Abstract
High sodium intake is known to regulate the renal renin-angiotensin system (RAS) and is a risk factor for the pathogenesis of obesity-related hypertension. The complex nature of the RAS reveals that its various components may have opposing effects on natriuresis and blood pressure regulation. We hypothesized that high sodium intake differentially regulates and shifts a balance between opposing components of the renal RAS, namely, angiotensin-converting enzyme (ACE)-ANG II-type 1 ANG II receptor (AT1R) vs. AT2-ACE2-angiotensinogen (Ang) (1–7)-Mas receptor (MasR), in obesity. In the present study, we evaluated protein and/or mRNA expression of angiotensinogen, renin, AT1A/BR, ACE, AT2R, ACE2, and MasR in the kidney cortex following 2 wk of a 8% high-sodium (HS) diet in lean and obese Zucker rats. The expression data showed that the relative expression pattern of ACE and AT1BR increased, renin decreased, and ACE2, AT2R, and MasR remained unaltered in HS-fed lean rats. On the other hand, HS intake in obese rats caused an increase in the cortical expression of ACE, a decrease in ACE2, AT2R, and MasR, and no changes in renin and AT1R. The cortical levels of ANG II increased by threefold in obese rats on HS compared with obese rats on normal salt (NS), which was not different than in lean rats. The HS intake elevated mean arterial pressure in obese rats (27 mmHg) more than in lean rats (16 mmHg). This study suggests that HS intake causes a pronounced increase in ANG II levels and a reduction in the expression of the ACE2-AT2R-MasR axis in the kidney cortex of obese rats. We conclude that such changes may lead to the potentially unopposed function of AT1R, with its various cellular and physiological roles, including the contribution to the pathogenesis of obesity-related hypertension.
Keywords: angiotensin receptors, angiotensin-converting enzymes, sodium, hypertension, obesity
the renin-angiotensin system (RAS) plays an important role in electrolyte and fluid homeostasis and blood pressure regulation (5). Changes in the expression and activity of various components of the RAS are implicated in the etiology of several cardiovascular and kidney diseases, including hypertension (40). ANG II is a major RAS peptide that exerts its actions via two receptors, namely, AT1R and AT2R, mediating opposing and counterbalancing effects (12, 41). AT1R mediates vasoconstriction, antinatriuresis, contributes to an increase in blood pressure, thirst, and release of vasopressin and aldosterone, fibrosis, cellular growth, and migration (12). AT2R mediates vasodilatation, inhibits cellular growth, promotes natriuresis, and potentially lowers blood pressure (4, 28). Angiotensin-converting enzyme (ACE) and ACE2 are two important enzymes that regulate the production of ANG II and other RAS peptides (25). ACE generates ANG II from its precursor ANG I, while ACE2 converts ANG I to angiotensinogen (1–9), i.e., Ang (1–9), and ANG II to Ang (1–7) (25). Ang (1–7), acting via the Mas receptor (MasR), is implicated in cardiovascular physiology and pathophysiology through actions that oppose AT1R functions (10, 12, 20, 22). Angiotensinogen serves as the primary RAS precursor, which is catalyzed to ANG I by renin activity.
The actions of RAS components are well balanced and tightly regulated; however, if the balance is disturbed due to genetic, environmental, and lifestyle factors, it contributes to various pathological effects including changes in blood pressure. Under pathological conditions, unbalanced and increased actions of renin, AT1R, and ACE can be deleterious if they are not balanced by the opposing actions of AT2R, ACE2 activity, and MasR. Since the level of ANG II may be affected by ACE vs. ACE2 activity and the cellular and physiological responses to ANG II may depend on the relative expression levels of AT1R, AT2R, and MasR, a comprehensive measurement of RAS components of opposing function may be better in predicting the role of the RAS in net pathophysiology. Furthermore, local RAS systems existing in different organ systems have unique physiological effects with independent regulation (11, 29). While a circulating RAS regulates systemic functions like vascular tone and blood pressure homeostasis, a local RAS system present in the kidney and heart operates in a complementary fashion with circulating RAS and has long-term effects on cardiovascular function and blood pressure control (29). In light of recent research developments, it is clear that while targeting AT1R, ACE, and renin to treat various renal and cardiovascular diseases, knowledge of the regulation of ACE2, AT2R, MasR, and Ang (1–7) may provide therapeutic relevance of these RAS components to renal and cardiovascular diseases (43).
Hypertension in obese humans and in animal models, including obese Zucker rats, has been attributed to increased activity of the RAS and to impairment of one or more renal mechanisms regulating sodium balance (1, 24, 42). While higher renal AT1R function is reported in obesity, upregulation of AT2R in the kidney cortical region and the protective role of AT2R against a blood pressure increase in obese Zucker rats have also been reported by our laboratory (15, 38). Inasmuch as dietary sodium is known to regulate renal RAS activity, a comprehensive regulation of RAS components in response to high sodium intake in obesity is not fully understood. We hypothesize that high sodium intake differentially regulates and shifts a balance between opposing components of the renal RAS, namely, ACE-ANG II-AT1R vs. AT2-ACE2-Ang (1–7)-MasR in obesity, thereby further contributing to the pathogenesis of hypertension. We tested this hypothesis in lean and obese Zucker rats placed on a high-sodium diet for 2 wk.
MATERIALS AND METHODS
Male lean and obese Zucker rats (11–12 wk of age) were obtained from Harlan (Indianapolis, IN) and housed in the University of Houston animal care facility. Various treatment groups included two groups of lean rats fed either a normal-sodium diet (NS; 0.4%) or a high-sodium diet (HS; 8% Na) and two groups of obese rats fed either NS or HS for 2 wk. The animals had free access to food and tap water and were maintained under a 12:12-h light-dark cycle. The Institutional Animal Care and Use Committee at the University of Houston approved the animal experimental protocols.
Blood Pressure
At the end of the 2-wk treatment, the rats were anaesthetized using Inactin (100 mg/kg body wt). Under anesthesia, the carotid artery was cannulated using PE-50 tubing for blood pressure measurement and connected to a data-acquisition system via a Grass PT 300 transducer. After 45 min of stabilization, the blood pressure was recorded.
