Abstract
Interstitial cystitis/painful bladder syndrome is a chronic bladder inflammatory disease of unknown etiology that is often regarded as a neurogenic cystitis. Interstitial cystitis is associated with urothelial lesions, voiding dysfunction, and pain in the pelvic/perineal area. In this study, we used a murine neurogenic cystitis model to identify genes participating in the development of pelvic pain. Neurogenic cystitis was induced by the injection of Bartha's strain of pseudorabies virus (PRV) into the abductor caudalis dorsalis (tail base) muscle of female C57BL/6J mice. Mice infected with PRV developed progressive pelvic pain. The sacral spinal cord was harvested on postinfection days (PID) 2 and 4, and gene expression was analyzed by microarrays and confirmed by quantitative RT-PCR. On PID 2, the overall expression profile was similar to that of uninfected sacral spinal cord; by PID 4, there were substantial differences in expression of multiple functional classes of genes, especially inflammation. Analysis of pain-signaling pathways at the dorsal horn suggested that Ca2+/calmodulin-dependent protein kinase II (CaMKII) contributes to neurogenic cystitis pelvic pain. Consistent with this, CaMKIIδ expression exhibited a mast cell-dependent increase in the sacral spinal cord at the mRNA level, and phospho-CaMKII immunoreactivity in the dorsal horn was increased on postinfection day (PID) 4 during PRV infection. Finally, intrathecal injection of the CaMKII inhibitor KN-93 attenuated the PRV pain response. These data suggest that CaMKII plays a functional role in pelvic pain due to neurogenic cystitis.
Keywords: transcriptome, pseudorabies virus
interstitial cystitis (ic) is a chronic bladder inflammatory disease with unknown etiology that afflicts as many as 1 million patients in the United States, with women comprising ∼90% of patients. IC is associated with severe pelvic pain and voiding dysfunction that includes urinary frequency, urgency, and nocturia (15, 31). IC is often considered a neurogenic cystitis due to both voiding dysfunction and the partial efficacy of sacral nerve stimulation or neuropharmacological therapies in some patients, which suggests a neural component (reviewed in Ref. 11). Supporting this idea, cats are susceptible to feline IC, a disease that closely mimics human IC and is associated with increased activity in the sympathetic nervous system (32).
A longstanding model of IC pathogenesis involves a positive feedback loop, whereby substance P-containing peripheral nerves stimulate mast cells, in turn releasing inflammatory mediators that induce urothelial inflammation (10). In this model, histamine released by mast cells feeds back onto peripheral nerves to cause sustained release of substance P and mast cell activation. Consistent with this model, patients with IC show elevated mast cell counts in the bladder lamina propria and increased levels of urinary histamine metabolites, and lamina propria mast cells are correlated with IC symptoms, supporting a role for mast cells in at least a subset of IC patients (1, 2, 9, 20, 30).
The precise mechanisms underlying pelvic pain in IC remain unclear, but recent studies using rodent models of neurogenic cystitis model are identifying mechanisms of bladder pathogenesis that may contribute to pelvic pain. Infection of rats with the attenuated Bartha's strain of pseudorabies virus (PRV) at the tail base muscle was shown to induce a cystitis accompanied by mast cell activation (13, 14). PRV-induced cystitis was not recapitulated by virus injection into the bladder, but was attenuated by either resection of bladder innervation or ablation of Barrington's nucleus, a brain center of bladder control, thus demonstrating that PRV induces a centrally mediated, neurogenic cystitis. In mice, PRV inoculation at the tail base muscle was found to promote mast cell trafficking to the bladder lamina propria and bladder pathology driven by mast cell production of tumor necrosis factor-α (3–5). PRV also induced allodynia specific to the pelvic region that was subject to colonic modulation, reminiscent of IC pelvic pain and exquisite sensitivity of many IC patients to specific dietary constituents (17, 18, 25, 27). Although pelvic allodynia was dependent on mast cells similar to PRV-induced pathology, allodynia was strictly dependent on histamine and interactions with histamine receptors H1R and H2R (24, 26). Together, these studies identify peripheral mechanisms contributing to pelvic pain in a neurogenic cystitis model that mimics findings in IC.
Here, we examined central events by quantifying PRV-induced transcriptional changes in the mouse sacral spinal cord, the site of integration for many pelvic inputs (31). Modeling of transcriptional responses suggested a role for Ca2+/calmodulin kinase II (CaMKII), and the δ-isoform was specifically induced by PRV. CaMKII phosphorylation was elevated in the sacral spinal cord dorsal horn, and a CaMKII inhibitor attenuated pelvic allodynia, suggesting a functional role for CaMKIIδ in neurogenic cystitis pain.
MATERIALS AND METHODS
Animals.
Adult female C57BL/6J mice (10–12 wk old) were purchased from Jackson Laboratory (Bar Harbor, ME). Mast cell-deficient KitW-sh/KitW-sh mice on the C57BL/6J that were originally purchased from Jackson Laboratory were bred at Northwestern. All experiments were performed using protocols approved by Northwestern University Animal Care and Use Committee. Mice were housed in containment facilities of the Center for Comparative Medicine and maintained on a regular 12:12-h light-dark cycle with food and water.
Induction of neurogenic cystitis.
