Abstract
In response to volume expansion, locally generated dopamine decreases proximal tubule reabsorption by reducing both Na/H-exchanger 3 (NHE3) and Na-K-ATPase activity. We have previously demonstrated that mouse proximal tubules in vitro respond to changes in luminal flow with proportional changes in Na+ and HCO3− reabsorption and have suggested that this observation underlies glomerulotubular balance. In the present work, we investigate the impact of dopamine on the sensitivity of reabsorptive fluxes to changes in luminal flow. Mouse proximal tubules were microperfused in vitro at low and high flow rates, and volume and HCO3− reabsorption (Jv and JHCO3) were measured, while Na+ and Cl− reabsorption (JNa and JCl) were estimated. Raising luminal flow increased Jv, JNa, and JHCO3 but did not change JCl. Luminal dopamine did not change Jv, JNa, and JHCO3 at low flow rates but completely abolished the increments of Na+ absorption by flow and partially inhibited the flow-stimulated HCO3− absorption. The remaining flow-stimulated HCO3− absorption was completely abolished by bafilomycin. The DA1 receptor blocker SCH23390 and the PKA inhibitor H89 blocked the effect of exogenous dopamine and produced a two to threefold increase in the sensitivity of proximal Na+ reabsorption to luminal flow rate. Under the variety of perfusion conditions, changes in cell volume were small and did not always parallel changes in Na+ transport. We conclude that 1) dopamine inhibits flow-stimulated NHE3 activity by activation of the DA1 receptor via a PKA-mediated mechanism; 2) dopamine has no effect on flow-stimulated H-ATPase activity; 3) there is no evidence of flow stimulation of Cl− reabsorption; and 4) the impact of dopamine is a coordinated modulation of both luminal and peritubular Na+ transporters.
Keywords: kidney proximal tubule, sodium chloride and bicarbonate transport, DA1 and DA2 receptor function, torque
kidney proximal tubules are responsible for reabsorption of ∼60% of filtered salt and water, and this fractional reabsorption is relatively constant, despite variations in the glomerular filtration rate (GFR). We have sought to understand the mechanism underlying this glomerulotubular balance (GTB) by measuring the impact of tubule perfusion rate on Na+ and HCO3− absorption in mouse proximal tubules in vitro. We have previously demonstrated that the afferent signal modulating Na+ and HCO3− transport is the flow-dependent torque (bending moment) exerted on brush border microvilli at their base (39) and that this impacts both NHE3-mediated Na+ and HCO3− absorption and H-ATPase-mediated H+ secretion (17, 19). The mouse proximal tubule cell was also used to examine the cellular mechanism of the flow-induced changes on NHE3 and H-ATPase expression and localization (20). In that system, fluid shear stress induces not only apical NHE3 and H-ATPase but also basolateral side Na-K-ATPase trafficking to the membrane surface. In addition, modulation of NHE3 and Na-K-ATPase was dependent on an intact actin cytoskeleton, but H-ATPase was dependent on the microtubule network.
Dopamine is the most important natriuretic signal to proximal tubule (3, 13). Urinary dopamine derives largely from circulating l-3,4-dihydroxyphenylalanine (l-DOPA; Refs. 6, 8), and proximal tubules are a source for this excreted dopamine (7). Within the proximal tubule, conversion from DOPA to dopamine is due to the enzyme l-amino acid decarboxylase (l-AADC), and the importance of the local generation of dopamine was underscored by the demonstration that maximal l-AADC activity is modulated by dietary salt intake (33). Proximal tubules are also a target for the locally generated dopamine, with inhibition of apical Na/H-exchange (NHE3) and basolateral Na-K-ATPase (3). A mouse model in which the intrarenal dopamine is deficient becomes hypertensive with increased NHE3, Na+-K+-2Cl− cotransporter 2 (NKCC2), Na+ -HCO3− contransporter (NBC), Na+-Cl− cotransporter (NCC), and aquaporin 2 (AQP2) mRNA expression in the kidney (45), consistent with dopamine downregulation of Na+ transport in the kidney. In opossum kidney (OK) cells, the dopamine-induced acute decrease in surface NHE3 is dependent on both DA1 and DA2 receptors, and dopamine-stimulated NHE3 endocytosis can be blocked by protein kinase A (PKA) inhibition or by mutation of PKA target serines on NHE3 (22).
In the present work, we study the effect of dopamine on Na+, Cl−, and HCO3− absorption in proximal tubules perfused at low and high flow rates, with specific attention to the changes due to increased flow. We examine the impact of the DA1 receptor antagonist SCH23390, the DA2 receptor antagonist sulpiride, the H-ATPase inhibitor bafilomycin, 8-bromo-cAMP (8-Br-cAMP), and the PKA inhibitor (H89) on flow-induced changes in transport. We find that dopamine completely blocks flow-stimulated Na+ transport and partially abolishes flow-stimulated HCO3− absorption, and the remaining portion of the increment of JHCO3 by flow is blocked by the H-ATPase inhibitor bafilomycin. The data also indicate a dopamine-dependent decrease in the sensitivity of the renal brush border to changes in microvillous torque. These results are consistent with the hypothesis that increased flow causes redistribution of NHE3 within the luminal membrane to a domain where it is active; this activation is essential for flow-stimulated Na+ and HCO3− absorption and is blunted by dopamine.
METHODS
Animals.
All animal work was conducted according to an Institutional Animal Care and Use Committee-approved protocol at Yale School of Medicine. Animals were maintained on a normal diet and tap water until the day of the experiment. Female mice, 5 to 8 wk, were used for isolated kidney proximal tubules and perfusion in vitro. Animals were anesthetized with intraperitoneal pentobarbital sodium (100 mg/kg).
Microperfusion of proximal tubules.
A standard method for isolated tubule perfusion was used as described previously (18). Briefly, fresh dissected proximal tubules (S2 segment) were perfused with an ultrafiltrate-like solution containing the following (in mM): 125 NaCl, 22 NaHCO3, 1 CaCl2, 1.2 MgSO4, 2 glutamine, 2 lactic acid, 10.5 glucose, 5 KCl, and 1.2 phosphoric acid. The bath medium consisted of the following (in mM): 101 NaCl, 22 NaHCO3, 1 CaCl2, 1.2 MgSO4, 2 glutamine, 2 lactic acid, 10.5 glucose, 5 KCl, 1.2 phosphoric acid, and 32.5 HEPES as well as 5 g/dl albumin. The perfusate and bath solutions were bubbled with 95% O2-5% CO2, the pH was adjusted to 7.4, and the osmolalities to 300 mosmol/kgH2O in both solutions. Bath fluid was continuously changed at a rate of 0.5 ml/min to maintain the constancy of pH and bath osmolality. The perfusion rate was calibrated and adjusted to the proper rate before each experiment and was also measured by timed fluid collections. Proximal tubules were perfused at either 5 or 20 nl/min, followed by four sample collections for measuring [3H]inulin and HCO3− concentrations. The order of low or high flow rate was used randomly. Net volume absorption (JV; nl·min−1·mm−1) was measured as the difference between the perfusion (V0) and collection (VL) rates (nl/min) normalized per millimeter of tubule length (L). Extensively dialyzed [3H]methoxy-inulin was added to the perfusate at a concentration of 30 μCi/ml as a volume marker. For each experimental period, four timed collections of tubular fluid were made, and 3H concentrations and total CO2 concentrations in perfusate and collected sample fluid were measured and the rates of fluid and HCO3− absorption were calculated by standard methods (38).
Dopamine (10−5 M), bafilomycin (10−7 M), DA1 antagonist SCH23390 (10−5 M), DA2 antagonist sulpiride (10−5 M), 8-Br-cAMP (10−4 M), and PKA inhibitor H89 (10−6 M) were added to the luminal perfusate, respectively. They were all purchased from Sigma (St. Louis, MO).
Sample measurement and analysis.
The rates of fluid (Jv) and HCO3− (JHCO3) absorption were calculated by measuring the concentrations of [3H]inulin and total CO2, as described previously (19). A calibrated collection pipette was used to obtain precise aliquots of initial perfusates and collection of samples to be analyzed for [3H]methoxy-inulin by liquid scintillation spectroscopy. The total CO2 concentration of both initial and collected fluids was measured by the nanoflow spectrometer (WPI). The rates of net fluid and HCO3− absorption were calculated as described previously (38) and expressed per millimeter tubular length.
