Abstract
Peroxisome proliferator-activated receptor-α (PPARα) activation attenuates cisplatin (CP)-mediated acute kidney injury by increasing fatty acid oxidation, but mechanisms leading to reduced renal triglyceride (TG) accumulation could also contribute. Here, we investigated the effects of PPARα and CP on expression and enzyme activity of kidney lipoprotein lipase (LPL) as well as on expression of angiopoietin protein-like 4 (Angptl4), glycosylphosphatidylinositol-anchored-HDL-binding protein (GPIHBP1), and lipase maturation factor 1 (Lmf1), which are recognized as important proteins that modulate LPL activity. CP caused a 40% reduction in epididymal white adipose tissue (WAT) mass, with a reduction of LPL expression and activity. CP also reduced kidney LPL expression and activity. Angptl4 mRNA levels were increased by ninefold in liver and kidney tissue and by twofold in adipose tissue of CP-treated mice. Western blots of two-dimensional gel electrophoresis identified increased expression of a neutral pI Angptl4 protein in kidney tissue of CP-treated mice. Immunolocalization studies showed reduced staining of LPL and increased staining of Angptl4 primarily in proximal tubules of CP-treated mice. CP also increased TG accumulation in kidney tissue, which was ameliorated by PPARα ligand. In summary, a PPARα ligand ameliorates CP-mediated nephrotoxicity by increasing LPL activity via increased expression of GPHBP1 and Lmf1 and by reducing expression of Angptl4 protein in the proximal tubule.
Keywords: peroxisome proliferator-activated receptor, angiopoietin-like protein 4, lipase maturation factor 1
cisplatin is an effective chemotherapeutic agent, but the development of nephrotoxicity is a major limiting factor associated with its use (3, 9, 35). In the kidney, cisplatin accumulates in the proximal tubule and activates complex signaling pathways, leading to tubular cell death (11, 10, 42). In addition, cisplatin activates a robust inflammatory response accompanied by vascular endothelial damage to kidney tissue, which ultimately contributes to ischemic injury, reduced glomerular filtration rate, and acute organ failure (22, 36).
We and others have documented the presence of metabolic abnormalities that coexist with the development of acute kidney injury (AKI) (5, 24, 26–29, 39–41). More specifically, hyperglycemia and insulin resistance have been associated with increased mortality during AKI in the intensive care unit setting (5). We have reported the accumulation of free fatty acids in serum, hyperglycemia, and hyperinsulinemia, in addition to the accumulation of neutral lipids in the proximal tubule in mice treated with cisplatin (40, 41). These findings support the presence of a systemic effect of cisplatin on lipid metabolism, a phenomenon previously documented in rats treated with cisplatin (1), as well as in patients surviving testicular cancer as a long-term complication of platinum-based chemotherapy (33). Currently, the mechanisms involved in cisplatin-mediated intracellular accumulation of triglycerides (TG) and its relationship to nephrotoxicity are not clear.
While adipose tissue has a unique capacity to store excess fatty acids in the form of TG in lipid droplets, non-adipose tissue such as renal tubular cells have a limited capacity for such storage of lipids (55). In hyperlipidemic states, accumulation of excess lipid in non-adipose tissues leads to cell dysfunction and/or cell death, a phenomenon known as lipotoxicity (49). Besides being important for storage and subsequent release of fatty acids, research over the last decade has shown that white adipose tissue also has an important endocrine function (2). Altered secretion of adipocyte-derived proteins contributes to the increased metabolic and cardiovascular risk found in obesity (15, 48). There are several pathways that allow the uptake of circulating lipids into cells. Cell surface receptors mediate the uptake of whole lipoproteins. The hydrolysis of circulating TG bound to chylomicrons and very low-density lipoproteins (VLDL) is catalyzed by the enzyme lipoprotein lipase (LPL), which is anchored to the capillary endothelium via heparin sulfate proteoglycans and is a key determinant of cellular fatty acid uptake (18, 54). LPL is expressed at high levels in tissues that depend on fatty acids as fuel (heart, skeletal muscle, kidney cortex) or synthesize fats for storage or secretion (adipose tissue, mammary tissue). The activity of LPL is regulated by various mechanisms (31, 54). In the present study, we examined the effects of cisplatin on the expression of kidney LPL activity and on the expression of several modulators of kidney LPL activity, including angiopoietin protein-like 4 (Angptl4) (20, 25), glycosylphosphatidylinositol-anchored-HDL-binding protein (GPIHBP1), and lipase maturation factor 1 (Lmf1). We demonstrate that 1) cisplatin significantly reduces epididymal white adipose tissue mass, leading to focal necrosis and increased lymphocytic infiltration; 2) cisplatin administration reduces the expression levels and enzyme activity of LPL in epididymal white adipose tissue as well as in kidney tissue; 3) cisplatin administration increases Angptl4 mRNA and protein levels in kidney tissue and reduces mRNA levels of kidney GPIHBP1 and Lmf1; and 4) treatment with a peroxisome proliferator-activated receptor-α (PPARα) ligand prevents cisplatin-mediated reduced expression and activity of LPL in kidney tissue. Our studies suggest that changes on the expression of kidney Angptl4, GPIHBP1, and Lmf1 gene expression may represent a cellular mechanism(s) involved in cisplatin-mediated regulation of kidney LPL.
METHODS
Animal Model of Cisplatin-Induced AKI
Experimental AKI was induced in 8- to 0-wk-old male mice (strain Sv129) using cisplatin administration. The animals used in these studies were housed at the Veterinary Medical Unit at the Central Arkansas Veterans Healthcare System (Little Rock, AR). When appropriate, animals were painlessly euthanized according to methods of euthanasia approved by the Panel on Euthanasia of the American Veterinary Medical Association. Our animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the Central Arkansas Veterans Healthcare System.
Animals were maintained on standard chow, and, as indicated, a group of animals was fed with a special diet containing WY-14643 (0.1%; WY) for 10 days before cisplatin administration. Cisplatin was administered by a single intraperitoneal injection of 20 mg/kg body wt. After the induction of renal failure, the animals were returned to their cages and allowed free access to food and water.
Cell Culture and Differentiation
To further examine the cellular effects of cisplatin on LPL activity in adipose tissue, we performed in vitro studies using an adipocyte cell line. 3T3-F442A cells were obtained from Dr. Howard Green (Harvard Medical School, Boston, MA). Cells were maintained in DMEM (GIBCO BRL) supplemented with 10% calf serum. For experiments, cells were grown to 70% confluence and stimulated to differentiate in DMEM containing 10% fetal bovine serum and 100 nM insulin for 14 days. Cells were treated with saline or the specified concentration of cisplatin for 24 h.
Gene Expression Studies
LPL, Angptl4, GPIHBP1, and Lmf1 mRNA levels were determined by quantitative real-time RT-PCR. Total RNA was extracted from cells or mouse kidney tissue and treated with RNase-free DNase before RT reaction. Real-time PCR was carried out using the StepOnePlus real-time PCR system (Applied Biosystems) with iTaqSYBR Green Supermix with Rox (Bio-Rad). In each experiment, triplicates of 50 ng cDNA (total RNA equivalent) of samples were amplified in a 20-μl reaction. Specificity of the amplified product was confirmed by melting curve analysis and agarose gel electrophoresis. For relative quantification, a standard curve was generated from a six-step cDNA dilution series. Samples were amplified with primers for LPL, Angptl4, GPIHBP1, Lmf1, and 18S rRNA. The relative expression of genes was calculated from the standard curve. Relative quantity was calculated by the ratio of the gene-specific and the appropriate 18S rRNA expression. The primer sequences in the real-time RT-PCR were the following: for LPL, 5′-CCT TCA CCC TGC CCG AGG TTT C-3′ (forward), 5′-GGC CAG CTG AAG TAG GAG TCG C-3′ (reverse); for Angptl 4, 5′-TAG ACC TCT TGG CCC CCA CGC-3′ (forward), 5′-GGC GGC CTG TGT AAG TGG GTG-3′ (reverse); for GPIHBP1, 5′-CCA CAG CGG AAC CGA CAA AGG-3′(forward), 5′-ACA GTG TGG ACT GGC AAC AGG TC-3′ (reverse); for Lmf1, 5′-GAA TCA TGC TTG GAG CGG GCC T-3′ (forward), 5′-GGC TAT CGG GTT GGG CAC GG-3′ (reverse); and for 18S rRNA, 5′- AGG AGT GGG CCT GCG GCT TA-3′ (forward), 5′-AAC GGC CAT GCA CCA CCA CC-3′ (reverse).