Neurogenic cystitis was induced by injection of 2.3 × 106 plaque-forming units of Bartha's PRV through the skin of isoflurane-anesthetized mice into the abductor caudalis dorsalis muscle using a 26-gauge Hamilton syringe. Ultraviolet-irradiated/heat-inactivated PRV stocks were employed as negative control (sham) inoculum in sham-treated mice, as previously reported (3).
Behavioral testing.
Mice were tested before PRV infection (baseline, PID 0), and on PID 1, 2, 3, and 4. Pelvic hyperalgesia and allodynia were quantified using von Frey filaments applied to the abdomen (19). Mice were tested in individual Plexiglas chambers (6 cm × 10 cm × 12 cm) with a stainless steel wire grid floor (mouse acclimation period of ∼10 min before testing). Frequency of withdrawal responses to the application of von Frey filaments to the abdomen was tested using five individual fibers with forces of 0.04, 0.16, 0.4, 1, and 4 g (Stoelting, Kiel, WI). Each filament was applied for ∼1 s with an interstimulus interval of 2–5 s for a total 10 times, and the filaments were tested in ascending order of force. Stimulation was confined to the lower abdominal area in the general vicinity of the bladder, and care was taken to stimulate different areas within this region to avoid desensitization or “wind up” effects. Three types of behaviors were considered as positive responses to pelvic stimulation: 1) sharp retraction of the abdomen; 2) immediate licking or scratching of the area of filament stimulation; or 3) jumping.
RNA preparation and microarrays.
The sacral spinal cord was dissected from mice immediately following euthanasia by cervical dislocation and stored at −80°C. All tissues were homogenized in ice-cold TRIzol with a homogenizer, and total RNA was purified according to manufacturer's instructions (Invitrogen, Carlsbad, CA). Gene expression was quantified using Affymetrix Mouse Genome 430 2.0 arrays that contain 45,101 probes and measure the expression level of 20,022 unique NCBI Entrez-identified genes. The data sets were preprocessed with normalization, variance stabilization, and log2 transformation. Student's t-tests were used to identify genes significantly differentially expressed (P < 0.05 and 2-fold) between PID 0, 2, and 4. Hierarchical clustering of those genes differentially expressed between groups was performed using BRB-Array Tools version 4.1 (Molecular Statistics and Bioinformatics Section, National Cancer Institute, Bethesda, MD) developed by Dr. R. Simon and A. Peng (http://linus.nci.nih.gov/BRB-ArrayTools.html). Average difference values were normalized to median over the arrays. The data were filtered so that only those genes that were adequately measured on 75% of the arrays were included. A class comparison protocol was used to identify genes whose degree of expression differed significantly by twofold or more among the three groups. To visualize whole genome expression level by function and pathway, the microarray data were analyzed with Ingenuity Systems Pathway Analysis (IPA; Ingenuity Systems, Redwood City, CA). IPA analysis identified canonical pathways differentially expressed (P < 0.05) between PID 0, 2, and 4.
Real-time RT-PCR.
To confirm the microarray results, the relative expression of eight inflammatory- and chemokine-associated genes was measured by real-time RT-PCR. RNA was reverse transcribed using an RT2 First Strand Kit (SABiosciences, Frederick, MD), according to manufacturer's directions. Quantitative real-time PCR analysis was performed using RT2 qPCR Mastermix (SABiosciences) in a MJ Research Chromo 4 thermocycler. The levels of mRNA were normalized to ribosomal protein L19 mRNA levels.
Immunohistochemistry and image analysis.
All mice were anesthetized with isoflurane and perfused with 4% paraformaldehyde in 1× phosphate-buffered saline (pH 7.4). Sacral spinal cords were rapidly dissected and postfixed in the same fixative overnight at 4°C. The tissues were soaked serially in 10% and 20% sucrose in phosphate-buffered saline for 1 h and 30% sucrose overnight, then frozen-sectioned on a sliding microtome at 5–10 μm. After blocking with 10% normal goat serum, sections were incubated with anti-phospho-CaMKII (sc-12886-R, Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Sections were then incubated with FITC-labeled goat anti-rabbit antibody (DAKO, Carpinteria, CA) for 1 h. Images were acquired using an epifluorescence and quantified using Volocity software (PerkinElmer, Waltham, MA).
Intrathecal injection of drugs.
Drug administration was performed in a volume of 5 μl by a 30-gauge needle connected to a 25-μl Hamilton syringe through an intervertebral space between L5 and L6, as described previously (12). Success of the intrathecal (IT) injection was verified by a lateral tail-flick. Mice were administered with KN-93 (15–45 nmol) or KN-92 (30 nmol) 1 h before allodynia testing on PID 4.
Statistical analysis.
Results were expressed as means ± SE and analyzed for statistical significance by the ANOVA, followed by a post hoc test comparison using Dunnet's multiple comparison, using the GraphPad Prism (GraphpPad, San Diego, CA). A value of P < 0.05 was considered statistically significant.
RESULTS
PRV induces pelvic pain.
