Abstract
The renal excretion of inorganic phosphate is regulated in large measure by three hormones, namely, parathyroid hormone, dopamine, and fibroblast growth factor-23. Recent experiments have indicated that the major sodium-dependent phosphate transporter in the renal proximal tubule, Npt2a, binds to the adaptor protein sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) and in the absence of NHERF-1, the inhibitory effect of these three hormones is absent. From these observations, a new model for the hormonal regulation of renal phosphate transport was developed. The downstream signaling pathways of these hormones results in the phosphorylation of the PDZ 1 domain of NHERF-1 and the dissociation of Npt2a/NHERF-1 complexes. In turn, this dissociation facilitates the endocytosis of Npt2a with a subsequent decrease in the apical membrane abundance of the transporter and a decrease in phosphate reabsorption. The current review outlines the experimental observations supporting the operation of this unique regulatory system.
Keywords: dopamine, FGF-23, parathyroid hormone
defense of plasma and cellular concentrations of inorganic phosphate is accomplished, in large measure, by rapid changes in the rates of reabsorption of phosphate in the proximal convoluted tubule of the kidney. This review summarizes recent studies that have sequentially examined the mechanism of action of three hormones that affect the renal tubular transport of phosphate, namely, parathyroid hormone (PTH), dopamine, and fibroblast growth factor-23 (FGF-23), with particular focus on the emerging role of the adaptor protein sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) that interacts not only with the PTH1 and dopamine D1-like receptors but also with the Npt2a and possibly the Npt2c sodium-dependent phosphate transporters. We initiate this review by summarizing a recently developed model of PTH-mediated inhibition of renal phosphate transport. The model proposes that NHERF-1 functions as a membrane-retention signal that extends the apical membrane lifetime of the Npt2a transporter and that phosphate uptake is decreased by endocytosis of Npt2a bound to NHERF after the disassociation of Npt2a/NHERF-1 complexes, a process initiated by activation of PKC and the phosphorylation of specific residues in the first PDZ domain of NHERF-1 (47, 54). As might have been anticipated, these experiments raised other important issues, including the need to better define the potential role of the cAMP/PKA pathway in the hormonal regulation of renal phosphate transport. In addition, we were interested in determining whether the model of PTH-mediated regulation of renal phosphate transport was specific for this hormone or whether it represented a more general model applicable to other hormones that affect phosphate transport. Accordingly, we next examined selective aspects of dopamine-mediated regulation of renal phosphate transport and studied the role of modifiable residues in the first PDZ domain of NHERF-1 on dopamine signaling and dopamine-mediated inhibition of phosphate transport (50). These experiments revealed an unanticipated interaction between the dopamine D1-like receptors and NHERF-1. They also indicated that dopamine was associated with phosphorylation of the same PDZ I residues of NHERF-1 as PTH. To expand the above studies, we then studied the role of NHERF-1 in FGF-23-mediated inhibition of renal phosphate transport, given that FGF-23 signals by a pathway involving MAPK but not PKC or PKA (52). These experiments revealed differences between the FGF-23 and the PTH/dopamine pattern of site-specific phosphorylation of the PDZ I domain of NHERF-1, suggesting a mechanism for modulating the effects of these hormones on phosphate excretion.
Filtered phosphate is reabsorbed exclusively in the proximal convoluted tubule of the kidney (33). The apical membrane contains three sodium-dependent phosphate transporters, Npt2a, Npt2c, and Pit-2, which collectively represent the rate-limiting step in transcellular phosphate transport. The exit step for phosphate across the basolateral membrane is not currently known but does not appear to be a regulated process, at least by the three hormones under discussion. Of the three apical membrane transporters, Npt2a, initially cloned and characterized by Murer and colleagues (29), is the best studied. In rodents, it is the major phosphate transporter, accounting for 75–80% of phosphate transport (5). Although the absence of Npt2c in mice has no effect on basal phosphate homeostasis due to an increase in the abundance of Npt2a, mutations in Npt2c have been identified in some patients with hereditary hypophosphatemic rickets with hypercalciuria, a disease characterized by renal phosphate wasting (7, 34). It is unclear at the present time why regulation of Npt2a could not compensate for the loss-of-function mutation of Npt2c in this disease state as it does when Npt2c is absent as in the null mice. This question is under active investigation in several laboratories.
Proximal tubule phosphate transport correlates with the apical membrane expression of Npt2a. As detailed by Murer and colleagues in an elegant series of studies over several years, an increase in the abundance of Npt2a in the apical membrane of the renal proximal tubule is associated with increased rates of phosphate transport (Reviewed in Refs. 10 and 29). Phosphate transport is decreased when Npt2a is endocytosed by a clathrin-mediated pathway and targeted for degradation in lysosomes (23, 39). Renal phosphate transport is inhibited by 30–50% within 30–45 min after treatment of renal proximal tubule cells with PTH, dopamine, or FGF-23, associated with a proportional decrease in the abundance of Npt2a in the apical membrane (10, 12, 15, 25, 26, 52). Npt2a removed from the apical membrane, unlike some other transporters, channels, and receptors, does not recycle back to the apical membrane (38). In this review, we focus on the inhibitory effects of PTH, dopamine, and FGF-23 on the Npt2a transporter, comparing and contrasting the mechanisms for regulation of Npt2a expression.
