Abstract
Fibroblast growth factor 23 (FGF23) significantly increases with declining renal function, leading to reduced renal tubular phosphate reabsorption, decreased 1,25-dihydroxyvitamin D, and increased left ventricular hypertrophy. Elevated FGF23 is associated with increased mortality. FGF23 is synthesized in osteoblasts and osteocytes; however, the mechanisms by which it is regulated are not clear. Patients with chronic kidney disease have decreased renal acid excretion leading to metabolic acidosis, which has a direct effect on bone cell activity. We hypothesized that metabolic acidosis would directly increase bone cell FGF23 production. Using cultured neonatal mouse calvariae, we found that metabolic acidosis increased medium FGF23 protein levels as well as FGF23 RNA expression at 24 h and 48 h compared with incubation in neutral pH medium. To exclude that the increased FGF23 was secondary to metabolic acidosis-induced release of bone mineral phosphate, we cultured primary calvarial osteoblasts. In these cells, metabolic acidosis increased FGF23 RNA expression at 6 h compared with incubation in neutral pH medium. Thus metabolic acidosis directly increases FGF23 mRNA and protein in mouse bone. If these results are confirmed in humans with chronic kidney disease, therapeutic interventions to mitigate acidosis, such as bicarbonate administration, may also lower levels of FGF23, decrease left ventricular hypertrophy, and perhaps even decrease mortality.
Keywords: osteoblasts, chronic kidney disease
the level of the phosphaturic hormone fibroblast growth factor 23 (FGF23) increases incrementally with declining renal function (29, 33), which results in decreased renal tubule inorganic phosphate (Pi) reabsorption (27, 33), a reduction of 1,25-dihydroxyvitamin D [1,25(OH)2D] (27, 33), and subsequent reduced intestinal Pi absorption (33). Elevated levels of FGF23 induce left ventricular hypertrophy (6) and are associated with a significant increase in mortality (12).
FGF23 is produced in osteocytes and osteoblasts (27); however, the mechanisms by which FGF23 is regulated are not clear. As patients progress from chronic kidney disease (CKD) stage 1 to stage 5, there is a significant incremental increase in FGF23, and patients on dialysis have the highest levels of FGF23 (29). The increase in both Pi retention and serum Pi during CKD may increase serum FGF23 (31). However, Pi may not be a primary regulator of FGF23 since there is little correlation between serum Pi and serum FGF23 in patients without CKD (26). 1,25(OH)2D has also been shown to directly increase FGF23 production and has been suggested as a “counterregulatory” hormone (24). However, in CKD, where levels of 1,25(OH)2D are low (23), 1,25(OH)2D is unlikely to be the proximate stimulus for the elevated FGF23 serum levels. Parathyroid hormone (PTH), which is elevated in patients with CKD with secondary hyperparathyroidism (23), stimulates FGF23 expression in osteoblasts (21). However, a large scale study in humans with CKD suggests that levels of FGF23 increase before those of PTH (11).
Patients with CKD have decreased renal net acid excretion (22). Cellular metabolism generates continual acid production so that as kidney function declines, metabolic acidosis (MET) develops and increases in severity. During MET the excess acid is buffered by bone, resulting in a release of mineral calcium (Ca) and Pi (22). In mouse organ cultures, MET not only increases osteoclastic bone resorption but also decreases osteoblastic bone formation (19).
Since MET regulates osteoblast activity in the process of stimulating osteoclastic bone resorption (16) and osteoblasts also produce FGF23 (31), we tested the hypothesis that MET would directly increase FGF23 production. We used neonatal mouse calvariae in culture and primary osteoblasts to ask whether a physiological model of MET would increase FGF23, and, if so, is the increase secondary to a MET-induced increase in bone Pi release? We found that MET stimulated FGF23 production not only in cultured mouse calvariae, where MET stimulates bone resorption, but also in isolated osteoblasts, where there is no release of mineral Pi.
METHODS
Organ culture of bone.
