureus and influenza virus that leads to necrotizing pneumonia. For this purpose, we established in vitro and in vivo experimental infection models that are based on costimulation with influenza virus and S. aureus PVL. Our results enabled us to propose a mechanistic model for the development of necrotizing pneumonia in influenza-infected patients undergoing a superinfection with PVL-producing S. aureus strains.
METHODS
Ethics Statement
Taking of blood samples from humans and cell isolation were conducted with approval of the local ethics committees (Ärztekammer Westfalen-Lippe und Medizinschen Fakultät der WWU, Az. 2008-034-f-S).
Mice were maintained under standard conditions and handled according to institutional (Helmholtz Center for Infection Research) and European guidelines (EU Directives 86/609/EEC). Animal experiments were approved by the ethical board LAVES, Oldenburg, Germany (permit number: 33.9-42502-04-10/0296).
Human Cells, Bacterial Strains, and Virulence Factors
A549 cells were cultured in Dulbecco's modified Eagle medium (DMEM), supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin, and maintained at 37°C with 5% carbon dioxide. Preparation of human neutrophils, generation of lukF-PV, lukS-PV, and phenol soluble modulins (PSMs), the bacterial strains used in this study, and the preparations of bacterial supernatants were previously described [9].
Influenza Virus
The human influenza virus A/Puerto-Rico/8/34 (H1N1) (PR8 wt) were propagated on Madin–Darby canine kidney cells. For infection, human cells were incubated with the virus at a multiplicity of infection as indicated and diluted in DMEM containing 0.2% bovine serum albumin and 100 U/mL penicillin/100 μg/mL streptomycin.
Western Blot
Lysing of cells and Western blot analysis were performed as described [16]. Antiserum sample against the influenza virus protein PB1 (vC-19) was from Santa Cruz Biotechnology. For loading controls an anti-ERK2 (C-14) rabbit polyclonal antibody (Santa Cruz) was used.
Plaque Titration
Supernatants of infected cells were collected at different times postinfection and used to assess the number of infectious particles (virus titers) in the samples. The plaque assay was performed as described [16].
Stimulation of Neutrophils and A549 Cells
After incubation of neutrophils with influenza virus and/or PVL, cells were stained with anti-CD11b allophycocyanin (APC)-labeled antibodies (eBioscience) to measure cell activation by flow cytometry (FACSCalibur; Becton Dickinson). For analysis of cell death, cells were incubated for the indicated time periods, stained with 10 µg/mL propidium iodide (Fluka), and analyzed by flow cytometry. To determine the number of adherent A549 cells, cells were trypsinized, resuspended in a defined volume, and counted by flow cytometry. To investigate the effect of neutrophil supernatant on A549 cells, neutrophils were stimulated and pelleted, and the supernatants were added to epithelial cells.
Human serum, heat-inactivated human serum, fetal bovine serum (PAA Laboratories GmbH), serum depleted of immunoglobulin G (Sunnylab), protease inhibitor cocktail set V (Calbiochem), C-reactive protein from human plasma, or serum amyloid P component (Sigma Aldrich) were added during incubation of neutrophils with PVL or during the incubation of A549 cells with neutrophil supernatants.
Real-Time Polymerase Chain Reaction
Real-time reverse transcription-polymerase chain reaction (RT-PCR), including complementary DNA synthesis, real-time amplification, and specific primers, was performed as described [17].
In Vivo Experiments
C57BL/6 mice aged 8–12 weeks were purchased from Harlan Winkelmann. Mice were briefly anesthetized with 3% isoflurane (Isoflo; Albrecht GmbH) and then instilled intranasally with 40 µL of supernatant. At 24 hours postinfection, mice were euthanized, and the lungs were expanded after administration of 300 µL of 10% formaldehyde in phosphate-buffered saline per tracheal cannulation. Lungs were then removed, fixed in 10% formaldehyde in phosphate-buffered saline, dehydrated, and wax-embedded according to standard protocols. Sections of 0.5 µm were cut and stained with hematoxylin and eosin and then examined microscopically.
Statistical Analysis
Unpaired Student t test was performed. A value of P < .05 was considered significant in all cases.