Npt2a and the PDZ Domain NHERF Adaptor Proteins
NHERF-1 and NHERF-2 are the first two members of a four-protein family of PDZ domain adaptor proteins (46, 57). NHERF-1 and NHERF-2 contain two tandem PDZ domains in the N-terminus of the protein and an ezrin, radixin, moesin, merlin (MERM) binding domain in the C-terminus. NHERF-1 and NHERF-2 are localized to the apical membrane of renal proximal tubule cells where they facilitate the formation of protein complexes (43, 44). NHERF-1 and NHERF-2 bind and affect the action of the PTH1 receptor (see below) while NHERF-1 also binds to the Npt2a transporter. By yeast-2 hybrid analysis, NHERF-2 is also capable of binding to Npt2a but NHERF-2 null mice have normal targeting of Npt2a to the apical membrane of proximal convoluted tubule cells and are capable of maintaining a normal serum phosphate concentration (14). The importance of NHERF-1 in phosphate metabolism was established from the initial studies of the NHERF-1 null mouse that exhibited hypophosphatemia and an increase in the renal excretion of phosphate associated with a decrease in the abundance of Npt2a in the apical membrane of renal proximal tubule cells (37). Subsequent studies using cultured cells and adenovirus vectors to rescue NHERF-1 null proximal tubule cells indicated that NHERF-1 increases the apical membrane abundance of Npt2a (13). In the context of this discussion, three sets of observations provided insights into the possible mechanism by which PTH inhibited phosphate transport in the kidney. First, under a number of experimental circumstances including alterations in the dietary intake of phosphate and PTH treatment, there were changes in the apical membrane abundance of Npt2a but not NHERF-1 (12.13, 16). Second, the phosphorylation of NHERF-1 was increased by PTH (16, 47). Third, despite the normal activation of PKA and PKC by PTH, phosphate transport in NHERF-1 null cells was resistant to the inhibitory effects of not only PTH itself but also activators of both PKC and PKA (13). The introduction of NHERF-1 into these NHERF-1 null cells increased basal phosphate transport and restored the inhibitory effect of PTH (13, 47). Collectively, these observations suggested that NHERF-1 functioned as a membrane retention signal for the Npt2a transporter, that NHERF-1 was the target of the down-stream protein kinase cascades activated by occupancy of the PTH1 receptor, and that PTH-mediated phosphorylation of NHERF-1 resulted in the dissociation of NHERF-1/Npt2a complexes.
PTH
Greenwald and Gross (19) were the first to demonstrate that parathyroid gland extract increased the urinary excretion of phosphate. The biochemical and molecular details of how PTH regulated renal phosphate transport, however, were unknown for many years. Renal proximal tubule cells express PTH1 receptors on both the apical and basolateral membrane of the cells. Earlier studies established that treatment of apical membranes with PTH activated PKC while PTH activated the cAMP/PKA pathway in basolateral membranes (22, 40). Resolution of this paradox was provided by Mahon and colleagues (27) and Friedman and coworkers (55), who demonstrated that the apical membrane PTH1 receptor bound to the PDZ domain adaptor proteins NHERF-1 and NHERF-2, also localized to the apical membrane of these cells. When bound to either NHERF-1 or NHERF-2, the apical receptor signaled by activation of PKC. In the absence of these adaptors, the receptors signaled by generation of cAMP and activation of PKA.
One of the targets of PKC and PKA is NHERF-1 (16, 17, 47). Curiously, Npt2a is likely not a target of these signaling pathways despite the presence of a number of consensus sequences for PKC phosphorylation (16). Using a variety of cellular assays coupled with confocal microscopy, we have advanced evidence that PTH results in the dissociation of Npt2a/NHERF-1 complexes (16, 47). Within second to minutes after the start of treatment with PTH, PKC is activated (51). This activation is associated with an increase in the lateral mobility of the Npt2a transporter in the apical membrane of proximal tubule cells in the ensuing 10 min, after which mobility returns to baseline (48). The increase in lateral mobility is dependent on the presence of NHERF-1 and the C-terminal PDZ binding motif of the Npt2a transporter. These findings suggest that this early increase in lateral mobility represents the dissociation of Npt2a from NHERF-1 while the return to baseline represents the association of Npt2a with other proteins that mediate its endocytosis. Thus, by 10–15 min after treatment, the apical membrane abundance of Npt2a but not the abundance of NHERF-1 begins to decrease, associated with a decrease in phosphate transport, which is maximal by 30–45 min (51).
Using sucrose density gradient ultracentrifugation, we have estimated that 35–50% of Npt2a in the apical membrane of proximal tubule cells is bound to NHERF-1 and that this pool of the transporter is the unique target of the downstream protein kinase cascades of the occupied PTH1 receptor (47). In the absence of NHERF-1, renal tubule cells are resistant to the inhibitory effects of PTH. These findings indicate either that NHERF-1 is the target of PKC and PKA or that in some manner; the presence of NHERF-1 facilitates the phosphorylation of Npt2a or other associated proteins. Given that Npt2a is not a phosphoprotein itself and that the phosphorylation of NHERF-1 is increased by PTH, the direct phosphorylation of NHERF-1 appears to be the central event in its dissociation from Npt2a (16, 47).