Calvariae were dissected from 4–6-day-old CD-1 mice (Charles River) and incubated in 2.8 ml of DMEM (Lonza) containing 15% heat-inactivated horse serum (Invitrogen), heparin (10 USP units/ml), and penicillin (100 U/ml) in 35-mm dishes (16). Medium was adjusted to neutral pH (NTL, pH ∼7.4) or physiologically acidic pH (MET, pH ∼7.1) by an addition of 2.4 N HCl to reduce [HCO3−], as a model of MET (16). The partial pressure of carbon dioxide (Pco2) was maintained at the physiological normal of ∼40 mmHg. To model respiratory acidosis, medium pH was adjusted by increasing Pco2 to ∼80 mmHg. All medium was preincubated at the appropriate Pco2 at 37°C. Immediately before two bones/dish were added, 1 ml of medium was removed to determine preincubation pH, Pco2, and total Ca and Pi concentration. Each determination presented for organ culture of calvariae represents the results from a pair of calvariae. At 24 h, medium was removed; again analyzed for pH, Pco2, Ca and Pi; and replaced with similar, fresh preincubated medium. After the second 24-h incubation, medium was analyzed for pH, Pco2, Ca, and Pi. Where indicated, an aliquot of medium was flash frozen in liquid N2 and stored at 70°C for subsequent measurement of FGF23. All animal protocols were approved by the University of Rochester Committee on Animal Resources.
Primary bone cell culture.
Primary bone cells, which are almost exclusively osteoblasts, were isolated from neonatal mouse calvariae (17). Bones were washed in phosphate-buffered saline containing 4 mM EDTA for 10 min at 37°C and then incubated in a HEPES buffer solution, consisting of 25 mM HEPES (pH 7.4), 70 mM NaCl, 30 mM KCl, 10 mM NaHCO3, 1.5 mM K2HPO4, 1 mM CaCl2, 60 mM sorbitol, 27.8 mM D(+)-glucose, and 1 mg/ml BSA and containing 2 mg/ml collagenase (Wako Pure Chemicals) and 90 μM Nα-tosyl-l-lysyl chloromethyl ketone for three sequential 20-min digestions at 37°C in a shaking water bath. After each digestion, released cells were collected and resuspended in the HEPES buffer with 1 mM MgSO4, and digests were pooled for plating on 60-mm Primaria plates (Falcon). Confluent, quiescent cells were then cultured in preequilibrated NTL or MET for 6 or 24 h.
pH, Pco2, Ca, and Pi.
Medium pH and Pco2 were determined with a blood-gas analyzer (ABL5, Radiometer), and the medium [HCO3−] was calculated. Initial experimental culture conditions for the ELISA experiments in Fig. 1 are presented in Table 1 and are representative of all of the experiments. Ca was measured by an ion-selective electrode (model 10, Nova Biomedical). Medium Pi was determined colorimetrically (Biovision) and was analyzed using a Benchmark Plus plate reader (Bio-Rad). Net ion flux was calculated as Vm([ion]f − [ion]i), where Vm is the medium volume and [ion]f and [ion]i are the final and initial medium concentrations, respectively, of Ca and Pi.
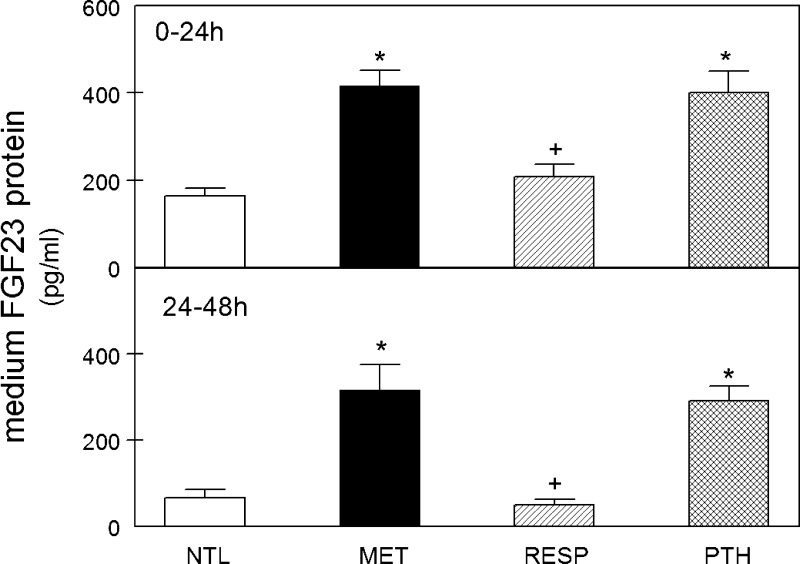
Fig. 1.
Medium fibroblast growth factor 23 (FGF23) concentration after incubation of calvariae in metabolic acidosis (MET) or parathyroid hormone (PTH). Calvariae were incubated in medium at neutral physiological pH (NTL), at reduced pH simulating metabolic acidosis (MET), reduced pH simulating respiratory acidosis (RESP), or with 10−8 M parathyroid hormone (PTH) in NTL for 24 h (0–24 h, top) or continued for an additional 24 h in fresh preequilibrated medium (24–48 h, bottom). Initial medium pH, Pco2, and [HCO3−] for ELISA experiments are shown in Table 1. Medium FGF23 concentration was assayed by ELISA at the end of the indicated incubation time (n = 4–29 pairs of calvariae/group). Values are means ± SE. *P < 0.05 vs. NTL; +P < 0.05 vs. MET.