Quantitative Real-Time RT-PCR
After blood pressure was measured, the rats were euthanized and the kidneys were extracted. The cortices, which contain kidney regions expressing all RAS components, were separated and used to isolate total RNA using an RNeasy kit (Qiagen, Valencia, CA). The integrity of isolated RNAs was checked on formaldehyde agarose gels, and concentrations were determined by measuring the absorbance at 260 nm (A260) in a U-2910 Hitachi Spectrophotometer (Hitachi High Technologies America). Total RNA (1 μg) was reverse-transcribed using random hexamers and multiscribe reverse transcriptase in a two-step RT-PCR reaction following the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA). Primers for angiotensinogen, renin, AT1A/BR; AT2R; ACE; ACE2, and MasR were designed using Primer Express software (Applied Biosystems, Foster City, CA) (Table 1). Quantitative PCR (qPCR) was performed using the SYBR Green detection system on an ABI Prism 7300 sequence detector (Applied Biosystems). Thermal cycling conditions included preincubation at 50°C for 2 min, DNA polymerase activation at 95°C for 1 min, and 40 PCR cycles for 15 s at 95°C and for 1 min at 60°C. The transcript levels were calculated at the cycle threshold values (CT), where the fluorescent signal was detected above background using the ABI Prism7300 SDS software (Version 1.4, Applied Biosystems). mRNA levels were normalized to a housekeeping gene, 18S rRNA, to control for RNA input and expressed in arbitrary units.
Table 1.
Primers used for quantitative real-time PCR (qRT-PCR)
| Gene | Gene ID | Forward | Reverse |
|---|---|---|---|
| AGT | NM_134432 | 5′-CCCTGAGCAGTCCGTTCCT-3′ | 5′-AAAGTGCAGCGCACCTGAGT-3′ |
| AT1A | NM_030985 | 5′-CAAGTCCCACTCAAGCCTGTC-3′ | 5′-TGTTATCCGAAGGCCGGTAA-3′ |
| AT1B | NM_031009 | 5′-CCTCCGCCGCACGAT-3′ | 5′-CCAGCCATTAGCCAGATGATG-3′ |
| AT2 | NM_012494 | 5′-TGCTGTTGTGTTGGCATTCA-3′ | 5′-ATCCAAGAAGGTCAGAACATGGA-3′ |
| ACE 1 | NM_012544 | 5′-TTTGCTACACAAATGGCACTTGT-3′ | 5′-CGGGACGTGGCCATTATATT-3′ |
| ACE 2 | NM_001012006 | 5′-TTGAACCAGGATTGGACGAAA-3′ | 5′-GCCCAGAGCCTACGATTGTAGT-3′ |
| Renin | NM_012642 | 5′-CAGGAACGATGACCTGTGCAT-3′ | 5′-CAGTGGGTGGTGGGATGTC-3′ |
| MasR | NM_001001506 | 5′-CACTGGCCCTCCTGATGAA-3′ | 5′-GGATGCCAGAATTGAACACAGA-3′ |
| 18 S | EU139318 | 5′-GTAGTCGCCGTGCCTACCAT-3′ | 5′-TCCGGAATCGAACCCTGA T-3′ |
| β-Actin | AF541940 | 5′-GGCCCAGTACGGCACAGT-3′ | 5′-CCACCGCCGTCTCACTCT-3′ |
AT1A, AT1B, and AT2: ANG II subtypes; ACE, angiotensin-converting enzyme; MasR, Mas receptor.
Western Blotting of RAS Components
The protein expressions of AT1R, AT2R, ACE, ACE2, and renin in the kidney cortex of various rat groups were determined by Western blotting. For this purpose, the kidney cortices were homogenized in the buffer containing 50 mM Tris, 10 mM EDTA, 1 mM PMSF, and a cocktail of protease inhibitors (aprotinin, calpain inhibitors, leupeptin, pepstatin, and trypsin inhibitor). Proteins in the homogenates were determined by the BCA method using a kit (Pierce, Rockford, IL). Equal amounts of protein, 30 μg for AT1R, 60 μg for AT2R, 50 μg for ACE and ACE2, and 30 μg for renin, from various rat groups were subjected to SDS-PAGE and electroblotted onto a polyvinylidene difluoride membranes. The blot was incubated with primary polyclonal antibodies for the AT1R, ACE, ACE2, renin, and AT2R, followed by washing and further incubating with horseradish peroxidase (HRP)-conjugated anti-rabbit or goat IgGs. The signal (densitometry of the band) was detected by the ECL system, recorded, and analyzed by FluorChem 8800 (Alpha Innotech, San Leandro, CA). For a loading control, the blots were stripped and reprobed with β-actin antibody. The resulting density values from each band were calculated for the significance among samples.
Sample Preparation for Liquid Chromatography/Mass Spectrometry
To determine ANG II levels quantitatively in the kidney cortex, 75 mg of tissue was homogenized in 500 μl of lysis buffer (10 mM Tris, pH 7.4) and centrifuged for 15 min at 4°C at 1,600 g. The resulting supernatant was loaded onto an C18-E (55 μm, 70 A) cartridge and equilibrated with 60% acetonitrile (ACN), 1% TFA, and 39% distilled water. After sample application, the C18-E cartridge was washed twice with 3 ml of 1% TFA. The column was eluted with the equilibrium buffer, and the eluent was collected in a 15-ml tube. The eluent was evaporated to dryness and reconstituted in 80% acetonitrile and 0.1% formic acid before liquid chromatography/mass spectrometry (LC-MS) analysis.
LC/MS Quantification
Protein samples were analyzed using the Ultra Performance LC System (ACQUITY, Waters, Milford, MA) coupled with a quadruple time-of-flight mass spectrometer (Q-TOF, Premier, Waters) with electrospray ionization (ESI) in both ESI+-MS and ESI+-MS/MS (SetMass without fragmentation) mode operated by Masslynx software (V4.1). Each sample, in H2O/acetonitrile, was directly injected into the ESI+ source at a flow rate of 50 μl/min with the mobile phase of 100% ACN or 50% ACN in water. The ion source voltages were set at 3 KV, the sampling cone at 37 V, and the extraction cone at 3 V. In both modes, the source and desolvation temperatures were maintained at 120 and 225°C, respectively, with the desolvation gas flow at 200 l/h. The TOF MS scanning was from 200 to 800 m/z at 1 s with a 0.1-s interscan delay using extended dynamic range acquisition with centriod data format. For real-time mass calibration, direct infusion of sodium formate solution (10% formic acid/0.1 M NaOH/ACN at a ratio of 1:1:8) at 1 s/10 s to the ion source at 2 μl/min was used. Ions of interest were analyzed for elemental composition using accurate mass measurement (<5 ppm error) and isotope modeling to identify the formula. Collision-induced dissociation (CID) by argon on precursor ions resulting in structural fragments further assisted the identification of selected ions.