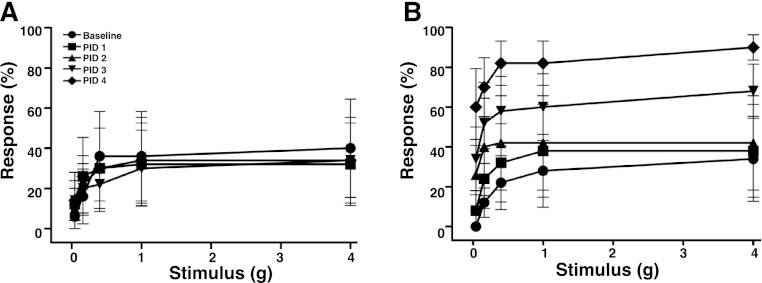
Pain originating from a visceral organ is typically referred to a corresponding “dermatome” on the skin that shares spinal innervations with the organ (28). Therefore, to quantify bladder-associated pelvic pain, we assessed allodynia of the pelvic region in response to mechanical stimulation with von Frey filaments. Stimulation of the pelvic area of female C57BL/6J (B6) mice evoked baseline responses (pelvic withdrawal, jumping, or pelvic licking/scratching), where the percentage of responses increased as a function of force applied using graded filaments (Fig. 1A). Following PRV infection, mice exhibited a progressively enhanced sensitivity to pelvic stimulation that became significant by PID 2, 3, and 4 (Fig. 1B). This allodynia is consistent with the development of pelvic pain specifically in response to active PRV, as our laboratory has shown previously (24–26).
Fig. 1.
Pseudorabies virus (PRV) induces pelvic pain in female mice. Pelvic allodynia was quantified with von Frey filaments. Responses to pelvic stimulus are shown for C57BL/6J mice infected with inactivated (sham) PRV (A) or active PRV (B) (n = 5 mice/group). Data represent mean ± SE response to 10 successive applications for each fiber. ANOVA analysis shows a significant increase in responses at all filaments tested in PRV-infected mice at postinfection days (PID) 2, 3, and 4 relative to baseline (P < 0.05).
Gene expression profile of sacral spinal cord after PRV infection.
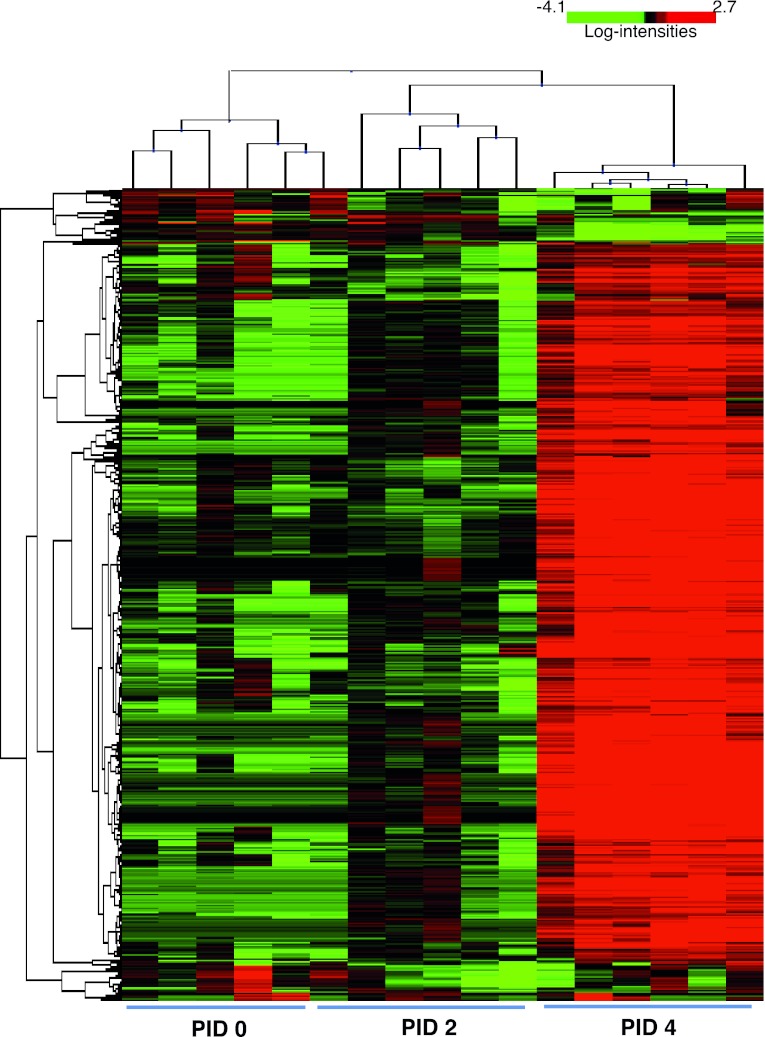
To identify molecular mediators of PRV-induced pelvic pain, we quantified transcriptional changes in the sacral spinal cord. RNA was purified from spinal cords from mice at PID 0, 2, and 4. Affymetrix mouse genome arrays were used to quantify the message level of 20,022 unique genes. After array normalization and initial expression filtering, 593 genes were identified level as being differently expressed among PID 0, 2, or 4 using BRB Array Tools version 4.1 (P < 0.05). The global changes in sacral spinal cord expression in response to PRV infection were identified by the hierarchical clustering of the variance-normalized expression profiles (Fig. 2). The expression profile on PID 2 was similar to that of PID 0, whereas, on PID 4, there were substantial differences in gene expression. IPA identified a significant increase of several intracellular signaling pathways that are important for antiviral responses and inflammation. The five most significant canonical pathways are shown in Table 1 and encompass responses characteristic of both chronic diseases and acute infection, including pattern recognition receptors and systemic lupus erythematous. Thus PRV infection induces a robust transcriptional response in the sacral spinal cord during the development of pelvic pain.