Protein Two-Dimensional Separation and Blotting
The cytoplasmic protein fraction was extracted from mouse kidneys using cell lysis buffer containing protease inhibitors (Sigma). Protein concentration was determined using a Bradford protein assay reagent (Bio-Rad). Protein samples (100 μg) were separated by two-dimensional (2D) gel electrophoresis [isoelectric focusing (IEF) as the first dimension and SDS-PAGE as the second]. Total protein was introduced in 125 μl of rehydration buffer (8 M urea, 2% CHAPS, 40 mM DTT, and 0.2% Biolyte). IEF was performed in a PROTEAN IEF Cell (Bio-Rad), following the voltage-gradient protocol as follows: S1, 250 V for 15 min; S2, 300 V for 15 min; S3, 500 V for 30 in; S4, at 4,000 V for 12,000 V-hours; and S5, 4,000 V for 2 h. The strips were first equilibrated for 15 min in 0.375 M Tris·Cl buffer (pH 8.80 containing 130 mM DTT, 6 M urea, 20% glycerol, and 2% SDS). A second equilibration step was carried out for 15 min in equilibration buffer containing 135 mM iodoacetamide instead of DTT. IEF strips were electrophoresed in 12% Invitrogen precast gels to separate and blotted onto nitrocellulose membranes.
Western Blotting and Quantification
Samples separated by 2D electrophoresis were blotted onto nitrocellulose membranes (Schleicher & Schuell). The membrane was blocked in 5% milk in TBS+0.1% Tween for 1 h at room temperature and incubated with Angptl4 antibody at 4°C overnight at a dilution of 1:1,000. We used a rabbit polyclonal antibody raised against full-length rat Angptl4. Secondary antibody was HRP-linked polyclonal anti-rabbit (1:5,000, Cell Signaling), incubated for 1 h at room temperature. Before and after secondary antibody incubations. membranes were washed four times with TBST to remove nonspecific binding. Chemiluminescence detection was performed using a SuperSignal Femto kit (Pierce, Rockford, IL). The blots were stripped and reprobed with actin antibody for protein load normalization control. Signal quantification was carried out using Bio-Rad imager software. For relative quantification, the integrated optical density value defined as the sum of total pixel value-background was determined for equal-sized regions drawn around spots of interest, with background values taken below each spot of interest to account for nonspecific antibody staining in the blot. The signal of Angptl4 was normalized to the actin signal, and Student's t-test was performed to calculate the significance of difference.
Biochemical Studies
Measurement of LPL activity.
LPL activities were determined in 4-h-fasted mice as described previously (44). Adipose or kidney tissue LPL was extracted from the tissue by homogenization in 100 μl of 50 mM Tris, pH 7.4, containing 0.2% Triton X-114, 10 U/ml of heparin, and 1 mM PMSF. The extractable LPL fraction was fractionated by centrifugation at 1,500 g for 15 min at 4°C, and the supernatant was diluted with four volumes of 50 mM Tris, pH 7.4, containing 10 U/ml heparin, and 10-μl aliquots were used for LPL activity measurements in duplicate. Given the diurnal variability in the measurements of LPL activity in the mouse, mice subjected to the experimental conditions described above were euthanized in the morning and LPL enzyme activity was measured immediately after kidney tissue was harvested. LPL catalytic activity was measured as previously described using a substrate containing [3H]triolein and fetal bovine serum as a source of apoC-II (32). Extracts from adipose or kidney tissue were incubated with substrate for 1 h at 37°C. The fatty acids released during the incubation were extracted using 3.25 ml of a mixture of methanol-chloroform-heptane 1.41:1.25:1 (vol/vol/vol) followed by 1.05 ml of 0.1 M potassium carbonate-borate buffer (pH 10.5). The methanol-water phase was separated by centrifugation at 3,000 g for 15 min at room temperature, and a 1-ml aliquot was counted using a Beckmann liquid scintillation counter. Enzyme activity was calculated and expressed as nanomoles of fatty acid released per hour per milligram protein. To measure post-heparin plasma LPL activity, mice were injected intraperitoneally (ip) with 1,000 U of heparin/kg body wt. After 15 min, the animals were euthanized and blood was collected for the isolation of plasma. LPL activity was assayed by triplicate measurements as the salt-inhibitable ability of plasma samples to hydrolyze an emulsion containing [3H]triolein as described above. To measure LPL activity in cultured adipocytes, cells were separated from the plate using a cell scraper and LPL activity was measured in 100 μl of extraction buffer as described above. Cell extracts were clarified by centrifugation at 5,000 g for 15 min. The supernatant (10 μl) was used for LPL activity measurements in duplicate as described above.
TG measurements.
Tissues samples were homogenized using 10 volumes of a mixture of hexane/2propanol (3/2); the suspension was filtered and evaporated to dryness. The TGs were dissolved in 100 mM Tris (pH 7.4), and total TG content was determined using a two-step TG assay kit (Sigma-Aldrich, St. Louis, MO) as described (45).
Oil Red O Staining
Frozen sections of kidney tissue obtained from various experimental conditions were used for oil red O staining, which was performed as previously described (40) to determine the renal accumulation of total neutral lipids.
Immunohistochemistry of LPL and Angptl4
Immunohistochemical staining was performed on paraffin-embedded tissue sections from mice treated with saline and cisplatin using a polyclonal anti-Angptl4 antibody obtained from Dr. S. S. Chugh, as well as a chicken polyclonal antibody against LPL provided by Dr. G. Olivecrona. We evaluated the presence of LPL and Angptl4 at 3 days after cisplatin injection in the presence of absence of the PPARα ligand WY.
Statistical Analysis
Results are presented as means ± SE. Statistical analysis was performed using an unpaired Student's t-test. A P value of <0.05 was considered to be statistically significant.
RESULTS
Changes in Renal Function After Cisplatin Treatment
Mice were fed either a regular diet or a diet containing 0.1% WY for 10 days before saline or cisplatin administration as described in methods. Kidney function was monitored for 3 days after intraperitoneal injection of saline or cisplatin by measuring blood urea nitrogen (BUN) and serum creatinine. Figure 1, A and B, presents the changes in BUN and creatinine seen in mice fed either a regular diet or a diet containing 0.1% WY. Comparison of the renal function between mice fed for 10 days either a regular diet or 0.1% WY-containing diet did not show differences in BUN and creatinine when treated with a single ip saline injection. Values for this control group, as shown in Fig. 1, A and B, were not significantly different on day 1 (BUN 24 mg/dl, creatinine 0.2 mg/dl) vs. day 3 (BUN 29 mg/dl, creatinine 0.3 mg/dl). Mice fed a regular diet developed acute kidney injury (AKI) on day 3. BUN increased from 26 to 132 mg/dl and creatinine increased from 0.2 to 1.3 mg/dl on day 3 after a single cisplatin injection (P < 0.001). The group of mice that received the WY diet and cisplatin did not develop significant AKI compared with mice treated with cisplatin alone (BUN increased from 23 on day 1 to 33 mg/dl on day 3, and creatinine increased from 0.2 on day 1 to 0.3 mg/dl on day 3).
Fig. 1.
Effects of cisplatin (CP) and peroxisome proliferator-activated receptor-α (PPARα) ligand Wy-14643 (WY) on renal function after single dose of CP. Mice were fed either a regular diet or a diet containing 0.1% WY for 10 days before CP administration, as described in methods. Blood urea nitrogen (BUN) and creatinine levels were measured every day for a period of 3 days after either saline (control) or CP intraperitoneal (IP) injection. Values are means ± SE of at least 4 independent experiments under each condition. A and B: changes in BUN and creatinine levels, respectively, after CP or saline administration in PPARα wild-type (WT) mice. *P < 0.001 compared with control. †P < 0.001 compared with CP by unpaired Student's t-test.