Table 1.
Initial medium pH, Pco2, and [HCO3−] for data shown in Fig. 1
| n | pH | Pco2, mmHg | [HCO3−], mM | |
|---|---|---|---|---|
| 0–24 h | ||||
| NTL | 29 | 7.46 ± 0.01 | 38.5 ± 0.3 | 27.1 ± 0.2 |
| MET | 30 | 7.13 ± 0.01* | 37.3 ± 0.3* | 12.2 ± 0.2* |
| RESP | 12 | 7.11 ± 0.01* | 89.8 ± 0.8* † | 27.5 ± 0.2† |
| PTH | 14 | 7.48 ± 0.01† | 35.6 ± 0.3* † | 26.2 ± 0.2* † |
| 24–48 h | ||||
| NTL | 10 | 7.48 ± 0.01 | 37.8 ± 0.8 | 27.8 ± 0.5 |
| MET | 12 | 7.18 ± 0.01* | 37.5 ± 0.7 | 13.4 ± 0.2* |
| RESP | 4 | 7.13 ± 0.01* | 87.6 ± 1.5* † | 28.0 ± 0.1† |
| PTH | 8 | 7.49 ± 0.01† | 35.2 ± 1.3 | 26.5 ± 0.2† |
Values are means ± SE; n, pairs of calvariae. Pco2, partial pressure of carbon dioxide; [HCO3−], bicarbonate concentration; NTL, neutral medium pH; MET, medium simulating metabolic acidosis; RESP, medium simulating respiratory acidosis; PTH, NTL medium with 10−8 M parathyroid hormone.
P < 0.05 compared with NTL;
P < 0.05 vs. MET, same time period.
Medium FGF23-excreted protein.
Medium from calvarial cultures was collected at the end of 0–24-h or 24–48-h incubations and immediately frozen at −70°C. The level of FGF23 was determined using an enzyme-linked immunoassay kit for mouse FGF23 COOH-terminus (Immutopics) and analyzed using a Benchmark Plus plate reader.
RNA isolation and quantitative real-time polymerase chain reaction.
Total RNA was isolated from pairs of calvariae or primary CD-1 calvarial cells using an RNeasy kit (Qiagen) (8). Calvariae were collected in RNAlater solution (Ambion) after 24 or 48 h and stored at −20°C until RNA was isolated. Cells were incubated for 6 h or 24 h in a CO2 incubator. Cells were then washed with cold phosphate-buffered saline and lysed in RLT buffer according to the Qiagen protocol using a Qiashredder. RNA was prepared using the Qiagen RNeasy kit. RNA (1 μg) was reverse transcribed to first-strand cDNA using an iScript cDNA synthesis kit (Bio-Rad), and specific transcript levels were determined by quantitative real-time polymerase chain reaction using iQ SYBR-green in an iCycler thermocycler and analyzed with MyIQ optical system software (Bio-Rad). Primers were synthesized by Integrated DNA Technologies for mouse FGF23, forward: 5′-CCT TCT CCC AGT TCC TGG C-3′ and reverse: 5′-GGG CGA ACA GTG TAG AAA TGC-3′; and for RPL13A, forward: 5′-GGA TCC CTC CAC CCT ATG ACA-3′ and reverse: 5′-CTG GTA CTT CCA CCC GAC CTC-3′. Standard curves were generated for each primer. Relative FGF23 expression levels were normalized to RPL13A RNA levels using the comparative threshold cycle method (25).
Statistical analyses.
All tests of significance were calculated using analysis of variance with a Bonferroni correction for multiple comparisons (Statistica, StatSoft). All values are expressed as means ± SE. P < 0.05 was considered significant.
RESULTS
Effect of MET on FGF23 protein and RNA in bone.
To determine whether MET stimulates bone FGF23 secretion, we incubated neonatal mouse calvariae in medium simulating physiological MET compared with incubation in medium at physiological NTL or incubation in medium simulating respiratory acidosis (RESP). As PTH has been shown to stimulate FGF23 production in bone (21), other calvariae incubated with 10−8 M PTH served as a positive control. To model MET, medium was acidified to a physiologically relevant pH by a reduction in bicarbonate concentration [HCO3−] at a constant Pco2, whereas the pH was reduced in RESP by increasing the Pco2 (Table 1). Only the physiological HCO3−/Pco2 buffer system was used in these experiments. After the initial 24-h incubation, there was a significant increase in medium FGF23 protein concentration in response to both MET and PTH, compared with NTL (Fig. 1, top) which continued to be significantly elevated in both MET and PTH for an additional 24 h of incubation (Fig. 1, bottom). RESP did not alter the level of FGF23 protein at either time point.
