Abstract
The mammalian cochlea is sophisticated in its function and highly organized in its structure. Although the anatomy of this sense organ has been well documented, the molecular mechanisms underlying its development have remained elusive. Information generated from mutant and knockout mice in recent years has increased our understanding of cochlear development and physiology. This article discusses factors important for the development of the inner ear and summarizes cochlear phenotypes of mutant and knockout mice, particularly Otx and Otx2. We also present data on gross development of the mouse cochlea.
The mammalian cochlea, the end organ of auditory function, is a truly remarkable structure. Its uniquely coiled shape, diversity of cell types, and intricate architecture are unmatched by any other organs in the body. The sensory component of the cochlea, the organ of Corti, consists of both sensory hair cells and supporting cells, and it spirals like a ribbon down the cochlear duct. The cochlea is tonotopically mapped so that the hair cells at each location along the cochlea are most sensitive to a particular frequency (for review, see ref. 1). Multiple structural features of the hair cells are organized in a gradient along the cochlea that could contribute to the differential frequency selectivity of the hair cells (for review, see ref. 2). For example, hair cells in the base of the cochlea have shorter cell bodies and their stereocilia are shorter and more abundant than those of hair cells in the apex. In addition, the width of the basilar membrane and the mass of the tectorial membrane also increase toward the apex of the cochlea. These overall structural gradients along the cochlea are largely conserved among different species but vary depending on the range of absolute frequencies detected and the most sensitive frequency range of an individual species. Little is known about the molecular mechanisms that establish the fine structural patterning of the cochlea or that underlie the tonotopic organization of the organ. Likewise, little is known about what makes a cochlea coil and what dictates the variation in the number of coils among different species (3). Recent gene targeting approaches in mice have provided insights by identifying a number of genes important for the shaping of the cochlea at both the gross and fine structural levels. Here, we summarize data from mutant and knockout mice with cochlear defects and highlight several features of the gross development of the cochlea that may pertain to its mature functions.
Gross Development of the Cochlea
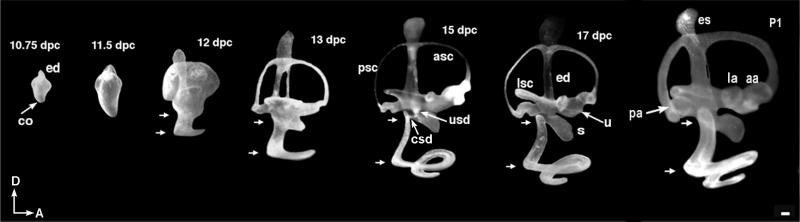
The mouse inner ear can be roughly divided into a dorsal vestibular and a ventral saccular and cochlear region. The cochlea develops from the ventral portion of the rudimentary otocyst. Fig. 1 illustrates a series of developing inner ears in which the lumen has been filled with a latex paint solution to reveal its gross anatomy. At 10.75 dpc (days postcoitium), the cochlear anlage becomes evident, and it first extends ventromedially and then anteriorly. As a result, the first turn of the cochlea is apparent at 12 dpc. Over time, there is a continual increase in length in the proximal region (Fig. 1, arrows) as well as coiling in the distal region of the cochlea to achieve its mature 1.75 turns by 17 dpc (4). Based on birth-dating studies, it has been proposed that the junction of the presumptive saccule and cochlea is the site of cochlear growth (5). Our paint-fill data confirmed that there is indeed a considerable increase in distance between the presumptive saccule and the location of the first turn of the cochlear duct over time (Fig. 1, distance between arrows). However, we cannot verify from these results whether the cochlea grows exclusively at the junction of the saccule and cochlea. Determining how the cochlea grows remains an important question because it may establish the basis for its tonotopic organization.
Figure 1.
Lateral views of paint-filled membranous labyrinths. Membranous labyrinths of inner ears from 10.75 dpc to postnatal day 1 were filled with latex paint solution as described. At 10.75 dpc, the protrusions of the endolymphatic duct in the dorsal and the cochlear anlage in the ventral portion of the otocyst are evident. By 17 dpc, the gross anatomy of the inner ear is mature. Arrowheads identify the proximal region of the cochlea. aa, anterior ampulla; asc, anterior semicircular canal; co, cochlea; csd, cochleosaccular duct; ed, endolymphatic duct; es, endolymphatic sac; la, lateral ampulla; lsc, lateral semicircular canal; pa, posterior ampulla; psc, posterior semicircular canal; s, saccule; u, utricle; usd, utriculosaccular duct. Orientation: D, dorsal; A, anterior. (Scale bar = 100 μm.) [Adapted with permission from Morsli et al. (4) (Copyright 1998, the Society for Neuroscience).]