Cisplatin Causes a Reduction in Total Body Weight and Epididymal White Adipose Tissue Weight
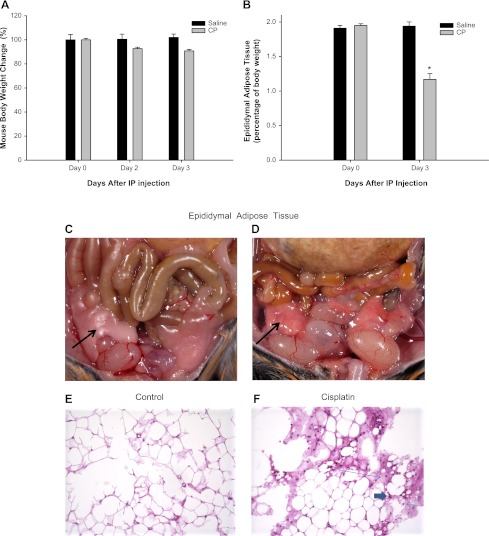
Wild-type mice fed a normal diet received a single ip injection of either saline solution or cisplatin. Total body weights were recorded daily for 3 days. As shown in Fig. 2, A and B, body weight, wet epididymal white adipose tissue, and epididymal white adipose tissue mass-to-body weight percentage did not change significantly in saline-treated mice. The average epididymal white adipose tissue weight was 416 mg at day 0 and 403 mg at day 3 after saline injection. This average weight for mouse epididymal white adipose tissue in our study is consistent with previous reports by Geloen et al. (16). At 3 days after cisplatin injection, there was a 10% reduction of total body weight as shown in Fig. 2A, but also a 40% reduction of epididymal white adipose tissue mass-to-body weight percentage (down from 1.95% at day 0 to 1.17% at day 3, P < 0.05) as shown in Fig. 2B. In addition, cisplatin caused a change in the appearance of the epididymal white adipose tissue, from white (saline-treated) to red (cisplatin-treated) as shown in Fig. 2, C and D.
Fig. 2.
Effect of CP on body weight (A), epididymal white adipose tissue mass-to-body weight percentage (B), gross appearance (C and D), and histological section (E and F) in saline- and CP-treated mice. There was a 40% reduction in wet weight of epididymal white adipose tissue in CP-treated mice (B) compared with saline-treated mice. Epididymal white adipose tissue (shown by black arrows) in CP-treated mice appears white in saline-treated compared with the red color in CP-treated mice. Histological changes in epididymal white adipose tissue after CP treatment are shown. Sections of epididymal white fat tissue obtained from saline (E)- vs. CP (F)-treated mice were stained with hematoxylin-eosin. E: saline-treated mice have unremarkable adipose tissue, ×100. F: epididymal fat tissue of CP-treated mice showed focal adipocyte necrosis (long thin arrow) with rare neutrophil (short thick arrow) and lymphocyte infiltration (short thin arrow), ×100. Error bars indicate SE. *P < 0.05 compared with day 0 by unpaired Student's t-test.
Cisplatin-Treated Mice Develop Inflammatory Changes in White Adipose Tissue
To investigate the histological changes in white epididymal adipose tissue, white epididymal adipose tissue mass was fixed in Bouin's solution for 24 h and then processed routinely through graded alcohols and xylene for 8 h; after that, it was embedded in paraffin. Three-micrometer sections were cut, and sections were stained with hematoxylin eosin. As shown in Fig. 2, E and F, the sections of white epididymal adipose tissue obtained from saline-treated mice showed adipocytes of normal size without any inflammatory infiltrate present. Hematoxylin-eosin-stained sections obtained from cisplatin-treated mice showed focal necrosis with loss of adipocytes, and infiltration of lymphocytes and neutrophils in the necrotic area.
Cisplatin and WY Effects on Adipose Tissue LPL mRNA Expression and Activity
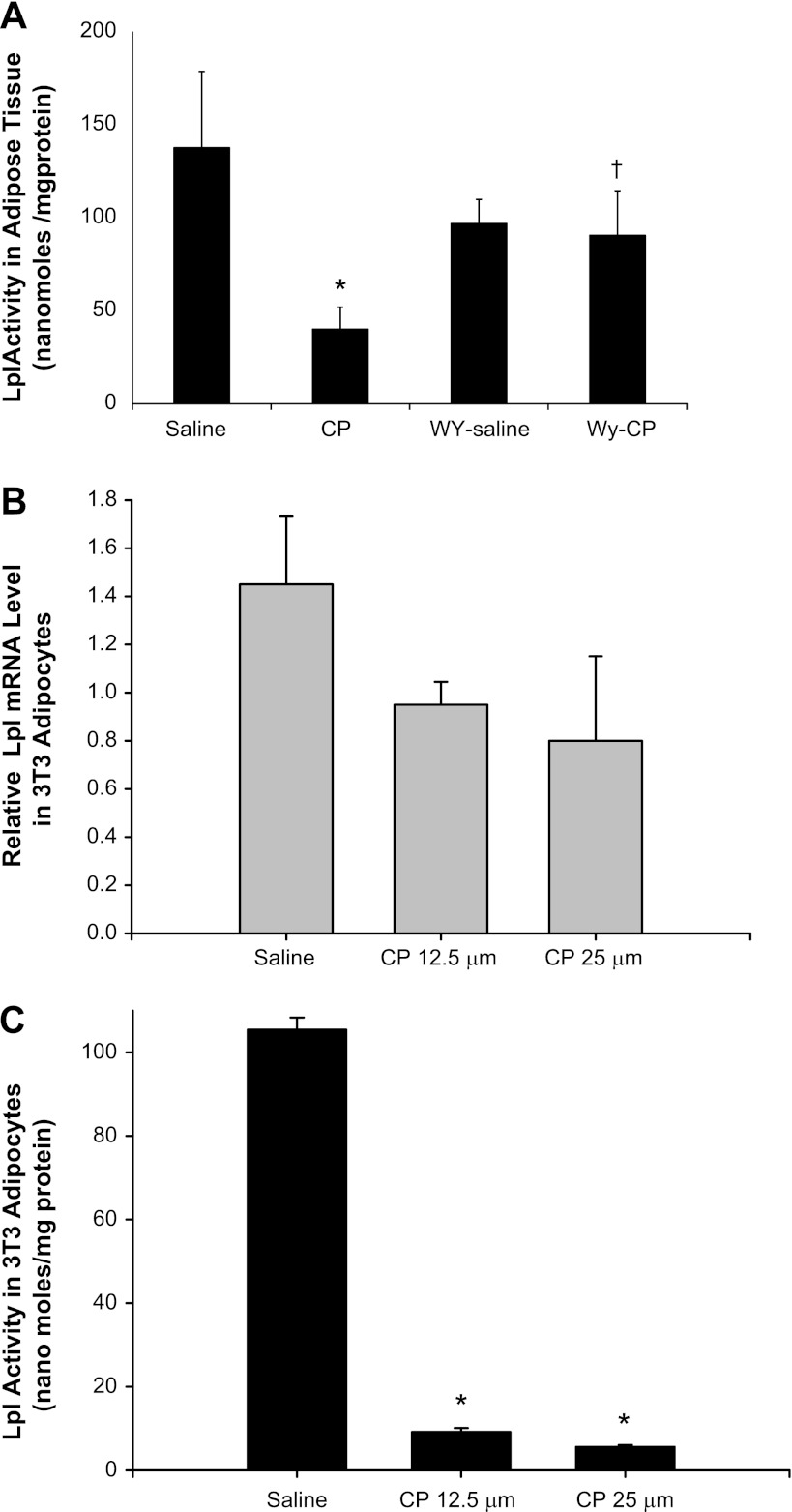
Since epididymal white adipose tissue mass was reduced by cisplatin treatment and lipoprotein lipase expressed in adipose tissue plays a critical role in the metabolism and transport of TG-containing lipoproteins, we next measured lipoprotein lipase activity in epididymal white adipose tissue isolated from saline-and cisplatin-treated mice. As shown in Fig. 3A, cisplatin inhibited LPL activity in epididymal white adipose tissues by 71% (P < 0.05). There was no significant change in epididymal white adipose tissue LPL activity in mice fed a WY-supplemented diet before cisplatin treatment, but pretreatment with WY prevented cisplatin-mediated inhibition of LPL activity in epididymal white adipose tissue (Fig. 3A). To determine whether this inhibition of LPL activity was a direct effect of cisplatin on white adipose tissue cells, we examined the effects of cisplatin on LPL mRNA levels and LPL activity using 3T3 adipocyte cells in culture. As shown in Fig. 3, B and C, cisplatin at 12.5 and 25 μM also induced a dose-dependent inhibition of both LPL mRNA and LPL activity in adipocyte cells in culture.