Chemicals
Primers for qRT-PCR were purchased from Integrated DNA Technologies (San Diego, CA). The primary polyclonal antibodies for the AT1R (sc-1173), ACE (sc-20791), ACE2 (sc-20998), renin (sc-27320), β-actin (sc-47778), and HRP-conjugated anti-rabbit and anti-goat IgGs used as secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and the antibody for AT2R was custom-raised by EZ Biolabs (Westfield, IL).
Statistics
Values are means ± SE. The data were analyzed using GraphPad Prism 4 (GraphPad Software, San Diego, CA) and subjected to one-way ANOVA with a Newman-Keuls post hoc test and Student's unpaired t-test; n = 6–7/group, as detailed in the figure legends. A P value of <0.05 was considered statistically significant.
RESULTS
Quantitative Gene and Protein Expression of RAS Components
Angiotensinogen and renin.
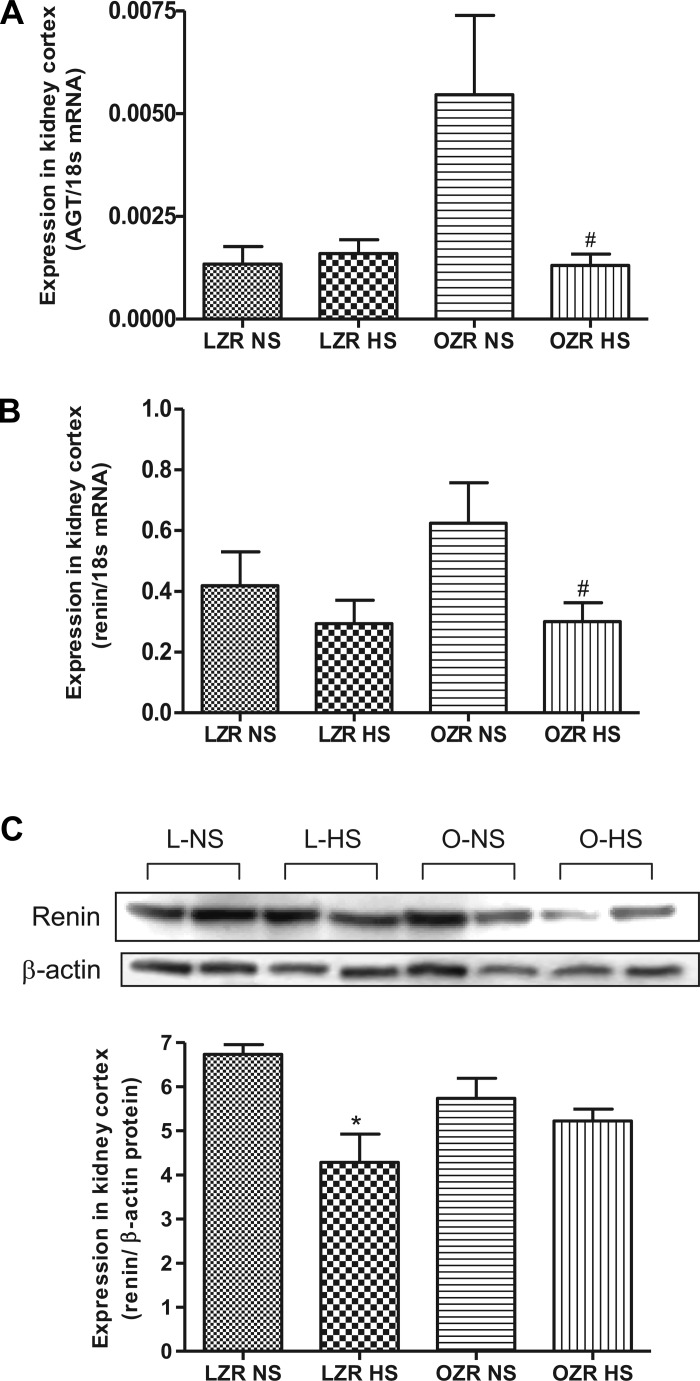
Angiotensinogen mRNA expression was significantly elevated in obese rats compared with lean rats. High sodium intake caused a significant reduction (P < 0.05) in angiotensinogen mRNA in obese rats but had no effect in lean rats (Fig. 1A). The renin mRNA expression showed no difference between lean and obese rats on the NS, but the HS diet had reduced (P < 0.05) the renin mRNA level in obese rats (Fig. 1B). In contrast, the renin protein expression was significantly reduced (P < 0.05) by HS diet in lean but not in obese rats (Fig. 1C).
Fig. 1.
Expression of angiotensinogen, renin in normal salt (NS)- and high salt (HS)-fed lean Zucker rats (LZR) and obese Zucker rats (OZR). A: quantitative expression level of angiotensinogen. B: quantitative expression of renin mRNA. C: protein expression of renin using Western blotting. Data were analyzed using 1-way ANOVA with a Newman-Keuls post hoc test; n = 6 to 7/group. #P < 0.05 compared with LZR NS.
ACE and ACE2.