RESULTS
The Proinflammatory and Cytotoxic Effect of PVL and/or Influenza Virus on Neutrophils and Lung Epithelial Cells
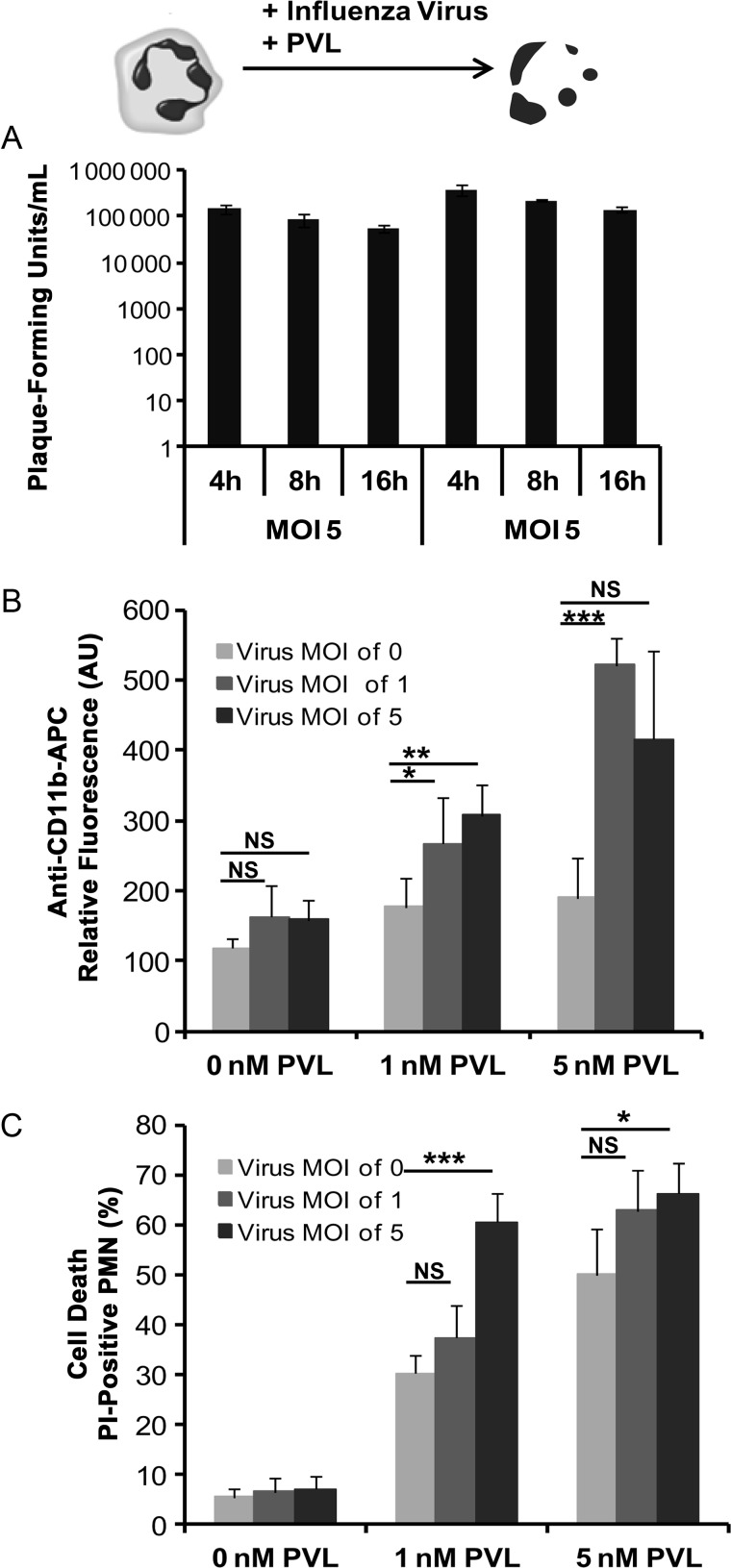
To test whether PVL-induced activation and cytotoxicity on neutrophils can be influenced by influenza virus, we costimulated freshly isolated human neutrophils with influenza virus and PVL and measured virus titer, activation, and cell death induction. Although we could detect viral RNA inside neutrophils by RT-PCR (data not shown), the supernatant fluids from the infected neutrophils did not show any increase in infectious particles, indicating that the residual virions remaining from the inoculum were unable to replicate (Figure 1A). Nevertheless, the surface expression of CD11b, which is a marker for neutrophil activation [18, 19], was significantly upregulated after incubation with both influenza virus and PVL but not after incubation with each of these components separately (Figure 1B). By contrast, neutrophil cell death was induced only by PVL, but coinfection with influenza virus significantly enhanced the rate of cell death (Figure 1C), particularly when low doses of PVL (1 nM) were used.
Figure 1.
The proinflammatory and cytotoxic effects of Panton–Valentine leukocidin (PVL) and/or influenza virus on polymorphonuclear neutrophils (PMNs). Human neutrophils were freshly isolated and infected with influenza virus (multiplicity of infection [MOI]: 0, 1, 5). Virus titers were determined in standard plaque assay 4, 8, and 16 hours postinfection (A). For analyzing the proinflammatory and cytotoxic effects of PVL and/or influenza virus, human neutrophils were freshly isolated, and 1 × 106 cells were infected with influenza virus (MOI: 0, 1, 5) for 3 hours and subsequently incubated with increasing concentrations of PVL (0, 1, 5 nM) for 1 hour. Cells were then washed and stained with allophycocyanin (APC)-labeled anti-CD11b antibodies to measure cell activation (B) or stained with propidium iodide (PI) to determine cell death (C) by flow cytometry. The values represent the mean ± standard deviation of at least 3 independent experiments. *P ≤ .05, **P ≤ .01, ***P ≤ .001 (independent t test). Abbreviations: NS, not significant, AU, arbitrary unit.