Fig. 3.
Effect of CP and WY on mouse lipoprotein lipase (LPL) activity in epididymal white adipose tissue (A) and on LPL mRNA levels (B) and LPL activity in 3T3 adipocytes (C). LPL activity was measured in epididymal white adipose tissue obtained from CP-, WY-, and WY+CP-treated mice (A) and in 3T3 adipocytes treated for 24 h with 12.5 and 25 μM CP (C). LPL mRNA expression was also measured in 3T3 adipocytes treated for 24 h with 12.5 and 25 μM CP. Values are means ± SE of at least 4 independent experiments under each condition. *P < 0.05 compared with saline-treated control mice. †P < 0.05 compared with CP-treated mice fed regular chow by unpaired Student's t-test.
Cisplatin and WY Effects on Kidney Tissue LPL mRNA, Protein Levels, and Activity
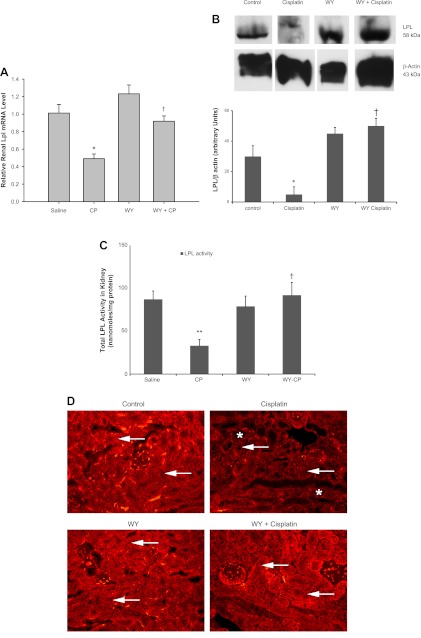
Previous studies have established the presence of kidney LPL in kidney tissue, but its regulation during AKI has not been previously studied. We examined the effects of cisplatin and the PPARα ligand WY on LPL mRNA, protein level, as well as on kidney LPL enzyme activity. As shown in Fig. 4, A–C, cisplatin caused a 50% decline in the mRNA expression of LPL in kidney tissue (Fig. 4A). Pretreatment with the PPARα ligand WY led to a minor increase in LPL mRNA (1.23-fold) but prevented cisplatin-mediated reduction of LPL mRNA levels. Similar effects were observed on LPL protein levels measured by Western blot analysis as shown in Fig. 4B. Cisplatin also inhibited LPL activity in kidney tissue by 40 ± 10% (P < 0.002) in mice fed normal chow, as seen in Fig. 4C. There was no significant change in renal LPL activity in mice fed a WY-supplemented diet before cisplatin treatment, and pretreatment with WY prevented cisplatin-mediated inhibition of LPL activity in kidney tissue.
Fig. 4.
Effect of CP and WY on LPL mRNA expression (A), protein levels (B), enzyme activity (C), and immunostaining (D) in mouse kidney tissues. In mice fed regular chow, following CP treatment, LPL mRNA expression (A), protein level (B), and activity (C) were inhibited in kidney tissue. LPL mRNA, LPL protein, and LPL activity were inhibited after CP treatment in mice fed a regular chow diet. There was no significant change in LPL mRNA, protein level, and activity in mice fed a WY-supplemented diet for 10 days before CP treatment. Values are means ± SE of at least 4 independent experiments under each condition. *P < 0.05, **P < 0.002 compared with saline-treated control mice. †P < 0.05 compared with CP-treated mice fed regular chow by unpaired Student's t-test. B: Western blot analysis of kidney LPL protein or β-actin. Equal quantities of protein from 4 different conditions, i.e., control, CP, WY, and WY+CP, were loaded and separated in the same gel and then subjected to Western blot analysis as described. D: immunostaining for kidney LPL. Positive LPL staining can be seen in the proximal tubules throughout the cortex in kidneys from control mice, which is significantly reduced after CP treatment. The staining in WY-pretreated animals is similar to control and it does not change after CP administration in the WY+CP-treated kidneys. WY pretreatment also protected kidney morphology, since necrotic tubules can be seen only in CP-treated mice (*) Arrows show that LPL immunostaining is present in proximal tubules.
Immunolocalization of LPL
As shown in Fig. 4D, positive LPL staining was detected in the proximal tubules throughout the cortex in kidneys from saline-injected control mice. This positive staining was significantly reduced in kidney samples obtained 3 days after cisplatin injection. WY pretreatment showed a similar staining pattern to the one seen in control mice, which was not reduced after cisplatin treatment since the positive LPL staining could be detected throughout the cortex of WY+cisplatin-treated animals.
Cisplatin Increases Angptl4 mRNA Levels in Kidney, Liver, and Adipose Tissue
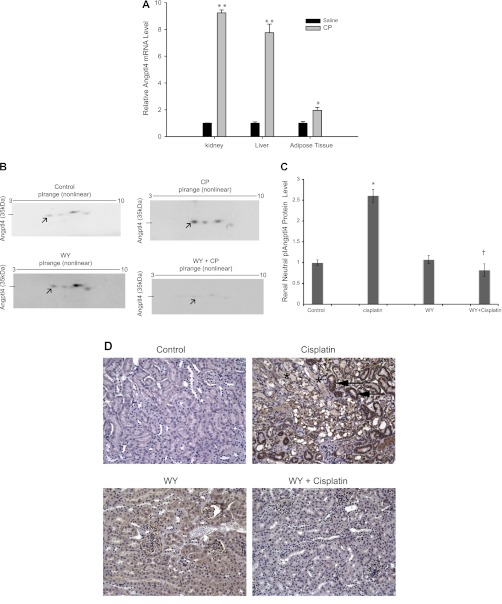
Our data indicate cisplatin and a PPARα ligand play an important role in regulating kidney tissue LPL expression and enzyme activity. Angptl4 has been identified recently as an important modulator of LPL activity. To further examine potential mechanisms of regulation of kidney LPL, we investigated the effects of cisplatin on mRNA levels of Angptl4 by quantitative real-time RT-PCR. Angptl4 mRNA levels in kidney tissue were increased by 9.24 ± 0.21-fold in cisplatin-treated mice compared with saline-treated mice (P < 0.001) as shown in Fig.. 5A. Similarly, Angptl4 mRNA level were increased by 7.76 ± 0.63 (P < 0.001)- in liver tissue and by 1.96 ± 0.38-fold in white adipose tissue (P < 0.05) of cisplatin-treated mice.
WY Prevents Cisplatin-Mediated Increased Angptl4 Protein Levels in the Mouse Kidney
We next examined the effects of cisplatin and fibrate (WY) on protein levels of Angptl4 measured by 2D gel separation and Western blot analysis. For these studies, we used Angptl4 antibodies produced by Dr. S .Chugh's laboratory rather than commercially available antibodies which did not give us consistent results. In our hands, Angptl4 protein was detected at very low levels in kidney tissue of saline-treated mice as several proteolytic fragments with a neutral pI and a 35-kDa molecular mass as shown by black arrows (Fig. 5). Figure 5C shows the quantification of Angptl4 protein levels normalized by actin levels demonstrating a significant increase (2.6-fold) in kidney tissue of cisplatin-treated mice. The use of the PPARα ligand WY inhibited the cisplatin-induced increase in Angptl4 protein levels. Our results suggest that PPARα ligand treatment prevents the cisplatin-induced increased expression of Angptl4 protein levels in kidney tissue.