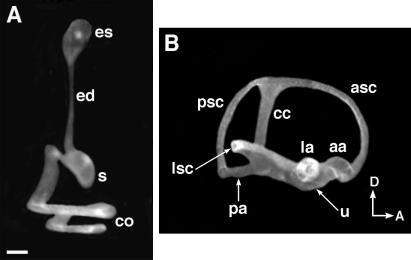
The development of the vestibular component in the dorsal portion of the otic vesicle occurs concurrently with ventral cochlear development. As morphogenesis progresses, there is a gradual restriction in the portion of the inner ear separating the dorsal and ventral regions. By 15 dpc, the utricle and saccule are separated by the utriculosaccular duct (Fig. 1, usd), which is continuous with the endolymphatic duct. By 16.5 dpc, when morphogenesis is more advanced, the utriculosaccular duct becomes progressively restricted, and the utricle and saccule are essentially two separate chambers (Fig. 2). As a result, a single injection of latex paint solution into the saccule, for example, invariably fills only four components of the labyrinth, including the endolymphatic sac and its duct, the saccule, and the cochlea (Fig. 2A). In contrast, single injections to either the utricle or ampulla fill the rest of the vestibular system (Fig. 2B). It is conceivable that the utricle and saccule are connected in the mature mouse inner ear by the utriculosaccular duct, but the lumen is too narrow for the passage of the paint solution. Nevertheless, these results suggest that there are largely two separate chambers in the membranous labyrinth of a mature mouse inner ear, and only the cochlea and the saccule are efficiently under fluid regulation by the endolymphatic sac. This gross anatomical finding is supported by the fact that the endolymph in the ampullae and cochlea have different ionic compositions and electrochemical potentials (6). In addition, a recent report of EphB2-knockout mice shows that loss of this member of Eph receptor tyrosine kinase family resulted in vestibular defects that may be associated with reduced endolymph production in the ampulla and canal regions (7). Despite the reduced size of vestibular membranous labyrinth, these mice have no apparent cochlear defects, and the membranous labyrinth of the cochlear region appeared normal (ref. 7 and B. Fritzsch, personal communication). Taken together, these results support the paint-fill data that the membranous labyrinth of a mature inner ear consists of two separate compartments. This difference in the properties of the endolymph within different inner ear components such as the cochlea and ampulla may play a direct role in facilitating the specific functions of each component. Furthermore, such functions may be affected in mutants in which the utricle and saccule fail to form separate chambers (8–11).
Figure 2.
Partially paint-filled mouse membranous labyrinths at 16.5 dpc. At 16.5 dpc, the membranous labyrinth largely consists of two compartments. (A) Latex paint solution injected into the endolymphatic sac fills the endolymphatic duct, saccule, and cochlea only. (B) Latex paint solution injected into the lateral ampulla fills the utricle, the three semicircular canals, and their ampullae. cc, common crus. Orientation as per Fig. 1. (Scale bar = 200 μm.)
Unlike the mouse, the endolymphatic duct in humans has a bifurcation connecting to both the utricle and saccule. Therefore, structures such as the ampullae and utricle in humans have a more direct access to fluid regulation by the endolymphatic sac than do these same structures in the mouse. This anatomical difference between humans and mice might be an important consideration when evaluating mouse models for human genetic disorders affecting fluid homeostasis.
Gross Patterning of the Cochlea
The normal development of the inner ear is thought to depend on multiple surrounding tissues, including the hindbrain, neural crest, mesenchyme, and possibly the notochord (for review, see refs. 12–16). Some of the genes involved have been identified, and the examination of mutants and knockout mice demonstrates that the absence of these gene products invariably affects the patterning of the cochlea as well (Table 1).
Table 1.