The phosphorylation of NHERF-1 has been under study for some years. Evidence to date suggests that site-specific phosphorylation of NHERF-1 may determine the extent of dimerization, the binding of target proteins such as CFTR to the PDZ II domain, and the association of the C terminus of NHERF-1 with its own PDZ II domain (31, 35, 36). The vast majority of NHERF-1 binding targets including Npt2a, however, involve the first PDZ domain. Cellular studies using protein phosphatase inhibitors and expressed NHERF-1 PDZ I domains indicated that serine77 was the major phosphate acceptor in the PDZ I domain while threonine95 was a secondary acceptor site (42). Quantitative pull-down studies showed that the phosphorylation of the PDZ I domain decreased the recovery of Npt2a. This suggested that phosphorylation of the PDZ I domain might regulate the apical membrane abundance of Npt2a and the rate of phosphate transport in renal proximal tubule cells. To test this more directly, we studied the effect of PTH on phosphate transport in NHERF-1 null cells infected with wild-type adenovirus-NHERF-1 or adenovirus-NHERF-1 containing a S77A mutation (47). While both constructs increased basal phosphate transport, mutation of serine77 to alanine blocked the inhibitory response to PTH. By contrast, mutation of serine77 to the phosphomimetic aspartic acid resulted in a lower rate of phosphate transport, a rate which approximated the effect of PTH on cells infected with wild-type adenovirus-NHERF-1 (47). Serine77 is in the α-helix forming part the protein binding groove of the PDZ-I domain of NHERF-1 (45). This residue does not face the groove directly, but our findings suggest that the conformational changes associated with phosphorylation of serine77 decreased its association with Npt2a. In later studies, we confirmed that mutation of serine77 to aspartic acid decreased the binding affinity for Npt2a (49).
The precise relationship between threonine95 and the binding groove of the PDZ I domain is not known since this residue was not included in the only available crystal structural analysis of NHERF-1 (45). Mutation of threonine95 to alanine or aspartic acid does not affect the binding affinity of Npt2a to NHERF-1 (49). Rather, phosphorylation of threonine95 or the phosphomimetic mutation T95D facilitates PKC-mediated phosphorylation of serine77. Mutation of threonine95 to alanine blocks the inhibition of phosphate transport by PTH (49). Expressed another way, there is cooperative phosphorylation of threonine95 and serine77 to affect the inhibition of phosphate transport by PTH.
For PTH, then, we propose the following mechanism to explain inhibition of renal phosphate transport. PTH interacts with PTH receptors in the apical and basolateral membranes of renal proximal tubule cells. PKC and PKA are activated. Most but not all studies would suggest that the rapid activation of PKC results in the phosphorylation of serine77 provided threonine95 is also phosphorylated, resulting in the dissociation of Npt2a/NHERF-1 complexes. The initial dissociation is manifest by an increase in the lateral mobility of the Npt2a transporter, followed by its reassociation with other proteins that decrease its mobility and facilitate its endocytosis from the apical membrane, a process that takes ∼10–15 min in a model cell system like opossum kidney (OK) cells. The decrease in the abundance of Npt2a, in turn, is associated with a decrease in the transport of phosphate. The inhibition of phosphate transport is maximal by 30–45 min after treatment with PTH, and the degree of inhibition averages 40–50% of the basal rate of transport, using a maximal concentration of the hormone (51).
In this model, the precise role of activation of PKA is uncertain. Using recombinant proteins and in vitro assays, PKC but not PKA can directly phosphorylate serine77 of NHERF-1 (47). In cellular assays, however, both PKC and PKA are capable of phosphorylating this residue, suggesting that PKA is acting through an indirect pathway. The potential role of PKA activation is further confused by the findings that pharmacological block of the cAMP/PKA pathway does not affect PTH-mediated inhibition of phosphate transport (11, 15). Suffice it to note that the role of PKA activation in PTH-mediated inhibition of renal phosphate transport is controversial at the present time and will require additional study (24, 25, 30). Nonetheless, we have speculated that activation of PKA by PTH may affect the duration or intensity of the PTH response, perhaps by activating an unknown protein phosphatase, but this speculation is not yet confirmed. However, it was this issue, i.e., understanding the role of PKA in mediating inhibition of phosphate transport, that led us to initiate studies of dopamine, another hormone that inhibits renal phosphate transport associated with activation of both PKC and PKA.
Dopamine
Dopamine interacts with two classes of receptors in the kidney. The D1-like receptors (D1 and D5) are associated with activation of PKA while the D2-like receptors (D2, D3, and D4) are associated with inhibition of adenylate cyclase (20). The D1 receptor, which is present in the renal proximal tubule, is also linked to activation of PKC (1). As in the case of PTH, the exact role of each pathway was uncertain but it was generally agreed that dopamine inhibited phosphate transport in the kidney by a PKA-mediated pathway (3, 26). In agreement with this model, we also found that the inhibitory effect of dopamine on phosphate transport could be blocked by pharmacological agents that disrupt the interaction of cAMP with PKA (15). However, the inhibitory effect of dopamine could also be blocked by inhibitors of PKC. Additional studies demonstrated that in mouse proximal tubule cells, activation of PKA activates PKC (15). Thus, in contrast to PTH which directly stimulated PKC, activation of PKC in response to dopamine required initial activation of PKA, which, in turn, stimulated PKC to inhibit phosphate transport. Tissue studies indicated that dopamine, like PTH, phosphorylated threonine95 and serine77 of the first PDZ domain of NHERF-1 and that an alanine substitution at either site blocked the inhibitory effect of dopamine on phosphate transport (49). A somewhat surprising finding was that stimulation of PKA and PKC in response to dopamine was absent in cultured proximal tubule cells from NHERF-1 null animals (50). Rescue of these cells using wild-type adenovirus-NHERF-1 restored the response to dopamine. Thus the D1-like receptors require NHERF-1, but unlike the PTH1 receptor, NHERF-2 cannot substitute for NHERF-1.