Fig. 5.
Effect of CP and WY on angiopoietin protein-like 4 (Angptl4) mRNA expression (A), protein levels (B), activity (C), and immunohistochemical localization of Angptl4 (D) in mouse kidney tissues. A: effects of CP on mouse Angptl4 mRNA levels in the kidney, liver, and epididymal white adipose tissues. B: representative autoradiograph of 2-dimensional gel electrophoresis and Western blot analysis from mouse kidney tissue homogenates. The arrows point toward a 35-kDa neutral pI Angptl4 protein in control, CP-, WY-, and WY+CP-treated mice. C: quantitative densitometry of the 35-kDa neutral pI Angplt4 protein shown in B after normalization with β-actin. Values are means ± SE of mRNA levels. Data were obtained from at least 4 independent experiments under each condition. *P < 0.05, **P < 0.001 compared with saline-treated WT mice. †P < 0.05 compared with CP-treated mice in unpaired Student's t-test. D: immunostaining for Angptl4 in kidney tissue. Angptl4 staining is mostly absent in control kidney tissue, and the arrows show that Angptl4 immunostaining is significantly increased in kidney tissue of CP-treated mice, and it is primarily localized to intact proximal tubules (primarily S1 and S2 segments). WY- and WY+CP-treated mice do not show significant staining for Angptl4 in the proximal tubule. Necrotic tubules can be seen only in CP-treated mice (*).
Immunolocalization of Angptl4 Protein in Mouse Kidney Tissue
Angptl4 staining was almost completely absent in kidney tissue of saline-treated control mice. Angptl4 staining was increased in kidney tissue of cisplatin-treated mice as shown in Fig. 5D and was primarily localized to intact proximal tubules (primarily S1 and S2 segments). WY- and WY+cisplatin-treated mice also did not show significant staining for Angptl4 in the proximal tubule.
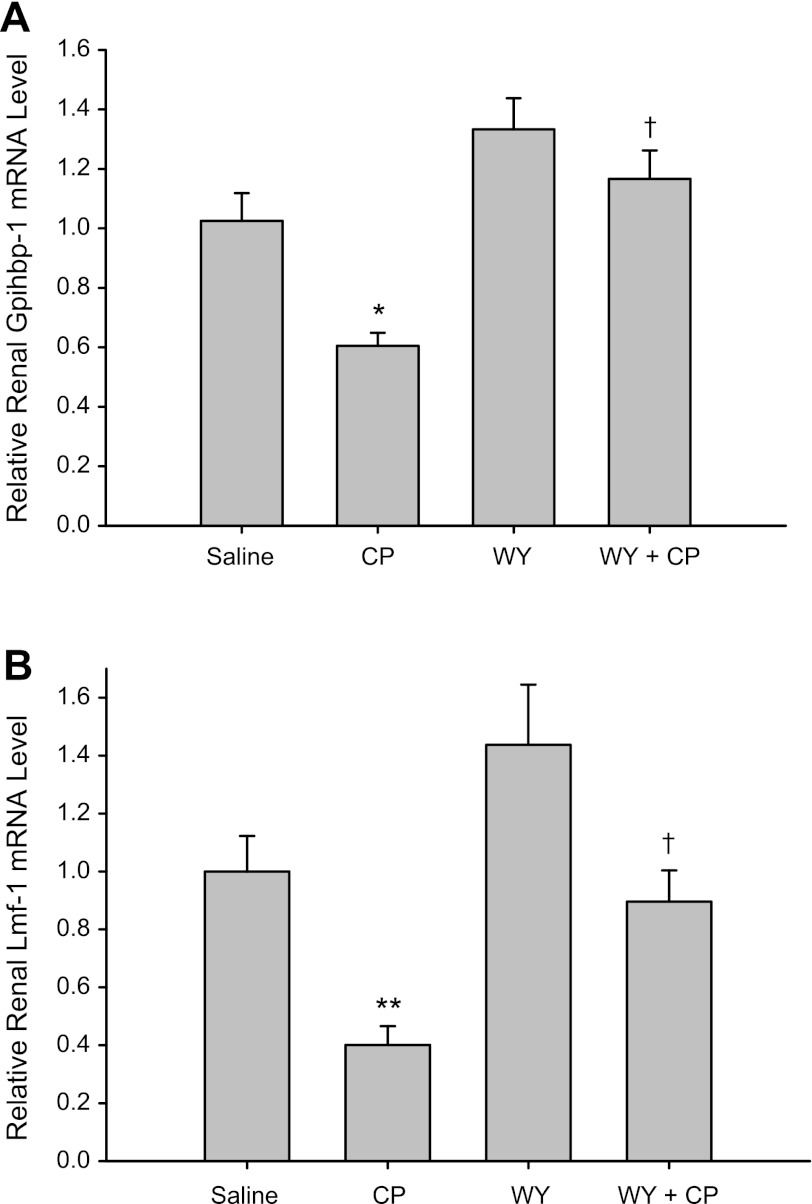
WY Reversed Cisplatin-Induced Downregulation of GPIHBP1 and Lmf1 mRNA Expression Levels
Recently, Lmf1 and GPIHBP1 have been identified as novel genes that play an important role in regulating LPL activity. We examined the effects of cisplatin and WY on renal GPIHBP1 and Lmf1 mRNA expression by quantitative real-time RT-PCR. As shown in Fig. 6, cisplatin caused a decline in the mRNA expression of GPIHBP1 and Lmf1 in kidney tissue. At day 3 after cisplatin administration, there was a 40% reduction in GPIHBP1 mRNA expression (P < 0.05) and a 60% reduction in Lmf1 mRNA expression (P < 0.01) compared with control mice. Pretreatment with WY led to a slight increase in GPIHBP1 and Lmf1 mRNA levels in wild-type mice (P > 0.05) but prevented the cisplatin-mediated reduction in kidney GPIHBP1 and Lmf1 mRNA levels.
Fig. 6.
Effect of CP and WY on renal glycosylphosphatidylinositol-anchored-HDL-binding protein (GPIHBP1; A) and lipase maturation factor 1 (Lmf1; B) mRNA levels in mouse kidney tissue. Real-time RT-PCR was performed using total RNA isolated from kidney tissue of mice fed a normal chow (group saline and CP) or WY-containing chow (groups WY and WY+CP). Tissues were collected at day 3 after either saline (group saline and WY) or CP (groups CP and WY+CP) IP injection. Values are means ± SE of mRNA levels. Data were obtained from at least 4 independent experiments under each condition. *P < 0.05, **P < 0.01 compared with saline-treated WT mice. †P < 0.05 compared with CP-treated WT mice by unpaired Student's t-test.