The JNa was calculated according to the rate of fluid absorption ([Na] * Jv), since the ratio of fluid and Na+ absorption is 1 in the proximal tubule (40). The JCl was estimated as JCl = JNa − JHCO3. The cell volume is identified with epithelial volume and is calculated as: [π·(OD/2)2 − π·(ID/2)2], where ID is inner tubular diameter and OD is outer tubular diameter. In this estimate, it is assumed that lateral intercellular space volume is a negligible fraction of epithelial volume. The total torque (bending moment) T on the microvilli due to fluid flow is described by an equation that we have derived previously (17).
where r is index to reference value; R is inner tubule radius with brush border; L is length of microvill (L = 2.5 μm); δ is microvilli tip interaction layer (δ = 150 nm); μ is fluid viscosity; and Q is flow rate in the tubule.
Statistics.
Data are presented as means ± SE. Student's t-test was used to compare control and experimental groups. ANOVA test was used for comparison of several experimental groups with a control group followed by Dunnett's test. The difference between the mean values of an experimental group and a control group will be considered significant if P < 0.05.
RESULTS
Effects of dopamine and DA receptor antagonist on flow-activated sodium transport.
The effects of dopamine and DA1 and DA2 receptor antagonists (SCH23390 and sulpiride) on flow-activated sodium transport were examined by microperfusion of proximal tubules in vitro under low (5 nl/min) and high (20 nl/min) perfusion rates. Prior studies in an OK cell line have shown that DA1 and DA2 inhibitors alone had no effect on NHE3 activity and NHE3 surface expression. We have used the same inhibitors and concentrations as reported previously (22, 42). Table 1 summarizes the tubule geometry from all groups of experiments, and Tables 2, 3, 4, and 5 summarize the changes of fluid and solute absorption produced by changes in perfusion rate. Figure 1 shows the effect of dopamine and its inhibitors on fluid, Na+, HCO3−, and Cl− absorption. As shown in Tables 2 and 4, there were no significant differences of Jv and JHCO3 between the DA1 inhibitor and the control group at both low and high flow rates. It may be noted, however, that the change in fractional volume reabsoption did achieve statistical significance. It is difficult to interpret this finding in the absence of secure changes in the component fluxes.
Table 1.
Effects of dopamine, DA1, DA2, PKA, and H-ATPase inhibitors and bafilomycin on flow-induced changes of cell volume and torque in mouse proximal tubules
| Group | n | Vo, nl/min | Length, mm | Cell Volume, μm3 | ID, μm | OD, μm | T/Tr |
|---|---|---|---|---|---|---|---|
| Control | 14 | 4.9 ± 0.41 | 0.78 ± 0.01 | 946.69 ± 21.48 | 11.61 ± 0.55 | 36.61 ± 0.55 | 1.00 ± 0.08 |
| 15 | 20.7 ± 0.72 | 0.77 ± 0.01 | 1121.81 ± 28.40c | 17.17 ± 0.46 | 41.50 ± 0.52 | 1.64 ± 0.06c | |
| Dopamine | 9 | 5.4 ± 0.39 | 0.87 ± 0.03 | 949.02 ± 23.14NS | 12.50 ± 0.42 | 36.94 ± 0.37 | 1.00 ± 0.11 |
| 12 | 20.3 ± 0.78 | 0.87 ± 0.02 | 1,063.56 ± 40.21NS,a | 17.29 ± 0.37 | 40.63 ± 0.63 | 1.78 ± 0.09NS,c | |
| Dopamine + bafilomycin | 7 | 5.1 ± 0.70 | 1.06 ± 0.07 | 910.92 ± 14.45NS | 12.25 ± 0.72 | 35.89 ± 0.45 | 1.00 ± 0.09 |
| 8 | 22.1 ± 1.43 | 1.03 ± 0.07 | 908.12 ± 18.83C,ns | 18.91 ± 0.60 | 38.91 ± 0.60 | 1.29 ± 0.06B,a | |
| Dopamine + SCH23390 | 11 | 5.1 ± 0.24 | 0.95 ± 0.04 | 874.20 ± 28.98NS | 11.36 ± 0.39 | 35.23 ± 0.53 | 1.00 ± 0.09 |
| 13 | 22.1 ± 0.79 | 0.94 ± 0.03 | 942.48 ± 30.81C,ns | 18.46 ± 0.35 | 39.23 ± 0.52 | 1.33 ± 0.04C,b | |
| Dopamine + sulpiride | 10 | 5.0 ± 0.36 | 1.05 ± 0.04 | 966.53 ± 14.50NS | 11.75 ± 0.38 | 37.00 ± 0.33 | 1.00 ± 0.04 |
| 12 | 21.4 ± 1.18 | 1.08 ± 0.04 | 870.48 ± 11.67C,c | 17.71 ± 0.37 | 37.71 ± 0.37 | 1.64 ± 0.04NS,c | |
| DA + SCH + sulpiride | 7 | 5.7 ± 0.37 | 0.86 ± 0.13 | 939.67 ± 19.83NS | 11.43 ± 0.51 | 36.43 ± 0.51 | 1.00 ± 0.09 |
| 13 | 22.9 ± 1.09 | 0.80 ± 0.04 | 894.15 ± 11.03C,a | 18.46 ± 0.35 | 38.46 ± 0.35 | 1.28 ± 0.03C,b | |
| 8-Bromo-cAMP | 7 | 5.4 ± 0.34 | 1.02 ± 0.05 | 953.70 ± 18.11NS | 11.79 ± 0.46 | 36.79 ± 0.46 | 1.00 ± 0.06 |
| 10 | 23.1 ± 0.98 | 1.02 ± 0.05 | 887.50 ± 16.76C,a | 18.25 ± 0.53 | 38.25 ± 0.53 | 1.54 ± 0.06NS,c | |
| Dopamine + H89 | 10 | 5.9 ± 0.44 | 0.89 ± 0.02 | 948.37 ± 18.83NS | 12.00 ± 0.20 | 36.75 ± 0.38 | 1.00 ± 0.04 |
| 13 | 21.7 ± 0.94 | 0.90 ± 0.02 | 907.82 ± 14.09C,ns | 18.40 ± 0.30 | 38.65 ± 0.36 | 1.36 ± 0.04C,c | |
| SCH23390 | 6 | 6.00 ± 0.43 | 0.97 ± 0.05 | 941.66 ± 25.99NS | 12.08 ± 0.42 | 36.67 ± 0.53 | 1.00 ± 0.05 |
| 8 | 22.6 ± 1.41 | 0.94 ± 0.05 | 878.51 ± 16.35C,ns | 17.66 ± 0.28 | 37.81 ± 0.39 | 1.54 ± 0.07NS,c |
Values are means ± SE; n = number of perfused tubules. Dopamine (10−5 M), bafilomycin (H-ATPase inhibitor, 10−7 M), SCH23390 (DA1 antagonist, 10−5 M), sulpiride (DA2 antagonist, 10−5 M), 8-bromo-cAMP (10−4 M), and H-89 (PKA inhibitor, 10−6 M) were added to the luminal perfusate, respectively. Vo, original perfusion rate; Length, tubular length; volume formula is π * (OD/2)2 * 1 − π * (ID/2)2 * 1, where 1 indicates the length or height is 1 μm; ID, inner tubular diameter; OD, outer tubular diameter; T, total torque; Tr, torque measured at the perfusion rate of 5 nl/min. ns: nonsignificant difference from low flow rate in the same group. a,b,cSignificant difference from low flow rates in the same group (aP < 0.05; bP < 0.01; cP < 0.001). NS: not significantly different compared with control at the similar flow rates; A,B,Csignificant difference compared with control at the similar flow rate (AP < 0.05; BP < 0.01; CP < 0.001).
Table 2.