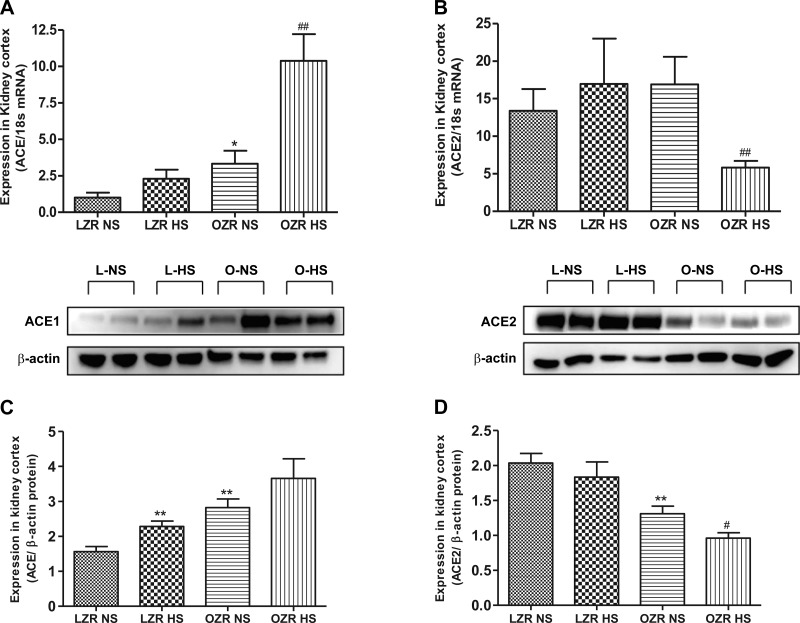
The expression of ACE mRNA was greater in obese than lean rats on NS (P < 0.05); HS intake further elevated (P < 0.0001) ACE level in obese rats (Fig. 2A). Similar to the mRNA expression, the ACE protein expression was higher (P < 0.01) in obese than lean rats on NS (Fig. 2B). Although the changes in protein expression of ACE in response to HS follows the same trend as gene expression, statistical analysis revealed that, unlike the mRNA level, ACE protein expression in response to HS was significantly elevated (P < 0.01) in lean rats but not in obese rats. The expression of ACE2 mRNA was significantly reduced (P < 0.001) in HS-fed obese rats (Fig. 2C). Compared with lean rats on NS, the expression of ACE2 protein in obese rats on NS was significantly lowered (P < 0.001) and was further reduced (P < 0.0001) in response to HS intake. Similarly, ACE2 mRNA expression in obese rats was reduced (P < 0.05) by HS intake (Fig. 2D).
Fig. 2.
Expression of angiotensin-converting enzyme (ACE) and ACE2 in NS- and HS-fed LZR and OZR. A: quantitative expression of the gene for ACE. B: quantitative expression of the gene for ACE2. C: expression of ACE protein using Western blotting. D: expression of ACE2 protein. Data were analyzed using 1-way ANOVA with a Newman-Keuls post hoc test; n = 6–7/group. *P < 0.05, **P < 0.01 compared with LZR NS. #P < 0.05, ##P < 0.01 compared with OZR NS.
AT1R, AT2R, and MasR.
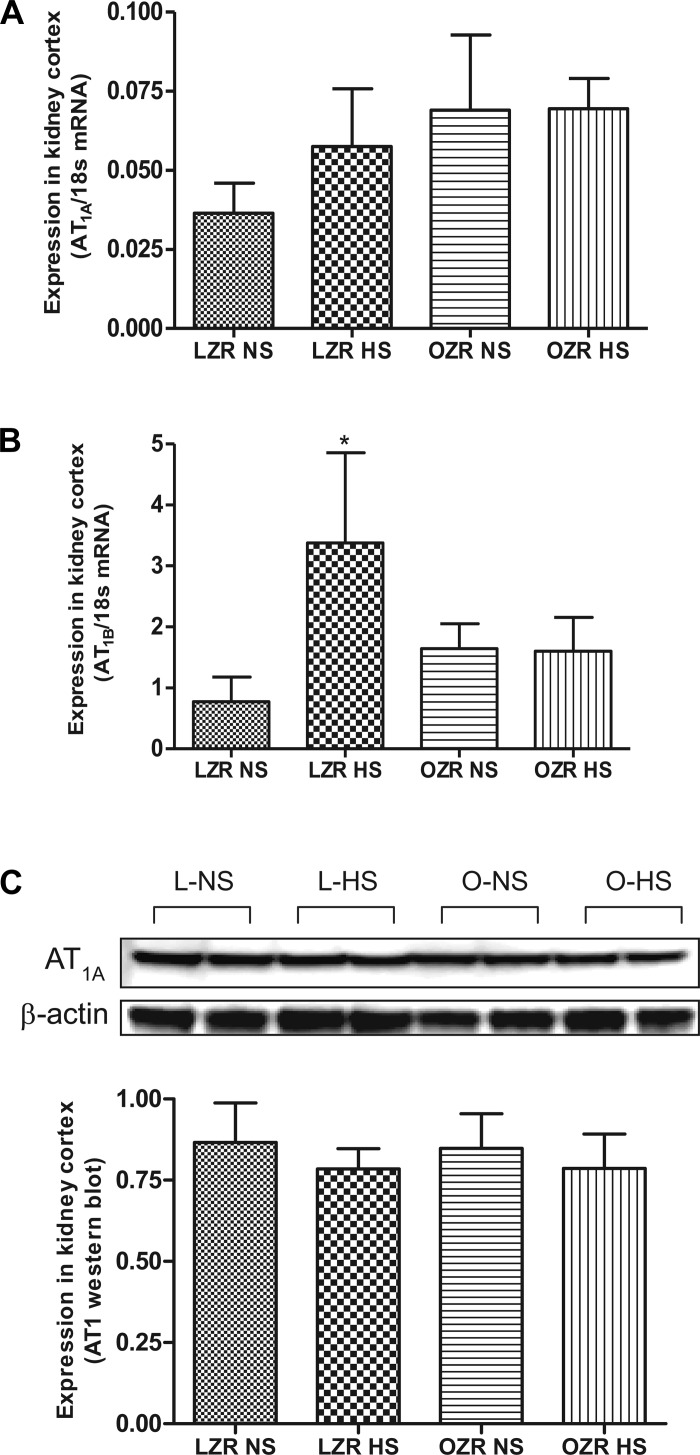
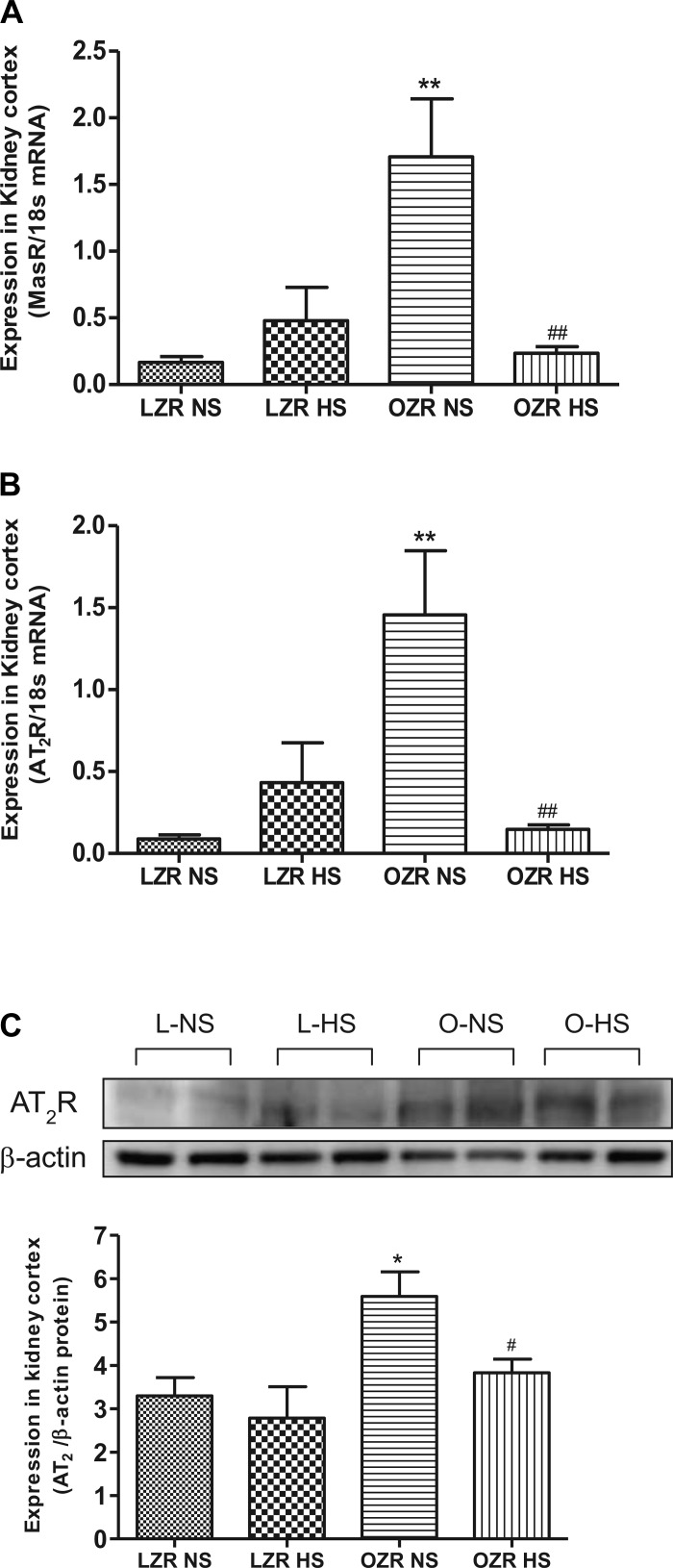