With few exceptions, experiments designed to examine the effects of dopamine on renal mineral and electrolyte transport have studied the effects of exogenous dopamine. Only a few studies have attempted to directly examine the effects of endogenous dopamine (2). Phosphate adaptation refers to the ability of the kidney to rapidly alter the rates of phosphate excretion in the urine in response to alterations in the dietary intake of phosphate (38). The sensing mechanism for this effect appears to be the cells of the small intestine (9). The signal between the gut and the kidney is not known, but evidence has been advanced to indicate that it does not involve PTH, vitamin D, FGF-23, or renal nerves. In an older and largely forgotten study in rats, Knox and colleagues (8) demonstrated that feeding a diet high in phosphate increased the renal excretion of dopamine. We have recently reexamined the role of dopamine in mediating the acute (24 h) response to feeding a high-phosphate diet (53). In mice, feeding a high-phosphate diet increased the renal content of dopamine as well as the urinary excretion of dopamine associated with activation of PKA and PKC, the downstream signaling pathways used by the dopamine D1-like receptors. In animals fed a high-phosphate diet, there was a significant increase in the abundance and activity of renal dopamine decarboxylase and significant reductions in the enzymes involved in dopamine degradation, namely, renalase, monoamine oxidase A, and monoamine oxidase B. Treatment with carbidopa to inhibit dopamine biosynthesis blocked the increase in the urinary excretion of dopamine in response to a high-phosphate diet and decreased the urinary excretion of phosphate. Although the signal by which alterations in the dietary intake of phosphate affect dopamine synthesis and degradation is not known, these studies indicated that endogenous dopamine plays an important role in the initial adaptation to a diet high in phosphate. To date, we have not specifically examined time periods exceeding 24 h of dietary phosphate adaptation but reasoned that the D1-like receptors would likely undergo time-dependent desensitization and that the sustained adaption to a high-phosphate diet would require non-dopamine-related processes, such as the generation of FGF-23.
FGF-23
The discovery of the biological effects of FGF-23 has been a major advance in the understanding of phosphate and vitamin D metabolism. The effects of FGF-23 are specific to this hormone, and no other member of the FGF family of proteins reproduces its biological effects. FGF-23 inhibits the renal tubule reabsorption of phosphate and also blocks the conversion of 25(OH)-vitamin D to 1,25(OH)2 vitamin D in the proximal tubule of the kidney (32). FGF-23 concentrations are increased in humans and animals ingesting a high-phosphate diet, increased in patients with renal insufficiency presumably in response to hyperphosphatemia, and suggested to be a biomarker for the progression of renal insufficiency and for the development of arterial disease. Elevations in FGF-23 concentrations are causative in clinical diseases such oncogenic hypophosphatemic osteomalacia, autosomal dominant hypophosphatemic rickets, and X-linked vitamin D-resistant rickets. In patients with oncogenic hypophosphatemic osteomalacia, removal of the FGF-23-producing tumors cures the metabolic defects (21, 32).
FGF-23 binds to a number of FGF receptor subtypes and requires klotho as a cofactor. The interaction between klotho and the FGF receptor confers tissue specificity to the actions of FGF-23 by allowing the hormone to bind its receptor complex with greater affinity. In cultured OK cells and in perfused rabbit renal proximal tubules, FGF-23 in the presence of heparin to stabilize the FGF-23/klotho receptor complex acutely inhibits phosphate transport (4, 56). The signaling pathway involves activation of MAPK but not PKC or PKA, and the inhibition of phosphate transport is associated with a decrease in the apical membrane abundance of Npt2a. In NHERF-1 null mice renal slices and cultured proximal tubule cells, FGF-23 does not affect phosphate transport despite normal activation of MAPK (52). Thus, like PTH and dopamine, FGF-23 targets the pool of Npt2a bound to NHERF-1 in the apical membrane of proximal tubule cells. In cultured cells, rescue of the cells with wild-type NHERF-1 increases the basal rate of phosphate transport and restores the inhibitory effect of FGF-23. Rescue of the NHERF-1 null cells with NHERF-1 containing a serine77-to-alanine mutation also increases basal phosphate transport but not the inhibitory effect of FGF-23 on phosphate transport. These findings are identical to those found in studies of PTH and dopamine and highlight the central role of serine77 and its interaction with Npt2a as a target of these three inhibitory hormones. In contrast to PTH and dopamine, which require the phosphorylation of threonine95 (or a T95D phosphomimetic mutation) to inhibit phosphate transport, rescue of NHERF-1 null cells with NHERF-1 containing a threonine95-to-aspartic acid mutation blocks the inhibitory effect of FGF-23 (52). The reasons for the differing role of threonine95 in PTH- and dopamine-mediated inhibition of phosphate transport compared with the inhibition of phosphate transport mediated by FGF-23 have not yet been explored. It remains possible that the T95D mutation is ineffective as a phosphomimetic substitution, although we think this unlikely since a T95A mutation yielded the same results as wild-type NHERF-1 in the FGF-23 studies but not in the PTH or dopamine experiments. One possible explanation may relate to the way PKC compared with MAPK accesses the serine77 site. At the present time, the inability to generate threonine95 and serine77 site-specific phosphoantibodies has been a major experimental issue in pursuing these questions.