Effects of dopamine and PKA inhibitors and bafilomycin on flow-induced changes of Jv under low and high perfusion rates in mouse proximal tubules
| 5 nl/min |
20 nl/min |
|||||
|---|---|---|---|---|---|---|
| Group | n | JVa, nl·min−1·mm−1 | n | JVb, nl·min−1·mm−1 | ΔJV (JVb − JVa) | ΔJV/JVa * 100 |
| Control | 14 | 0.88 ± 0.03 | 15 | 1.35 ± 0.04c | 0.48 ± 0.05 | 54.31 ± 4.10 |
| Dopamine | 9 | 0.85 ± 0.08NS | 12 | 0.92 ± 0.06ns,C | 0.07 ± 0.10C | 7.69 ± 7.53C |
| DA + bafilomycin | 7 | 0.84 ± 0.05NS | 8 | 0.96 ± 0.04ns,C | 0.12 ± 0.06C | 14.05 ± 5.3C |
| DA + SCH | 11 | 0.97 ± 0.04NS | 13 | 1.73 ± 0.05c,C | 0.77 ± 0.06C | 79.53 ± 5.20C |
| DA + Sul | 10 | 0.83 ± 0.06NS | 12 | 1.12 ± 0.04c,C | 0.28 ± 0.07A | 33.88 ± 4.28B |
| DA + SCH + Sul | 7 | 1.12 ± 0.13A | 13 | 1.66 ± 0.14a,A | 0.54 ± 0.19NS | 48.50 ± 12.20NS |
| 8-Bromo-cAMP | 7 | 0.69 ± 0.03C | 10 | 1.02 ± 0.04c,C | 0.33 ± 0.05NS | 46.96 ± 5.26NS |
| DA + H89 | 10 | 0.82 ± 0.09NS | 13 | 1.44 ± 0.06c,NS | 0.62 ± 0.11NS | 75.12 ± 6.81B |
| SCH | 6 | 0.79 ± 0.04NS | 8 | 1.36 ± 0.03c,NS | 0.57 ± 0.05NS | 72.34 ± 3.56B |
Values are means ± SE; n = number of perfused tubules. Jv, rate of fluid reabsorption; ΔJv, differences of Jv between low (JVa) and high (JVb) perfusion rates (5 and 20 nl/min); ΔJv/Jva * 100, percent changes in fluid reabsorption from low flow rate; Sul, sulpiride; SCH, SCH23390. ns: not significantly different from low flow rate in the same group; a,b,csignificant difference from low flow rate in the same group (aP < 0.05; bP < 0.01; cP < 0.001). NS: nonsignificant difference compared with control at the similar flow rate; A,B,Csignificant difference compared with control at the similar flow rate (AP < 0.05; BP < 0.01; CP < 0.001).
Table 3.
Effects of dopamine and PKA inhibitors and bafilomycin on sodium absorption under low and high perfusion rates in mouse proximal tubules
| 5 nl/min |
20 nl/min |
|||||
|---|---|---|---|---|---|---|
| Group | n | JNaa, pmol·min−1·mm−1 | n | JNab, pmol·min−1·mm−1 | ΔJNa (JNab − JNaa) | ΔJNa/JNaa * 100 |
| Control | 14 | 128.51 ± 3.99 | 15 | 198.31 ± 5.27b | 69.80 ± 6.64 | 54.31 ± 4.10 |
| Dopamine | 9 | 124.73 ± 11.66NS | 12 | 134.33 ± 9.39ns,e | 9.60 ± 14.74e | 7.69 ± 7.53e |
| DA + bafilomycin | 7 | 123.55 ± 6.63NS | 8 | 140.91 ± 6.57ns,e | 17.36 ± 9.33e | 14.05 ± 5.31e |
| DA + SCH | 11 | 141.64 ± 5.96NS | 13 | 254.29 ± 7.37b,e | 112.65 ± 9.56e | 79.53 ± 5.20e |
| DA + Sul | 10 | 122.31 ± 8.61NS | 12 | 163.75 ± 5.23b,e | 41.44 ± 9.86c | 33.88 ± 4.28d |
| DA + SCH + Sul | 7 | 163.97 ± 18.59c | 13 | 243.51 ± 20.01a,c | 79.53 ± 27.61NS | 48.50 ± 12.20NS |
| 8-Bromo-cAMP | 7 | 101.89 ± 5.04e | 10 | 149.74 ± 5.36b,e | 47.85 ± 7.40c | 46.96 ± 5.26NS |
| DA + H89 | 10 | 120.57 ± 13.18NS | 13 | 211.15 ± 8.21b,NS | 90.58 ± 15.07NS | 75.12 ± 6.81d |
| SCH2 | 6 | 115.88 ± 5.57NS | 8 | 199.7 ± 4.12b,NS | 83.83 ± 6.78NS | 72.34 ± 3.56d |
Values are means ± SE; n = number of perfused tubules. JNa, rate of sodium reabsorption; ΔJNa: the differences of JNa between low (JNaa) and high (JNab) perfusion rates (5 and 20 nl/min); ΔJNa/JNaa * 100, percent changes in sodium reabsorption from low flow rate. ns: not significant different from low flow rate in the same group; a,bsignificant difference from low flow rate in the same group (aP < 0.05; bP < 0.001). NS: not significant different compared with control at the similar flow rate; c,d,esignificant difference compared with control at the similar flow rate (cP < 0.05; dP < 0.01; eP < 0.001).
Table 4.
Effects of dopamine and PKA inhibitors and bafilomycin on bicarbonate absorption under low and high perfusion rates in mouse proximal tubules
| 5 nl/min |
20 nl/min |
|||||
|---|---|---|---|---|---|---|
| Group | n | JHCO3a, pmol·min−1·mm−1 | n | JHCO3b, pmol·min−1·mm−1 | ΔJHCO3 (JHCO3b − JHCO3a) | ΔJHCO3/ JHCO3a*100 |
| Control | 14 | 61.72 ± 2.41 | 15 | 128.53 ± 3.79a | 66.81 ± 4.52 | 108.24 ± 6.14 |
| Dopamine | 9 | 61.02 ± 4.75NS | 12 | 102.11 ± 5.15a,d | 41.09 ± 7.05d | 67.34 ± 8.44d |
| DA + bafilomycin | 7 | 47.27 ± 2.03c | 8 | 57.49 ± 6.55ns,d | 10.21 ± 7.04d | 21.61 ± 13.86d |
| DA + SCH | 11 | 59.06 ± 2.78NS | 13 | 126.65 ± 3.93a,NS | 67.59 ± 4.88NS | 114.44 ± 6.66NS |
| DA + Sul | 10 | 53.03 ± 2.58b | 12 | 110.91 ± 2.24a,d | 57.89 ± 3.39NS | 109.16 ± 4.22NS |
| DA + SCH + Sul | 7 | 80.22 ± 4.46d | 13 | 146.91 ± 6.42a,b | 66.69 ± 8.22NS | 83.14 ± 8.00b |
| 8-Bromo-cAMP | 7 | 52.09 ± 2.98b | 10 | 107.28 ± 2.68a,d | 55.20 ± 3.97NS | 105.98 ± 5.15NS |
| DA + H89 | 10 | 60.00 ± 2.73NS | 13 | 135.19 ± 3.32a,NS | 75.18 ± 4.35NS | 125.30 ± 5.54b |
| SCH | 6 | 68.11 ± 2.27NS | 8 | 136.18 ± 2.52a,NS | 68.07 ± 3.42NS | 99.94 ± 3.70NS |
Values are mean ± SE; n = number of perfused tubules; JHCO3, the rate of bicarbonate reabsorption; ΔJHCO3, the differences of JHCO3 between low (JHCO3a) and high (JHCO3b) perfusion rates (5 and 20 nl/min); ΔJHCO3/JHCO3a * 100, percentage changes in bicarbonate reabsorption between high and low flow rates. ns: not significant different from low flow rate in the same group; asignificant difference from low flow rate in the same group (aP < 0.001). NS: not significant different compared with control at the similar flow rate; b,c,dsignificant difference compared with control at the similar flow rate (bP < 0.05; cP < 0.01; dP < 0.001).
Table 5.
