Abstract
Ectonucleoside triphosphate diphosphohydrolase-1 hydrolyzes extracellular ATP and ADP to AMP. Previously, we showed that CD39 is expressed at several sites within the kidney and thus may impact the availability of type 2 purinergic receptor (P2-R) ligands. Because P2-Rs appear to regulate urinary concentrating ability, we have evaluated renal water handling in transgenic mice (TG) globally overexpressing hCD39. Under basal conditions, TG mice exhibited significantly impaired urinary concentration and decreased protein abundance of AQP2 in the kidney compared with wild-type (WT) mice. Urinary excretion of total nitrates/nitrites was significantly higher in TG mice, but the excretion of AVP or PGE2 was equivalent to control WT mice. There were no significant differences in electrolyte-free water clearance or fractional excretion of sodium. Under stable hydrated conditions (gelled diet feeding), the differences between the WT and TG mice were negated, but the decrease in urine osmolality persisted. When water deprived, TG mice failed to adequately concentrate urine and exhibited impaired AVP responses. However, the increases in urinary osmolalities in response to subacute dDAVP or chronic AVP treatment were similar in TG and WT mice. These observations suggest that TG mice have impaired urinary concentrating ability despite normal AVP levels. We also note impaired AVP release in response to water deprivation but that TG kidneys are responsive to exogenous dDAVP or AVP. We infer that heightened nucleotide scavenging by increased levels of CD39 altered the release of endogenous AVP in response to dehydration. We propose that ectonucleotidases and modulated purinergic signaling impact urinary concentration and indicate potential utility of targeted therapy for the treatment of water balance disorders.
Keywords: ectonucleotidases, extracellular nucleotides, arginine vasopressin, nitric oxide, nucleoside triphosphate diphosphohydrolase-1, prostaglandins, desmopressin
extracellular nucleotides, acting through type 2 (P2) purinergic receptors, regulate renal tubular transport of water, sodium, and urea and thereby urinary concentrating ability (9, 13, 17, 21–23). P2 receptor signal modulation is tightly controlled by the availability and concentrations of extracellular ATP and related nucleotides, which are physiologically in low micromolar concentrations. This is achieved by regulated release of nucleotides from the cells and then rapid hydrolysis of released nucleotides by ectonucleotidases (12, 16). Furthermore, the activities of ectonucleotidases are often coupled with the activity of ecto-5′-nucleotidases (CD73). CD73 releases adenosine, which is a potent agonist of P1 receptors. In many tissues, activation of P1 receptors opposes the cellular responses elicited by the stimulation of P2 receptors (10).
Several types of ectonucleotidases have been cloned and characterized (3, 24). Nucleoside triphosphate diphosphohydrolases (NTPDases) are a family of surface-located enzymes that sequentially hydrolyze extracellular nucleotides and thus limit P2 receptor activities and desensitization. NTPDase1 is identical to CD39 and hydrolyzes ATP and ADP to AMP, whereas NTPDase2 (CD39-like protein-1; CD39L1) preferentially converts ATP to ADP. Previously, we localized the mRNA and protein expression of NTPDase1 (CD39) and NTPDase2 (CD39L1) in murine (rat and mouse) kidneys, using the approach of in situ hybridization and immunohistochemistry, respectively. We documented that NTPDase1 is expressed in vasculature and several tubular structures in the kidney (8). In the renal cortex, NTPDase1 is expressed by vascular smooth muscle cells and endothelium in interlobular arteries, afferent glomerular arterioles, and peritubular capillaries. Within the renal medulla, NTPDase1 is expressed in ascending thin limbs of Henle's loop (juxtaposed to collecting ducts), ducts of Bellini, and pelvic wall but not in vasa recta (8). Since NTPDase1 is an ectonucleotidase expressed on the surface of cells with its catalytic domain oriented to the exterior of the cells, it can effectively hydrolyze extracellular ATP or other nucleotides when they are released into the tiny extracellular spaces.
Since the distribution pattern of NTPDase1 in the kidney largely parallels to the known distribution of P2 receptors in the kidney, we hypothesized that altered expression of NTPDase1 (CD39) may affect the function of P2 receptors by modulating the availability of extracellular nucleotides. To address this possibility, we utilized a transgenic mouse model developed by us, where human CD39 (hCD39) gene has been globally overexpressed (4). Based on the known effects of purinergic signaling mediated by extracellular nucleotides in the kidney (9, 13, 21–23), we postulated that overexpression of hCD39 should result in increased urinary concentrating ability in the transgenic (TG) mice compared with the wild-type (WT) mice. However, contrary to our expectations, we found that overexpression of hCD39 actually impaired the urinary concentrating ability of the TG mice. Here, using the approach of whole animal physiology and analysis of urine and kidney tissue, we document the impaired urinary concentrating ability in hCD39 TG mice and provide significant insights into the apparent cause(s) for that impairment. This study also suggests that native or engineered ectonucleotidases might be considered to modulate urinary concentration defect in clinically relevant conditions.
METHODS
Experimental Animals
Generation and characterization of TG mice overexpressing hCD39 were reported previously (4). Briefly, these mice were generated using a construct containing the murine H-2kb (MHC class) promoter to drive expression of hCD39 in C57BL/6 mice. Immunohistochemical examination revealed widespread expression of hCD39 in the kidney and other organs. CD39 activity was also significantly higher in different organs of the TG mice. Breeding colonies of hCD39 TG and WT mice were established in the Veterinary Medical Unit of the Veterans Affairs (VA) Salt Lake City Health Care System. TG mice bred were confirmed by PCR on tail DNA using primer pairs specific for hCD39. Breeding and animal procedures described in this communication were approved by the Institutional Animal Care and Use Committee of the VA Salt Lake City Health Care System.
Study Protocols
Mice aged between 2 and 4 mo were used in the studies. To evaluate the urinary concentrating ability, the mice were studied under eight types of experimental conditions as follows.
Basal conditions.
WT and TG mice (n = 10 mice/genotype) were acclimiated to metabolic cages for 3 consecutive days (1 mouse/cage), with free access to drinking water and standard chow. Urine output and osmolality during the last 24 h were recorded.
Stable hydrated conditions.