Whatever the explanations for the differences between the inhibitory effect of PTH and FGF-23 on phosphate transport, it seems clear that these two potent hormones affect phosphate transport differently, which raises the question of how these hormones might interact with one another. In this regard, there was a clinical report that indicated that the hypophosphatemia associated with elevated levels of FGF-23 could be abrogated by treatment with drugs that inhibited the release of PTH (18). The serum concentrations of phosphate and 1,25-dihydroxy vitamin D are determinants of both PTH and FGF-23 levels, and perturbations in the levels of PTH might be predicted to affect the concentration and/or metabolic actions of FGF-23. In addition, evidence has been advanced that FGF-23 directly inhibits PTH synthesis and release from parathyroid glands (6). Recent studies from our laboratory, however, have suggested a heretofore unrecognized interaction between these hormones directly at the level of renal proximal tubule cells. In renal slices from the mouse kidney, FGF-23 and PTH, in doses that individually had no effect on PKC, PKA, or MAPK activity and did not affect phosphate transport in the kidney, dramatically inhibited phosphate transport when cells were treated with low concentrations of both hormones simultaneously (52). Moreover, the combined low doses of PTH and FGF-23 stimulated PKC and PKA activity but not MAPK. These findings would suggest that FGF-23, in some manner, sensitizes renal proximal tubule cells to PTH. The biochemical nature of the interaction between the two hormones is not known at the present time, but, given the protocol of the experiments and the time frame of the response, it would appear that the interaction between these hormones does not necessarily engage long-term adaptive pathways involving tissues other than the kidney.
Summary and Conclusions
Regulation of the serum and tissue concentrations of inorganic phosphate requires fine control of the rates of excretion of phosphate in the urine, a process mediated by the three hormones under discussion. PTH, dopamine, and FGF-23; all acutely decrease the abundance of the major sodium-dependent phosphate transporter in the apical membrane of the proximal convoluted tubule of the kidney and inhibit the renal tubular reabsorption of phosphate (Fig. 1). The PTH receptors as well as the dopamine D1-like receptors interact with the adaptor protein NHERF-1. Although an interaction between NHERF-1 and the PTH1 receptor is clearly documented, in some species such as the mouse, the presence of NHERF-2 in the apical membrane may be sufficient to support near normal activity of this receptor (12, 27, 28). By contrast, NHERF-1 is absolutely required for signaling by dopamine D1-like receptors in the kidney (50). This is a somewhat surprising finding given that the C-terminal sequences of D1 and D5 would not necessarily predict a PDZ domain interaction. It is certainly possible that the binding of NHERF-1 to the D1-like receptors is indirect involving other proteins or represents a mode of binding not involving the PDZ domains. An initial study of FGF-23 signaling in renal tissue, on the other hand, indicates that NHERF-1 is not required.
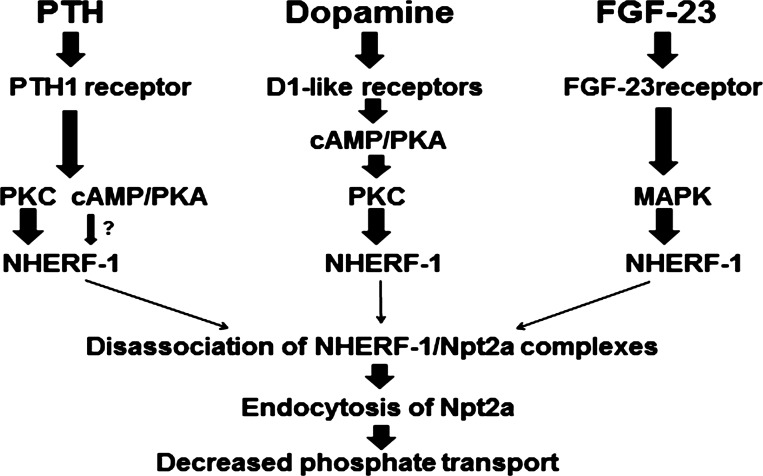
Fig. 1.
Summary of the biochemical pathways for parathyroid hormone (PTH)-, dopamine-, and FGF-23-mediated inhibition of phosphate transport in the renal proximal convoluted tubule. PTH interacts with the PTH1 receptor in renal proximal cells to activate PKC and PKA, resulting in the phosphorylation of sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) and the dissociation of the proximal tubule sodium-dependent phosphate transporter (Npt2a) from NHERF-1. The unbound Npt2a transporter then interacts with other proteins that facilitate its endocytosis from the apical membrane, resulting in decreased phosphate transport. Dopamine interacts with renal D1-like receptors to activate the cAMP/PKA pathway, which, in turn, activates PKC. Like PTH, activated PKC phosphorylates NHERF-1 and dissociates Npt2a/NHERF-1 complexes, resulting in decreased phosphate transport. FGF-23 activates MAPK and phosphorylates NHERF-1. The site-specific phosphorylation of NHERF-1 by FGF-23, however, differs from that of PTH and dopamine (Fig. 2).
NHERF-1 binds to the C terminus of Npt2a and, by mechanisms still under study, to Npt2c (41). The Npt2a/NHERF-1 protein complexes appear to represent a unique pool of Npt2a subject to acute regulation by PTH, dopamine, and FGF-23 (Fig. 2). Based on initial studies using pharmacological inhibitors of protein phosphatases and later studies using mutated forms of NHERF-1 expressed in NHERF-1 null proximal tubule cells, serine77 of the first PDZ domain was identified as the major phosphate acceptor in the PDZ I domain and a critical determinant of Npt2a binding. The phosphorylation of serine77 results in decreased binding of the transporter and dissociation of Npt2a/NHERF-1 complexes. The dissociation of Npt2a from NHERF-1, in turn, is a necessary event allowing Npt2a to be endocytosed from the apical membrane of proximal tubule cells, resulting in reduced reabsorption of filtered phosphate. Thus, from the initial observation that phosphate transport in NHERF-1 null proximal tubule cells was resistant to inhibition by PTH, we have proposed a new model whereby PTH, dopamine, and FGF-23 target the PDZ I domain of NHERF-1 to modify serine77 in the α-helix of the putative binding domain of Npt2a, causing the dissociation of Npt2a from NHERF-1. The freeing of the Npt2a transporter from NHERF-1 permits its association with other proteins that facilitate its retrieval, reduce its abundance at the apical membrane of proximal tubule cells, and reduce the renal tubular reabsorption of phosphate.