Fig. 2.
Hierarchical cluster analysis of the expression levels of 593 genes differentially expressed at PID 0, PID 2, and PID 4. Clustering was done with the BRB-Array Tools by sample and by gene with the Complete Linkage Algorithm. Genes are represented horizontally, and the individual sacral spinal cord samples are represented vertically. Colors indicate expression level. Genes expressed above average are represented in red; genes expressed below average are shown in green. The degree of red or green intensity indicates the degree of up- or downregulation relative to the average level of expression across all samples.
Table 1.
Five most-significant Ingenuity Pathways Analyses canonical pathways induced by pseudorabies virus
| Pathway | Ratio | P Value |
|---|---|---|
| Role of pattern recognition receptors in recognition of bacteria and viruses | 0.421 (16/38) | 6.45E-14 |
| Cross talk between dendritic cells and natural killer cells | 0.4 (16/40) | 1.71E-13 |
| Systemic lupus erythematosis signaling | 0.292 (19/65) | 6.54E-13 |
| Dendritic cell maturation | 0.253 (21/83) | 8.91E-13 |
| Type 1 diabetes mellitus signaling | 0.273 (18/66) | 9.95E-12 |
Pathways are associated where constituent genes exhibited expression elevated at postinfection day (PID) 4 relative to PID 0. Ratio is the number of genes expressed in the pathway divided by the total number of genes in the pathway.
Validation of microarray findings by real-time PCR.
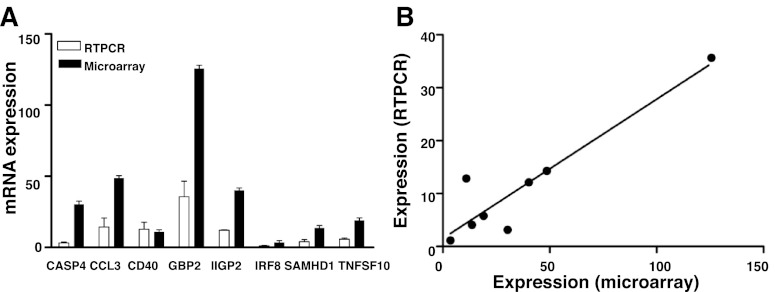
To confirm our microarray findings, we performed quantitative RT-PCR analysis on sacral spinal cord from PID 0, 2, and 4. Since inflammatory responses were highly induced, we chose eight inflammation-associated genes as targets for validation that represented a wide range of response, from 4-fold to 125-fold induction. Like the microarray studies, RT-PCR analyses confirmed that each of the eight validation targets was induced (Fig. 3A). Moreover, RT-PCR and microarray data were significantly correlated (Fig. 3B, R2 = 0.8649), suggesting that microarray data are appropriate for modeling using pathway analysis tools.
Fig. 3.
Quantitative RT-PCR (qRT-PCR) confirms microarray findings. A: the ratio of normalized transcription level on PID 4 relative to PID 0, of select genes identified as differentially expressed by microarray, were validated by quantitative RT-PCR. These genes were caspase 4 (CASP4), chemokine (C-C motif) ligand 3 (CCL3), CD40 antigen (CD40), guanylate nucleotide binding protein 2 (GBP2), interferon inducible GTPase 2 (IIGP2), interferon regulatory factor 8 (IRF8), SAM domain and HD domain 1 (SAMHD1), and tumor necrosis factor (ligand) superfamily, member 10 (TNFSF10). For each gene in the qRT-PCR assay, the three biological replicates were analyzed in duplicates and normalized to the L19 mean value. Shown are means ± SE. B: scatter plot representation of the ratio of qRT-PCR data to microarray data (R2 = 0.86).
Mast cell-dependent induction of CaMKII.