AT1AR mRNA expression was similar among four diet groups (Fig. 3A). AT1BR mRNA expression showed a significant increase (P < 0.05) in lean rats on HS compared with lean rats on NS (Fig. 3B). Similar to mRNA expression, AT1R protein expression did not differ among diet groups (Fig. 3C). AT1BR expression in obese rats on NS was similar to lean rats on NS and was not affected by HS. MasR mRNA expression was higher (P < 0.05) in obese than lean rats on NS, and the expression was significantly decreased (P < 0.05) in HS obese rats (Fig. 4A). Similar to MasR, the expression of AT2R (both mRNA and the protein) was significantly elevated (P < 0.05) in the obese rats on NS compared with lean rats on NS, and the expression was reduced (P < 0.05) in HS obese rats (Fig. 4, B and C).
Fig. 3.
Expression of ANG II receptors (AT1AR and AT1BR) in NS- and HS-fed LZR and OZR. A: quantitative gene expression of AT1AR. B: quantitative gene expression of AT1BR. C: expression of AT1R protein by Western blotting. Data were analyzed using 1-way ANOVA with a Newman-Keuls post hoc test; n = 6–7/group. *P < 0.05 compared with LZR NS.
Fig. 4.
Expression of Mas receptor (MasR) and AT2R in NS- and HS-fed LZR and OZR. A: quantitative expression pattern of MasR gene. B: quantitative gene expression of AT2R. C: Western blot analysis of AT2R. Data were analyzed using 1-way ANOVA with a Newman-Keuls post hoc test; n = 6–7/group. *P < 0.05, **P < 0.01 compared with LZR NS. #P < 0.05, ##P < 0.01 compared OZR NS.
ANG II and Ang (1–7)
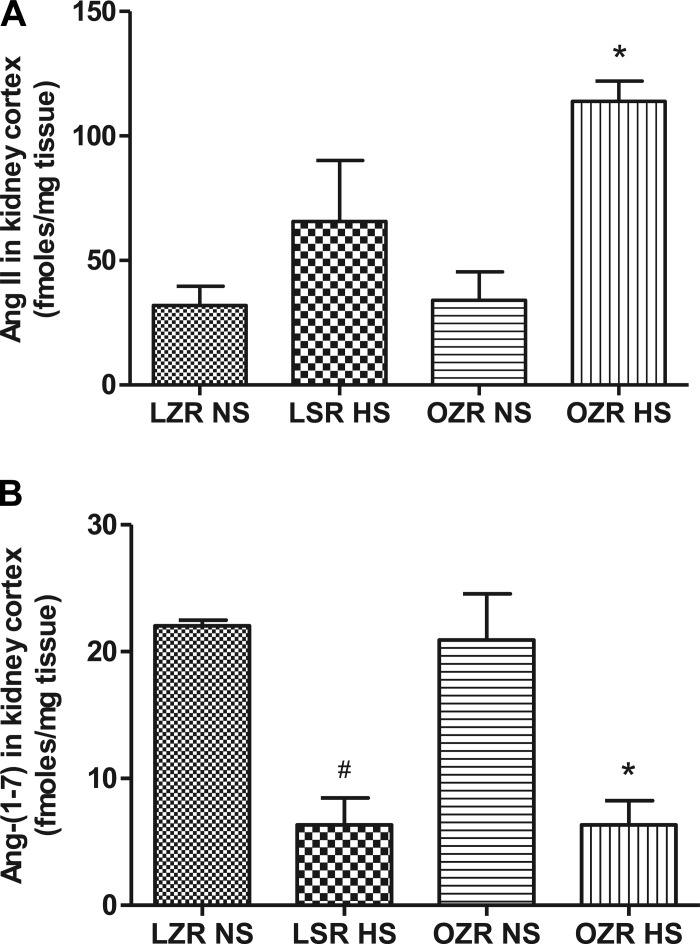
The level of ANG II in the lean and obese rats on NS was similar. However, the ANG II levels in the kidney cortex were significantly elevated (P < 0.0001) in obese rats on HS than in obese rats on NS (Fig. 5A). The Ang-(1–7) level was similar in lean and obese rats on NS but was significantly reduced (P < 0.0001) in both rat strain groups on HS (Fig. 5B).