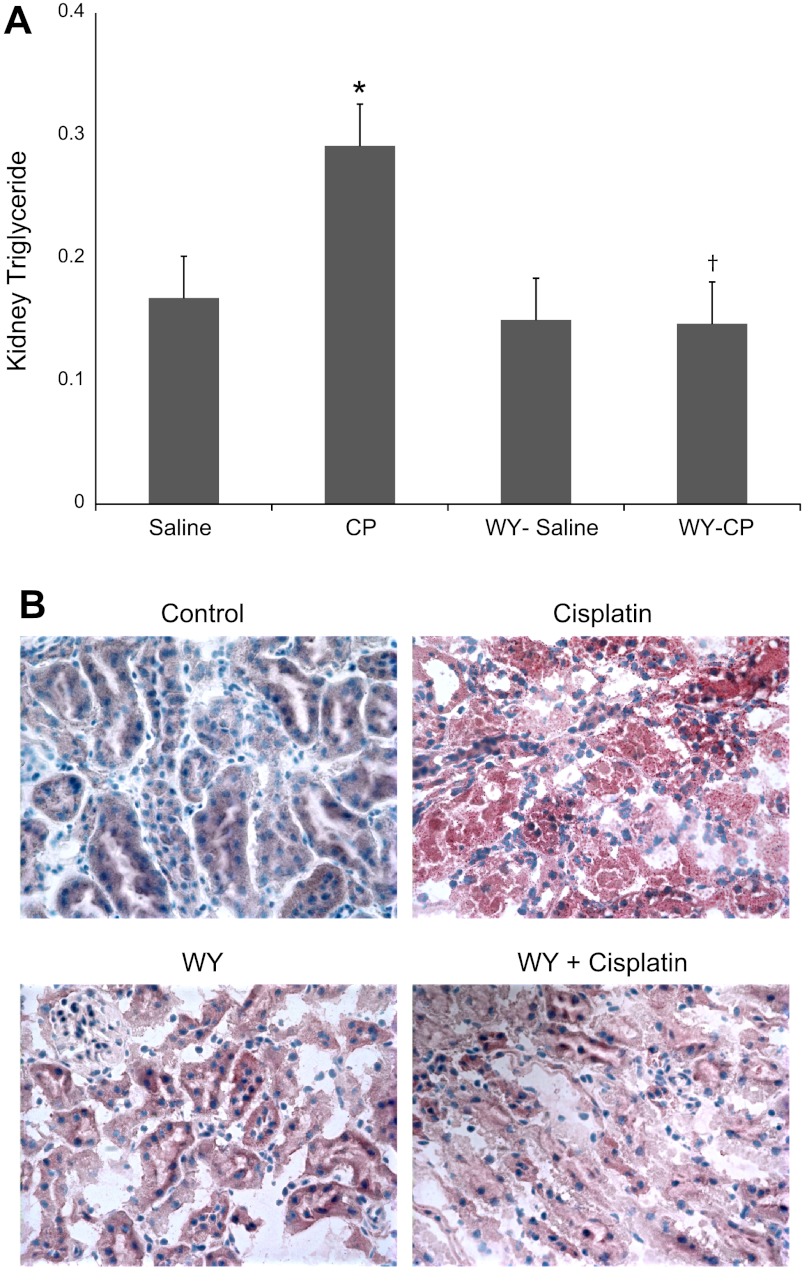
Effects of Cisplatin and PPARα Ligand on Kidney Tissue TG Levels
Our previous studies have shown that cisplatin causes neutral lipid accumulation in the kidney cortex (39). We next examined the effect of cisplatin and WY on TG levels in kidney tissue. As shown in Fig. 7A, cisplatin-treated mice exhibited a twofold increase in TG levels in kidney tissue (P < 0.05). Pretreatment of mice with WY alone did not have a significant effect on renal TG level, but it prevented the increase in renal TG levels induced by cisplatin. We also examined the effects of cisplatin and a PPARα ligand on neutral lipid accumulation by performing intracellular stain of neutral lipids using the oil red O stain in frozen sections of kidney tissue. As shown in Fig. 7B, cisplatin treatment induced a significant accumulation of neutral lipids, which was detected as an increased intracellular red stain compared with saline-treated mice. This accumulation of neutral lipids was more pronounced than the chemical measurements of TGs given the fact that oil red O stain detects not only TGs but also nonesterifed fatty acids and cholesterol accumulation after cisplatin treatment. The oil red O stain was predominantly present in the region corresponding to the proximal tubules in the kidney cortex. Pretreatment of mice with the PPARα ligand WY did not have a significant effect on neutral lipid staining but prevented the accumulation of neutral lipids induced by cisplatin.
Fig. 7.
Effect of CP and WY on triglyceride content (A) and neutral lipid accumulation (B) in mouse kidney tissue. Mice were fed a regular chow or a WY-supplemented diet for 10 days before they were treated with CP as described in methods. Triglyceride content is expressed as milligrams triglyceride per milligram tissue protein (A). B: frozen sections were obtained from animals subjected to 4 experimental conditions, processed, and stained with oil red O dye as described in methods. Depicted are pictures obtained from oil red O staining of kidney sections from control, CP-, WY-, and WY+CP-treated mice. Data were obtained from at least 4 independent experiments under each condition. *P < 0.05 compared with saline-treated WT mice. †P < 0.05 compared with CP-treated WT mice by unpaired Student's t-test.
DISCUSSION
The current studies expand on previous observations and address potential mechanism(s) by which cisplatin induces the accumulation of TG and neutral lipids in kidney tissue (40). In our new studies, we found that cisplatin directly mediates white adipose tissue breakdown by significantly reducing the white epididymal fat pad mass which is accompanied by increased inflammation and cell death as shown in Figs. 2 and 3. The mechanisms responsible for these systemic effects of cisplatin on white adipose tissue are not entirely clear but seem to be specific for this nephrotoxin. Ischemia for 45 min followed by 24 h of reperfusion injury did not cause significant changes in the weight of the white epididymal fat mass (results not shown). White adipose tissue stores fat as a source of energy in the case of fluctuations in food availability, but also serves as an endocrine organ secreting leptin and other hormones that signal energy status to the brain and regulate appetite and insulin sensitivity (2, 15, 48). We speculate that reduced epididymal white fat pad mass is part of a systemic inflammatory response induced by cisplatin. In fact, previous studies have reported that cisplatin-mediated nephrotoxicity is accompanied by activation of proinflammatory cytokines and chemokines (27, 43). Ramesh et al. (43) found that cisplatin injection increases the levels of TNF-α in the serum, kidney tissue, and urine and that the inhibition of TNF-α, using TNF-α-deficient mice or TNF-α inhibitors, ameliorated cisplatin-induced renal dysfunction. TNF-α in turn has been shown to stimulate lipolysis in white adipose tissue and could represent one of the factors involved in reduced adipose tissue mass (23, 56). A recent study investigated the direct effects of uremic serum on human adipocyte function (4). Using this in vitro system, the authors measured released glycerol as well as mRNA levels of the lipid-associated protein perilipin (PLIN). Axelsson et al. (4) concluded that undefined circulating factors in chronic kidney disease (CKD) patients increase basal lipolysis in human adipocytes in vitro, probably by attenuating the expression of the lipolytic regulator PLIN. Therefore, the presence of white adipose tissue lipolysis mediated by increased serum levels of TNF-α and the presence of circulating factors caused by uremia during AKI could explain the effects of cisplatin on reducing white adipose tissue mass. Our studies also show that, in addition to reducing epididymal white adipose tissue mass, cisplatin reduces epididymal white adipose tissue LPL activity. Although we did not explore in detail the mechanisms involved in the inhibition of LPL activity in epididymal white adipose tissue, our data suggest that both reduced epididymal white adipose tissue mass as well as increased expression of Angptl4 could contribute to the observed inhibition of adipose LPL activity.
Next, we investigated the effects of cisplatin and the PPARα ligand WY on expression and enzyme activity of kidney tissue LPL and Angptl4, a protein that appears to play a major role in LPL function. LPL is the enzyme responsible for the hydrolysis of core TGs in chylomicrons, producing chylomicron remnants and intermediate-density lipoproteins (14, 18, 54). This enzyme is primarily expressed in tissues that use fatty acids for fuel or store large amounts of TGs, such as parenchymal cells of the adipose tissue, skeletal muscle, heart, and kidney cortex, and is secreted to the endothelium of local blood vessels (53). Previous studies by Olivecrona's group (46) demonstrated that LPL is present in mouse kidney proximal tubular epithelial cells; however, the regulation of LPL enzyme activity or its expression during acute kidney injury has not been previously examined. We found that similar to the effects of reducing epididymal white adipose tissue mass and LPL activity, cisplatin also inhibited the expression and enzyme activity of kidney LPL. Our Western blot analysis demonstrated reduced expression of LPL protein in kidney tissue of cisplatin-treated mice, and immunohistochemical studies further identified reduced proximal tubule LPL protein after cisplatin treatment. We also found that cisplatin-mediated inhibition of kidney tissue LPL activity was accompanied by increased expression of Angptl4 mRNA and protein levels. In addition, we show by immunohistochemical studies that increased expression of Angptl4 protein by cisplatin occurs predominantly in proximal tubules. Our studies are the first to show a correlation between inhibition of kidney tissue LPL activity and increased expression of Angptl4 protein in this model of cisplatin-mediated AKI. This would be logical because the tubular epithelial cells consume much energy and derive it mainly from fatty acids. Experiences with other tissues indicate that LPL is usually produced by the cells that will take up and metabolize fatty acids. Our studies in the cisplatin model as well as in the ischemia-reperfusion injury model document inhibition of kidney tissue LPL activity during AKI. This is in contrast to what has been described in the ⅚ nephrectomy model of CKD, where Vaziri et al. (50–53) found a significant reduction of gene expression, protein abundance, and enzyme activity of LPL in non-kidney tissues including adipose tissue, skeletal muscle, and myocardium. Those findings in the animal model of CKD could also explain why patients with CKD develop hypertriglyceridemia, impaired clearance of VLDL and chylomicrons, TG enrichment of LDL, and HDL (19, 51).