Effects of dopamine and PKA inhibitors and bafilomycin on chloride absorption under low and high perfusion rates in mouse proximal tubules
| 5 nl/min |
20 nl/min |
|||||
|---|---|---|---|---|---|---|
| Group | n | JCla, pmol·min−1·mm−1 | n | JClb, pmol·min−1·mm−1 | ΔJCl (JClb − JCla) | ΔJCl/JCla * 100 |
| Control | 14 | 66.79 ± 1.58 | 15 | 69.79 ± 6.99ns | 2.99 ± 7.28 | 4.48 ± 10.47 |
| Dopamine | 9 | 63.71 ± 7.72NS | 12 | 32.21 ± 5.87b,e | −31.49 ± 9.51d | −49.43 ± 9.21e |
| DA + bafilomycin | 7 | 76.28 ± 6.69NS | 8 | 83.42 ± 5.48ns,NS | 7.14 ± 8.59NS | 9.36 ± 7.18NS |
| DA + SCH | 11 | 82.59 ± 4.19e | 13 | 127.65 ± 5.35b,e | 45.06 ± 6.86e | 54.56 ± 6.48e |
| DA + Sul | 10 | 69.29 ± 7.54NS | 12 | 52.84 ± 5.1ns,NS | −16.45 ± 8.95NS | −23.74 ± 7.38c |
| DA + SCH + Sul | 7 | 83.76 ± 15.72NS | 13 | 96.60 ± 15.20ns,NS | 12.84 ± 21.76NS | 15.33 ± 18.14 NS |
| 8-Bromo-cAMP | 7 | 49.80 ± 7.31d | 10 | 42.45 ± 5.79ns,c | −7.35 ± 9.13NS | −14.76 ± 11.62NS |
| DA + H89 | 10 | 60.57 ± 12.45 NS | 13 | 75.96 ± 7.41ns,NS | 15.39 ± 14.03NS | 25.42 ± 12.23NS |
| SCH | 6 | 47.77 ± 5.92e | 8 | 63.53 ± 3.97a,NS | 15.76 ± 6.93NS | 32.99 ± 8.30NS |
Values are means ± SE; n = number of perfused tubules; JCl: the rate of chloride reabsorption; ΔJCl: the differences of JCl between low (JCla) and high (JClb) perfusion rates (5 and 20 nl/min); ΔJCl/ JClb*100: percentage changes in chloride reabsorption from low flow rate; ns, not significant different from low flow rates in the same group; a,bsignificant difference from low flow rate in the same group (aP < 0.05; bP < 0.001). NS, not significant different compared with control at the similar flow rates; c,d,esignificant difference compared with control at the similar flow rates (cP < 0.05; dP < 0.01; eP < 0.001).
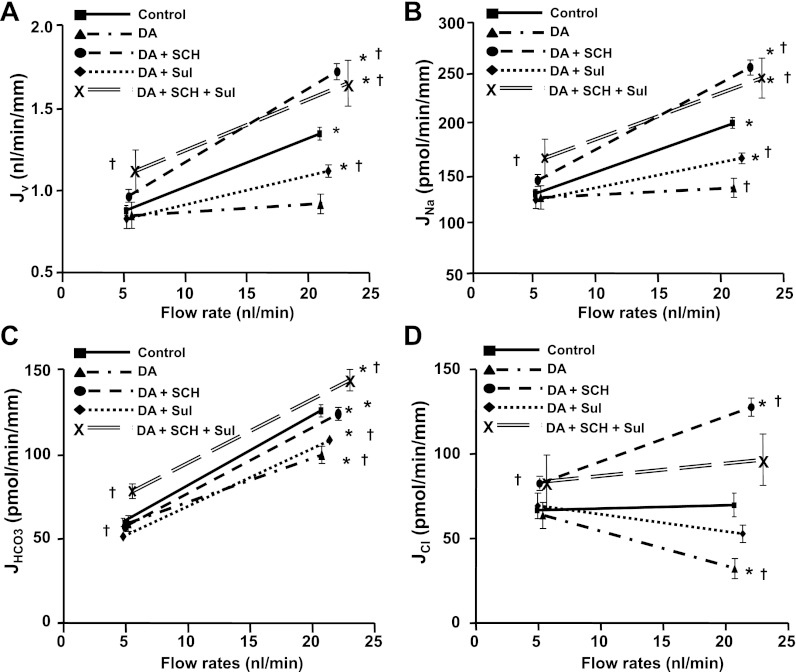
Fig. 1.
Effects of dopamine and its antagonists on flow-induced changes in fluid (A), sodium (B), bicarbonate (C), and chloride (D) absorption (Jv, JNa, JHCO3, and JCl) in mouse proximal tubules. Jv, JNa, JHCO3, and JCl were measured at low and high perfusion rates in the absence and presence of dopamine (10−5 M), SCH23390 (SCH; DA1 inhibitor, 10−5 M) and sulpiride (Sul; DA2 inhibitor, 10−5 M), *P < 0.05, compared with low flow rates in the same group; †P < 0.05, compared with the control at a similar flow rate.
As shown in Fig. 1, the increasing perfusion rate from 5 to 20 nl/min increased fluid and Na+ absorption by 54% in the control group, which is similar to our previous study (19). Addition of dopamine did not change Jv, JNa, or JHCO3 at the low flow rate: Jv was 0.85 vs. 0.88 nl/min, JNa was 125 vs. 129, JHCO3 was 61.0 vs. 61.7, and JCl was 63.7 vs. 66.8 pmol·min−1·mm−1, respectively, in the presence of dopamine compared with control. The increment of Jv and JNa due to the increase in perfusion rate was completely abolished by dopamine (Fig. 1). Dopamine reduced the flow-stimulated fluid and Na+ absorption, so that the fractional increase of Jv and JNa by flow was reduced from 54 to 7.7% by addition of dopamine in the luminal perfusate (Tables 2 and 3). This observation complements the results of a prior report (9) on the perfusion of isolated rabbit tubules that dopamine had no effect on proximal tubule transport in unstimulated conditions but abolished the norepinephrine-induced increase in Na+ absorption.
At least five isoforms of dopamine receptors (DA1 to DA5) have been identified, and they have all been found in the mammalian kidney (29). The DA1 receptor is expressed in kidney proximal tubules at both the apical and basolateral sides, and the DA2 receptor is also detected in the proximal tubule and distal nephron segments (29). Previous studies (11, 22) show both DA1 and DA2 receptors regulate Na+ transport in proximal tubule and proximal tubule cells. We examined whether the effect of dopamine on flow-stimulated Na+ absorption is due to a DA1 and/or a DA2 receptor-mediated mechanism. Figure 1 illustrates that when the DA1 receptor antagonist and dopamine are added together to the luminal perfusate, the effect of dopamine was completely abolished by the DA1 antagonist SCH23390. Addition of the DA2 antagonist sulpiride only partially blocked the effect of dopamine, and addition of both DA1 and DA2 antagonists together produced an additive effect of inhibited dopamine activity. As shown in Fig. 1, Jv and JNa increased significantly at low flow rate when DA1 and DA2 blockers were added together, but the Jv and JNa at the high flow rate were similar to that of the DA1 inhibitor alone. These results are consistent with the previous observation those DA1 is the primary receptor for dopamine activation and the DA2 receptor synergistically enhances the effect of DA1 on regulation of NHE3 (22). It should be noted that when the DA1 receptor is blocked, Jv and JNa at the high flow rate are significantly higher than those of control. The fractional increase of Jv and JNa by increasing flow from 5 to 20 nl/min was 54% in control and 80% in the presence of the DA1 inhibitor (Tables 2 and 3), indicating that Na+ transport is more sensitive to axial flow when the DA1 receptor is blocked.
Effect of dopamine and DA receptor antagonists on flow-activated bicarbonate transport.
The effect of flow-induced changes in bicarbonate absorption was examined by measuring the JHCO3 at low and high flow rates in the absence and presence of dopamine or its receptor antagonist, SCH23390, to block DA1 and sulpiride to block DA2 receptor, respectively. As shown in Fig. 1C, increasing axial flow rate doubled JHCO3 in the control group, similar to our previous reports (19). Addition of dopamine had no effect on JHCO3 at the low flow rate but reduced JHCO3 significantly at the high flow rate, consistent with dopamine inhibition of Na+ and HCO3− absorption in the stimulated condition. However, in contrast to complete block by dopamine of flow-stimulated Jv and JNa, the flow stimulated JHCO3 was only partially inhibited by dopamine. As shown in Table 4, the fractional increase of JHCO3 was reduced only 38% by dopamine, from 108.2 to 67.3%. JHCO3 still increased significantly when flow rate is increased in the presence of dopamine. Figure 1 shows that the inhibitory effect of dopamine on JHCO3 at the high flow rate was completely blocked by the DA1 antagonist and was partially blocked by the DA2 receptor antagonist and that addition of DA1 and DA2 antagonists together had an additive effect on the inhibition of dopamine. These results suggest that the DA1 receptor is the primary receptor for dopamine and the DA2 receptor synergistically enhances the effect of DA1.