Fig. 5.
Liquid chromatography/mass spectrometry (LC/MS) quantification of ANG II and angiotensinogen (1–7) [Ang (1–7)] in the kidney cortex of NS- and HS-fed LZR and OZR. Data were analyzed using 1-way ANOVA with a Newman-Keuls post hoc test; n = 3–7/group. *P < 0.05 compared with OZR NS. #P < 0.05 compared with LZR NS.
Mean Arterial Pressure
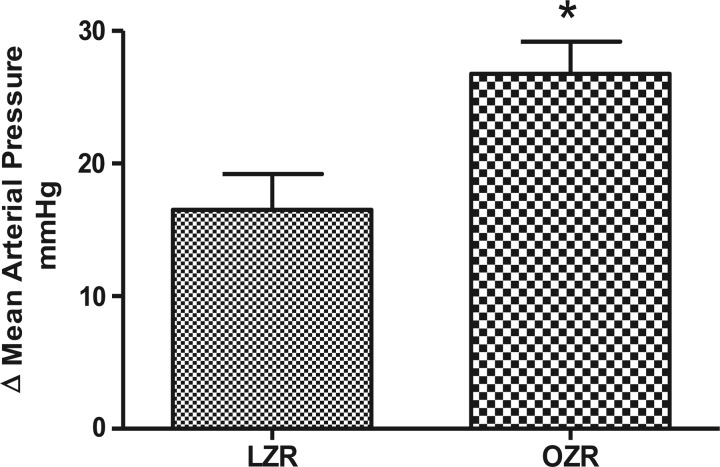
Mean arterial pressure (MAP) in the lean rats on NS was 94 ± 3.5 mmHg, which was increased by 16 mmHg in the HS group. The blood pressure in the obese rats on NS was 126 ± 2 mmHg and was 153 ± 3 mmHg in the HS-fed group. However, the net change in MAP between the obese rats on NS and obese rats on HS (27 ± 2.4) was higher (P < 0.05) than that between lean rats on NS and lean rats on HS (16.5 ± 2.7) (Fig. 6).
Fig. 6.
Change in the mean arterial blood pressure in NS- and HS-fed LZR and NS- and HS-fed OZR. Data were analyzed using 1-way ANOVA with a Newman-Keuls post hoc test; n = 6–7/group. *P < 0.05 compared with LZR NS.
DISCUSSION
Hyperactivity of the RAS, genetic or in response to external changes influencing blood pressure, has been observed in a number of animal models, including obese Zucker rats (23, 26, 30, 34). However, in a multicomponent system such as RAS, hyperactivity may not be a straightforward increase in expression/activity of its components but rather a shift in the net balance of components having potentially harmful consequences and those mediating protective actions. A local RAS expressed in the kidneys can operate independently of a systemic RAS and is believed to contribute to long-term blood pressure control and renal injury associated with obesity (11, 29, 31). In pursuit of understanding renal RAS regulation, we measured various RAS components and compared their levels in lean and obese Zucker rats fed NS and HS diets.
Long-term blood pressure regulation is a multihormonal/factorial phenomenon. For example, reduced functions of atrial natriuretic factor (47) and the dopamine D1 receptor (18) and enhanced function of renal β-adrenergic receptors (1), the AT1 receptor (42), and the AT2 receptor in obesity have been reported (16). However, the RAS is a major player in obesity-related blood pressure control, and changes in RAS activity correlates well with blood pressure changes (2, 9). The goal of this study is to evaluate the expression of various components, not just the changes in renin expression, to predict the net outcome of RAS activity and its potential correlation with blood pressure changes in response to high sodium intake, which is a known risk factor for the pathogenesis of hypertension. We observed blood pressure in lean and obese rats that were fed a HS diet for 2 wk to be significantly elevated, the greater increase being in obese rats. While the increase in blood pressure correlates with the expression profiles of specific RAS components, different sets of RAS components are changing in lean rats and obese rats. In lean rats, renin expression is significantly lowered, possibly creating renin deficiency, which could have resulted in reduced ANG II production. However, the renin deficiency and unchanged ANG II production in lean rats on HS appears to be compensated by a simultaneous increase in ACE expression, shifting the ACE/ACE2 ratio, which is predominantly responsible for the net conversion of ANG I to ANG II and ANG II to Ang (1–7). Interestingly, despite the unchanged expression of ACE2 and its substrate ANG II, there was a significant decrease in Ang (1–7) in lean rats on HS intake. This unexpected decrease in Ang (1–7) levels can be explained by a possible decrease in a non-ACE2 pathway, producing Ang (1–7), such as neprilysin, prolyl-endopetidase, thimetoligopeptidase (35, 44, 46), and an increased breakdown to Ang (1–5) by the increased ACE expression as observed in this study. In terms of these hormone targets, we observed that AT1BR expression is increased in response to HS intake in lean rats, while AT2R and MasR remained unaffected. AT1BR cannot be pharmacologically distinguished from AT1AR, but knockout studies have revealed that the signaling mechanism and function of AT1BR are similar to human AT1R or rodent AT1AR (45). Similar to AT1AR, AT1BR has been implicated in vasoconstriction and hypertension (14, 27). Therefore, in the case of lean rats, we speculate that enhanced AT1BR with normal ligand ANG II concentration and reduced ligand Ang (1–7) with normal MasR expression following HS feeding disrupt the critical balance between ANG II-AT1R function and Ang (1–7)-MasR function and contribute to elevated blood pressure in lean animals (Table 2).