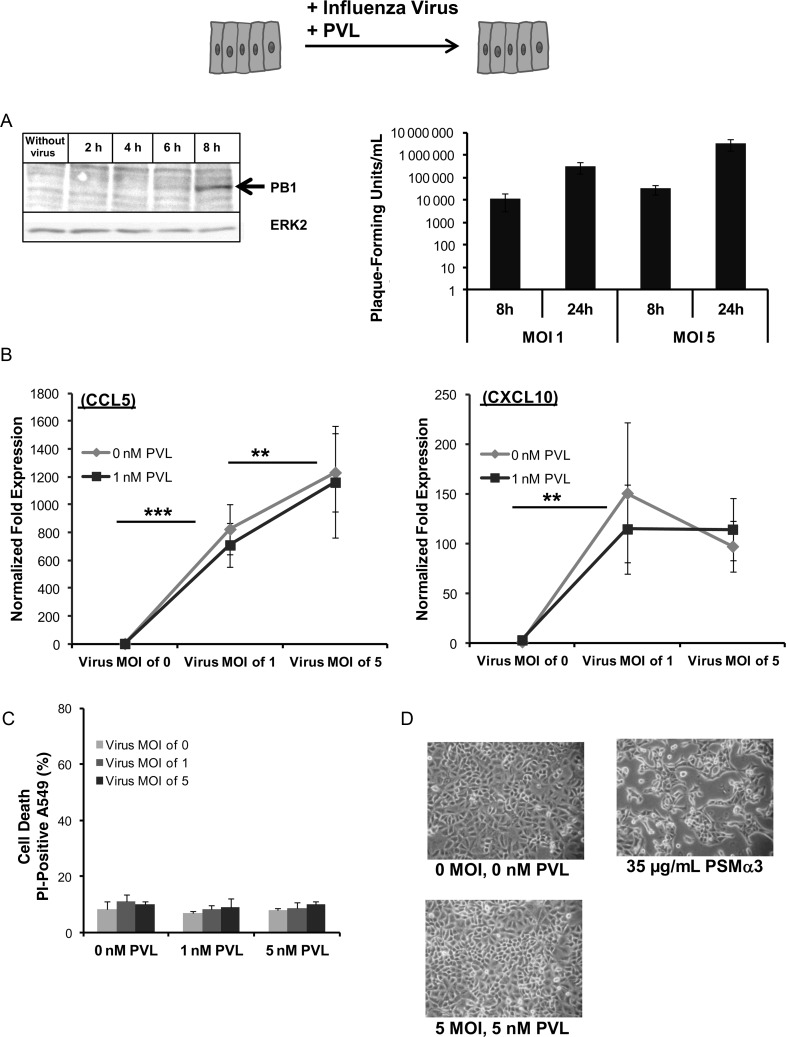
Next, we tested the effect of influenza infection and PVL on confluent monolayers of epithelial A549 cells, either after 18 hours incubation with influenza virus or after 18 hours of incubation with influenza virus followed by a challenge with 1 nM PVL for 2 hours. The supernatant fluids from infected A549 cells, assayed at 24 hours postinfection, contained approximately 2 orders of magnitude more virus particles than the supernatant at 8 hours postinfection, showing viral replication within epithelial cells. Additionally, viral PB1-protein accumulation was detected by Western blot analysis 8 hours postinfection. (Figure 2A). Infection with influenza alone induced a remarkable upregulation of the chemokines CCL5/RANTES and CXCL10/IP-10 (Figure 2B), which are known for their potential to attract immune cells [13]. The viability (Figure 2C) of A549 cells and the integrity of the cell monolayer (Figure 2D) were not affected by treatment with influenza alone, PVL alone, or both. By contrast, S. aureus PSMs induce cell death in epithelial cells and largely damage the epithelial cell monolayer when used at doses of ≥35 µg/mL (Figure 2D).
Figure 2.
The effect of Panton–Valentine leukocidin (PVL) and/or influenza virus on lung epithelial cells. Lung epithelial A549 cells were infected with influenza virus (multiplicity of infection [MOI]: 0, 1, 5) 2, 4, 6, and 8 hours postinfection. Viral PB1-protein accumulation was detected by Western blot analysis, equal protein loads were verified using anti-ERK2 antibodies, and virus titers were determined in standard plaque assay 8 and 24 hours postinfection (A). For analyzing the effect of PVL and/or influenza virus on the A549 cells, the cells were infected with influenza virus (MOI: 0, 1, 5) for 18 hours and subsequently incubated with different concentrations of PVL (0, 1, 5 nM) or phenol soluble modulins (PSMs) (35 µg/mL) for 2 hours. Cells were then washed, and whole RNA was extracted to measure the expression of the chemokine CCL5 (RANTES) and CXCL10 (IP-10) (B). Additionally, cells were stained with propidium iodide (PI) to determine cell death by flow cytometry (C) and examined by light microscopy to determine the integrity of the cell monolayer (D). The values represent the mean ± standard deviation of at least 3 independent experiments. **P ≤ .01, *** P ≤ .001 (independent t test).
Effect of Supernatants from PVL-Treated and/or Influenza-Infected Human Neutrophils on Epithelial Cell Monolayers
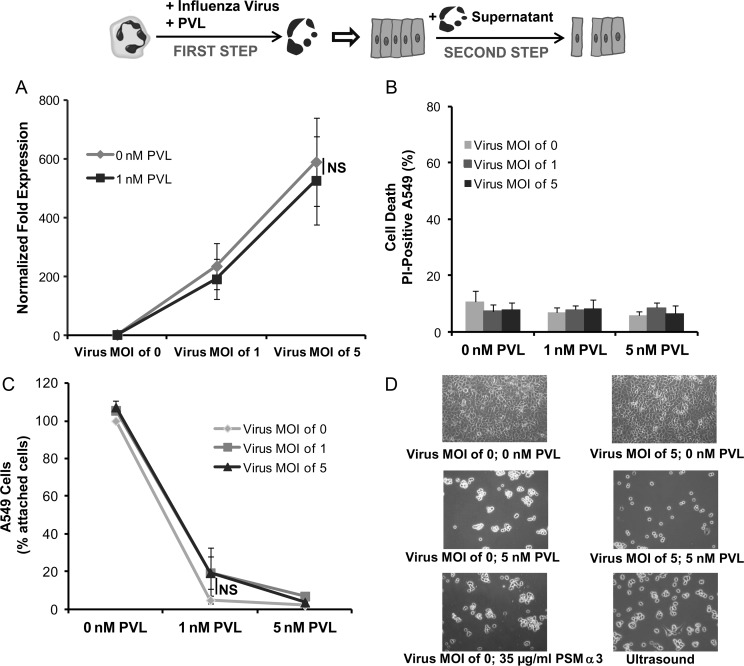
A 2-step cell culture model that mimics the conditions of an S. aureus superinfection in influenza-infected tissue was then established to investigate the mechanisms inducing epithelial cell damage during necrotizing pneumonia. In the first step, human neutrophils were infected with different doses of influenza virus in the presence or absence of PVL. In the second step, the supernatants of these cultures were removed and used to stimulate confluent epithelial monolayers. As shown in Figure 3A, supernatant from influenza-infected neutrophils significantly upregulated the expression of CCL5 in epithelial cells. Interestingly, treatment with supernatant from influenza-infected and/or PVL-treated neutrophils did not affect the viability of the epithelial cells (Figure 3B) but induced epithelial cell detachment and disintegration of the cell monolayer (Figure 3C and D). The level of monolayer disintegration was proportional to the amount of PVL used to stimulate the human neutrophils and therefore to the rate of neutrophil cell death (Figure 1C). Supernatant from neutrophils lysed by PSMs (35 µg/mL) or disrupted by ultrasound was also effective at disintegrating the epithelial monolayers, suggesting that mediators released by the dying neutrophils are responsible for this effect (Figure 3D).