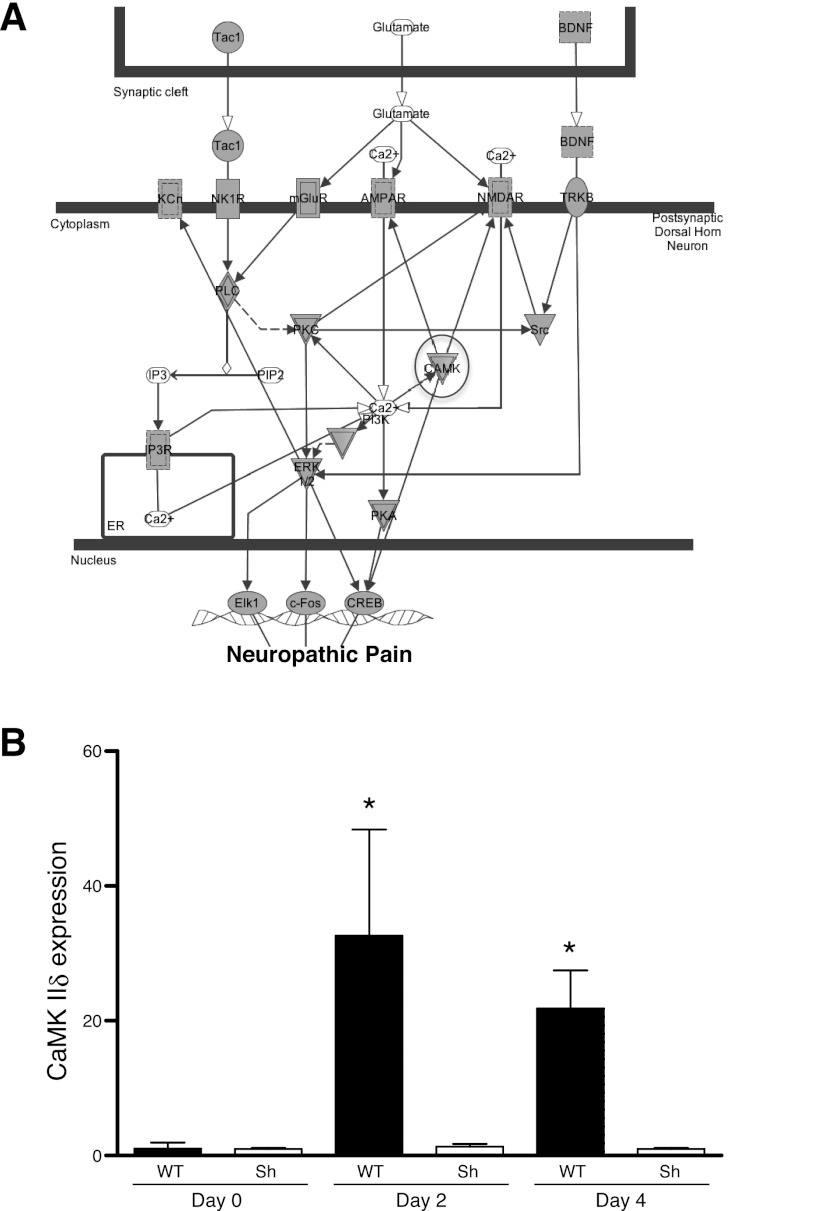
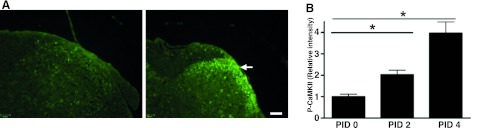
Pelvic allodynia occurred at PID 2, yet major changes in gene expression were largely observed at PID 4 (Figs. 1–3). This suggested that subtle changes in gene expression mediating pain responses occurred by PID 2. IPA modeling of dorsal horn neuropathic pain signaling pathways in PID 2 and 4 identified a central role for CaMKII (Fig. 4A). CaMKII belongs to a family of multifunctional serine/threonine kinases activated in response to increases in intracellular calcium (reviewed in Ref. 34). There are four CaMKII genes, α, β, γ, and δ, and each gene yields several isoforms through alternative splicing. CaMKII isoforms assemble into homo- or heteromultimeric holoenzymes composed of 8–12 subunits. The binding of Ca2+/calmodulin to its regulatory domain releases its autoinhibitory effect and activates the kinase. The activated CaMKII then undergoes autophosphorylation at a highly conserved threonine residue to render the kinase constitutively active. The microarray data showed that CaMKIIδ mRNA increased during PRV infection, whereas other isoforms remained constant; therefore, we focused on CaMKIIδ and confirmed expression by quantitative RT-PCR (Fig. 4B). CaMKIIδ expression induced a 32.6-fold increase in sacral spinal cord at PID 2 and a 21.8-fold increase at PID 4. This was consistent with the microarray data, where CaMKIIδ was increased 1.7-fold at PID 2 and 4.4-fold at PID 4. Since we previously found that mast cells mediate allodynia in PRV cystitis (24), we examined CaMKIIδ expression in mast cell-deficient KitW-sh/KitW-sh “sash” mice during PRV infection. In contrast to wild-type mice, PRV did not induce CaMKIIδ expression in mast cell-deficient sash mice, suggesting that CaMKIIδ induction is a response to peripheral events that depend on mast cells, rather than a direct effect of PRV on CaMKIIδ gene expression within the central nervous system. Phosphorylated CaMKII was then examined by immunohistochemistry to evaluate potential activation of this pathway by PRV. Phospho-CaMKII immunoreactivity was localized to the superficial dorsal horn, and quantitative analyses indicated that phospho-CaMKII accumulated from PID 0 to PID 4 (Fig. 5). Thus the increase in CaMKII in RNA and CaMKII phosphorylation in the dorsal horn suggest a potential role for CaMKII in pain, as predicted by IPA, and the absence of CaMKII induction in mast cell-deficient mice supports a requirement for peripheral events in allodynia of neurogenic cystitis.
Fig. 4.