Table 2.
Relative changes in RAS component expression in lean and obese rats in response to HS
| Group | Renin | Angiotensinogen | ACE | ANG II | AT1A/B | AT2 | ACE2 | Ang (1–7) | MasR | Blood Pressure |
|---|---|---|---|---|---|---|---|---|---|---|
| Lean rats | ↓ | ↑ | ↑ | ↓ | ↑ | |||||
| Obese rats | ↑ | ↑↑ | ↓ | ↓ | ↓ | ↓ | ↑↑ |
RAS, renin-angiotensin system; HS, high salt intake; ↓= decrease; ↑= increase; ↑↑ = more pronounced increase. The indicated changes are compared with respective groups on NS.
The increase in blood pressure with a greater net change in MAP in obese rats appears to be far more complex (Table 2). It is likely that elevated blood pressure in HS-fed obese rats is due to elevated ACE expression with a concomitant decrease in the expression of AT2R, ACE2, and MasR. Many renal RAS components including AT2R play a role in lowering blood pressure in obese rats (17, 38). Recently, our laboratory has demonstrated that enhanced expression of AT2R in the kidney plays a compensatory and protective role in long-term blood pressure regulation in obese rats by promoting natriuresis and lowering kidney renin expression (38). Beneficial effects of AT2R have been observed in AT2R-lacking mice that have elevated basal blood pressure and an exaggerated pressor response to exogenous ANG II infusion compared with wild-type controls (19). Agonistic stimulation of AT2R is reported to decrease arterial pressure in normal rats and increase it in the presence of an AT2R-specific antagonist (3). While AT2R seems to have an important role in blood pressure regulation, the exact nature and extent under pathological conditions are unclear. However, we speculate that despite the lack of change in expression of AT1R, decreased AT2R expression in the kidney cortex may be causing a functional imbalance between AT1R and AT2R actions, leading to elevated blood pressure in HS-fed obese rats. Such an imbalance appears to be more significant in light of the fact that the AT2Rs have a protective role against a blood pressure increase in obese rats (34). In other words, although the reduction in AT2R expression in obese animals on HS did not fall below the expression in lean rats on NS, any reduction in AT2R expression may have potentially diminished its protective role against a blood pressure increase in obese rats on HS.
Reduced ACE2 protein expression in obese rats observed in our study is of particular significance since ACE2 is a negative regulator of blood pressure (8, 21, 32). Studies by Crackower et al. (7) using three different models of hypertension have shown an inverse relationship between ACE2 mRNA/protein levels and blood pressure. Additionally, since ACE and ACE2 are involved in generating and/or degrading the bioactive peptides of the RAS, levels of these peptidases predicted the occurrence of vasoconstriction over dilatation or the dominance of pathophysiological stimuli over beneficial conditions (6, 37). Analysis of angiotensin fragments suggested that greater expression of ACE or a lower expression of ACE2 might have resulted in lowering of Ang (1–7) and increasing of ANG II in the HS-fed obese rats. Thus the increase in bioavailability of ANG II for AT1R in the face of decreased expression of AT2R and MasR with reduced Ang (1–7) in HS-fed obese rats could potentially cause a greater impact on the blood pressure increase in obese rats.
Considering the changes in RAS components in lean and obese rats together, it is apparent that the net increase in AT1R function may have been the major outcome of these changes. However, the net increase in AT1R function would be more dramatic in the face of a reduction in the entire opposing axis of the RAS, i.e., AT2R-ACE2-Ang (1–7)-MasR, in obese rats on HS. This shift may have contributed to a greater blood pressure increase in obese animals than in lean animals on HS. There is evidence showing interregulation among RAS components (36). It has been shown that ANG II via AT1R decreases ACE2 expression in myocytes and fibroblasts (13), and an AT1R antagonist increases cardiac ACE2 expression and activity in normotensive animals (10). Also, there is evidence suggesting that blocking AT1R leads to an increase in cardiac AT2R expression (39). In our study, it is likely that the dramatic decrease in renal ACE2 expression in obese rats on HS may have been due to a pronounced increase in ANG II levels/AT1R function in the kidneys of these animals. Similarly, increased ANG II levels/AT1R function observed in our study might have caused a reduction in AT2R expression in obese rats on HS. Overall, it appears that the higher levels of ANG II may be the primary trigger leading to changes in other RAS components and shifting the balance toward prohypertensive mechanisms. Meanwhile, the causes of a high increase in ANG II levels in obese rats in response to HS remain a subject for further investigation. A study conducted by Riazi et. (33) in obese Zucker rats also supports that a HS diet increases blood pressure in obese rats; however, they did not report any change in blood pressure in lean Zucker rats on a HS diet. The discrepancy may lie in the fact that we measured blood pressure under anesthesia and placed animals on higher sodium (8%) diet for a longer period (14 days), whereas Riazi et al. measured blood pressure by telemetry in conscious animals placed on 4% sodium diet for 4 days.
In summary, a comprehensive profile of RAS component expression in the kidney cortex suggests that HS intake differentially regulates renin, ACE, ACE2, AT1R, AT2R, and MasR in lean rats and obese Zucker rats. Specifically, reduced cortical expression of the ACE2-AT2R-MasR axis and a concomitant increase in ANG II levels in obese rats constitute the basis of a shift in the functional aspects of AT1R, a pro-blood pressure increase component of RAS. Additionally, a significant reduction in AT2R and MasR expression in obese animals may affect other cellular changes such as cell growth and apoptosis, which may have more than the opposing hemodynamic effect of AT1R. Our findings warrant functional studies to correlate the changes in various RAS components and elucidate the underlying mechanism of changes in obesity.
GRANTS
This work was supported by the Advanced Research Program-Higher Education Coordinating Board (Texas) and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-61578 (to T. Hussain).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: P.S. and T.H. provided conception and design of research; P.S., Q.A., R.S., and Y.W. performed experiments; P.S. and Q.A. analyzed data; P.S. and Q.A. prepared figures; P.S. drafted manuscript; P.S. and T.H. edited and revised manuscript; Q.A., Y.W., and T.H. interpreted results of experiments; T.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors carried out the major part of this study during their tenure at the Department of Pharmacological and Pharmaceutical Sciences, University of Houston College of Pharmacy (Houston, TX).