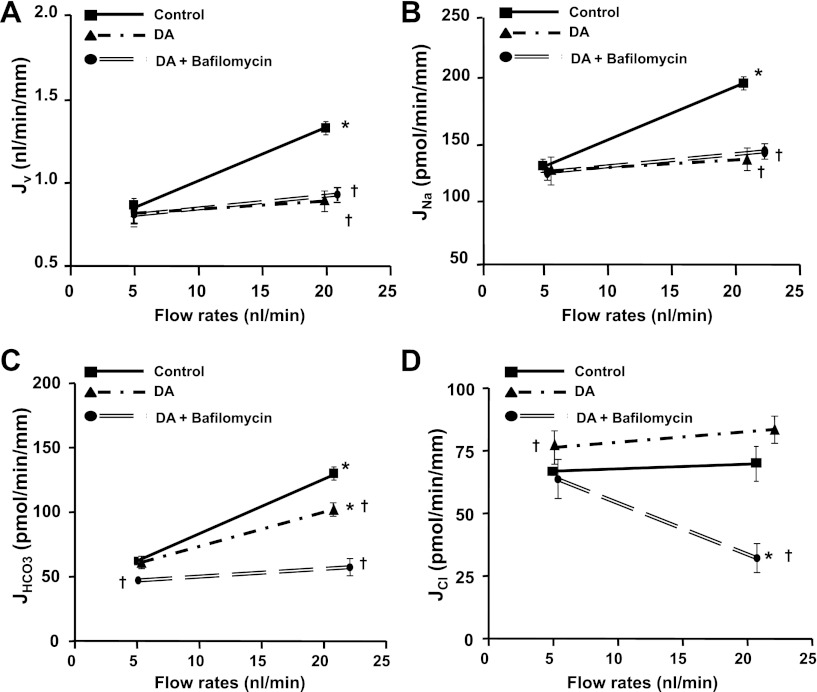
Since H-ATPase in the apical membrane is responsible for absorbing 30% of filtered HCO3− in the proximal tubule and H-ATPase activity is also regulated by axial flow (19), we asked whether the remaining flow-stimulated JHCO3 in the presence of dopamine is via an H-ATPase mediated mechanism. As shown in Fig. 2, addition of bafilomycin and dopamine together did not produce any additive effect on JNa compared with either control or dopamine alone, but the HCO3− absorption was extensively reduced when dopamine and bafilomycin were added together compared with control and dopamine alone. Addition of dopamine and bafilomycin together significantly reduced JHCO3 at both low and high flow rates, and the remaining flow-stimulated HCO3− absorption was completely abolished by bafilomycin (Fig. 2). The value of JHCO3 measured at low and high flow rates in the presence of both dopamine and bafilomycin is similar to our previous data (19), which showed that both NHE3 and H-ATPase mediated HCO3− absorption were inhibited when EIPA and bafilomycin were combined. These results indicate that dopamine inhibits NHE3-mediated but not H-ATPase-mediated HCO3− absorption in the proximal tubule.
Fig. 2.
Effects of dopamine and bafilomycin on flow-induced changes in fluid (A), sodium (B), bicarbonate (C), and chloride (D) absorption (Jv, JNa, JHCO3, and JCl) in proximal tubules. Jv, JNa, JHCO3, and JCl absorption were measured at low and high perfusion rates in absence and presence of dopamine (10−5 M) and the H-ATPase inhibitor bafilomycin (10−7 M). *P < 0.05, compared with low flow rates in the same group; †P < 0.05, compared with the control at a similar flow rate.
Flow-induced changes in chloride transport and cell volume in proximal tubules.
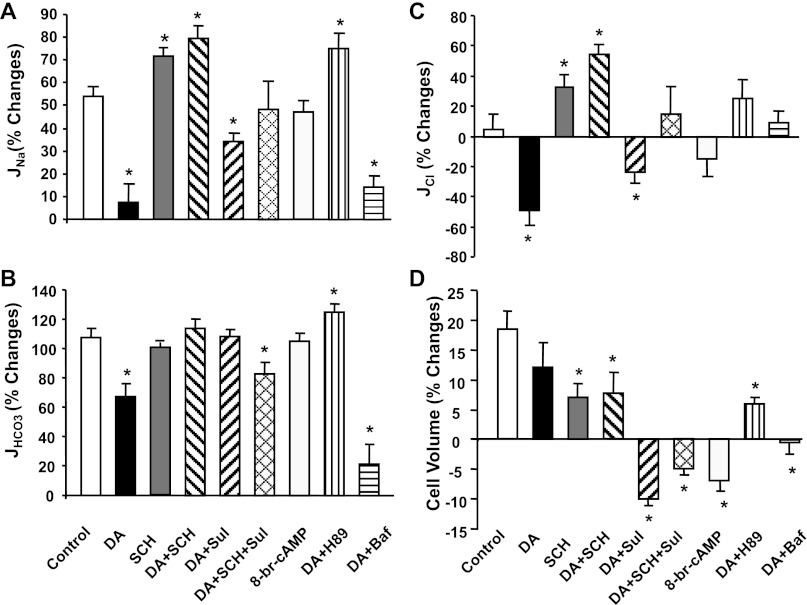
The flow-induced changes in Cl− absorption were calculated according to the balance of Na+ and Cl− plus HCO3− transport under all experimental conditions. Data summarized in Table 5 show that increasing flow rate from 5 to 20 nl/min did not stimulate Cl− absorption. JCl was 66.8 pmol·min−1·mm−1 at both low and high flow rates (Table 5 and Fig. 1D). The fractional changes of JCl due to flow (see Fig. 4) depend directly on the change of JNa (which provides a driving force for Cl− absorption), and inversely upon JHCO3 (which provides driving force for Cl− backflux into the lumen). For example, dopamine eliminated the flow-dependent increase of Na+ absorption by flow but only slightly reduced JHCO3. On balance, the net change of JCl by increasing flow in the presence of dopamine became negative (−49%; Fig. 4C). When the flow-dependent increase in JHCO3 is eliminated by bafilomycin, then the flow-dependent decrease in JCl is eliminated.
Fig. 4.
Effect of dopamine, dopamine antagonist, bafilomycin, 8-Br-cAMP, and PKA inhibitor on flow-induced changes in sodium (A), bicarbonate (B), chloride (C) reabsorption (JNa, JHCO3, and JCl), and cell volume (D) under low and high perfusion rates in proximal tubules. % Change is the percent difference from the low flow rate of Na+, HCO3−, and Cl− absorption shown in Tables 3 (JNa), 4 (JHCO3), and 5 (JCl). *P < 0.05, compared with the change in control group.
The flow-induced changes in cell volume are shown in Fig. 4D and Table 1. Increasing perfusion rate from 5 to 20 nl/min increased cell volume by 18% in control, suggesting a small increase in inflow over outflow until a new steady state is reached at the high flow condition. Dopamine had no impact on the cell volume, either at low or high flows. Given the fact that flow increased fluid and Na+ absorption by 54% and dopamine reduced the fractional increase of Jv and JNa by 86%, the flow-induced changes in cell volume are small, compared with the changes in flow-stimulated Na+ absorption. Cell volumes were slightly increased or reduced by flow under application of different receptor inhibitors. The changes are associated with the changes of JNa, but the variations are relatively small, compared with the changes of Na+ absorption (Fig. 4).
Role of PKA in regulation of Na+ and HCO3− transport.