PRV induces Ca2+/calmodulin-dependent protein kinase II (CaMKII) δ transcription in sacral spinal cord. A: Ingenuity Pathways Analyses identifies CaMKII as a candidate regulatory node in neurogenic cystitis pain. Genes that met the twofold change threshold and were mapped in the Ingenuity Pathways Knowledge are circled. Genes that were not mapped in the Ingenuity Pathways Knowledge are represented by white nodes. Nodes are displayed with various shapes that represent the functional class of the gene product. Nodes with double edges represent a group or a complex. Tac1, tachykinin 1; BDNF, brain derived neurotrophic factor; TRKB, tyrosine kinase receptor B; NMDAR, N-methyl-d-aspartate receptor; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor; mGluR, metabotropic glutamate receptor; NK1R, neurokinin 1 receptor; CAMK, Ca2+/calmodulin-dependent protein kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; PI3K, phosphatidylinositol 3-kinase; ER, estrogen receptor; CREB, cAMP response element binding protein. B: CaMKIIδ mRNA expression level increased in sacral spinal cord of B6 mice [wild type (WT)] at PIDs 2 and 4, compared with PID 0 (n = 4–6). *P < 0.05. CaMKIIδ mRNA expression level was not significantly increased in sacral spinal cord of mast cell-deficient C57BL6/J Wsh/Wsh mice (Sh) at PIDs 2 or 4, compared with PID 0 (n = 4–6, P > 0.05). Values are means ± SE.
Fig. 5.
CaMKII is induced during the neurogenic cystitis. Immunostaining of sacral spinal cord before and after PRV infection is shown. A: transverse sections from PID 0 (left) and PID 4 (right) were stained with anti-phopho-CaMKII (P-CaMKII) antibody. The arrow indicates P-CaMKII-positive profiles located in dorsal horn. Scale bar, 100 μm. B: immunostaining pixel intensity was measured by Volocity software (n = 20 slices/5 mice). Values are means ± SE. *P < 0.05.
CaMKII contributes to pelvic pain of neurogenic cystitis.
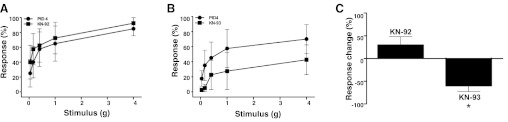
We sought to determine whether CaMKII plays a functional role in pelvic allodynia induced by PRV. PRV-infected C57BL/6 female mice were treated by IT administration of a selective CaMKII inhibitor, KN-93, that competes with Ca2+/calmodulin for binding to CaMKII (29). KN-93 blocks activity of all CaMKII isoforms and has been shown previously to attenuate allodynia in mouse and rat pain models (7, 8). To determine whether PRV-induced visceral pain is similarly responsive to KN-93 as models employed in prior studies, we initially performed a dose-response experiment in the PRV model. Similar to previous studies (6), we observed the greatest attenuation of allodynia at 30 μM (Table 2). Sham-treated mice were administered the inactive control compound KN-92, and KN-92-treated mice showed no significant change in allodynia. In contrast, mice treated with KN-93 exhibited significantly decreased pain responses 1 h after treatment (Fig. 6). These data demonstrate that pelvic pain of neurogenic cystitis can be significantly attenuated by a CaMKII antagonist.
Table 2.
Dose-response evaluation of intrathecal KN-93 in visceral pain
| KN-93 Concentration, μM | Inhibition of Allodynia, % | P Value |
|---|---|---|
| 15 | 21.59 ± 1.9 | |
| 30 | 59.27 ± 10.04 | <0.01 |
| 45 | 35.45 ± 10.34 | <0.05 |
Values are means ± SE. Inhibition is expressed as ratio of allodynia following KN-93 to allodynia preceding treatment at day 4 following pseudorabies virus.
Fig. 6.
CaMKIIδ antagonist reduces pelvic allodynia. Mice were infected with PRV and then treated intrathecally with KN-92 (A) or KN-93 (B) (n = 6 mice). Pelvic allodynia was assessed before and 1 h after intrathecal treatment. C: mice treated with KN-93 exhibited significantly diminished pelvic allodynia relative to mice treated with KN-92. Values are means ± SE. *P < 0.05.
DISCUSSION
The purpose of the present study was to identify genes underlying pain in a murine model of IC induced by PRV Bartha. IPA analysis identified a significant increase of several pathways important for the microbial responses and inflammation (e.g., role of pattern recognition receptors in recognition of bacteria and viruses, cross talk between dendritic cells and natural killer cells, and dendritic cell maturation). This is in agreement with studies of rat brains infected with PRV 152, a derivative of PRV Bartha, that revealed the host transcriptional response after 96 h was dominated by induction of immune and proinflammatory genes (23). In addition, many immune response genes are upregulated in pig brain naturally infected with PRV (33). Here, we extend these observations by highlighting a potential signaling pathway mediating pain during PRV infection.
IPA analysis identified a potential candidate gene in neurogenic cystitis pain, CaMKII. Compared with PID 0, a significant increase of phospho-CaMKII immunoreactivity was observed in the superficial dorsal horn of the sacral spinal cord after PRV infection. The increase of phospho-CaMKII immunoreactivity appeared on PID 2, coupled with accumulation of CaMKII mRNA. Since phospho-CaMKII may activate its own transcription and translation, it may act in a positive-feedback loop in the dorsal horn during neurogenic cystitis (16, 21). Consistent with this, we note that Yuan and colleagues identified that CaMKII was upregulated, along with calcium signaling pathways, in PRV-infected pig brain and lung (33). Thus CaMKII is a potential diagnostic marker and therapeutic target for pelvic pain and other chronic pain syndromes.