Groups of TG and WT mice were acclimated to metabolic cages (1 mouse/cage) and fed a gelled diet containing a fixed proportion of nutrients and water as the sole ration for 7 days while they remained in the metabolic cages. The gel contained all of the nutrients and normal content of water (60% by weight of the gel) by having a defined quantity of PMI Micro-Stabilizer Rodent Powdered Diet (LD101; LabDiet, Richmond, IN) reconstituted, as described previously (29). Twenty-four-hour urine output and osmolalities on days 6 and 7 were recorded and the data averaged. Data from three independent experiments were pooled to obtain n = 15 mice/genotype.
Acute water loading.
WT and TG mice were deprived of water overnight so that acute water loading could be performed the next morning. This ensured a comparable state of hydration in all mice with empty urinary bladders just prior to acute water loading. On the morning of the experimental day, each mouse was injected intraperitoneally with 2 ml of sterile water and then placed on wire mesh in a small custom-made plastic metabolic cage (1 mouse/cage) that contained no food or water for 6 h. The lower chamber contained a layer of light mineral oil to enable the collection of small drops of urine that fall down. Urine drops accumulated in the lower chamber under the oil were collected every 2 h for up to 6 h and stored in separate microtubes yielding three pools corresponding to the time periods of 0–2, 2–4, and 4–6 h. The volume and osmolality of each pool of urine sample collected were determined. Based on the urine output and osmolality, the osmolar excretion was calculated for each time period. Data from two independent experiments were pooled to obtain n = 9 for WT and n = 10 for TG mice.
Chronic water loading.
After the mice were acclimated to metabolic cages for 2 days (1 mouse/cage), chronic water loading was achieved by feeding WT and TG mice with a gel diet containing high water content (78% by weight of the gel) as the sole source of food and water for 7 days while the mice remained in the metabolic cages. To make the mice eat this gel diet in sufficient quantities needed to maintain their nutritional status, it was flavored with bacon fat. Daily water intake through the gel, urine output, and urine osmolality were monitored, and the data obtained on the last 2 days were averaged.
Water restriction.
WT and TG mice (n = 5 mice/genotype) were acclimated to metabolic cages (1 mouse/cage) and fed a normal gel diet for 7 days to attain stable hydrated conditions as described above under the protocol. Following the stabilization period, the diet was changed to a low water-containing (47% by weight of the gel) gelled diet for 3 consecutive days. Daily consumption of gel (and thus water intake), urine output, and urine osmolality were monitored all through the experimental period while the mice remained in the metabolic cages.
Water deprivation.
Groups of WT and TG mice (n = 10 mice/genotype) kept in metabolic cages (1 mouse/cage) were allowed to have free access to standard solid chow and drinking water for 3 days. After collecting 24-h baseline urine samples during the last 2 days, the water bottles were removed for a 24-h period, leaving the mice to sustain on the solid food only. Twenty-four-hour urine samples were also collected during the water deprivation period.
Subacute effect of desamino-8-d-arginine vasopressin treatment.
Osmolalities of spot urine samples collected under basal conditions or following the injection of desamino-8-d-arginine vasopressin (dDAVP) were used to study the subacute effect of dDAVP treatment. Groups of WT and TG mice were injected subcutaneously with sterile normal saline, dDAVP, or desmopressin (1 mg/kg body wt; Sigma Chemical, St. Louis, MO). Then, each mouse was kept on wire mesh in a custom-made plastic metabolic cage for 4 h (1 mouse/cage) for periodic collection of urine samples under light mineral oil. Samples collected at different time points were stored in separate microtubes, and their osmolalities were determined. Data from two independent experiments were pooled to obtain n = 12 mice/genotype.
Chronic dDAVP infusion.
Groups of WT and TG mice (n = 5 mice/genotype) were kept in metabolic cages (1 mouse/cage) for 2 days. Baseline 24-h urine samples were collected during this period. Then, the mice were chronically infused with dDAVP (desmopressin; 1 ng/h) for 5 days by surgical implantation of osmotic minipumps under isofluorane anesthesia. Mice were returned to metabolic cages (1 mouse/cage) after surgery. Twenty-four-hour urine samples were collected on each day until day 5 postimplantation of the pumps, and the increase in urine osmolality was monitored. All through the experimental period the mice had free access to solid chow and drinking water.
Sample Collection and Analysis
Consumption of food and water, urine output, and urine osmolality were monitored as described above. At the end of the experimental period, mice were euthanized and blood was collected. Kidneys were removed, and cortices and medullas were dissected out, flash-frozen in liquid nitrogen, and then stored at −80°C for analysis. Aliquots of urine samples were centrifuged to obtain clear supernatants. Osmolality of the clear urine supernatants were determined by vapor pressure method (Wescor, Logan, UT). Serum and urine sodium and potassium levels were measured on EasyElectrolytes (Medica, Bedford, MA). Total nitrate/nitrite contents of urine samples were determined by a commercial kit (Cayman Chemical, Ann Arbor, MI). Urinary excretion of prostaglandin E2 (PGE2) was determined using a prostaglandin E metabolite EIA kit (Cayman Chemical), as described previously (27, 28). Urinary excretion of AVP was determined using an EIA kit (Enzo Biochemicals, Farmingdale, NY). Serum and urine creatinine levels were determined by QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA). Fractional excretion of sodium was calculated using the formula FENa = 100 × (UNa × Pcr)/(PNa × Ucr), where UNa and PNa are urinary and plasma sodium levels and Pcr and Ucr are plasma and urinary creatinine levels. Electrolyte-free water clearance (CH2O[e]) was computed using the formula CH2O[e] = V (1 − UNa + UK/PNa), where V is urine volume, UNa and UK are urine sodium and potassium, respectively, and PNa is plasma sodium. A negative value for CH2O[e] indicates electrolyte-free water absorption, and a positive value indicates electrolyte-free water excretion by the kidney (1).
Western Blot Analysis of Tissue Samples
This was performed as described previously (27–29). Briefly, whole medullary tissue (outer + inner medulla) samples were homogenized in a buffer containing protease inhibitors. After the protein concentrations were determined, the homogenates were solubilized in Laemmli sample buffer. Quality of tissue sample preparation was assessed by staining loading gels with Coomassie blue (Gelcode Blue; Pierce Endogen, Rockford, IL). For immunoblotting, samples were loaded into individual lanes of 12% polyacrylamide gels and size fractionated by electrophoresis. After electrotransfer to nitrocellulose membranes, blots were probed with our own rabbit polyclonal antibodies to aquaporin-2 (AQP2) (29). Loading accuracy was evaluated by probing nitrocellulose membranes with β-actin monoclonal antibody (Biolegend, San Diego, CA). Chemilumnescence reaction was used to capture sites of antigen/antibody reaction on the X-ray films. Images were digitized, and pixel densities of the protein bands were determined using Un-Scan-It software (Silk Software, Orem, UT). Pixel densities of AQP2 bands were normalized to the pixel densities of the β-actin bands.