REFERENCES
- 1. Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension 28: 1047–1054, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Aneja A, El-Atat F, McFarlane SI, Sowers JR. Hypertension and obesity. Recent Prog Horm Res 59: 169–205, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Carey RM, Howell NL, Jin XH, Siragy HM. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension 38: 1272–1277, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Carey RM, Padia SH. Angiotensin AT2 receptors: control of renal sodium excretion and blood pressure. Trends Endocrinol Metab 19: 84–87, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 24: 261–271, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Castro-Chaves P, Cerqueira R, Pintalhao M, Leite-Moreira AF. New pathways of the renin-angiotensin system: the role of ACE2 in cardiovascular pathophysiology and therapy. Expert Opin Ther Targets 14: 485–496 [DOI] [PubMed] [Google Scholar]
- 7. Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Der Sarkissian S, Huentelman MJ, Stewart J, Katovich MJ, Raizada MK. ACE2: a novel therapeutic target for cardiovascular diseases. Prog Biophys Mol Biol 91: 163–198, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med (Berl) 79: 21–29, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 111: 2605–2610, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Fleming I, Kohlstedt K, Busse R. The tissue renin-angiotensin system and intracellular signalling. Curr Opin Nephrol Hypertens 15: 8–13, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Fyhrquist F, Saijonmaa O. Plasma renin activity: an assay with ongoing clinical relevance. Clin Chem 54: 1400, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol 295: H2373–H2379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gurley SB, Le TH, Coffman TM. Gene-targeting studies of the renin-angiotensin system: mechanisms of hypertension and cardiovascular disease. Cold Spring Harb Symp Quant Biol 67: 451–457, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension 45: 270–275, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol 290: F503–F508, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hussain T. Renal angiotensin II receptors, hyperinsulinemia, and obesity. Clin Exp Hypertens 25: 395–403, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Hussain T, Beheray SA, Lokhandwala MF. Defective dopamine receptor function in proximal tubules of obese Zucker rats. Hypertension 34: 1091–1096, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, Niimura F, Ichikawa I, Hogan BL, Inagami T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature 377: 748–750, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1–7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 289: H1013–H1019, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Ingelfinger JR. Angiotensin-converting enzyme 2: implications for blood pressure and kidney disease. Curr Opin Nephrol Hypertens 18: 79–84, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 43: 970–976, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens 18: 431–440, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Kurtz TW, Morris RC, Pershadsingh HA. The Zucker fatty rat as a genetic model of obesity and hypertension. Hypertension 13: 896–901, 1989 [DOI] [PubMed] [Google Scholar]
- 25. Mizuiri S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis 51: 613–623, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Nicholls MG, Richards AM, Agarwal M. The importance of the renin-angiotensin system in cardiovascular disease. J Hum Hypertens 12: 295–299, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Oliverio MI, Best CF, Kim HS, Arendshorst WJ, Smithies O, Coffman TM. Angiotensin II responses in AT1A receptor-deficient mice: a role for AT1B receptors in blood pressure regulation. Am J Physiol Renal Physiol 272: F515–F520, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Padia SH, Kemp BA, Howell NL, Fournie-Zaluski MC, Roques BP, Carey RM. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension 51: 460–465, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Pinto YM, Buikema H, van Gilst WH. Hyperactive tissue renin-angiotensin systems in cardiovascular dysfunction: experimental evidence and clinical hypotheses. Clin Exp Hypertens 17: 441–468, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension 45: 9–14, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Raizada MK, Ferreira AJ. ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol 50: 112–119, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Riazi S, Khan O, Hu X, Ecelbarger CA. Aldosterone infusion with high-NaCl diet increases blood pressure in obese but not lean Zucker rats. Am J Physiol Renal Physiol 291: F597–F605, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Rothermund L, Paul M. Hypertension and the renin-angiotensin system–evidence from genetic and transgenic studies. Basic Res Cardiol 93, Suppl 2: 1–6, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Schulz R, Sakane Y, Berry C, Ghai R. Characterisation of neutral endopeptidase 3.42411 (NEP) in the kidney: comparison between normotensive, genetically hypertensive and experimentally hypertensive rats. J Enzyme Inhib 4: 347–358, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Schunkert H, Ingelfinger JR, Hirsch AT, Pinto Y, Remme WJ, Jacob H, Dzau VJ. Feedback regulation of angiotensin converting enzyme activity and mRNA levels by angiotensin II. Circ Res 72: 312–318, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Shi L, Mao C, Xu Z, Zhang L. Angiotensin-converting enzymes and drug discovery in cardiovascular diseases. Drug Discov Today 15: 332–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siddiqui AH, Ali Q, Hussain T. Protective role of angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension 53: 256–261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sim MK, Chen WS. Effects of losartan on angiotensin receptors in the hypertrophic rat heart. Regul Pept 137: 140–146, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Siragy HM. The angiotensin II type 2 receptor and the kidney. J Renin Angiotensin Aldosterone Syst 11: 33–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siragy HM, de Gasparo M, Carey RM. Angiotensin type 2 receptor mediates valsartan-induced hypotension in conscious rats. Hypertension 35: 1074–1077, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Tallam LS, Jandhyala BS. Significance of exaggerated natriuresis after angiotensin AT1 receptor blockade or angiotensin- converting enzyme inhibition in obese Zucker rats. Clin Exp Pharmacol Physiol 28: 433–440, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Tan Z, Wu J, Ma H. Regulation of angiotensin-converting enzyme 2 and Mas receptor by Ang-(1–7) in heart and kidney of spontaneously hypertensive rats. J Renin Angiotensin Aldosterone Syst 12: 413–419, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Velez JC, Bland AM, Arthur JM, Raymond JR, Janech MG. Characterization of renin-angiotensin system enzyme activities in cultured mouse podocytes. Am J Physiol Renal Physiol 293: F398–F407, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Wang CH, Li F, Takahashi N. The renin angiotensin system and the metabolic syndrome. Open Hypertens J 3: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Welches WR, Brosnihan KB, Ferrario CM. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci 52: 1461–1480, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Zeigler DW, Patel KP. Reduced renal responses to an acute saline load in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 261: R712–R718, 1991 [DOI] [PubMed] [Google Scholar]