Figure 3.
Effect of supernatants from Panton–Valentine leukocidin (PVL) and/or influenza-treated neutrophils on epithelial A549 cells. In a 2-step cell culture model, human neutrophils were stimulated with influenza virus (multiplicity of infection [MOI]: 0, 1, 5) and PVL (0, 1, 5 nM) (step 1) and the obtained supernatants were used to incubate epithelial cell monolayers for 18 hours (step 2). Epithelial cells were then washed and whole RNA was extracted to measure the expression of the chemokine CCL5 (RANTES) (A). Additionally, all cells (attached and detached) were stained with propidium iodide (PI) to determine cell death by flow cytometry (B). Furthermore, the remaining attached cells were detached by trypsinisation and counted by flow cytometry (C). Attached epithelial cells were examined by light microscopy to determine the integrity of the cell monolayer (D). The values represent the mean ± standard deviation of at least 3 independent experiments. Abbreviations: NS, not significant, PSM, phenol soluble modulin.
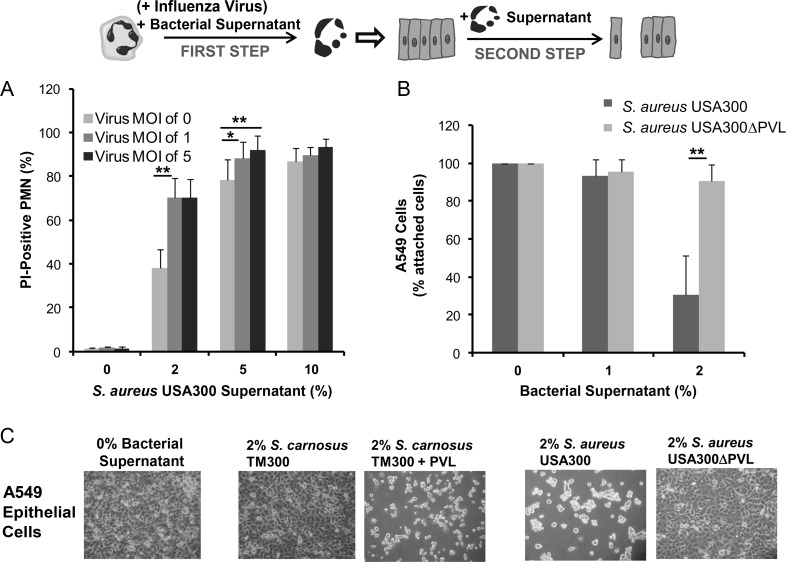
Next, we investigated whether the action of recombinant PVL can be reproduced using PVL-expressing S. aureus. For this purpose, the viability of human neutrophils was determined after treatment with culture medium from S. aureus strain USA300 (PVL-positive) with or without preceding influenza infection. As shown in Figure 4A, culture medium from S. aureus USA300 exerted a cytotoxic effect in human neutrophils in a dose-dependent manner. Furthermore, an additional infection with influenza virus significantly increased the rate of neutrophil cell death, particularly when low doses of supernatants (2%–5%) were used (Figure 4A). We then incubated neutrophils with culture medium from S. aureus USA300 or from the PVL-deficient USA300ΔPVL and subsequently used the cell culture supernatant to stimulate epithelial monolayers. Supernatant from neutrophils incubated with media generated with strain USA300 induced much more detachment of epithelial cells than that from neutrophils incubated with media obtained with the corresponding knockout mutant USA300ΔPVL (Figure 4B and C), which required higher doses (>10%). In general, bacterial supernatants of virulent S. aureus strains exerted a destructive effect on host cells, including neutrophils and epithelial cells, when used at high doses (>10%) (Supplementary Figure 1), which can be attributed to other staphylococcal cytolytic components, such as PSMs. By contrast, supernatant from neutrophils treated with culture medium from the avirulent Staphylococcus carnosus TM300, which lacks most staphylococcal virulence factors, did not affect the integrity of the cell monolayer (Figure 4C; Supplementary Figure 1). However, this effect could be reversed after complementation of S. carnosus TM300 with a plasmid encoding the genes for PVL (TM300 + PVL), which render the bacterial supernatants very destructive (Figure 4C; Supplementary Figure 1).
Figure 4.
Bacterial supernatants from Panton–Valentine leukocidin (PVL)–producing strains kill neutrophils and have a destructive effect on epithelial cell monolayers. First, human polymorphonuclear neutrophils (PMNs) were infected with influenza virus (multiplicity of infection [MOI]: 0, 1, 5) for 3 hours and were subsequently incubated for 30 minutes with bacterial culture medium (2%, 5%, 10%) obtained from different bacterial strains including TM300, TM300 + PVL, USA300, and USA300ΔPVL (step 1). Cells were then stained with propidium iodide (PI) to determine cell death by flow cytometry (A); data only shown for strain USA300. The supernatants from treated neutrophils were collected and used to incubate epithelial cell monolayers for 18 hours (step 2). The numbers of remaining attached epithelial cells were counted by flow cytometry (B). Attached epithelial cells were examined by light microscopy to determine the integrity of the cell monolayer (C). The values represent the mean ± standard deviation of at least 3 independent experiments. *P ≤ .05, ** P ≤ .01, *** P ≤ .001 (independent t test). Abbreviations: S. aureus, Staphylococcus aureus; S. carnosus, Staphylococcus carnosus.