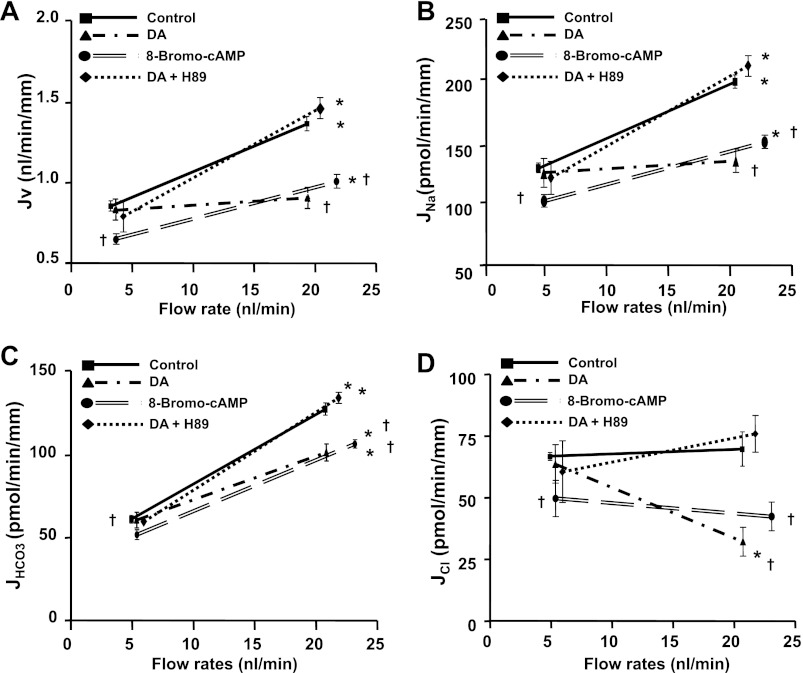
Previous studies have indicated that dopamine regulates NHE3 activity within the luminal cell membrane via a PKA-mediated mechanism; this is manifested either as NHE3 endocytosis in OK cells (22) or NHE3 redstribution to the coated pit region of the membrane in vivo (25). We have examined the effect of the PKA inhibitor H89 on dopamine-induced changes in flow-stimulated proximal tubule transport. Hu et al. (22) showed that H89 (10−6 M) alone had no effect on NHE3 surface expression but abolished the reduction in NHE3 expression by dopamine. In addition, Murtazina et al. (31) showed that 1 μm of H89 had no effect on NHE3 function, but it completely abolished the effect of cAMP on NHE3 activity. In this study, we have used the same concentration of H89 as reported previously. As shown in Fig. 3, H89 completely blocked the effect of dopamine on flow-stimulated Jv, JNa, and JHCO3, consistent with the prior reports (22, 25) that the dopamine-induced inactivation of NHE3 is PKA-dependent. The effect of 8-Br-cAMP on proximal tubule transport was also investigated under low and high perfusion rates. Unlike dopamine, which had no effect on Na+ and HCO3− absorption in unstimulated conditions, 8-Br-cAMP reduced JNa and JHCO3 significantly at both low and high flow rates. This result is consistent with the prior finding that cAMP inhibits NHE3 activity (44); it suggests that the dopamine effect is not simply uniform cAMP generation but contingent on the prevailing stimuli to Na+ transport.
Fig. 3.
Effects of dopamine, 8-bromo-cAMP (8-Br-cAMP), and PKA inhibitor on flow-induced changes in fluid (A), sodium (B), bicarbonate (C), and chloride (D) absorption (Jv, JNa, JHCO3, and JCl) in proximal tubules. Jv, JNa, JHCO3, and JCl were measured at low and high perfusion rates in absence and presence of dopamine (10−5 M), 8-Br-cAMP (10−4 M), and PKA inhibitor H89 (10−6 M) respectively. *P < 0.05, compared with low flow rates in the same group; †P < 0.05, compared with the control at a similar flow rate.
Effect of dopamine on flow-induced changes in torque and ion transport.
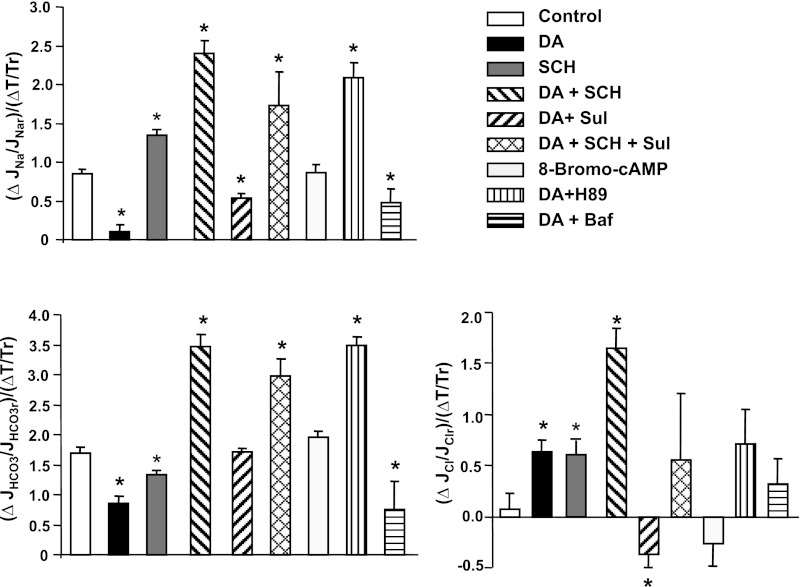
We (17, 19) have previously reported that the flow-induced change in Na+ and HCO3− absorption is dependent on microvillous torque (bending moment). Specifically, the fluid induced bending moment on microvilli increases proportionally with axial flow rate and decreases inversely as tubule diameter squared (see governing equation). Increases in flow can lead to significant increases in tubule diameter, which serves to reduce the flow mediated torque on the microvilli (39). In this study, we investigated the effect of dopamine and its inhibitors on flow-induced changes in torque and torque-mediated ion transport. As shown in Table 1, increasing flow rate from 5 to 20 nl/min increased torque by 64 and 78% in the absence and presence of dopamine (P > 0.05), indicating dopamine itself did not significantly affect the flow-induced change in torque. Although 8-Br-cAMP inhibits NHE3 activity and reduced Na+ and HCO3− absorption in both low and high flow rates, 8-Br-cAMP similarly did not influence the flow-induced changes in torque. We next examined the ion transport sensitivity to torque by computing the ratio of flow-induced change in Na+, HCO3−, and Cl− absorption, relative to the flow-induced change in microvillous torque (Fig. 5). In the control group, the ratio of flow-induced change in Na+ transport to torque is close to 1 and close to 2 for HCO3− absorption (Fig. 5). This ratio is reduced by dopamine and increased by the DA1 receptor antagonist and the PKA antagonist; it was unchanged by 8-Br-cAMP. Again, changes in torque-dependent Cl− transport depend directly on the changes in Na+ transport and indirectly on changes in HCO3− transport (Fig. 5). Since dopamine reduces microvillous expression of NHE3, our results suggest that the sensitivity of torque-mediated Na+ and HCO3− transport depends on the availability of NHE3 within the brush border.
Fig. 5.
Effect of dopamine, dopamine antagonist, bafilomycin, 8-br-cAMP, and PKA inhibitor on flow-induced changes in torque (T) and sodium (A), bicarbonate (B), chloride (C) reabsorption in proximal tubules. Data are represented as the ratio of changes in ion absorption and changes in torque by flow; r is the reference value obtained from low flow rate. *P < 0.05, compared with the change in control group.
DISCUSSION
Dopamine is an important natriuretic signal to proximal tubule (3, 13), and proximal tubules are a target for locally generated dopamine. Dopamine inhibits sodium reabsorption in the isolated perfused straight tubule (10); in proximal convoluted tubules (in vitro), dopamine blunts norepinephrine-stimulated sodium reabsorption (9). RNA message for the DA1 dopamine receptor was demonstrated in proximal tubule (43), and subsequently the protein was identified on both luminal and peritubular cell membranes (32). In cell culture, cell membrane expression of the DA1 receptor is increased both by dopamine itself (12), as well as by inhibition of the angiotensin receptor (AT1R) by losartan (27). In the rat in vivo, dopamine downregulates AT1R (14), and conversely, blockade of DA1 receptor attenuates the antihypertensive effect of losartan (27). What is reported here is the impact of dopamine to blunt the increases in proximal reabsorption that accompany increases in luminal flow. The physiologic significance of this effect seems straightforward: when glomerular filtration rate increases, perhaps in response to a humoral signal, but in the absence of volume expansion, the flow-dependent increase in reabsorptive fluxes will provide a balanced increase in tubular reabsorption; when the increase in GFR occurs in response to volume expansion, the increase in local dopamine generation will abrogate this glomerulotubular balance, favoring increased Na+ excretion. Put another way, in the absence of dopamine, GTB by itself will tend to blunt the impact of increases in GFR on distal delivery; however, when there is volume expansion, and increased dopamine generation, there will be a greater impact of GFR increases on distal delivery.