Our studies show that cisplatin-mediated inhibition of LPL expression and enzyme activity correlates with increased expression of Angptl4 and increased accumulation of intracellular TG in kidney tissue and, more specifically, in the proximal tubule. Since reduced kidney LPL activity by cisplatin is expected to reduce the amount and TG and fatty acids delivered to kidney tissue, we propose that despite inhibiting renal LPL by upregulation of Angptl4 protein, cisplatin treatment raises TG levels in kidney tissue by impairing fatty acid oxidation, which can be prevented by administration of a PPARα agonist. Indeed, our previous studies have demonstrated that ischemia-reperfusion and cisplatin-mediated AKI inhibit fatty acid oxidation in kidney tissue, leading to the accumulation of neutral lipids including nonesterified fatty acids and TGs. We also showed in those studies that the use of a PPARα ligand or proximal tubule PPARα transgenic mice ameliorates AKI by reducing proximal tubule cell injury and lipid accumulation (26, 28, 29).
A variety of proteins that regulate LPL activity have been identified including apo CII, apo CIII, apo A5, and angiopoietin-like proteins 3 and 4 (7, 8, 17, 21, 30, 47). Angptl4 has been shown to convert catalytically active LPL to inactive monomers (47). In recent years the angiopoietin-like proteins Angptl3 and Angptl4 have emerged as novel modulators of LPL activity. Studies in transgenic animals supported by in vitro experiments have demonstrated that Angptl3 and Angptl4 impair TG clearance by inhibiting LPL activity. In humans, genetic variation within the Angptl3 and Angptl4 genes contributes to variation in plasma TG and HDL levels, thereby validating the importance of angiopoietin-like proteins in the regulation of lipoprotein metabolism in humans (30). Although our study shows that increased expression of Angptl4 in the proximal tubule is associated with reduced kidney tissue LPL activity during cisplatin-mediated AKI, additional studies are needed to demonstrate a cause-effect relationship in kidney tissue between increased Angptl4 expression and reduced kidney LPL activity. In a recent study by Clement et al. (12), the authors injected rats with nephrotoxic serum and demonstrated increased expression of Angptl4 in podocytes. In addition, these authors showed that injection of a single dose of puromycin aminonucleoside or use of the model of passive Heymann nephritis led to a significant increase in the expression of Angptl4 protein in podocytes. To further examine the consequences of increased Angptl4 expression in podocytes, the authors developed podocyte-specific Angptl4 transgenic rats, as well as adipose tissue-specific Angptl4 transgenic rats. Podocyte-specific transgenic overexpression of Angptl4 in rats induced nephrotic range proteinuria, loss of glomerular basement membrane charge, and foot process effacement, whereas transgenic expression specifically in the adipose tissue resulted in increased circulating Angptl4 but not proteinuria. In addition, these authors noted that Angptl4 secreted from podocytes in some forms of nephrotic syndrome lacked normal sialylation, and that the use of sialic acid precursor N-acetyl-d-mannosamine in podocyte-specific Angptl4 transgenic rats increased Angptl4 sialylation and reduced albuminuria (12). Altogether, these results suggest that Angptl4 plays a key role in the pathogenesis of nephrotic syndrome. Although we did not measure Angptl4 protein expression in the serum of cisplatin-treated mice, the observed systemic response of mice receiving cisplatin with increased expression of Angptl4 in adipose tissue, liver, and kidney tissue again suggests the presence of a generalized metabolic response in various organ systems after cisplatin injection. Additional studies using proximal tubule Angptl4 transgenic mice as well as Angptl4 −/− mice are necessary to further understand the function of increased Angptl4 expression in the proximal tubule and its effect on inhibiting kidney LPL activity after cisplatin injury.
Finally, we examined the effects of cisplatin injury on the expression of kidney GPHBP1 and Lmf1, two important modulators of LPL activity. The function of GPIHBP1 in lipolysis was clarified when it was shown that GPIHBP1 knockout mice on a chow diet have milky plasma with high plasma TG levels (6). By immunohistochemistry, GPIHBP1 is located in capillary endothelial cells, and GPIHBP1-expressing cells bind LPL avidly. Furthermore, Davies et al. (13) showed that GPIHBP1 shuttles LPL from the interstitial spaces of tissues where it is secreted by parenchymal cells into the capillary lumen, the site where it needs to be to hydrolyze TGs in lipoproteins. A recent study using 124I-labeled GPIHBP1-specific monoclonal antibodies, along with PET scanning, yielded new and unexpected insights into GPIHBP1 expression and function (34). Significant amounts of GPIHBP1 were found in the lungs, liver, and kidney, tissues that are not thought to have important roles in LPL-mediated processing of TG-rich lipoproteins. Lmf1 has been identified as the gene responsible for the combined lipase deficiency in mice with severe hypertriglyceridemia (37, 38). This chaperone is involved in the posttranslational maturation of lipase polypeptide chains within the endoplasmic reticulum, allowing the assembly of lipase monomers into active homodimers or in the stabilization of dimers already formed. We find that GPIBP1 and Lmf1 mRNA levels are both reduced by cisplatin injury. Use of a PPARα ligand restored the expression of GPIBP1 and Lmf1 in kidney tissue back to normal levels.
In summary, our studies demonstrate reduced lipolytic processing of kidney tissue LPL after cisplatin injury. We also present evidence for increased expression of proximal tubule Angptl4 and reduced expression of GPIHBP1 and Lmf1 as potential cellular mechanisms responsible for cisplatin-mediated inhibition of kidney tissue LPL activity. Additional studies, including in situ hybridization and immunolocalization studies as well as functional studies of GPIHBP1 and Lmf1 activities in kidney tissue, are needed to further understand the role of reduced LPL activity in kidney tissue during AKI.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK75976, a Veterans Affairs Merit Award, and a REAP award. S. M. Ali was supported by NIDDK Grant T3DK061921.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.L., K.N., G.R., S.M.A., B.S., S.A., S.K., and D.P. performed experiments; S.L., K.N., G.R., N.G., S.A., J.M., and D.P. prepared figures; S.L. and D.P. drafted manuscript; S.L. and D.P. approved final version of manuscript; N.G., J.M., G.O., S.S.C., S.K., and D.P. interpreted results of experiments; D.P. provided conception and design of research; D.P. analyzed data; D.P. edited and revised manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank G. Olivecrona (Umea University, Umea, Sweden) for sharing chicken anti-LPL polyclonal antibodies, S. S. Chugh (University of Alabama) for sharing rabbit anti-Angptll4 polyclonal antibodies, and Cindy Reid for help with manuscript preparation.