Quantitative Real-Time RT-PCR Assay of Tissue Samples
These were performed as per the methods established in our laboratory (26–28). Briefly, total RNA from whole medullary tissue samples was extracted by TRIzol method (Invitrogen, Carlsbad, CA). Traces of genomic DNA were removed, and RNA samples were transcribed by SuperScript Reverse Transcriptase II (Invitrogen) to obtain cDNA samples. Target genes in the cDNA samples were amplified (40 cycles) in Applied Biosystems 7500 Real-Time PCR system (Foster City, CA) using AmpliTaq Gold, and SYBR Green was used for detection. Table 1 shows the sequences of the primers, annealing temperatures, and amplicon sizes. Specificity of amplifications was assessed by sequencing the PCR products in the DNA core facility of the University of Utah and blasting them in the National Center for Biotechnology Information nucleotide database. Expression of target genes was computed relative to the expression levels of the housekeeping gene β-actin.
Table 1.
Nucleotide sequences of primer pairs used in PCR
| Gene | Accession No. | Primer Position | Primer Sequence | Annealing Temperature, °C | Amplicon Size, bp | Source |
|---|---|---|---|---|---|---|
| P2Y1 | NM_008772 | 1,263–1,282 | ACGTCCAATGATTACCTGCG | 60 | 288 | A |
| 1,532–1,551 | CCCTGTCGTTGAAATCACAC | |||||
| P2Y2 | NM_008773 | 802–822 | GCGTTTCCTCTTCTACACCAA | 60 | 156 | B |
| 940–957 | ACCAGCACCCACACAACC | |||||
| P2Y4 | NM_020621 | 200–219 | GCCAGAAGAAGCAGCAGAAC | 60 | 207 | B |
| 386–407 | TCAGAGGCAACAGGATGAACT | |||||
| P2Y6 | NM_183168 | 825–846 | TGCTTGGGTAGTGTGTGGAGTC | 60 | 338 | A |
| 1,142–1,163 | TGGAAAGGCAGGAAGCTGATGG | |||||
| A1 | NM_001008533 | 1,356–1,375 | TCCTGGCTCTGCTTGCTATT | 60 | 183 | B |
| 1,519–1,538 | AGGCTTGTTCCACCTCACTC | |||||
| A2a | NM_009630 | 695–715 | GTCCTCACGCAGAGTTCCAT | 60 | 181 | B |
| 858–876 | AGTTGTTCCAGCCCAGCAT | |||||
| A2b | NM_007413 | 303–321 | CCTTTGCCATCACCATCAG | 60 | 244 | B |
| 528–546 | CCCAGGAACGGAGTCAATC | |||||
| A3 | NM_009631 | 127–147 | GAGACCTGCATCCTCCAGGTT | 60 | 70 | C |
| 177–197 | GGCCTGTTACAGGACCATCAA | |||||
| β-Actin | NM_007393.2 | 1,034–1,054 | GCTCTGGCTCCTAGCACCATG | 60 | 73 | D |
| 1,089–1,108 | GCCACCGATCCACACAGAGT |
Statistical Analysis
Quantitative data are expressed as means ± SE. Differences between the means of two groups were determined by unpaired or paired t-test. Where applicable, Mann-Whitney nonparametric method was used. P values <0.05 were considered significant.
RESULTS
Basal Conditions
First, we studied the urinary concentrating ability of the TG mice under basal conditions free of experimental manipulations. Figure 1 shows water balance and AQP2 protein abundance in the kidney in WT and TG mice (n = 10 mice/genotype) under basal conditions with free access to solid chow and drinking water. As shown, the mean values of water intake and urine output were significantly higher (P < 0.04 and 0.002, respectively), and urine osmolality was significantly lower (P < 0.002) in TG mice compared with the WT mice. These alterations in water balance in TG mice were associated with a significant decrease in the protein abundance of AQP2 water channel in the cortex and medulla (P < 001) compared with the WT mice. Table 2 presents blood and urine parameters. Serum sodium and potassium levels in TG mice were significantly different from the corresponding values in WT mice (P = 0.019 and 0.027, respectively), although the observed differences were minor, and values were within the physiological limits. Despite the increased urine output, the electrolyte-free water excretion in TG mice is not significantly different from that in WT mice (P = 0.220). This may be due to the fact that the TG mice have a tendency for higher urinary excretion of sodium, although the differences between the WT and TG mice did not reach statistical significance (P = 0.067). As shown in Fig. 3, there were no significant differences in the urinary excretion of AVP between the WT and TG mice (P = 0.27), but the urinary excretion of PGE2 in TG mice has a tendency for lower mean value compared with the WT mice (P = 0.067). On the other hand, TG mice showed a significant, twofold higher urinary excretion of total nitrates/nitrites compared with the WT mice (P < 0.002).
Fig. 1.
Water balance, urinary parameters, and aquaporin-2 (AQP2) protein abundance in the kidneys of wild-type (WT) and human CD39 (hCD39)-transgenic (TG) mice under basal conditions. WT and TG mice were allowed to feed on standard solid chow with free access to drinking water in metabolic cages. After acclimation to the housing conditions for 2 days, 24-h water consumption, urine output, and urine osmolalities were recorded. Mice were euthanized, and kidney cortical and whole medullary (outer + inner) tissue samples were processed separately for semiquantitative immunoblotting for AQP2 protein and β-actin. A–C: water intake (A), urine ouput (B), and urine osmolality (C) in the WT and TG mice (n = 10 mice/genotype). D: representative immunoblots of AQP2 and β-actin in cortical and medullary tissue samples. Total AQP2 band densities (29 kDa native + 35–50 kDa glycosylated form) were determined for each mouse and normalized to the corresponding β-actin band densities. Values are converted to %mean values in WT mice and are shown in bar graphs adjacent to the corresponding immunoblots. Statistical analysis was performed by unpaired t-test, and the P values are shown above the bars (n = 5 mice/bar).
Table 2.