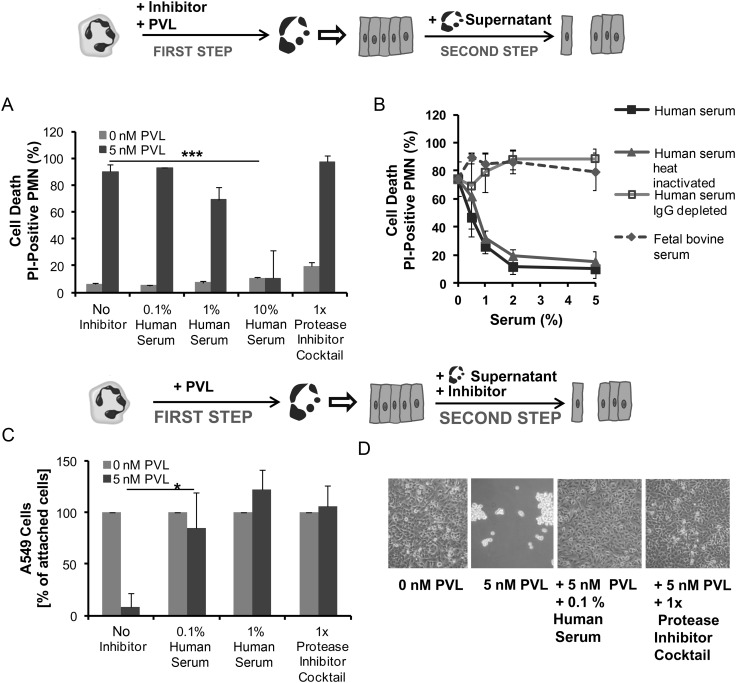
Human Serum and Proteases Inhibitors Can Prevent the Destructive Effect of PVL
Proteases are stored in large quantities in neutrophil granules [20]. To determine if specific inhibition of proteases could prevent the destructive effect of PVL-treated neutrophils on epithelial monolayers, a protease inhibitor cocktail and different serum preparations were used. First, we added each of these components to the media when neutrophils were challenged with PVL. Whereas the protease inhibitors did not impede the destructive effect of PVL on neutrophils, increasing concentrations of human serum prevented PVL-induced cytotoxicity, with maximal effect observed at concentrations ≥2% (Figure 5A and B). Furthermore, we tested fetal calf serum and different human serum preparations (heat-inactivated serum, serum depleted of antibodies). Fetal calf serum and human serum depleted of antibodies were not effective at inhibiting the cytotoxic action of PVL on neutrophils, indicating that antibodies in human serum mediated protection against PVL (Figure 5B). The protective role of antibodies was further supported by the finding that serum obtained from 6 different donors varied in inhibitory function against PVL and the levels of protection correlated with the amount of antibodies measured in the sera (Supplementary Figure 2). By contrast, heat-inactivated serum was equally efficient at preventing PVL-mediated cytotoxicity, indicating that complement factors are not involved in protection of neutrophils (Figure 5B). Similarly, serum amyloid, C-reactive protein, and bovine serum albumin (serum components that are known to bind toxins) did not confer protection (Supplementary Figure 3).
Figure 5.
Human serum and protease inhibitors can prevent the destructive effect of Panton–Valentine leukocidin (PVL). First, human neutrophils (PMN) were stimulated with PVL (5 nM) in the presence or absence of serum or a protease inhibitor cocktail. Cells were then stained with propidium iodide (PI) to determine cell death by flow cytometry (A). In a second experiment, neutrophils were killed with 5 nM PVL, and different human serum preparations and fetal bovine serum were tested for their ability to inhibit the cytotoxic effect (B) (step 1). Supernatants from PVL-treated neutrophils were used to incubate epithelial cell monolayers for 18 hours with or without human serum or a protease inhibitor cocktail as indicated (step 2). The numbers of remaining attached epithelial cells were counted by flow cytometry (C) and examined by light microscopy to determine the integrity of the cell monolayer (D). The values represent the mean ± standard deviation of at least 3 independent experiments. *P ≤ .05, *** P ≤ .001 (independent t test). Abbreviation: IgG, immunoglobulin G.
In a second set of experiments, protease inhibitors and human serum were added to the epithelial cell monolayers during incubation with the supernatant from PVL-treated and destroyed neutrophils. Here, destruction of the epithelial cell monolayer could be prevented by adding very small amounts (≥0.1%) of human serum (Figure 5C and D). A similar protective effect was observed when a protease inhibitor cocktail was used. Both agents completely abrogated destruction of epithelial cell monolayers caused by PVL or PVL-producing strains (Figure 5C and D). These results indicate that proteases released by PVL-damaged neutrophils are mainly responsible for lung injury during infection with PVL-producing strains.