REFERENCES
- 1. Abdel-Gayoum AA, El-Jenjan KB, Ghwarsha KA. Hyperlipidemia in cisplatin-induced nephrotic rats. Hum Exp Toxicol 18: 454–459, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab 11: 327–332, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Arany I, Safirstein RL. Cisplatin nephrotoxicity. Sem Nephrol 23: 460–464, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Axelsson J, Astrom G, Sjolin E, Qureshi AR, Lorente-cebrian S, Stenvinkel P, Ryden M. Uraemic sera stimulate lipolysis in human adipocytes: role of perilipin. Nephrol Dial Transplant 26: 2485–2491, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Basi S, Pupim LB, Simmons EM, Sezer T, Shyr Y, Freedman S, Chertow GM, Mehta RL, Paganini E, Himmelfarb J, Ikizler TA. Insulin resistance in critically ill patients with acute renal failure. Am J Physiol Renal Physiol 289: F259–F264, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Beigneux AP, Davies BS, Gin P, Weinstein MM, Farber E, Qiao X, Peale P, Bunting S, Walzem RL, Wong JS. Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab 5: 279–291, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calandra S, Priore Oliva C, Tarugi P, Bertolini S. APOA5 and triglyceride metabolism, lesson from human APOA5 deficiency. Curr Opin Lipidol 17: 122–127, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Catapano AL. Apolipoprotein C-II and lipoprotein lipase activity. Ric Clin Lab 12: 35–40, 1982 [DOI] [PubMed] [Google Scholar]
- 9. Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Pérez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem 7: 3–18, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol 61: 223–242, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Ciarimboli G, Deuster D, Knief A, Sperling M, Holtkamp M, Edemir B, Pavenstadt H, Lanvers-Kamisnky C, Zenhoff-dinnesen A, Scinkel AH, Koepsell H, Jurgens H, Schlatter E. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is target for protective interventions. Am J Pathol 176: 1169–1180, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clement LC, Avila-Casado C, Mane C, Soria E, Bakker WW, Kersten S, Chugh SS. Podocyte-secreted angiopoietin-like 4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nature Med 17: 117–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davies BS, Beigneux AP, Barnes RH, Tu Y, Gin P, Weinstein MM, Nobumori C, Nyren R, Goldberg G, Olivecrona G. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab 12: 42–52, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med 320: 1060–1068, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316: 129–139, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Géloën A, Trayhurn P. Regulation of the level of uncoupling protein in brown adipose tissue by insulin require the mediation of the sympathetic nervous system. FEBS Lett 267: 265–267, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Ginsberg HN, Le NA, Goldberg IJ, Gibson JC, Rubinstein A, Wang-Iverson P, Norum R, Brown WV. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and A1. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J Clin Invest 78: 1287–1295, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldberg IJ, Merkel M. Lipoprotein lipase: physiology, biochemistry, and molecular biology. Front Biosci 6: D388–D405, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Horkko S, Huttunen K, Korkonen T, Kesaniemi YA. Decreased clearance of low-density lipoprotein in patients with chronic renal failure. Kidney Int 45: 561–570, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Kersten S, Mandard S, Tang NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 275: 28488–28493, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Köster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, Heuer JG, Jaskunas SR, Eacho P. Transgenic angipoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology 146: 4943–4950, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Launay-Vacher V, Rey JB, Isnard-Bagnis C, Deray G, Daouphars M. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol 61: 903–909, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Laurencikiene J, van Harmelen V, Arvidsson Nordstrom E, Dicker A, Blomqvist L, Naslund E, Langin D, Arner P, Ryden M. NF-kB is important for TNF-α-induced lipolysis in human adipocytes. J Lipid Res 48: 1069–1077, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Leverve XM, Cano NJ. Nutritional management in acute illness and acute kidney insufficiency. Contrib Nephrol 156: 112–118, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Li C. Genetics and regulation of angiopoietin-like proteins 3 and 4. Curr Opin Lipidol 17: 152–156, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Li S, Basnakian A, Bhatt R, Megyesi J, Gokden N, Shah SV, Portilla D. PPAR-α ligand ameliorates acute renal failure by reducing cisplatin-induced increased expression of renal endonuclease G. Am J Physiol Renal Physiol 287: F990–F998, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Li S, Gokden N, Okusa MD, Bhatt R, Portilla D. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am J Physiol Renal Physiol 289: F469–F480, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Li S, Nagothu KK, Desai V, Lee T, Branham W, Moland C, Megyesi JK, Crew MD, Portilla D. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-alpha in mice confers protection during acute kidney injury. Kidney Int 76: 1049–1062, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li S, Wu P, Yarlagadda P, Vadjunec NM, Proia AD, Harris RA, Portilla D. PPAR α ligand protects during cisplatin-induced acute renal failure by preventing inhibition of renal FAO and PDC activity. Am J Physiol Renal Physiol 286: F572–F580, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Mattijssen F, Kersten S. Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochim Biophys Acta 1821: 782–789, 2012 [DOI] [PubMed] [Google Scholar]
- 31. Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med (Berl) 80: 753–769, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Nilsson-Ehle P, Schotz MC. A stable radioactive substate emulsion for assay of lipoprotein lipase. J Lipid Res 17: 536–541, 1976 [PubMed] [Google Scholar]
- 33. Oh JH, Baum DD, Pham S, Cox M, Nguyen ST, Ensor J, Chen I. Long-term complications of platinum-based chemotherapy in testicular cancer survivors. Med Oncol 24: 175–181, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Olafsen T, Yopung SG, Davies BS, Beigneux AP, Kenanova VE, Voss C, Young G, Wong KP, Tu Y. Unexpected expression pattern for glycosylphosphatidylinositol-anchored HDL-binding protein GPIHBP1 in mouse tissues revealed by positron emission tomography scanning. J Biol Chem 285: 39239–39248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Perazella MA, Moeckel GW. Nephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology, and prevention/therapy. Semin Nephrol 30: 570–581, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Peterfy M, Ben-Zeev Mao HZ, Weisglass-Volkov D, Aouizerat BE, Pullinger CR, Frost PH, Kane JP, Malloy MJ, Reue K, Pajukanta P, Doolittle MH. Mutations in LMF1 cause combined lipase deficiency and severe hypertriglyceridemia. Nat Genet 39: 1483–1487, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Peterfy M. Lipase maturation factor 1: a lipase chaperone involved in lipid metabolism. Biochim Biophys Acta 1821: 790–794, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Portilla D, Dai G, McClure T, Bates L, Kurten R, Megyesi J, Price P, Li S. Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int 62: 1208–1218, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Portilla D, Li S, Nagothu KK, Megyesi J, Kaissling B, Schnackenberg L, Safirstein RL, Beger RD. Metabolomic study of cisplatin-induced nephrotoxicity. Kidney Int 69: 2194–2204, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Portilla D. Energy metabolism and cytotoxicity. Semin Nephrol 23: 432–438, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 33: 9–23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramesh G, Reeves WB. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ranganathan G, Li C, Kern PA. The translational regulation of lipoprotein lipase in diabetic rats involves the 3′-untranslated region of lipoprotein lipase mRNA. J Biol Chem 275: 40986–40991, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Ranganathan S, Kern PA. Thiazolidinediones inhibit lipoprotein lipase activity in adipocytes. J Biol Chem 273: 26117–26122, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Ruge T, Neuger L, Sukonina V, Wu G, Barath S, Gupta J, Frankel B, Christophersen B, Nordstoga K, Olivecrona T, Olivecrona G. Lipoprotein lipase in the kidney: activity varies widely among animal species. Am J Physiol Renal Physiol 287: F1131–F1139, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA 103: 17450–17455, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 60: 329–339, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Unger RH. Lipotoxic diseases. Annu Rev Med 53: 319–336, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Vaziri ND, Liang K. Down-regulation of tissue lipoprotein lipase expression in experimental chronic renal failure. Kidney Int 50: 1928–1935, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Vaziri ND, Navab M, Fogelman AM. HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol 6: 287–296, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Vaziri ND, Wang XQ, Liang K. Secondary hyperparathyroidism downregulates lipoprotein lipase expression in chronic renal failure. Am J Physiol Renal Physiol 273: F925–F930, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol 290: F262–F272, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 297: E271–E288, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Weinberg JM. Lipotoxicity. Kidney Int 70: 1560–1566, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-α stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes 51: 2929–2935, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.