Blood and urine parameters under basal conditions
| Parameter | Wild Type | hCD39-TG | P Value |
|---|---|---|---|
| Serum sodium, mmol/l (n = 6) | 149 ± 0.2 | 148 ± 0.3 | 0.019* |
| Serum potassium, mmol/l (n = 6) | 3.6 ± 0.07 | 3.9 ± 0.10 | 0.027* |
| Urine sodium, μmol/day (n = 10) | 185 ± 12 | 217 ± 16 | 0.067 |
| Urine potassium, μmol/day (n = 10) | 670 ± 42 | 736 ± 53 | 0.169 |
| FENa, % (n = 6) | 1.09 ± 0.04 | 1.16 ± 0.06 | 0.158 |
| CH2O[e], μl/min (n = 6) | −1.703 ± 0.232 | −1.970 ± 0.238 | 0.220 |
Values are means ± SE.
hCD39, human CD39; FENa, fractional excretion of sodium; CH2O[e], electrolyte-free water clearance.
Significantly different by unpaired t-test.
Fig. 3.
Urinary excretion of analog of vasopressin (AVP), prostaglandin E2 (PGE2), and metabolite and total nitrates/nitrites in WT and hCD39-TG mice under basal conditions and during water deprivation. After acclimation to housing conditions in metabolic cages, 24-h urine samples were collected from WT and TG mice under basal conditions (free access to chow and drinking water) and during the subsequent 24-h water deprivation period (free access to chow, but no drinking water). Data from 2 independent experiments were pooled (n = 10 mice/genotype). Urinary excretion of AVP (A), PGE2 metabolite (B), and total nitrates/nitrites (C) was determined and normalized to 20 g body wt to correct for small variations in the body weights among different mice. Statistical analysis was performed by unpaired t-test, and P values are shown above the bars. NO, nitric oxide.
Effect of Water Deprivation
To determine the peak concentrating ability of the TG mice, we subjected the WT and TG mice to water deprivation for 24 h. As shown in Fig. 2, 24-h water deprivation decreased urine output and increased urine osmolality in both WT and TG mice (n = 10 mice/genotype), but the differences in these variables between the genotypes remained significant (P < 0.001). Water deprivation also resulted in significantly lower responses in urinary AVP and PGE2 (P < 0.02 and 0.04, respectively) and a higher response in urinary total nitrate/nitrite (P < 0.03) in the TG mice compared with the WT mice (Fig. 3).
Fig. 2.
Effect of water deprivation on urine output and urine osmolality in WT and hCD39-TG mice. The effect of 24-h water deprivation in WT and TG mice was studied as described in methods. Data collected from 2 independent experiments were pooled (n = 10 mice/genotype). A and B: urine output and urine osmolality were monitored prior to and during 24-h water deprivation. Basal, data collected prior to water deprivation; WD, data collected during water deprivation. Statistical analysis was performed by unpaired t-test, and P values are shown above the bars.
Effect of Acute Water Loading
We determined the ability of TG mice to excrete an acute load of water as a function of time. As shown in Fig. 4 (A–C), following an acute water loading, during the first 2 h TG mice had significantly higher urine output and osmolar excretion compared with the WT mice (P < 0.04). The mean differences between these two genotypes during subsequent time periods were not significantly different despite the tendency for higher urine output and osmolality in some TG mice during the final 4- to 6-h period. As a result, the cumulative mean values of urine output, urine osmolality, and osmolar excretion between the WT and TG mice were not significantly different (Fig. 4, D–F).
Fig. 4.
Effect of acute water loading in WT and hCD39-TG mice. Acute water loading was performed in WT and TG by injecting 2 ml of sterile water intraperitoneally after an overnight water deprivation to ensure a comparable state of hydration and empty bladders, as described in methods. Data from 2 independent experiments were pooled (n = 9 WT mice and 10 TG mice). Urine output, urine osmolality, and osmolar excretion were monitored over a 6-h period. A–C: urine output (A), urine osmolality (B), and osmolar excretion (C) over a 6-h period in blocks of 2 h. D–F: cumulative values over a 6-h period for urine output (D), urine osmolality (E), and osmolar excretion (F). It should be noted that although 9 or 10 mice/group were used, all mice did not void urine during the period of every time block. Therefore, the no. of responding mice in each time block is shown in parentheses over the bars in A. Statistical significance for relevant pairs of data was assessed by Mann-Whitney nonparametric method, and the P values are shown above the bars.
Effect of Stable Hydrated Conditions
We compared the urinary concentrating ability of TG mice with WT mice (n = 15 mice/genotype) under stable hydrated conditions achieved by feeding a gelled diet with normal water content as the sole source of nutrition for 7 days. Figure 5 presents the water balance and AQP2 protein abundance in the medulla of WT and TG mice subjected to stable hydrated conditions. Under these conditions, the TG mice consumed significantly less amounts of water (P < 0.02), but had similar urine output as the WT mice (P = 0.37). However, the TG mice still had modest but significant decreases in urine osmolality (P < 0.04). In line with the water balance data, the mean values of AQP2 protein abundance in medulla were not significantly different between the WT and TG mice. Moreover, under stable hydrated conditions there were no significant differences in the urinary excretion of AVP or PGE2 between the WT and TG mice (data not shown here).
Fig. 5.
Water balance, urinary parameters, and AQP2 protein abundance in the medulla of WT and hCD39-TG mice under stable hydrated conditions. WT and TG mice were acclimated to metabolic cages and fed a gelled diet containing fixed proportions of nutrients and normal water content as the sole ration for 7 days. Twenty-four-hour water consumption, urine output, and urine osmolalities were monitored, and the values obtained during the last 2 days (days 6 and 7) were averaged and used in the computation of the results. Data from 3 independent experiments were pooled (n = 15 mice/genotype). A–C: water intake (A), urine ouput (B), and urine osmolality (C) in the WT and TG mice (n = 15 mice/group). Representative immunoblots of AQP2 and β-actin proteins in the medullary tissue and densitometric values are shown in A. Total AQP2 band densities (29 kDa native + 35–50 kDa glycosylated form) were determined for each mouse and normalized to the corresponding β-actin band densities. Values obtained for TG mice were converted to %mean values in WT mice and are shown (n = 5 mice/ggenotype). Statistical analysis was performed by unpaired t-test, and P values are shown above the bars.