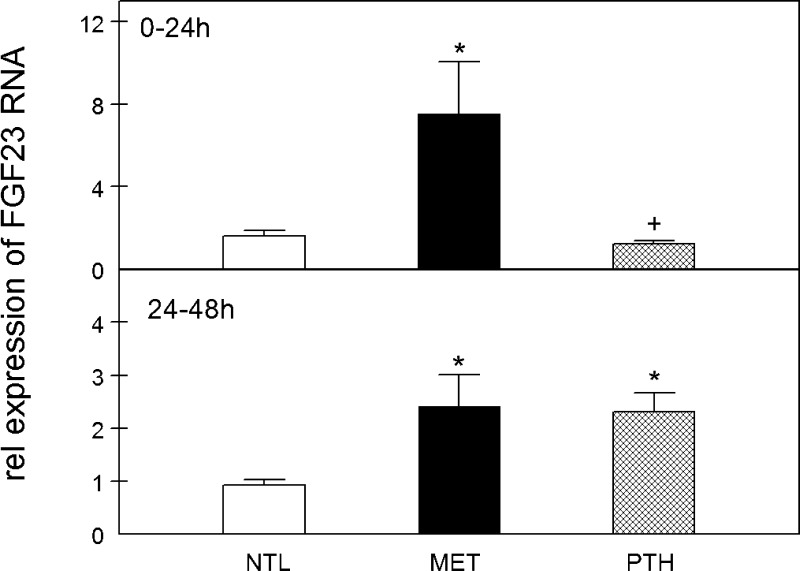
FGF23 RNA expression in the calvariae was measured in comparable experiments after 24- and 48-h incubations. FGF23 RNA expression was also increased at both 24 and 48 h in MET compared with NTL (Fig. 2, top and bottom, respectively), whereas PTH stimulated FGF23 RNA expression only after 48 h of culture (Fig. 2, bottom).
Fig. 2.
FGF23 mRNA expression after incubation of calvariae in MET or PTH. Calvariae were incubated in medium at neutral physiological pH (NTL), at reduced pH simulating metabolic acidosis (MET), or with 10−8 M parathyroid hormone (PTH) in NTL for 24 h (0–24 h, top) or continued for an additional 24 h in fresh preequilibrated medium (24–48 h, bottom). Initial medium pH, Pco2, and [HCO3−] were not different from those shown in Table 1 for ELISA experiments. FGF23 mRNA expression in calvariae was assayed by real-time PCR at the end of the indicated incubation time (n = 8–13 pairs of calvariae/group). Rel, relative. Values are means ± SE. *P < 0.05 vs. NTL; +P < 0.05 vs. MET, same time period.
Net Ca and Pi efflux from calvariae in response to MET or PTH.
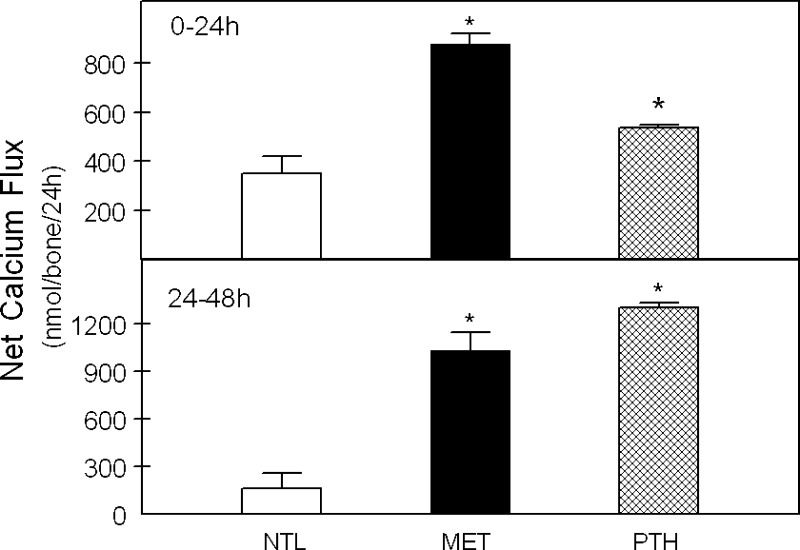
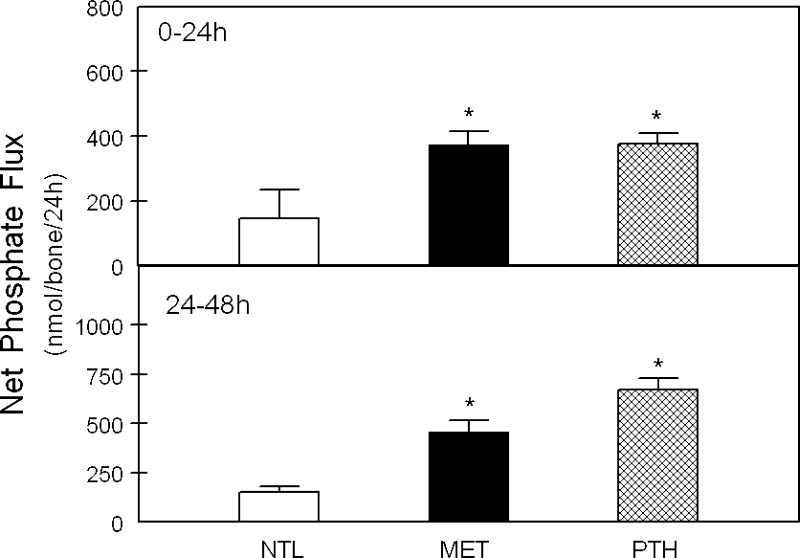
Net Ca efflux from calvariae was significantly increased with incubation in MET and with PTH at both 24 and 48 h in culture compared with NTL (Fig. 3, top and bottom, respectively), as we have previously reported (16). In response to both MET and PTH, there was also a significant increase in net Pi efflux from bone at both 24 h and 48 h compared with NTL (Fig. 4, top and bottom). MET induced a relatively greater Ca than Pi efflux from bone. This is consistent with our previous observation that the medium is in equilibrium with the mineral calcium carbonate (CaCO3) (3) and suggests that both CaCO3 and a CaPi mineral phase are released from bone in response to MET.