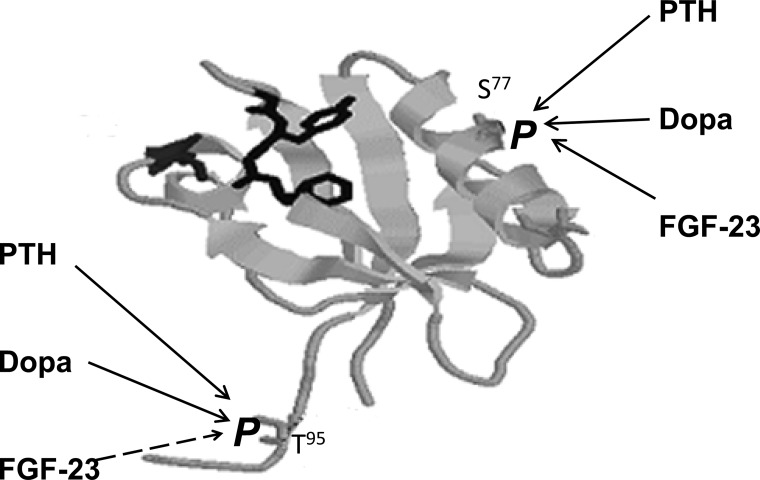
Fig. 2.
Proposed model of PTH-, dopamine (dopa)-, and FGF-23-mediated phosphorylation of serine77 (S77) and threonine95 (T95) in the first PDZ domain of NHERF-1. The PDZ I domain is represented by a ribbon diagram showing the β sheets, one of the two α-helixes, and the signature GYGF sequence (in bold). The position of S77 was determined by X-ray crystallography. The location of T95, on the other hand, is not known and is shown for illustrative purposes only. PTH, dopamine, and FGF-23 all require the phosphorylation of serine77 to inhibit phosphate transport in renal proximal convoluted tubule cells. PTH and dopamine also require the phosphorylation of threonine95 to inhibit phosphate transport, whereas FGF-23 requires that threonine95 not be phosphorylated.
Our recent studies also suggest an important role for threonine95 in the first PDZ domain of NHERF-1. Biochemical studies indicate that the phosphorylation of threonine95 of NHERF-1 affects the phosphorylation of serine77 by PKC (49). The inhibitory effect of PTH and dopamine on renal phosphate transport requires the phosphorylation of threonine95. Modification of this residue does not directly affect Npt2a binding to NHERF-1 but is required for PTH and dopamine to phosphorylate serine77. By contrast, the inhibitory effect of FGF-23 on renal phosphate transport is blocked nearly completely when threonine95 is phosphorylated. The details of the differences between the role of threonine95 modifications and the actions of PTH and dopamine compared with FGF-23 are not known, but it is of interest to speculate that threonine95 acts as an on-off switch or molecular rheostat to coordinate the effects of three hormones critically involved in phosphate homeostasis. When threonine95 is phosphorylated, the actions of PTH and dopamine would be enhanced while that of FGF-23 would be reduced. Under circumstances where threonine95 is not phosphorylated, the inhibitory effect of PTH and dopamine on phosphate transport would be blunted while that of FGF-23 would be facilitated. This postulate, however, has to be reconciled with our recent studies indicating that very low concentrations of FGF-23 and PTH synergize in renal proximal convoluted tubule cells to directly to inhibit phosphate transport. The interaction between low concentrations of PTH and FGF-23 involves the downstream signaling pathway of the PTH but not the FGF-23 receptor, which suggests a therapeutic strategy for the treatment of patients with FGF-23-mediated hypophosphatemia. Comparison of the differences between the biochemical events involved in PTH-mediated and dopamine-mediated inhibition of phosphate transport and that mediated by FGF-23 indicates a new level of complexity in the hormonal regulation of renal phosphate excretion. It would be predicted that understanding of these differences might uncover additional regulatory pathways and suggest new therapeutic strategies.
GRANTS
E. J. Weinman is supported by grants from the National Institutes of Health and the Department of Veterans Affairs. E. D. Lederer is supported by a grant from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.J.W. and E.D.L. prepared figures; E.J.W. and E.D.L. drafted manuscript; E.J.W. and E.D.L. edited and revised manuscript; E.J.W. and E.D.L. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors appreciate the helpful comments of Dr. Paul Light, University of Maryland School of Medicine.