As with angiotensin, dopamine impacts transporters of both luminal and peritubular cell membranes, most notably NHE3 and the Na-K-ATPase. When renal cortical tissue is incubated with dopamine or DA1 agonists, Na+/H+ exchange in brush border membrane vesicles is diminished, and this effect is blocked by inhibitors of either adenylate cyclase or PKA (21). In OK cells, dopamine (via DA1) and PKA phosphorylates NHE3 on identical sites (42). In large measure, the decrease in Na+/H+ exchange reflects a dopamine-mediated decrease in NHE3 within the luminal cell membrane (5). With respect to other luminal transporters, dopamine also induces internalization of the Na+-phosphate cotransporter (4). The impact of dopamine on the Na-K-ATPase is demonstrable as a decrease of enzyme activity in micro-dissected tubules (2) or as a decrease in oxygen consumption by proximal tubules in suspension (34). The dopamine-mediated decrease in proximal tubule cellular Na-K-ATPase activity has been shown to occur in association with endocytosis of the enzyme (15). An early event in the endocytic process is PKC-dependent phosphorylation of the α-subunit of the Na-K-ATPase (16). Complementary to the decrease in luminal NHE3, dopamine also blunts peritubular HCO3− exit via the Na+-3 HCO3− cotransporter (26). In the perfused proximal straight tubule of the rabbit, the net impact of dopamine is to increase cytosolic Na+ concentration, so that the peritubular effect appears dominant (26).
The current work is the first examination of the interaction of dopamine with the machinery responsible for flow dependence of proximal Na+ reabsorption. The primary measurements of this study are tubular transport of volume and HCO3−, luminal diameter, and cell height; estimated variables are tubular transport of Na+ and Cl−, and microvillous torque. The data confirm earlier observations that increasing luminal flow increases reabsorption of Na+ and HCO3−, with only a small increase in cell height, and no effect on Cl− reabsorption. The new findings are 1) Dopamine has no impact on Na+ reabsorption at the low perfusion rate but blocks the increase in Na+ flux with increasing luminal flow; this increase is eliminated by blockade of DA1 or inhibition of PKA. Indeed, with DA1 block there is actually an enhanced flow-dependent increase in Na+ transport. 2) Dopamine partially blocks the increase in HCO3− flux with increasing perfusion; there is complete elimination of the increase in HCO3− reabsorption (and of the decrease in Cl− reabsorption) when luminal H-ATPase has been inhibited. The straightforward conclusion from these findings is that increasing luminal flow augments transport across luminal membrane NHE3 and that this increase is blocked by application of exogenous dopamine. Furthermore, DA1 blockade enhances the sensitivity of NHE3 transport to increases in luminal flow, without impacting fluxes under low flow. In view of the fact that the dopamine precursor l-DOPA is not provided to the tubules perfused in vitro, the absence of effect of the DA1 inhibitor is not surprising and cannot be construed as an argument against a physiologic role of dopamine in regulating proximal transport. How the flow-dependent augmentation of NHE3 flux can be understood in terms of cellular events requires considerably more speculation. It may be the case that DA1 inhibition upregulates angiotensin receptor density and thus enhances the impact of flow to activate angiotensin-dependent increases in NHE3 membrane density.
The hypothesis offered by this laboratory has been that microvillous torque (bending moment) is the afferent signal to the cell to adjust its transporter configuration in response to changes in luminal flow rate. Important evidence for torque as the mechanical stimulation is the response of the tubule to increases in luminal fluid viscosity to increase bending moment (with no change in axial flow rate; Ref. 17). Additional supporting evidence is that changes in transport scale linearly with torque (i.e., using the formula encompassing the change in internal diameter), and for sufficiently large diameter change, a change in flow can produce negligible change in transport (19, 39). As a consequence of this effect of luminal diameter, changes in microvillous torque for tubules perfused in vitro may be considerably smaller than for tubules in vivo and thus considerably smaller than the fractional change in luminal flow rate. In particular, the 60% change in volume flux that was observed with a fourfold increase in perfusion rate cannot be construed as violating glomerulotubular balance. In the past, the sensitivity of the change in transport of either Na+ or HCO3− to changes in microvillous torque has been ∼1.0 (19); (unpublished observations). In this work, that observation is reproduced under control conditions; however, with DA1 block or PKA inhibition, we report sensitivities of ∼2 for Na+ transport and ∼3 for HCO3− transport (Fig. 5). This observation suggests that the DA1 receptor and downstream events provide tonic inhibition of NHE3 activity. Physical interactions between the DA1 receptor and AT1R and the Na-K-ATPase have been recognized, and these interactions appear to have functional consequences (24). The findings in Fig. 5 prompt the question as to whether such interactions can impact transport flow sensitivity.
The cellular events that accompany an increase in luminal flow are largely unknown, although the integrity of the actin cytoskeleton is a prerequisite for the increase in Na+ resabsorption (17). In cultured proximal tubule cells, an increase in apical shear stress provokes redistribution of NHE3 and the H-ATPase toward the apical cell membrane, as well as Na-K-ATPase toward the basolateral cell membrane (20). In a mathematical model of the proximal tubule, robust increases in Na+ flux cannot be achieved with only perturbations in luminal membrane transporter density; furthermore, solitary increases in NHE3 activity provoke sizable increases in cell volume (41). What the present work provides to this discussion is the observation that under control conditions, the 54% increase in Na+ reabsorption that accompanies rapid luminal flow occurs with only an 18% increase in cell volume; with DA1 blockade increased luminal flow provoked an 80% increase in Na+ reabsorption with an insignificant (8%) increase in cell volume; and with PKA inhibition, the flow-derived increase in Na+ flux was 75%, while cell volume decreased 6%. These findings suggest that activation of peritubular exit pathways is a critical component of the cellular response to increases in luminal membrane shear stress and that changes in cytosolic composition (i.e., lower Na+ concentration and/or acidification) may well accompany increased luminal flow.
The translational significance of the proximal tubule response to dopamine has focused largely on the diabetic kidney. It was an early observation in humans that type-1 diabetics showed a blunted natriuresis in response to Na+ infusion, along with a blunted increase in urinary dopamine excretion; control subjects, but not diabetics, showed a small decrease in fractional lithium clearance during the infusion, which prompted the conclusion that the natriuresis was attributable to proximal tubule (35). In streptozotocin-treated diabetic rats, who were hyperglycemic, there was diminished renal responsiveness to a DA1 agonist (blunted decrease in Na-K-ATPase) in association with decreased DA1 receptor number (28). With insulin treatment of diabetic rats, DA1 number and the functional response to dopamine or Na+ load were restored (30). On the opposite end of the spectrum, obese rats also showed decreased dopamine receptor binding, and diminished down-stream dopamine effects, including cAMP generation, PKA activation, and inhibition luminal membrane vesicle Na+/H+ exchange (23). Similar findings were observed in a hyperinsulinemic rat model (1). Vallon and colleagues (36, 37) have provided micropuncture evidence that the hyperfiltration of early diabetes derives from enhanced proximal Na+ reabsorption and diminished distal delivery which activates tubuloglomerular feedback. Of note, these workers stopped short of attributing the increase in proximal Na+ transport to failure of the cellular dopamine machinery. What the present work contributes to this discussion is the observation that defective DA1 or PKA inhibition may suffice to increase proximal Na+ reabsorption and that additional diabetic defects in proximal tubule may not be required to rationalize the abnormal transport.
In summary, flow-stimulated Na+ reabsorption and H+ secretion are essential ingredients of glomerulotubular balance; there appears to be no direct effect of flow on Cl− reabsorption. In the isolated perfused tubule, dopamine abrogates the flow stimulation of both Na+ transport and the non H-ATPase component of H+ secretion. With DA1 blockade, or with PKA inhibition, the sensitivity of Na+ reabsorption and H+ secretion to changes in flow increases to levels not previously reported. These dramatic changes in transport occur with no perceptible change in cell volume, suggesting coordinated modulation of luminal and peritubular membrane transporters driven by changes in luminal flow.
GRANTS
This investigation was supported by National Institute of Diabetes and Digestive and Kidney Diseases Public Health Service Grants RO1-DK-62289 (to T. Wang) and RO1-DK-29857 (to A. M. Weinstein).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.D. performed experiments; Z.D., Q.Y., A.M.W., and T.W. analyzed data; Z.D., L.W., S.W., A.M.W., and T.W. edited and revised manuscript; Z.D., Q.Y., L.W., S.W., A.M.W., and T.W. approved final version of manuscript; L.W. and T.W. prepared figures; S.W., A.M.W., and T.W. interpreted results of experiments; A.M.W. and T.W. conception and design of research; T.W. drafted manuscript.