Expression of P2Y and Adenosine Receptors
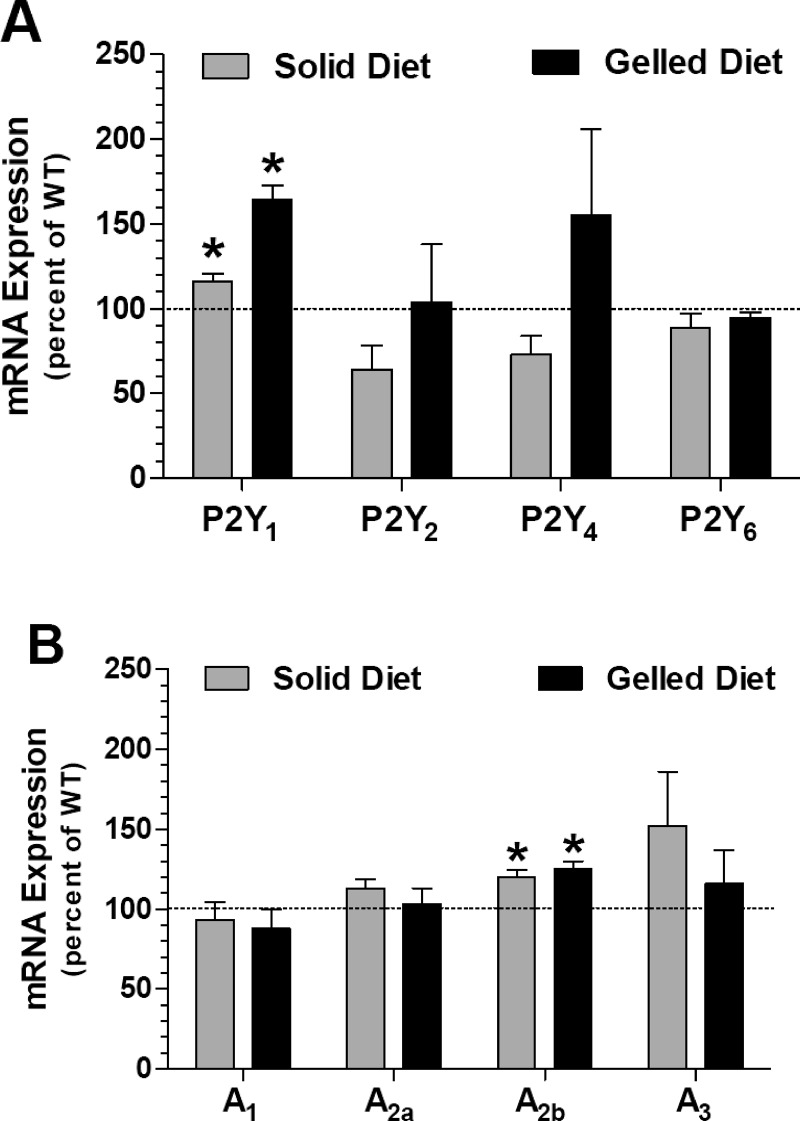
In view of the observed differences between the WT and TG mice under basal conditions and blunting of these differences under stable hydrated conditions, we determined the relative mRNA expression of P2Y receptors (P2Y1, P2Y2, P2Y4, and P2Y6) and adenosine receptors (A1, A2a, A2b, and A3) in the medullas of WT and TG mice (n = 5 mice/genotype) subjected to these two conditions. As shown in Fig. 6, only P2Y1 and A2b receptor expression were significantly higher in TG mice compared with WT mice, irrespective of the experimental conditions (P < 0.05 and 0.002 for P2Y1, for solid diet and gelled diet; P < 0.01 for A2b, for both solid and gelled diets). Thus, the experimental conditions (solid diet vs. gelled diet) per se have no significant effect on the expression of these receptors in TG mice vs. WT mice.
Fig. 6.
Relative mRNA expression of P2Y and adenosine receptors in the medulla of WT and hCD39-TG mice under basal conditions (solid diet) or stable hydrated conditions (gelled diet). WT and TG mice were allowed to feed on standard solid chow, with free access to drinking water in metabolic cages, or fed a gelled diet, as described in methods. Mice were euthanized, and kidney cortical and whole medullary (outer + inner) tissue samples were processed separately for RNA extraction, followed by real-time RT-PCR for P2Y receptors and adenosine receptors, as described in methods. The mRNA expression of target genes was expressed relative to the mRNA expression of β-actin in the samples. Means ± SE in TG mice were plotted as %corresponding mean values in WT mice. Statistical analysis was performed by unpaired t-test, and the P values are shown above the bars (n = 5 mice/genotype).
Effect of Water Restriction
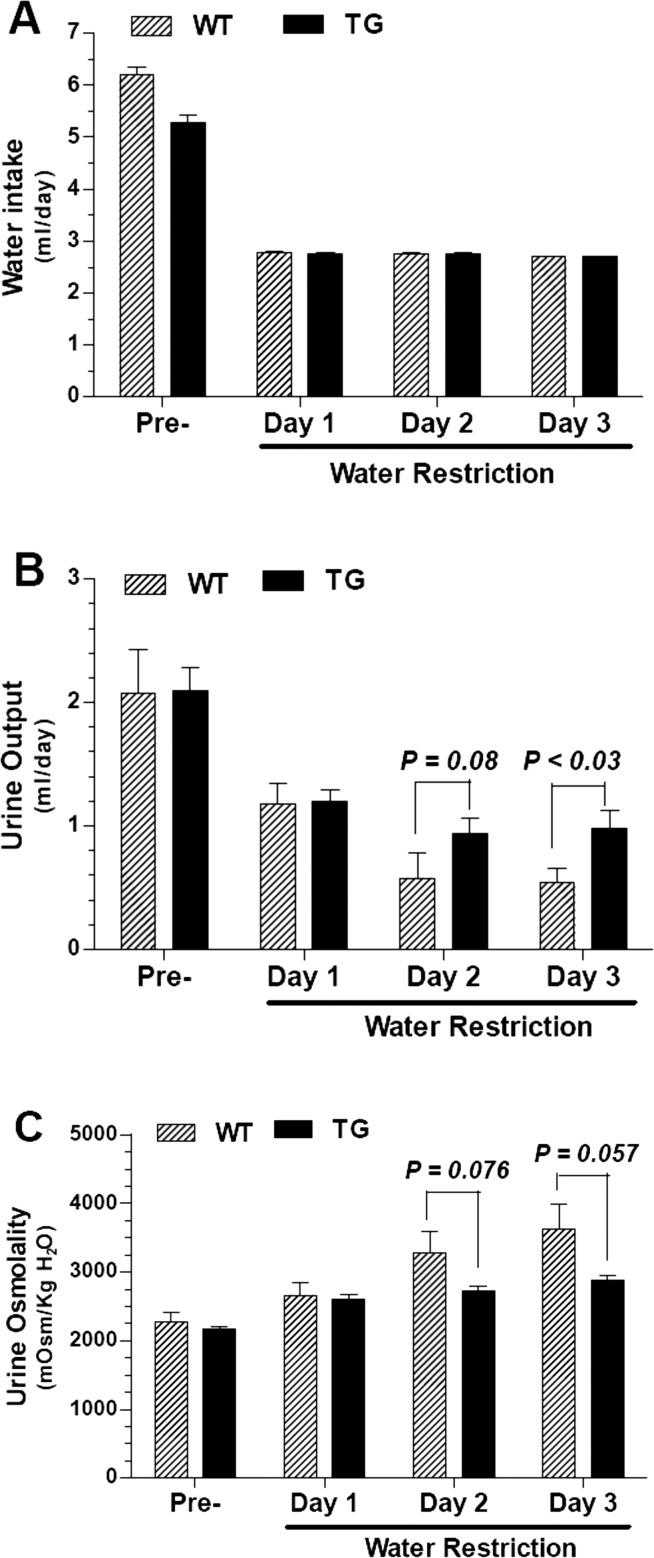
To examine the adaptation of the TG mice to reduced water intake, we subjected the WT and TG mice (n = 5 mice/genotype) to water restriction. Water restriction was achieved by reducing the water content of the gelled food after attaining stable hydrated conditions. As shown in Fig. 7, when subjected to water restriction, the TG mice showed a tendency for impaired urinary concentration, which is evident on day 3 as significantly higher urine output (P < 0.03), associated with a marginal increase in urine osmolality (P = 0.057).