Fig. 3.
Net calcium (Ca) efflux from calvariae in response to MET or PTH. Calvariae were incubated in medium at neutral physiological pH (NTL), at reduced pH simulating metabolic acidosis (MET), or with 10−8 M parathyroid hormone (PTH) in NTL for 24 h (0–24 h, top) or continued for an additional 24 h in fresh preequilibrated medium (24–48 h, bottom). Initial medium pH, Pco2, and [HCO3−] were not different from Table 1. The Ca fluxes for 6–13 pairs of calvariae/group are means ± SE. *P < 0.05 vs. NTL.
Fig. 4.
Net inorganic phosphate (Pi) efflux from calvariae in response to MET or PTH. Calvariae were incubated in medium at neutral physiological pH (NTL), at reduced pH simulating metabolic acidosis (MET), or with 10−8 M parathyroid hormone (PTH) in NTL for 24 h (0–24 h, top) or continued for an additional 24 h in fresh preequilibrated medium (24–48 h, bottom). Initial medium pH, Pco2, and [HCO3−] were not different from Table 1. The Pi fluxes for 10–15 pairs of calvariae/group are means ± SE. *P < 0.05 vs. NTL.
Direct stimulation of FGF23 by MET in osteoblasts.
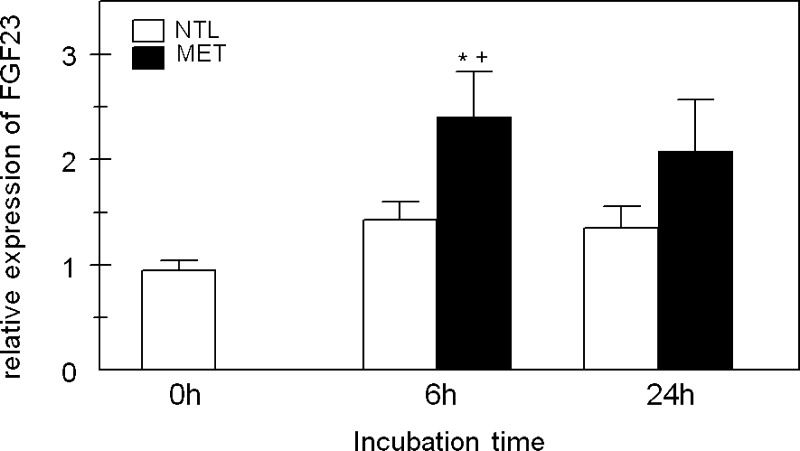
To determine whether the MET-induced release of bone Pi was necessary for MET to increase FGF23, we used primary osteoblasts isolated from neonatal mouse calvariae (17). The absence of a mineral phase will eliminate any significant contribution of Pi to the response to MET. Osteoblasts were incubated in NTL or MET for 6 or 24 h. After 6 h of incubation in MET, there was a significant increase in FGF23 mRNA levels compared with incubation in NTL and to the baseline at 0 h (Fig. 5). However, after 24 h of incubation in MET, there was no significant difference in FGF23 compared with NTL.