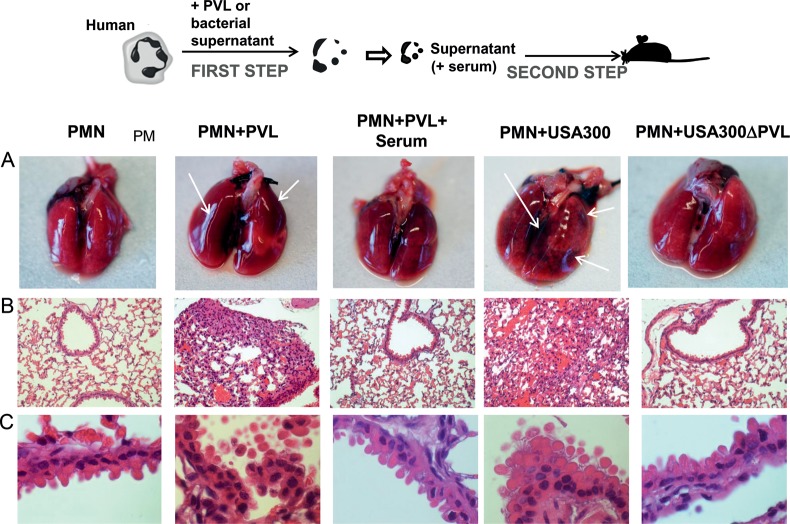
Supernatants of PVL-Treated Human Neutrophils Induce Tissue Damage in the Lungs of Mice After Intranasal Instillation
To test whether the destructive effect of PVL-treated neutrophils can also take place in an in vivo system, we challenged mice via the intranasal route with 40 µL of supernatants from neutrophils treated with either PVL, culture medium from strain USA300, or culture medium from strain USA300ΔPVL or from neutrophils left untreated (1 × 106 neutrophils/mouse). After 24 hours, mice were euthanized, and the lungs were removed, photographed, and subjected to histopathological evaluation. Macroscopically, the lungs challenged with supernantant from PVL- or USA300-treated neutrophils revealed hemorrhages in different parts of the organ (Figure 6A, arrows). Histopathological analysis of these lungs showed extensive peribronchiolar infiltrates and cellular inflammation extended into the airspaces of the surrounding alveolar parenchyma (Figure 6B and C). In contrast, mice challenged with supernatants from untreated neutrophils or neutrophils treated with either PVL plus human serum or culture medium from strain USA300ΔPVL did not show signs of bleeding (Figure 6A) or inflammatory or tissue destructive processes (Figure 6B and C). The histopathological findings of this murine model closely resemble the clinical picture of necrotizing pneumonia [6].
Figure 6.
In vivo model of necrotizing pneumonia. Freshly isolated human neutrophils (PMNs) (1 × 106 cells) were incubated for 30 minutes with Panton–Valentine leukocidin (PVL) (5 nM) or with 2% of bacterial culture medium obtained from strains USA300 or USA300ΔPVL in the presence or absence of human serum (1%). Subsequently, 40 µL of the supernatants of treated neutrophils were instilled intranasally in the lung of mice. After 24 hours, mice were killed, and the lungs were macroscopically evaluated (A) and were subjected to histopathological examination (B and C).
DISCUSSION
A 2-step cell culture model with neutrophils and epithelial cells was developed in this study to analyze postinfluenza S. aureus pneumonia caused by PVL-expressing strains. Most studies investigating coinfection with influenza virus and S. aureus have been performed in mice [21–23]. Although these studies have provided important information regarding immune responses and pathogen virulence, they are highly limited for the investigation of pathogenic mechanisms at the cellular level. Additionally, murine models do not necessarily reflect the situation in humans because the action of PVL is species specific and restricted to human neutrophils [9, 24]. To overcome these limitations, we developed an experimental model system that provides relevant advantages: it is based on cells of human origin, and it includes well-defined cell types, such as neutrophils and epithelial cells, which are both essentially involved in the pathogenesis of pneumonia, thus enabling us to study separately the effect of the pathogens on the different cell types.
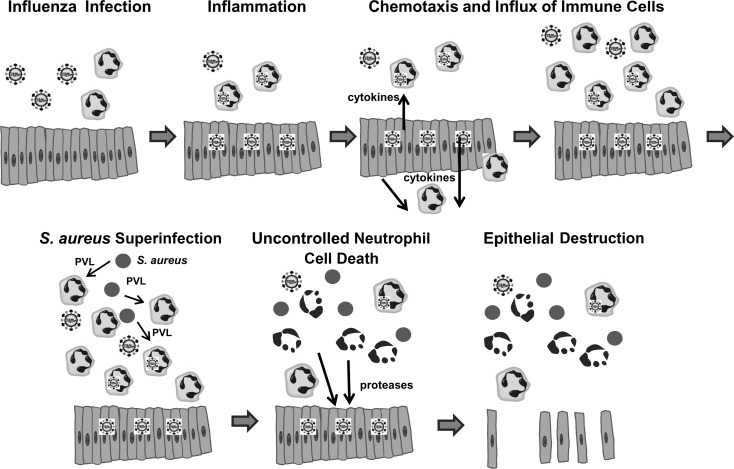
Our results show aspects that can play a crucial role during influenza pneumonia followed by superinfection of PVL-expressing S. aureus strains (Figure 7). First, we found that influenza virus exerts a strong proinflammatory reaction in epithelial cells (Figure 2), involving upregulation of important chemokines that are known to enhance chemotaxis of immune cells, including neutrophils [25]. We did not detect cell death induction by influenza virus infection in epithelial cells. Hence, following viral infection, the lung tissue is most likely intact although inflamed and filled with granulocytes, monocytes, and macrophages. Analogous findings have been obtained from many murine models of influenza infection [21, 26, 27].
Figure 7.
Schematic model of the proposed pathogenic mechanisms leading to the development of necrotizing pneumonia. Abbreviations: PVL, Panton–Valentine leukocidin; S. aureus, Staphylococcus aureus.