Fig. 7.
Effect of water restriction in WT and hCD39-TG mice. The effect of water restriction for 3 days in WT and TG mice was performed by switching the mice from stable hydrated conditions (normal gelled diet) to a low-water-containing gelled diet, as described in methods. A–C: differences in water intake (A), urine output (B), and urine osmolality (C) in WT and TG mice prior to (Pre-) and during the 3 days of water restriction. Statistical analysis was performed by unpaired t-test, and the P values are shown above the bars (n = 5 mice/genotype).
Effect of Chronic Water Loading
To examine the adaptation of the TG mice to increased water intake, we subjected the WT and TG mice (n = 5 mice/genotype) to chronic water loading. Chronic water loading was achieved by feeding the high water-containing gelled diet as the sole ration for 7 days. As shown in Fig. 8, chronic water loading had a similar effect on WT and TG mice, with no significant differences in urine output or urine osmolality.
Fig. 8.
Effect of chronic water loading in WT and hCD39-TG mice. Chronic water loading was performed in WT and TG mice by giving a high-water-containing gelled diet as the sole ration for 7 days, as described in methods. Water intake, urine output, and urine osmolality were monitored. A–C: data collected on the last 2 days were averaged for each mouse and adjusted to 20 g body wt to correct for small variations in the body weights (n = 5 mice/genotype).
Effect of Subacute dDAVP Administration
To examine the response of the kidneys of TG mice for short-term or subacute effect of vasopressin, we injected the WT and TG mice (n = 12 mice/genotype) with dDAVP (desmopressin), a V2 receptor-specific analog of vasopressin (AVP). Figure 9 shows that both WT and TG mice responded to dDAVP with comparably significant increases in their urine osmolalities.
Fig. 9.
Effect of subacute desamino-8-d-arginine vasopressin (dDAVP) administration. The subacute effect of dDAVP over a 4-h period was evaluated in WT and TG mice by determining the increase in urine osmolalities of spot urine samples following the administration of dDAVP compared with the osmolalities of baseline spot urine samples. Data from 2 independent experiments were pooled (n = 12 mice/genotype). Mean values of osmolalities of spot urine samples under basal conditions (baseline) and the highest values attained for osmolality within 4 h of dDAVP administration (+dDAVP) in WT and TG mice are shown. Each bar represents the mean ± SE of 10–12 spot urine samples. It should be noted that baseline osmolalities of spot urine samples collected during the daytime are generally low compared with the osmolalities of 24-h urine collections (Fig. 1). Statistical analysis was performed by Mann-Whitney nonparametric method, and P values are shown above the bars.
Effect of Chronic dDAVP Administration
To examine the response of the kidneys of TG mice for long-term effect of vasopressin, we subcutaneously infused WT and TG mice (n = 5 mice/genotype) with dDAVP (desmopressin) for 5 days. As shown in Fig. 10, both WT and TG mice responded equally well to chronic dDAVP administration, with no significant differences in dDAVP-induced increases in urine osmolalities.
Fig. 10.
Effect of chronic dDAVP infusion. The chronic effect of dDAVP on urinary concentrating ability was evaluated in WT and TG mice by infusing dDAVP at a rate of 1 ng/h for 5 days. Osmolalities of urine samples were monitored daily. Mean urine osmolalities in WT and TG mice on day 0 (prior to the start of dDAVP infusion) and on days 3-5 of dDAVP infusion expressed as %changes over respective mean values on day 0 (100%; n = 5 mice/genotype) are shown.
DISCUSSION
In this communication, we demonstrate that global and widespread overexpression of NTPDase1/hCD39 results in defective renal water handling and failure to concentrate urine under conditions of water deprivation. Based on the outcome of different experimental manipulations to which these transgenic mice were subjected, it appears that alterations in both renal and central mechanisms may be responsible for the observed defective renal water handling in the TG mice. This is the first report of impaired urinary concentrating ability due to overexpression of an ectonucleotidase and opens up the possibility of exploring therapeutic potential of targeting ectonucleotidases for the treatment of water balance disorders.
Although in this study we have not documented that overexpression of hCD39 has actually led to an increased breakdown of extracellular ATP in renal tissue, it is reasonable to assume that this might be occurring for the following reasons. Our previous work on these TG mice showed increased levels of AMP and adenosine in the plasma following a proinflammatory insult (4), suggesting increased activity of hCD39 in these mice. We also showed that CD39 activity in the heart, lung, and pancreatic islests of TG mice was 1.8- to 2.4-fold higher compared with the wild-type mice (4). Furthermore, kidneys that overexpress hCD39 are protected from ischemia-reperfusion injury through adenosine A2a receptor-mediated mechanism (2), thus implicating adenosine generated from increased nucleotide hydrolysis as the effector molecule.