Fig. 5.
FGF23 expression in primary osteoblasts after incubation in MET. Primary osteoblasts were isolated from neonatal mouse calvariae. At confluence, some plates were collected for a baseline measurement of FGF23 expression (0 h), and the remaining cells were incubated at neutral physiological pH (NTL; white bars) or at reduced pH simulating metabolic acidosis (MET; black bars) for 6 or 24 h. Initial medium pH, Pco2, and [HCO3−] were not different from Table 1. At the end of the incubation period, cells were collected for RNA isolation and FGF23 expression was assayed by real-time PCR. Data are means ± SE for 10–20 samples/group. *P < 0.05 vs. 0; +P < 0.05 vs. NTL, same time period.
DISCUSSION
Our results demonstrate that MET directly stimulates FGF23 in neonatal mouse calvariae and primary mouse osteoblasts. In response to MET, there was an increase in FGF23 protein secreted from the bones in organ culture as well as an increase in FGF23 RNA expression in both whole calvariae and cultured osteoblasts. Further evidence for the specificity of the response to MET is the lack of stimulation of FGF23 with an isohydric reduction of pH in RESP. Since the increased expression of FGF23 was also observed in the isolated osteoblasts, the response to MET was not secondary to Pi released from bone mineral. Given that we found significant stimulation of FGF23 RNA within 6-h incubation of primary osteoblasts in response to MET, it is possible that a significant response in the intact calvariae also occurred earlier than at 24 h. However, we do not observe a significant cell-mediated stimulation of Ca release before 24 h in the calvarial cultures, so this was the time point at which we examined an effect on FGF23. In addition, the level of FGF23 protein secreted from the osteoblasts into the culture medium at 6 h was too low to be measurable.
FGF23 is a bone-derived hormone that regulates renal Pi excretion and active vitamin D metabolism (27). FGF23 directly decreases renal tubular Pi reabsorption by decreasing the sodium phosphate cotransporters NaPi-2a and NaPi-2c, leading to hyperphosphaturia (27, 33). FGF23 lowers levels of 1,25(OH)2D by inhibiting renal CYP27B1, which catalyzes the hydroxylation of 25(OH)D to 1,25(OH)2D and by stimulating CYP24A1, which metabolizes 1,25(OH)2D to inactive hormones (27, 33). Through its effects to decrease serum levels of 1,25(OH)2D, FGF23 indirectly inhibits intestinal Pi absorption (27).
Although there is significant understanding of the systemic regulation of FGF23, the major factors regulating FGF23 synthesis in osteoblasts and osteocytes have yet to be definitively determined. During early CKD, there are changes in Pi, 1,25(OH)2D, and PTH, all of which have been suggested as regulators of serum FGF23 (27). Although dietary Pi increases serum FGF23 in mice (30) and 1,25(OH)2D (31) and PTH (21) stimulate FGF23 expression in osteocytes and/or osteoblasts, these effects are variable and may not be direct (31). In CKD, the elevation in serum Pi is correlated with an elevation of FGF23 (29) and the binding of intestinal Pi decreases FGF23 in these patients (5). However, extracellular Pi does not directly stimulate FGF23 mRNA levels in osteoblasts (24), and there is conflicting evidence as to whether dietary Pi directly regulates FGF23. In a rat model of progressive CKD, the administration of a Pi binder decreased urinary Pi excretion but had no effect on FGF23, PTH, or 1,25(OH)2D levels (28). A small study of healthy young men demonstrated that 5 days of a low Pi diet together with Pi binders decreased FGF23, whereas a high Pi diet increased FGF23 (7). In contrast, an epidemiologic study of elderly patients without CKD found that serum Pi did not correlate with FGF23 levels, although there was an association between FGF23 and PTH (26, 33).