The second major finding of our study is that the proinflammatory and cytotoxic action of PVL on neutrophils is enhanced by a preceding infection with influenza virus. Although neutrophils were unable to produce infectious progeny, which has already been described [28], we found a huge proinflammatory effect on neutrophils induced by an influenza infection. This proinflammatory capacity of influenza virus (Figure 1A) could promote neutrophil extravasation, which further increases neutrophil accumulation at the infection focus. Additionally, influenza virus enhances cell death in neutrophils so that very low doses of PVL (1 nM) are already sufficient to damage the majority of a neutrophil population. By contrast, the effect of other staphylococcal cytotoxic components (eg, PSMs) was not augmented by influenza virus and required doses ≥35 µg/mL. The cytotoxic role of PVL during bacterial infections is further strengthened by results obtained with diluted supernatants of PVL-expressing strains (Figure 4). Because PVL expression was found to be high in abscesses and in the presence of neutrophils [29], it is very likely that cytotoxic levels are reached within infected tissues.
Neutrophil cell death induced by PVL is rapid and largely lacks apoptotic features [9]. However, necrosis is an unfavorable mechanism of cell death, whereby the potent antimicrobial molecules spill uncontrolled within the host tissue and cause tissue damage [30]. In our 2-step cell culture experiments and in the in vivo testing in murine lungs, we could demonstrate a massive destructive effect of PVL-treated neutrophils on epithelial cells and on lung tissue. Neutrophils contain granules loaded with proteases and nonenzymatic defensins. For both components, a destructive effect on epithelial cell monolayers is described, whereas proteases induce detachment of epithelial cells, and defensins cause cell lysis [31]. In our experimental setting, PVL-treated neutrophils exerted detachment of epithelial cells rather than cell lysis. This detrimental effect was completely abrogated by the addition of a protease inhibitor cocktail, indicating neutrophil proteases are responsible factors. Besides PVL, other staphylococcal components, such as PSMs, can also contribute to uncontrolled neutrophil death and subsequent epithelial damage, particularly when applied at high doses. Nevertheless, because the effect of strain USA300 was significantly stronger than the destructive action of strain USA300ΔPVL in vitro and in vivo, a major role for PVL must be assumed in this background.
Most interesting, low doses of human serum added at different steps of the experiment were highly effective at preventing the damaging action of PVL (Figure 5). First, human serum (≥2%) abolished PVL-mediated cytotoxicity on human neutrophils, which is due to antibodies against PVL in human serum. Second, very low doses of human serum (≥0.1%) completely preserved the integrity of the epithelial cell monolayer and lung tissue during exposure to supernatants of PVL-treated neutrophils. This effect can be attributed to the protease inhibitors in the serum and further strengthens our hypothesis that PVL-induced tissue destruction is mainly mediated through uncontrolled release of proteases from damaged neutrophils. The dual-protective role of human serum could explain why PVL-associated necrotizing diseases mainly develop under serum-free conditions, such as in pulmonary alveoli. Our in vivo experiments in murine lungs revealed that the alveolar surfactant, which also contains proteases inhibitors [32], did not confer sufficient protection against supernatants from PVL-treated neutrophils. PVL-producing strains are also frequently found in necrotizing skin infections, such as furunculosis and cabuncles [33], in which the onset of inflammation takes place under serum-free conditions within infected skin glands. The dependency of PVL-mediated pathology on serum and/or protease-free environment could also explain the discrepant results recently reported using rabbit models of USA300-induced skin infections. In the model reported by Lipinska et al [34], bacteria were injected intradermally between the layers of the skin, where the poor vasculature and the lack of serum components enables the induction of tissue necrosis that could be assigned to the effect of PVL [35]. By contrast, in the subcutaneous model reported by Kobayashi et al [36], much higher bacterial doses were required to cause skin lesions because in well-perfused tissue the action of PVL, but not of serum-independent toxins, such as PSMs or α-hemolysin, is most likely inhibited.
In summary, we present a model in which influenza virus and PVL-producing S. aureus act together to induce inflammation and tissue damage in the respiratory epithelium. Influenza infection activates the epithelium, which induces a massive influx of neutrophils within the lungs. A superinfection with PVL-producing S. aureus results in extensive neutrophil lysis and massive release of granule proteases. Disintegration of the epithelial airway is accompanied by hemorrhage and tissue damage, leading to the development of necrotizing pneumonia. Our finding, that the cytotoxic action of PVL is efficiently inhibited by serum antibodies and protease inhibition, can have an important impact for improvement of therapeutic interventions in patients affected by S. aureus necrotizing pneumonia. Fast inactivation of PVL, dampening of neutrophil recruitment, and/or inactivation of the released neutrophil contents could provide therapeutic benefits as has been shown for other pathologies, such as in chronic obstructive pulmonary disease [37].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Michaela Brück and Brigitte Schuhen for their excellent technical assistance.