Under basal conditions, with free access to solid chow and drinking water, the TG mice clearly exhibited an impaired ability to void concentrated urine compared with the WT mice. This impaired urinary concentrating ability in TG mice under basal conditions is associated with a significant decrease in the protein abundance of AVP-regulated AQP2 water channel in renal medulla and cortex. However, the urinary excretion of AVP is not significantly different between the WT and TG mice, suggesting a renal but not a central mechanism for the observed impaired urinary concentrating ability. Furthermore, it is apparent that under basal conditions the TG mice had higher osmolar excretion, suggesting higher osmolar intake through food. However, such an increased osmolar load should have been handled by an increase in urine output, without a concomitant decrease in urine osmolality, if the kidneys of TG mice had intact concentrating ability. Therefore, the possibility that increased osmolar intake contributes to increased urine flow with decreased concentrating ability can be excluded. To explain this non-AVP-dependent mechanism of impaired urinary concentrating ability in TG mice, we determined the urinary excretion of PGE2 metabolite and total NO3/NO2. Both nitric oxide (NO) and PGE2 are known to modulate renal water handling (reviewed in Ref. 5). Although we did not find significant alterations in the urinary excretion of PGE2 in TG mice under basal conditions, the urinary total NO3/NO2 in TG mice showed a significant twofold increase compared with the WT mice. Evidence suggests that NO induces a cAMP-specific phosphodiesterase in rat pulmonary artery smooth muscle cells (1a). Based on this, one can expect that the increased production of NO in hCD39 TG mice should result in increased cellular cAMP levels, which in turn should increase the AQP2 protein abundance and urinary concentration. However, we observed that TG mice have an impaired ability to concentrate urine associated with significantly decreased AQP2 protein abundance in the kidney. Therefore, obviously the increased NO production in TG mice cannot explain the observed defect in urinary concentrating ability. On the other hand, it has been reported that endogenously produced NO may exert a tonic inhibitory effect on the expression of major sodium transporters such as Na-K-ATPase, NHE3, and NCC (7). Interestingly, in this context, we observed modest nonsignificant increases in both urinary sodium excretion and fractional excretion of sodium (FENa) in TG mice vs. WT mice. In view of this, it is possible that TG mice may have an impaired ability to generate medullary osmotic gradients to the same extent as the WT mice. However, although this aspect needs to be investigated further, it may not explain the decreased abundance of AQP2 protein in the kidneys of TG mice.
To gain further insights, we determined the mRNA expression of P2Y or P1 (adenosine) receptor subtypes and found significant increases in P2Y1 and adenosine A2b receptors in the TG mice. P2Y1 receptor expression in the renal medulla and in tubular cells has been confirmed in previous reports (13). It has been reported that activation of adenosine A2b receptor enhances chloride secretion in the mIMCD-K2 cell line, a murine model of immortalized renal inner medullary collecting duct cells (14). Those authors proposed that it is possible that in vivo setting signaling through the A2b receptor can result in a significant amount of NaCl secretion in the inner medullary collecting duct, especially during high dietary NaCl intake. The latter is known to elevate renal medullary adenosine levels. Hence, it is possible that increased production of adenosine coupled with upregulation of adenosine A2b receptor in TG mice might have contributed to the increased urinary excretion of sodium in the TG mice. This possibility needs to be investigated by approaches that are beyond the scope of this study.
Interestingly, when mice were subjected to stable hydrated conditions by feeding them a gelled diet with normal water content, the observed differences between the TG and WT mice, including the protein abundance of AQP2 in the medulla, were obliterated. However, the TG mice on the gelled diet continued to have a modest yet significant impairment in urinary concentrating ability.
Water deprivation is a method by which one can assess the intactness of both central and renal abilities and their cross-talk in water conservation. When water-deprived for 24 h, the TG mice were not able to reduce urine output or raise urine osmolality to the same extent as the WT mice. Although the possibility of higher osmolar intake by the TG mice accounting for more urine flow during water deprivation cannot be excluded in this experimental protocol, it should be noted that the differences in urine output and osmolalities between the WT and TG mice persisted to some extent even when the mice were subjected to water restriction. Interestingly, these significant differences in urine output and urine osmolalities were associated with significant decreases in urinary excretion of AVP and PGE2 and a modest but significant increase in urinary total NO3/NO2. Based on these observations, it is conceivable that TG mice may have an impaired central response in AVP release when subjected to water deprivation. AVP is produced by the magnocellular neurons (MCN) in the supraoptic nuclei (SON) and paraventricular nuclei of hypothalamus. Several G protein-coupled receptors, including the purinergic receptors, expressed in these structures potentially regulate the synthesis and/or release of AVP (reviewed in Ref. 18). Of particular interest, the P2Y1 receptor is expressed prominently in SON, whereas P2Y2 and P2Y4 receptors are expressed in a few SON neurons (18, 20). It has been shown that the activation of the P2Y1 receptor by a selective agonist, 2-methylthio-ADP, induces release of Ca2+ from intracellular stores associated with transient increases in AVP and oxytocin release (19, 20). Furthermore, based on the observation that P2Y receptors activate TRPV1 (transient receptor potential cation channel subfamily V, member 1), it has been suggested that the potential for ATP activation of P2Y receptors to modulate activity of TRPV1 channels may be physiologically important as a mechanism allowing MCNs to integrate osmotic and hemodynamic information (18). In view of these, it is possible that heightened nucleotide scavenging in hCD39 TG mice might have impaired their ability to release AVP in response to water deprivation. If established in further studies, these observations may open the possibility of therapeutic usage of soluble and engineered ectonucleotidases in dilutional hyponatremia due to excessive AVP release.
Acute water loading by intraperitoneal injection of sterile water allows us to assess the ability of the kidneys to excrete the water load by overcoming both central and renal conditions. Although there were no significant differences between the WT and TG mice in the cumulative (over a 6-h period) urine output, urine osmolality, or osmolar excretion, nevertheless, the TG mice exhibited a tendency to excrete water load more quickly than the WT mice in terms of both significantly higher mean urine output and a higher number of mice responding during the 0- to 2- and 2- to 4-h periods. Thus, it appears that the kidneys of TG mice are conditioned to excrete water more readily than the WT mice. However, chronic water loading did not reveal significant differences between the WT and TG mice in urinary parameters, suggesting that TG mice have the ability to adapt to chronic conditions.
Finally, the WT and TG mice responded comparably when treated with dDAVP either subacutely over a 4-h period or chronically over 5 days. These finding suggest that the collecting ducts of TG mice are as sensitive to exogenous dDAVP (or AVP) as the WT mice and are capable of overcoming the local or systemic factors in responding to dDAVP.