The periphery may play a key role in the central pathways mediating pain during PRV infection. Our laboratory previously demonstrated that bladder mast cells mediate both pain and bladder pathology in neurogenic cystitis (3, 18, 24–26). Apoptotic lesions of the bladder urothelium were abrogated in mast cell-deficient mice, and inhibiting bladder mast cell trafficking with antibodies against the mast cell chemokine RANTES (regulated upon activation normal T-cell expressed and secreted) attenuated urothelial apoptosis and stabilized transepithelial resistance characteristic of intact urothelium (4, 5). However, pelvic allodynia associated with PRV proved independent of the TNF-mediated bladder pathology and was instead dependent on mast cell histamine, demonstrating a requirement for peripheral inputs in pelvic pain during PRV-induced neurogenic cystitis (24, 26). CaMKII mRNA accumulation peaks at PID 2 and precedes accumulation of phospho-CaMKII immunoreactivity at PID 4, whereas mast activation and cell trafficking are detectable at PID 2 and complete by PID 3. Since PRV-induced allodynia is progressive, these differential kinetics suggest that CaMKII activation in the dorsal horn is a response to mast cell activation in the periphery. Indeed, mast cell-deficient mice displayed impaired CaMKII mRNA induction, suggesting CaMKII activation results from peripheral inputs. Together, these findings clarify the sequence of events in neurogenic cystitis and suggest that central activation of bladder efferents leads to bladder mast cell activation through neurokinin receptor 1 (26). Mast cell activation and secretion of histamine, in turn, leads to excitation of bladder sensory afferents through histamine receptors H1R and H2R (24, 26) and, subsequently, to central induction of CaMKII as a result of the mast cell-dependent peripheral signal. Our laboratory previously reported that PRV allodynia was modulated by the colon (25). Since PRV cystitis is not associated with colonic pathology (25), this colonic modulation is likely due to the effects of summation mechanisms on the convergence of gastrointestinal inputs with bladder inputs within the spinal cord (17, 18). Thus central activation of bladder mast cells triggers both bladder pathology and pelvic pain. Additional experiments are required to determine whether colonic modulation of pain is associated with corresponding modulation of CaMKII and whether CaMKII is differentially involved in the establishment and/or maintenance of pain.
Since PRV infection induced expression and phosphorylation of CaMKII in spinal cord, and IPA analysis indicated a potential role of CaMKII in dorsal horn pain signaling, we examined the functional role of CaMKII in pelvic pain. KN-93, a specific CaMKII inhibitor, competitively blocks Ca2+/calmodulin binding to the CaMKII, and thus prevents activation. KN-93 has been shown previously to attenuate allodynia in a rat model of mononeuropathy induced by the chronic constriction injury of the sciatic nerve (8). Similarly, KN-93 attenuates allodynia in a mouse pain model induced by subcutaneous formalin injection into the plantar surface of the hind paw (7). PRV-infected mice treated with IT KN-93 exhibited significantly decreased pelvic allodynia, suggesting that CaMKII contributes to initiation and/or maintenance of pelvic pain of PRV neurogenic cystitis. Therefore, CaMKII is central to pain responses in multiple and diverse pain models and thus represents a therapeutic target. Indeed, trifluoperazine is an orally available antipsychotic drug that inhibits CaMKII. Trifluoperazine has recently been shown to reverse mechanical allodynia and thermal hyperalgesia induced in spinal nerve ligation and complete Freund's adjuvant pain models (6, 22). This raises the possibility of targeting CaMKII at the dorsal horn with the convenience of oral therapies for pain.
In summary, our findings suggest that activation of CaMKII in sacral spinal cord plays an important role in the process of generating and maintaining pelvic pain of neurogenic cystitis. Specific inhibitors of CaMKII are potential therapeutic agents for the treatment of neuropathic pain, including IC.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases award DK066112 (D.J. Klumpp).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
W.Y., C.N.R., E.H., and D.J.K. conception and design of research; W.Y., C.N.R., and E.H. performed experiments; W.Y., C.N.R., E.H., J.D.D., and D.J.K. analyzed data; W.Y., C.N.R., E.H., S.A.A., and D.J.K. interpreted results of experiments; W.Y. and D.J.K. prepared figures; W.Y. drafted manuscript; W.Y., S.A.A., J.D.D., and D.J.K. edited and revised manuscript; W.Y., C.N.R., E.H., S.A.A., J.D.D., and D.J.K. approved final version of manuscript.