Financial support. This project was supported by a Deutsche Forschungsgemeinschaft (DFG) grant (HA3177/2-1 and SFB1009/B1 to S. N., G. P., and B. L.), by a Bundesministerium für Bildung und Forschung (BMBF) grant (01KI1104 to B. L. and C. E.), and an Interdisziplinäres Zentrum für Klinische Forschung Münster (IZKF) grant (Löf2/030/10).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson L, Caley JP, Moore J. Importance of Staphylococcus aureus in pneumonia in the 1957 epidemic of influenza A. Lancet. 1958;2:233–6. doi: 10.1016/s0140-6736(58)90060-6. [DOI] [PubMed] [Google Scholar]
- 3.Bhat N, Wright JG, Broder KR, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med. 2005;353:2559–67. doi: 10.1056/NEJMoa051721. [DOI] [PubMed] [Google Scholar]
- 4.Randolph AG, Vaughn F, Sullivan R, et al. Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics. 2011;128:e1450–8. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton–Valentine leukocidin. Lab Invest. 2007;87:3–9. doi: 10.1038/labinvest.3700501. [DOI] [PubMed] [Google Scholar]
- 6.Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton–Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–9. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 7.Gillet Y, Vanhems P, Lina G, et al. Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton–Valentine leukocidin. Clin Infect Dis. 2007;45:315–21. doi: 10.1086/519263. [DOI] [PubMed] [Google Scholar]
- 8.Woodin AM. Purification of the two components of leucocidin from Staphylococcus aureus. Biochem J. 1960;75:158–65. doi: 10.1042/bj0750158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Löffler B, Hussain M, Grundmeier M, et al. Staphylococcus aureus Panton–Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szmigielski S, Prevost G, Monteil H, Colin DA, Jeljaszewicz J. Leukocidal toxins of staphylococci. Zentralbl Bakteriol. 1999;289:185–201. doi: 10.1016/s0934-8840(99)80105-4. [DOI] [PubMed] [Google Scholar]
- 11.Lam WY, Yeung AC, Chu IM, Chan PK. Profiles of cytokine and chemokine gene expression in human pulmonary epithelial cells induced by human and avian influenza viruses. Virol J. 2010;7:344. doi: 10.1186/1743-422X-7-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SZ, Forsyth KD. The interaction of neutrophils with respiratory epithelial cells in viral infection. Respirology. 2000;5:1–10. doi: 10.1046/j.1440-1843.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- 13.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77:568–75. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–39. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 15.Diep BA, Chan L, Tattevin P, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton–Valentine leukocidin–induced lung inflammation and injury. Proc Natl Acad Sci U S A. 2010;107:5587–92. doi: 10.1073/pnas.0912403107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seyer R, Hrincius ER, Ritzel D, et al. Synergistic adaptive mutations in the hemagglutinin and polymerase acidic protein lead to increased virulence of pandemic 2009 H1N1 influenza A virus in mice. J Infect Dis. 2012;205:262–71. doi: 10.1093/infdis/jir716. [DOI] [PubMed] [Google Scholar]
- 17.Tuchscherr L, Medina E, Hussain M, et al. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med. 2011;3:129–41. doi: 10.1002/emmm.201000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liles WC, Thomsen AR, O'Mahony DS, Klebanoff SJ. Stimulation of human neutrophils and monocytes by staphylococcal phenol-soluble modulin. J Leukoc Biol. 2001;70:96–102. [PubMed] [Google Scholar]
- 19.Wang R, Braughton KR, Kretschmer D, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–4. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 20.Korkmaz B, Moreau T, Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90:227–42. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Iverson AR, Boyd KL, McAuley JL, Plano LR, Hart ME, McCullers JA. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis. 2011;203:880–8. doi: 10.1093/infdis/jiq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MH, Arrecubieta C, Martin FJ, Prince A, Borczuk AC, Lowy FD. A postinfluenza model of Staphylococcus aureus pneumonia. J Infect Dis. 2010;201:508–15. doi: 10.1086/650204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tashiro M, Ciborowski P, Klenk HD, Pulverer G, Rott R. Role of Staphylococcus protease in the development of influenza pneumonia. Nature. 1987;325:536–7. doi: 10.1038/325536a0. [DOI] [PubMed] [Google Scholar]
- 24.Lowy FD. How Staphylococcus aureus adapts to its host. N Engl J Med. 2011;364:1987–90. doi: 10.1056/NEJMp1100251. [DOI] [PubMed] [Google Scholar]
- 25.Mahalingam S, Karupiah G. Chemokines and chemokine receptors in infectious diseases. Immunol Cell Biol. 1999;77:469–75. doi: 10.1046/j.1440-1711.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- 26.McAuley JL, Hornung F, Boyd KL, et al. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host Microbe. 2007;2:240–9. doi: 10.1016/j.chom.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrone LA, Plowden JK, Garcia-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4:e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassidy LF, Lyles DS, Abramson JS. Synthesis of viral proteins in polymorphonuclear leukocytes infected with influenza A virus. J Clin Microbiol. 1988;26:1267–70. doi: 10.1128/jcm.26.7.1267-1270.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loughman JA, Fritz SA, Storch GA, Hunstad DA. Virulence gene expression in human community-acquired Staphylococcus aureus infection. J Infect Dis. 2009;199:294–301. doi: 10.1086/595982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anwar S, Prince LR, Foster SJ, Whyte MK, Sabroe I. The rise and rise of Staphylococcus aureus: laughing in the face of granulocytes. Clin Exp Immunol. 2009;157:216–24. doi: 10.1111/j.1365-2249.2009.03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Wetering S, Mannesse-Lazeroms SP, Dijkman JH, Hiemstra PS. Effect of neutrophil serine proteinases and defensins on lung epithelial cells: modulation of cytotoxicity and IL-8 production. J Leukoc Biol. 1997;62:217–26. doi: 10.1002/jlb.62.2.217. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Verdugo I, Descamps D, Chignard M, Touqui L, Sallenave JM. Lung protease/anti-protease network and modulation of mucus production and surfactant activity. Biochimie. 2010;92:1608–17. doi: 10.1016/j.biochi.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton–Valentine leukocidin–producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 34.Lipinska U, Hermans K, Meulemans L, et al. Panton–Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: a rabbit model. PLoS One. 2011;6:e22864. doi: 10.1371/journal.pone.0022864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cribier B, Prevost G, Couppie P, Finck-Barbancon V, Grosshans E, Piemont Y. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study. Dermatology. 1992;185:175–80. doi: 10.1159/000247443. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi SD, Malachowa N, Whitney AR, et al. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204:937–41. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korkmaz B, Horwitz MS, Jenne DE, Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev. 2010;62:726–59. doi: 10.1124/pr.110.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.