In conclusion, this study shows that global overexpression of hCD39 1) impairs urinary concentrating ability of the kidney despite normal AVP levels, 2) impairs AVP response during water deprivation, and 3) does not affect the response of the collecting duct to subacute or chronic dDAVP treatment. Although the exact mechanism of the impaired urinary concentrating ability of the kidney despite normal AVP levels needs to be investigated further, at this stage it appears that heightened nucleotide scavenging by the overexpression of hCD39 might impair the AVP release response in hypothalamus. Thus, these studies, while unraveling new findings on the role of ectonucleotidases in urinary concentrating ability, also opened the avenue for exploration of therapeutic utility of soluble or engineered ectonucleotidases for the treatment of water-retaining conditions, such as dilutional hyponatremia due to excessive and inappropriate release of AVP (SIADH), especially in combination with V2 receptor blockers (aquaretics).
GRANTS
This work has been supported primarily by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R21DK081041; to B. K. Kishore and S. C. Robson) supplemented by funds from the National Kidney Foundation of Utah and Idaho (to B. K. Kishore) and resources and facilities at the VA Salt Lake City Health Care System, Salt Lake City, UT.
DISCLOSURES
Parts of this work were presented at the 43rd Annual Meeting of the American Society of Nephrology, November 2010, Denver, CO, and appeared as a printed abstract in the proceedings of that meeting (25).
AUTHOR CONTRIBUTIONS
Y.Z., S.C.R., and B.K.K. did the conception and design of the research; Y.Z., K.L.M., S.K.S., and B.K.K. performed the experiments; Y.Z., K.L.M., S.K.S., and B.K.K. analyzed the data; Y.Z., K.M.D., K.E., S.C.R., and B.K.K. interpreted the results of the experiments; Y.Z. and B.K.K. prepared the figures; Y.Z., K.M.D., K.E., S.C.R., and B.K.K. edited and revised the manuscript; Y.Z., K.L.M., S.K.S., K.M.D., K.E., S.C.R., and B.K.K. approved the final version of the manuscript; S.C.R. and B.K.K. drafted the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Carolyn M. Ecelbarger for critical reading of the manuscript and for thoughtful suggestions.
REFERENCES
- 1. Berl T, Schrier RW. Disorders of water metabolism. In: Renal and Electrolyte Disorders, edited by Schrier RW. Philadelphia, PA: Lippincott Williams Wilkins, 2010, p. 16 [Google Scholar]
- 1a. Busch CJ, Liu H, Graveline AR, Bloch KD. Nitric oxide induces phosphodiesterase 4B expression in rat pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 290: L747–L752, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Crikis S, Lu B, Murray-Segal LM, Selan C, Robson SC, D'Apice AJ, Nandurkar HH, Cowan PJ, Dwyer KM. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant 10: 2586–2595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv Pharmacol 61: 301–332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, Fisicaro N, Mysore TB, Kaczmarek E, Cowan PJ, d'Apice AJ. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest 113: 1440–1446, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Jia Z, Zhang A, Zhang H, Dong Z, Yang T. Deletion of microsomal prostaglandin E synthase-1 increases sensitivity to salt loading and angiotensin II infusion. Circ Res 99: 1243–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Kim JS, Choi KC, Jeong MH, Kim SW, Oh Y, Lee J. Increased expression of sodium transporters in rats chronically inhibited of nitric oxide synthesis. J Korean Med Sci 21: 1–4, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kishore BK, Isaac J, Fausther M, Tripp SR, Shi H, Gill PS, Braun N, Zimmermann H, Sévigny J, Robson SC. Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol 288: F1032–F1043, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y(2) receptors and water transport in the kidney. Purinergic Signal 5: 491–499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kukulsko F, Lévesque SA, Sévigny J. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv Pharmacol 61: 263–299, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Lukashev DE, Smith PT, Caldwell CC, Ohta A, Apasov SG, Sitkovsky MV. Analysis of A2a receptor-deficient mice reveals no significant compensatory increases in the expression of A2a, A1, and A3 adenosine receptors in lymphoid organs. Biochem Pharmacol 65: 2081–2090, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol 61: 221–261, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Prætorius HA, Leipiziger J. Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72: 377–393, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Rajagopal M, Pao AC. Adenosine activates A2b receptors and enhances chloride secretion in kidney inner medullary collecting duct cells. Hypertension 55: 1123–1128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwiebert EM. Extracellular Nucleotides and Nucleosides: Release, Receptors and Physiological and Pathophysiological Effects. Current Topics in Membranes (Vol. 54). Boston, MA: Academic, 2003 [Google Scholar]
- 17. Schwiebert EM, Kishore BK. Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol 280: F945–F963, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Sladek CD, Song Z. Diverse roles of G-protein coupled receptors in the regulation of neurohypophyseal hormone secretion. J Neuroendocrinol 24: 554–565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song Z, Gomes DA, Stevens W. Role of purinergic P2Y1 receptors in regulation of vasopressin and oxytocin secretion. Am J Physiol Regul Integr Comp Physiol 297: R478–R484, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Song Z, Vijayaraghavan S, Sladek CD. ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. Am J Physiol Regul Integr Comp Physiol 292: R423–R431, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Wildman SS, Marks J, Turner CM, Yew-Booth L, Peppiatt-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19: 731–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wildman SS, Kang ES, King BF. ENaC, renal sodium excretion and extracellular ATP. Purinergic Signal 5: 481–489, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783: 673–694, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Zhang Y, Enjyoji K, Morris KL, Sparrow SK, Dwyer KM, Robson SC, Kishore BK. Renal phenotype of transgenic mice over-expressing hCD39/NTPDase1 (Abstract). J Am Soc Nephrol 21: 598a, 2010. 20133478 [Google Scholar]
- 26. Zhang Y, Listhrop R, Ecelbarger CM, Kishore BK. Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion. Am J Physiol Renal Physiol 300: F657–F668, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Kohan DE, Nelson RD, Carlson NG, Kishore BK. Potential involvement of P2Y2 receptor in diuresis of postobstructive uropathy in rats. Am J Physiol Renal Physiol 298: F634–F642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Nelson RD, Carlson NG, Kamerath CD, Kohan DE, Kishore BK. Potential role of purinergic signaling in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 296: F1194–F1201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol 295: F1715–F1724, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]