While 1,25(OH)2D directly stimulates FGF23 expression in bone, FGF23 suppresses 1,25(OH)2D production, suggesting a closed regulatory feedback loop (31). As there is evidence to suggest there is production of 1,25(OH)2D by osteoblasts (1), we cannot rule out the possibility that local production of 1,25(OH)2D could mediate the response to MET. However, in patients with CKD (23), as well as animal models of CKD (36), circulating levels of 1,25(OH)2D are significantly reduced as kidney function decreases, yet their serum levels of FGF23 are elevated (33, 36), making it unlikely that 1,25(OH)2D induces the elevated FGF23 in this disease state. Indeed, the high levels of FGF23 during CKD may be the proximate cause of 1,25(OH)2D deficiency in CKD (10, 27). PTH has been shown to stimulate FGF23 in mouse bone and osteocytes in vivo and in mouse osteoblasts and osteocytes in vitro (14, 21, 34); thus we used the effect of PTH in the calvariae as a positive control for comparison with the response to MET. However, others have found no stimulation of FGF23 by PTH in normal or uremic rats (35) or in rat osteosarcoma cells (24) or rat calvariae (35). The difference in responses to PTH may be due to the different species or different model systems used.
The results presented here support the observation that PTH stimulates FGF23 in mouse calvariae (Fig. 1). FGF23 protein and RNA were stimulated by MET at 24 h, but PTH only increased FGF23 protein and not RNA at 24 h, suggesting that PTH induced preformed FGF23 protein release before there was stimulation of RNA, in contrast to MET. At 48 h, both MET and PTH induced an increase in both FGF23 RNA and protein. Even after the medium was concentrated, the levels of FGF23 in cultures of primary osteoblasts are too low to be reliably detected by an ELISA assay. Thus we cannot determine whether FGF23 protein increased before mRNA in the osteoblast cultures.
Although PTH increases FGF23, FGF23 inhibits PTH production (2), which should limit the ability of PTH to further increase FGF23. Perhaps most importantly, in early CKD, FGF23 appears to increase before there are measurable increases in Pi or PTH or decreases in 1,25(OH)2D (10, 11, 13, 29), making primary regulation by any of these parameters of mineral metabolism less likely.
In humans, the kidney must excrete the daily endogenous acid production or MET will develop (22). In patients with progressive CKD, acid production continues while excretion diminishes, leading to MET. During MET, bone buffers the increase in hydrogen ions through direct physicochemical dissolution (4) and also by inhibiting osteoblastic bone formation and stimulating osteoclastic bone resorption (19), leading to Ca and Pi release into the systemic circulation (22). The MET-induced increase in FGF23, shown in this study, would facilitate renal excretion of this additional Pi.
We and others have shown that MET increases osteoclastic bone resorption by a mechanism that is mediated through stimulation of osteoblastic prostaglandin E2 production (8, 18, 20, 32). This response is initiated by activation of Ca signaling in the osteoblast through a specific proton receptor (9, 15). We do not yet know whether the effect of MET to increase FGF23 uses the same signaling pathway or another novel mechanism.
The results of this study, in cultured mouse bone and isolated osteoblasts, suggest that the elevated levels of FGF23 in CKD may be due, at least in part, to MET directly stimulating osseous production of FGF23. Understanding the regulation of FGF23 in CKD is critically important as FGF23 has been shown to induce left ventricular hypertrophy (6), and there is a clear association between increased serum levels of FGF23 and mortality (12). If the results of this study, which were obtained in mouse bone, can be confirmed in humans, therapeutic interventions, including the provision of oral bicarbonate to mitigate the CKD-induced acidosis, have the potential to lower FGF23 levels, decrease left ventricular hypertrophy, and even lessen mortality.
GRANTS
This study was supported in part by National Institutes of Health Grants AR-46289 and DK-75462 (both to D. A. Bushinsky) and a grant from the Renal Research Institute (to N. S. Krieger).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.S.K. and D.A.B. conception and design of research; N.S.K., C.D.C., and D.A.B. analyzed data; N.S.K. and D.A.B. interpreted results of experiments; N.S.K. prepared figures; N.S.K. drafted manuscript; N.S.K. and D.A.B. edited and revised manuscript; N.S.K. and D.A.B. approved final version of manuscript; C.D.C. and K.K.-S. performed experiments.