REFERENCES
- 1. Aldenborg F, Fall M, Enerback L. Mast cells in interstitial cystitis. Ann Urol (Paris) 23: 165–166, 1989 [PubMed] [Google Scholar]
- 2. Boucher W, el-Mansoury M, Pang X, Sant GR, Theoharides TC. Elevated mast cell tryptase in the urine of patients with interstitial cystitis. Br J Urol 76: 94–100, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Chen MC, Blunt LW, Pins MR, Klumpp DJ. Tumor necrosis factor promotes differential trafficking of bladder mast cells in neurogenic cystitis. J Urol 175: 754–759, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Chen MC, Keshavan P, Gregory GD, Klumpp DJ. RANTES mediates TNF-dependent lamina propria mast cell accumulation and barrier dysfunction in neurogenic cystitis. Am J Physiol Renal Physiol 292: F1372–F1379, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Chen MC, Mudge CS, Klumpp DJ. Urothelial lesion formation is mediated by TNFR1 during neurogenic cystitis. Am J Physiol Renal Physiol 291: F741–F749, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Chen Y, Luo F, Yang C, Kirkmire CM, Wang ZJ. Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice. J Pharmacol Exp Ther 330: 650–659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi SS, Seo YJ, Shim EJ, Kwon MS, Lee JY, Ham YO, Suh HW. Involvement of phosphorylated Ca2+/calmodulin-dependent protein kinase II and phosphorylated extracellular signal-regulated protein in the mouse formalin pain model. Brain Res 1108: 28–38, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dai Y, Wang H, Ogawa A, Yamanaka H, Obata K, Tokunaga A, Noguchi K. Ca2+/calmodulin-dependent protein kinase II in the spinal cord contributes to neuropathic pain in a rat model of mononeuropathy. Eur J Neurosci 21: 2467–2474, 2005 [DOI] [PubMed] [Google Scholar]
- 9. el-Mansoury M, Boucher W, Sant GR, Theoharides TC. Increased urine histamine and methylhistamine in interstitial cystitis. J Urol 152: 350–353, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Elbadawi A. Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology 49: 14–40, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Hanno P. Bladder pain syndrome (interstitial cystitis) and related disorders. In: Campbell-Walsh Urology (10th ed.), edited by Wein A, Kavoussi L, Novick A, Partin A, Peters C. Philadelphia, PA: Elsevier, 2012, p. 357–401 [Google Scholar]
- 12. Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 67: 313–316, 1980 [DOI] [PubMed] [Google Scholar]
- 13. Jasmin L, Janni G, Manz HJ, Rabkin SD. Activation of CNS circuits producing a neurogenic cystitis: evidence for centrally induced peripheral inflammation. J Neurosci 18: 10016–10029, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jasmin L, Janni G, Ohara PT, Rabkin SD. CNS induced neurogenic cystitis is associated with bladder mast cell degranulation in the rat. J Urol 164: 852–855, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology 49: 2–9, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Kennedy MB, Bennett MK, Bulleit RF, Erondu NE, Jennings VR, Miller SG, Molloy SS, Patton BL, Schenker LJ. Structure and regulation of type II calcium/calmodulin-dependent protein kinase in central nervous system neurons. Cold Spring Harb Symp Quant Biol 55: 101–110, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Klumpp DJ, Rudick CN. Dietary sensitivity of interstitial cystitis: bane and opportunity. Eur Urol 4: 54–56, 2009 [Google Scholar]
- 18. Klumpp DJ, Rudick CN. Summation model of pelvic pain in interstitial cystitis. Nat Clin Pract Urol 5: 494–500, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 92: 335–342, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Leiby BE, Landis JR, Propert KJ, Tomaszewski JE. Discovery of morphological subgroups that correlate with severity of symptoms in interstitial cystitis: a proposed biopsy classification system. J Urol 177: 142–148, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Lisman J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci 17: 406–412, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Luo F, Yang C, Chen Y, Shukla P, Tang L, Wang LX, Wang ZJ. Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther 325: 267–275, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Paulus C, Sollars PJ, Pickard GE, Enquist LW. Transcriptome signature of virulent and attenuated pseudorabies virus-infected rodent brain. J Virol 80: 1773–1786, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ. Mast cell-derived histamine mediates cystitis pain. PLoS One 3: e2096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rudick CN, Chen MC, Mongiu AK, Klumpp DJ. Organ crosstalk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol 293: R1191–R1198, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Rudick CN, Schaeffer AJ, Klumpp DJ. Pharmacologic attenuation of pelvic pain in a murine model of interstitial cystitis. BMC Urol 9: 16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shorter B, Lesser M, Moldwin RM, Kushner L. Effect of comestibles on symptoms of interstitial cystitis. J Urol 178: 145–152, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Sturge WA. The phenomena of angina pectoris, and their bearing upon the theory of counter-irritation. Brain 5: 492–510, 1883 [Google Scholar]
- 29. Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun 181: 968–975, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Tomaszewski JE, Landis JR, Russack V, Williams TM, Wang LP, Hardy C, Brensinger C, Matthews YL, Abele ST, Kusek JW, Nyberg LM. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology 57: 67–81, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Wesselmann U. Neurogenic inflammation and chronic pelvic pain. World J Urol 19: 180–185, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Westropp JL, Buffington CA. In vivo models of interstitial cystitis. J Urol 167: 694–702, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Yuan JF, Zhang SJ, Jafer O, Furlong RA, Chausiaux OE, Sargent CA, Zhang GH, Affara NA. Global transcriptional response of pig brain and lung to natural infection by Pseudorabies virus. BMC Microbiol 9: 246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res 63: 476–486, 2004 [DOI] [PubMed] [Google Scholar]