REFERENCES
- 1. Ahmad Banday A, Lokhandwala MF. Defective renal dopamine D1 receptor function contributes to hyperinsulinemia-mediated hypertension. Clin Exp Hypertens 28: 695–705, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Aperia A, Bertorello A, Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol Renal Fluid Electrolyte Physiol 252: F39–F45, 1987 [DOI] [PubMed] [Google Scholar]
- 3. Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol 62: 621–647, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bacic D, Capuano P, Baum M, Zhang J, Stange G, Biber J, Kaissling B, Moe OW, Wagner CA, Murer H. Activation of dopamine D1-like receptors induces acute internalization of the renal Na+/phosphate cotransporter NaPi-IIa in mouse kidney and OK cells. Am J Physiol Renal Physiol 288: F740–F747, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bacic D, Kaissling B, McLeroy P, Zou L, Baum M, Moe OW. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int 64: 2133–2141, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baines AD, Chan W. Production of urine free dopamine from DOPA; a micropuncture study. Life Sci 26: 253–259, 1980 [DOI] [PubMed] [Google Scholar]
- 7. Baines AD, Drangova R, Hatcher C. Dopamine production by isolated glomeruli and tubules from rat kidneys. Can J Physiol Pharmacol 63: 155–158, 1985 [DOI] [PubMed] [Google Scholar]
- 8. Ball SG, Gunn IG, Douglas IH. Renal handling of dopa, dopamine, norepinephrine, and epinephrine in the dog. Am J Physiol Renal Fluid Electrolyte Physiol 242: F56–F62, 1982 [DOI] [PubMed] [Google Scholar]
- 9. Baum M, Quigley R. Inhibition of proximal convoluted tubule transport by dopamine. Kidney Int 54: 1593–1600, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bello-Reuss E, Higashi Y, Kaneda Y. Dopamine decreases fluid reabsorption in straight portions of rabbit proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 242: F634–F640, 1982 [DOI] [PubMed] [Google Scholar]
- 11. Bertorello A, Aperia A. Inhibition of proximal tubule Na+-K+-ATPase activity requires simultaneous activation of DA1 and DA2 receptors. Am J Physiol Renal Fluid Electrolyte Physiol 259: F924–F928, 1990 [DOI] [PubMed] [Google Scholar]
- 12. Brismar H, Asghar M, Carey RM, Greengard P, Aperia A. Dopamine-induced recruitment of dopamine D1 receptors to the plasma membrane. Proc Natl Acad Sci USA 95: 5573–5578, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carey RM. Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension 38: 297–302, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Cheng HF, Becker BN, Harris RC. Dopamine decreases expression of type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest 97: 2745–2752, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chibalin AV, Katz AI, Berggren PO, Bertorello AM. Receptor-mediated inhibition of renal Na+-K+-ATPase is associated with endocytosis of its α- and β-subunits. Am J Physiol Cell Physiol 273: C1458–C1465, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Chibalin AV, Pedemonte CH, Katz AI, Feraille E, Berggren PO, Bertorello AM. Phosphorylation of the catalyic alpha-subunit constitutes a triggering signal for Na+,K+-ATPase endocytosis. J Biol Chem 273: 8814–8819, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Du Z, Duan Y, Yan Q, Weinstein AM, Weinbaum S, Wang T. Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci USA 101: 13068–13073, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du Z, Ferguson W, Wang T. Role of PKC and calcium in modulation of effects of angiotensin II on sodium transport in proximal tubule. Am J Physiol Renal Physiol 284: F688–F692, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T. Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillus bending moments. Am J Physiol Renal Physiol 290: F289–F296, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Duan Y, Weinstein AM, Weinbaum S, Wang T. Shear stress-induced changes of membrane transporter localization and expression in mouse proximal tubule cells. Proc Natl Acad Sci USA 107: 21860–21865, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Felder CC, Campbell T, Albrecht F, Jose PA. Dopamine inhibits Na+-H+ exchanger activity in renal BBMV by stimulation of adenylate cyclase. Am J Physiol Renal Fluid Electrolyte Physiol 259: F297–F303, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem 276: 26906–26915, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Hussain T, Becker M, Beheray S, Lokhandwala MF. Dopamine fails to inhibit Na,H-exchanger in proximal tubules of obese Zucker rats. Clin Exp Hypertens 23: 591–601, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Khan F, Spicarova Z, Zelenin S, Holtback U, Scott L, Aperia A. Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol 295: F1110–F1116, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol 289: F249–F258, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Kunimi M, Seki G, Hara C, Taniguchi S, Uwatoko S, Goto A, Kimura S, Fujita T. Dopamine inhibits renal Na+:HCO3− cotransporter in rabbits and normotensive rats but not in spontaneously hypertensive rats. Kidney Int 57: 534–543, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Li D, Scott L, Crambert S, Zelenin S, Eklof AC, Di Ciano L, Ibarra F, Aperia A. Binding of losartan to angiotensin AT1 receptors increases dopamine D1 receptor activation. J Am Soc Nephrol 23: 421–428, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marwaha A, Banday AA, Lokhandwala MF. Reduced renal dopamine D1 receptor function in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol 286: F451–F457, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Moreira-Rodrigues M, Quelhas-Santos J, Serrao P, Fernandes-Cerqueira C, Sampaio-Maia B, Pestana M. Glycaemic control with insulin prevents the reduced renal dopamine D1 receptor expression and function in streptozotocin-induced diabetes. Nephrol Dial Transplant 25: 2945–2953, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Murtazina R, Kovbasnjuk O, Zachos NC, Li X, Chen Y, Hubbard A, Hogema BM, Steplock D, Seidler U, Hoque KM, Tse CM, De Jonge HR, Weinman EJ, Donowitz M. Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) null mice. cAMP inhibition is differentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J Biol Chem 282: 25141–25151, 2007 [DOI] [PubMed] [Google Scholar]
- 32. O'Connell DP, Botkin SJ, Ramos SI, Sibley DR, Ariano MA, Felder RA, Carey RM. Localization of dopamine D1A receptor protein in rat kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1185–F1197, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Seri I, Kone BC, Gullans SR, Aperia A, Brenner BM, Ballermann BJ. Influence of Na+ intake on dopamine-induced inhibition of renal cortical Na+-K+-ATPase. Am J Physiol Renal Fluid Electrolyte Physiol 258: F52–F60, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Seri I, Kone BC, Gullans SR, Aperia A, Brenner BM, Ballermann BJ. Locally formed dopamine inhibits Na+-K+-ATPase activity in rat renal cortical tubule cells. Am J Physiol Renal Fluid Electrolyte Physiol 255: F666–F673, 1988 [DOI] [PubMed] [Google Scholar]
- 35. Stenvinkel P, Saggar-Malik AK, Wahrenberg H, Diczfalusy U, Bolinder J, Alvestrand A. Impaired intrarenal dopamine production following intravenous sodium chloride infusion in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 34: 114–118, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Vallon V, Blantz RC, Thomson S. Glomerular hyperfiltration and the salt paradox in early [corrected] type 1 diabetes mellitus: a tubulo-centric view. J Am Soc Nephrol 14: 530–537, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Vallon V, Huang DY, Deng A, Richter K, Blantz RC, Thomson S. Salt-sensitivity of proximal reabsorption alters macula densa salt and explains the paradoxical effect of dietary salt on glomerular filtration rate in diabetes mellitus. J Am Soc Nephrol 13: 1865–1871, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Wang T, Chan YL. Mechanism of angiotensin II action on proximal tubular transport. J Pharmacol Exp Ther 252: 689–695, 1990 [PubMed] [Google Scholar]
- 39. Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol Renal Physiol 299: F1220–F1236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weinstein AM, Stephenson JL. Models of coupled salt and water transport across leaky epithelia. J Membr Biol 60: 1–20, 1981 [DOI] [PubMed] [Google Scholar]
- 41. Weinstein AM, Weinbaum S, Duan Y, Du Z, Yan Q, Wang T. Flow-dependent transport in a mathematical model of rat proximal tubule. Am J Physiol Renal Physiol 292: F1164–F1181, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Wiederkehr MR, Di Sole F, Collazo R, Quinones H, Fan L, Murer H, Helmle-Kolb C, Moe OW. Characterization of acute inhibition of Na/H exchanger NHE-3 by dopamine in opossum kidney cells. Kidney Int 59: 197–209, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Yamaguchi I, Jose PA, Mouradian MM, Canessa LM, Monsma FJ, Jr, Sibley DR, Takeyasu K, Felder RA. Expression of dopamine D1A receptor gene in proximal tubule of rat kidneys. Am J Physiol Renal Fluid Electrolyte Physiol 264: F280–F285, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Yun CH, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA 94: 3010–3015, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest 121: 2845–2854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]