REFERENCES
- 1. Armando I, Villar VA, Jose PA. Dopamine and renal function and blood pressure regulation. Comp Physiol 1: 1075–1117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baines AD, Drangova R. Regulation of sodium transport by endogenous dopamine production in proximal tubular and OK cells. Clin Exp Hypertens 19: 87–91, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Baines AD, Drangova R. Does dopamine use several signal pathways to inhibit Na-Pi transport in OK cells? J Am Soc Nephrol 9: 1604–1612, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Baum M, Schiavi S, Dwarakanath V, Quigley R. Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int 68: 1148–1153, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA 95: 5372–5377, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78: 179–192, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berndt TJ, MacDonald A, Walikonis R, Chinnow S, Dousa TP, Tyce GM, Knox FG. Excretion of catecholamines and metabolites in response to increased dietary phosphate intake. J Lab Clin Med 122: 80–84, 1993 [PubMed] [Google Scholar]
- 9. Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ, Kumar R. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci USA 104: 11085–11090, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biber J, Hernando N, Forster I, Murer H. Regulation of phosphate transport in proximal tubules. Pflügers Arch 458: 39–52, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Cole JA, Forte LR, Eber S, Poelling RE. Regulation of sodium-dependent phosphate transport by parathyroid hormone in opossum kidney cells: adenosine 3′,5′-monophosphate-dependent and -independent mechanisms. Endocrinology 122: 2981–2989, 1988 [DOI] [PubMed] [Google Scholar]
- 12. Cunningham R, E X, Steplock D, Shenolikar S, Weinman EJ. Defective PTH regulation of sodium-dependent phosphate transport in NHERF-1-/- renal proximal tubule cells and wild-type cells adapted to low-phosphate media. Am J Physiol Renal Physiol 289: F933–F938, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Cunningham R, Steplock D, E X, Biswas RS, Wang F, Shenolikar S, Weinman EJ. Adenoviral expression of NHERF-1 in NHERF-1 null mouse renal proximal tubule cells restores Npt2a regulation by low phosphate media and parathyroid hormone. Am J Physiol Renal Physiol 291: F896–F901, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Cunningham R, Esmaili A, Brown E, Biswas RS, Murtazina R, Donowitz M, Dijkman HB, van der Vlag J, Hogema BM, De Jonge HR, Shenolikar S, Wade JB, Weinman EJ. Urine electrolyte, mineral, and protein excretion in NHERF-2 and NHERF-1 null mice. Am J Physiol Renal Physiol 294: F1001–F1007, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Cunningham R, Biswas R, Brazie M, Steplock D, Shenolikar S, Weinman EJ. Signaling pathways utilized by PTH and dopamine to inhibit phosphate transport in mouse renal proximal tubule cells. Am J Physiol Renal Physiol 296: F355–F361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Déliot N, Hernando N, Horst-Liu Z, Gisler SM, Capuano P, Wagner CA, Bacic D, O'Brien S, Biber J, Murer H. Parathyroid hormone treatment induces dissociation of type IIa Na+-Pi cotransporter-Na+/H+ exchanger regulatory factor-1 complexes. Am J Physiol Cell Physiol 289: C159–C167, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Fouassier L, Nichols MT, Gidey E, McWilliams RR, Robin H, Finnigan C, Howell KE, Housset C, Doctor RB. Protein kinase C regulates the phosphorylation and oligomerization of ERM binding phosphoprotein 50. Exp Cell Res 306: 264–273, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Geller JL, Khosravi A, Kelly MH, Riminucci M, Adams JS, Collins MT. Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res 22: 931–937, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Greenwald I, Gross J. The effect of the administration of a potent parathyroid extract upon the excretion of nitrogen, phosphorus, calcium, and magnesium, with some remarks on the solubility of calcium phosphate in serum and on the pathogenesis of tetany. J Biol Chem 66: 217–227, 1925 [Google Scholar]
- 20. Jose PA, Eisner GM, Felder RA. Renal dopamine and sodium homeostasis. Curr Hypertens Rep 2: 174–183, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Jüppner H. Phosphate and FGF-23. Kidney Int 121: S24–S27, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaufmann M, Muff R, Stieger B, Biber J, Murer H, Fischer JA. Apical and basolateral parathyroid hormone receptors in rat renal cortical membranes. Endocrinology 134: 1173–1178, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Keusch I, Traebert M, Lötscher M, Kaissling B, Murer H, Biber J. Parathyroid hormone and dietary phosphate provoke a lysosomal routing of the proximal tubular Na/Pi-cotransporter type II. Kidney Int 54: 1224–1232, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Khundmiri SJ, Rane MJ, Lederer ED. Parathyroid hormone regulation of type II sodium-phosphate cotransporters is dependent on an A kinase anchoring protein. J Biol Chem 278: 10134–10141, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Lederer ED, McLeish KR. P2 purinoceptor stimulation attenuates PTH inhibition of phosphate uptake by a G protein-dependent mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 269: F309–F316, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Lederer ED, Sohi SS, McLeish KR. Dopamine regulates phosphate uptake by opossum kidney cells through multiple counter-regulatory receptors. J Am Soc Nephrol 9: 975–985, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Mahon MJ, Donowitz M, Yun CC, Segre GV. Na+/H+ exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signaling. Nature 417: 858–861, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Mahon MJ, Cole JA, Lederer ED, Segre GV. Na+/H+ exchanger-regulatory factor 1 mediates inhibition of phosphate transport by parathyroid hormone and second messengers by acting at multiple sites in opossum kidney cells. Mol Endocrinol 17: 2355–2364, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Murer H, Hernando N, Forster I, Biber J. Proximal tubular phosphate reabsorption: molecular mechanisms. Physiol Rev 80: 1373–1409, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Nagai S, Okazaki M, Segawa H, Bergwitz C, Dean T, Potts JT, Jr, Mahon MJ, Gardella TJ, Jüppner H. Acute down-regulation of sodium-dependent phosphate transporter NPT2a involves predominantly the cAMP/PKA pathway as revealed by signaling-selective parathyroid hormone analogs. J Biol Chem 286: 1618–1626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li J, Poulikakos PI, Dai Z, Testa JR, Callaway DJ, Bu Z. Protein kinase C phosphorylation disrupts Na+/H+ exchanger regulatory factor 1 autoinhibition and promotes cystic fibrosis transmembrane conductance regulator macromolecular assembly. J Biol Chem 282: 27086–27089, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Quarles LD. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol 8: 276–286, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samiy AH, Hirsch PF, Ramsay AG. Localization of phosphaturic effect of parathyroid hormone in nephron of dog. Am J Physiol 208: 20873–20877, 1965 [DOI] [PubMed] [Google Scholar]