REFERENCES
- 1. Anderson PH, Atkins GJ. The skeleton as an intracrine organ for vitamin D metabolism. Mol Aspects Med 29: 397–406, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bushinsky DA, Lechleider RJ. Mechanism of proton-induced bone calcium release: calcium carbonate-dissolution. Am J Physiol Renal Fluid Electrolyte Physiol 253: F998–F1005, 1987 [DOI] [PubMed] [Google Scholar]
- 4. Bushinsky DA, Wolbach W, Sessler NE, Mogilevsky R, Levi-Setti R. Physicochemical effects of acidosis on bone calcium flux and surface ion composition. J Bone Miner Res 8: 93–102, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Cancela AL, Oliveira RB, Graciolli FG, dos Reis LM, Barreto F, Barreto DV, Cuppari L, Jorgetti V, Carvalho AB, Canziani ME, Moyses RM. Fibroblast growth factor 23 in hemodialysis patients: effects of phosphate binder, calcitriol and calcium concentration in the dialysate. Nephron Clin Pract 117: 74–82, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St, John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90: 1519–1524, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Frick KK, Bushinsky DA. Metabolic acidosis stimulates RANK ligand RNA expression in bone through a cyclooxygenase dependent mechanism. J Bone Miner Res 18: 1317–1325, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Frick KK, Krieger NS, Nehrke K, Bushinsky DA. Metabolic acidosis increases intracellular calcium in bone cells through activation of the proton receptor OGR1. J Bone Miner Res 24: 305–313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jueppner HW, Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CAM, Lash JP, Hsu Cy Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez O, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu Cy Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients With chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ix JH, Shlipak MG, Wassel CL, Whooley MA. Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol Dial Transplant 25: 993–997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol 18: 2683–2688, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Krieger NS, Bushinsky DA. Pharmacologic inhibition of intracellular calcium release blocks acid-induced bone resorption. Am J Physiol Renal Physiol 300: F91–F97, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krieger NS, Frick KK, Bushinsky DA. Mechanism of acid-induced bone resorption. Curr Opin Nephrol Hypertens 13: 423–436, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Krieger NS, Hefley TJ. Differential effects of parathyroid hormone on protein phosphorylation in two osteoblast-like cell populations isolated from neonatal mouse calvaria. Calcif Tissue Int 44: 192–199, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Krieger NS, Parker WR, Alexander KM, Bushinsky DA. Prostaglandins regulate acid-induced cell-mediated bone resorption. Am J Physiol Renal Physiol 279: F1077–F1082, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Krieger NS, Sessler NE, Bushinsky DA. Acidosis inhibits osteoblastic and stimulates osteoclastic activity in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 262: F442–F448, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Krieger NS, Frick KK, LaPlante Strutz K, Michalenka A, Bushinsky DA. Regulation of COX-2 mediates acid-induced bone calcium efflux in vitro. J Bone Miner Res 22: 907–917, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol 299: F882–F889, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Lemann J, Jr, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans. Am J Physiol Renal Physiol 285: F811–F832, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol 17: 1305–1315, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Marsell R, Grundberg E, Krajisnik T, Mallmin H, Karlsson M, Mellstrom D, Orwoll E, Ohlsson C, Jonsson KB, Ljunggren O, Larsson TE. Fibroblast growth factor-23 is associated with parathyroid hormone and renal function in a population-based cohort of elderly men. Eur J Endocrinol 158: 125–129, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Martin A, David V, Quarles LD. Regulation and function of the FGF23/Klotho endocrine pathways. Physiol Rev 92: 131–155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moe SM, Radcliffe JS, White KE, Gattone VH, Seifert MF, Chen X, Aldridge B, Chen NX. The pathophysiology of early-stage chronic kidney disease-mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J Bone Miner Res 26: 2672–2681, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Pande S, Ritter CS, Rothstein M, Wiesen K, Vassiliadis J, Kumar R, Schiavi SC, Slatopolsky E, Brown AJ. FGF-23 and sFRP-4 in chronic kidney disease and post-renal transplantation. Nephron Physiol 104: 23–32, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146: 5358–5364, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118: 3820–3828, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rabadjija L, Brown EM, Swartz SL, Chen CJ, Goldhaber P. H+-stimulated release of prostaglandin E2 and cyclic adenosine 3′,5′-monophosphoric acid and their relationship to bone resorption in neonatal mouse calvaria cultures. Bone Miner 11: 295–304, 1990 [DOI] [PubMed] [Google Scholar]
- 33. Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol 5: 611–619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49: 636–643, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saji F, Shigematsu T, Sakaguchi T, Ohya M, Orita H, Maeda Y, Ooura M, Mima T, Negi S. Fibroblast growth factor 23 production in bone is directly regulated by 1α,25-dihydroxyvitamin D, but not PTH. Am J Physiol Renal Physiol 299: F1212–F1217, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Stubbs JR, He N, Idiculla A, Gillihan R, Liu S, David V, Hong Y, Quarles LD. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J Bone Miner Res 27: 38–46, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]