- 34. Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K. Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol 20: 104–113, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shenolikar S, Weinman EJ. NHERF: targeting and trafficking membrane proteins. Am J Physiol Renal Physiol 280: F389–F395, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Shenolikar S, Minkoff CM, Steplock D, Chuckeree C, Liu MZ, Weinman EJ. N-terminal PDZ domain is required for NHERF dimerization. FEBS Lett 489: 233–236, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Shenolikar S, Voltz J, Minkoff CM, Wade J, Weinman EJ. Targeted disruption of the mouse gene encoding a PDZ domain-containing protein adaptor, NHERF-1, promotes Npt2 internalization and renal phosphate wasting. Proc Natl Acad Sci USA 99: 11470–11475, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tröhler U, Bonjour JP, Fleisch H. Inorganic phosphate homeostasis. Renal adaptation to the dietary intake in intact and thyroparathyroidectomized rats. J Clin Invest 57:264–73, 1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Traebert M, Roth J, Biber J, Murer H, Kaissling B. Internalization of proximal tubular type II Na-Pi cotransporter by PTH: immunogold electron microscopy. Am J Physiol Renal Physiol 278: F148–F154, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Traebert M, Völkl H, Biber J, Murer H, Kaissling B. Luminal and contraluminal action of 1–34 and 3–34 PTH peptides on renal type IIa Na-Pi cotransporter. Am J Physiol Renal Physiol 278: F792–F798, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Villa-Bellosta R, Barac-Nieto M, Breusegem SY, Barry NP, Levi M, Sorribas V. Interactions of the growth-related, type IIc renal sodium/phosphate cotransporter with PDZ proteins. Kidney Int 73: 456–464, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Voltz JW, Brush M, Sikes S, Steplock D, Weinman EJ, Shenolikar S. Phosphorylation of PDZ1 domain attenuates NHERF-1 binding to cellular targets. J Biol Chem 282: 33879–33887, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Wade JB, Welling P, Donowitz M, Shenolikar S, Weinman E. Differential renal distribution of NHERF isoforms and their co-localization with NHE3, Ezrin, and ROMK. Am J Physiol Cell Physiol 280: C192–C198, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Wade JB, Liu J, Coleman RA, Cunningham R, Steplock D, Lee-Kwon W, Pallone TL, Shenolikar S, Weinman EJ. Localization and interaction of NHERF isoforms in the renal proximal tubule of the mouse. Am J Physiol Cell Physiol 285: C1494–C1504, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Webster G, Leung T, Karthikeyan S, Birrane G, Ladias JA. Crystallographic characterization of the PDZ1 domain of the human Na+/H+ exchanger regulatory factor. Acta Crystallogr D Biol Crystallogr 57: 714–716, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Weinman E, Steplock D, Wang Y, Shenolikar S. Characterization of a protein co-factor that mediates protein kinase A regulation of the renal brush border membrane Na+-H+ exchanger. J Clin Invest 95: 2143–2149, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weinman EJ, Biswas RS, Peng Q, Shen L, Turner CL, E X, Steplock D, Shenolikar S, Cunningham R. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor-1. J Clin Invest 117: 3412–3420, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48. Weinman EJ, Steplock D, Cha B, Kovbasnjul O, Frost NA, Cunningham R, Shenolikar S, Blanpied TA, Donowitz M. PTH transiently increases the percent mobile fraction of Npt2a in OK cells as determined by FRAP. Am J Physiol Renal Physiol 297: F1560–F1565, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weinman EJ, Steplock D, Zhang Y, Biswas R, Bloch RJ, Shenolikar S. Cooperativity between the phosphorylation of Thr95 and Ser77 of NHERF-1 in the hormonal regulation of renal phosphate transport. J Biol Chem 285: 25134–25138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weinman EJ, Biswas R, Steplock D, Douglass TS, Cunningham R, Shenolikar S. Sodium-hydrogen exchanger regulatory factor 1 (NHERF-1) transduces signals that mediate dopamine inhibition of sodium-phosphate co-transport in mouse kidney. J Biol Chem 285: 13454–13460, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weinman EJ, Steplock D, Shenolikar S, Blanpied TA. Dynamics of PTH-induced disassembly of Npt2a/NHERF-1 complexes in living OK cells. Am J Physiol Renal Physiol 300: F231–F235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weinman EJ, Steplock D, Shenolikar S, Biswas R. Fibroblast growth factor-23-mediated inhibition of renal phosphate transport in mice requires sodium-hydrogen exchanger regulatory factor-1 (NHERF-1) and synergizes with parathyroid hormone. J Biol Chem 286: 37216–37221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weinman EJ, Biswas R, Steplock D, Wang P, Lau YS, Desir GV, Shenolikar S. Increased renal dopamine and the acute renal adaptation to a high phosphate diet. Am J Physiol Renal Physiol 300: F1123–F1129, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weinman EJ, Lederer ED. PTH-mediated inhibition of the renal transport of phosphate. Exp Cell Res 318: 1027–1032, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wheeler D, Garrido JL, Bisello A, Kim YK, Friedman PA, Romero G. Regulation of parathyroid hormone type 1 receptor dynamics, traffic, and signaling by the Na+/H+ exchanger regulatory factor-1 in rat osteosarcoma ROS 17/2.8 cells. Mol Endocrinol 22:1163–1170, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yamashita T, Konishi M, Miyake A, Inui K, Itoh N. Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem 277: 28265–28270, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse C, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA 94: 3010–3015, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]