Genes affecting cochlear patterning
| Gene | Type of protein | Distribution in the inner ear and surrounding structures | Mutant or knockout phenotype | Ref. |
|---|---|---|---|---|
| Brn4 | Pou domain transcription factor | Periotic mesenchyme | Defects in fibroblasts of spiral ligament; shortened cochlea | 48–51 |
| Dlx 5 | Homeobox transcription factor | Dorsal region of otic vesicle; semicircular canals and endolymphatic duct | No anterior or posterior canal; reduced lateral canal; abnormal endolymphatic duct and cochlea | 25, 26 |
| Eya 1 | Transcriptional coactivator | Ventralmedial otic vesicle; VIIIth ganglion; vestibular and cochlear sensory regions; periotic mesenchyme | No VIIIth ganglion; amorphic inner ear | 18, 22, 52, 53 |
| Fgf3 | Growth factor | r5 and r6; prospective otic placode region; neurogenic and sensory regions | No endolymphatic duct or sac; reduced spiral ganglion; enlarged membranous labyrinth | 47, 54–57 |
| Fgfr2 (IIIb) | Growth factor receptor | Otic placode; dorsal and medial wall of otic vesicle; nonsensory regions of the inner ear | Dysgenesis of membranous labyrinth; rudimentary sensory patches and VIIIth ganglion; 50% of mutants lack endolymphatic duct | 47 |
| Hoxa1 | Homeobox transcription factor | 8 dpc: r3/4 boundary to spinal cord | No endolymphatic duct or sac; amorphic inner ear; no organ of Corti; reduced VIIIth ganglion | 58–61 |
| Hoxa1/b1 | Homeobox transcription factors | Hoxb1: 8 dpc: r3/4 boundary to spinal cord; 9 dpc: expression up-regulated in r4 | Amorphic inner ear; more severe phenotype than Hoxa1−/− alone | 62 |
| Hoxa2 | Homeobox transcription factor | r1/2 boundary to spinal cord; expression upregulated in r3 and r5 | Membranous labyrinth appeared enlarged; scala vestibuli lacking or collapsed | 63, 64 |
| Kreisler | bZIP Transcription factor | r5 and r6 | Misplaced otocyst; inner ear usually cyst-like; endolymphatic duct is often missing | 56, 65–67 |
| ngn 1 | bHLH transcription factor | Anteroventrolateral otic vesicle | No VIIIth ganglion; fusion of utricle and saccule; shortened cochlea | 11, 40 |
| Otx1 | Transcription factor | Lateral wall of otic vesicle; lateral canal and ampulla; lateral wall of saccule and cochlea | No lateral canal or ampulla; no lateral crista; incomplete separation of utricle and saccule; misshapen saccule and cochlea | 10, 29 |
| Otx2 | Transcription factor | Ventral tip of otic vesicle; lateral wall of saccule and cochlea | Otx1−/−, Otx2+/−: more severe saccular and cochlear phenotype than Otx1−/− | 10 |
| Pax2 | Paired-box transcription factor | Medial wall of otic vesicle; endolymphatic duct and sac; cochlea | Agenesis of the cochlea and spiral ganglion | 17, 19, 68 |
| Pax3 | Paired-box transcription factor | Dorsal half of neural tube | Splotch mouse: aberrant endolymphatic duct; misshapen cochlear and vestibular components | 69–73 |
Genes Expressed in the Otic Epithelium.
Several genes expressed in the otic epithelium are important for the normal development of the cochlea (Table 1). For example, the absence of Pax2, a paired-box transcription factor, leads to agenesis of the cochlea (17). The development of inner ears of Eya1 (eyes absent)-knockout mice arrest at the otic vesicle stage (18). In these mice, the endolymphatic duct is either absent or malformed, and the VIIIth ganglion fails to form. Both Otx1 and Otx2 are expressed in the otocyst and are important for cochlear and vestibular development. As such genetic information accumulates, it is important to determine when and where these genes act along the developmental pathway. This task is complicated by the fact that often several members of a single gene family, which may share redundant functions, are expressed in the inner ear during development. For example, Pax2 and -8 are both expressed in the otic epithelium (18–20). However, no inner ear phenotypes have been reported for Pax8-knockout mice so far (21). Eya1 and -2 are both expressed in the VIIIth ganglion (22). Dlx2, -3, -5, and -6 are all expressed in the mouse inner ear (23–26). However, only the knockout of Dlx5 has been reported to display inner ear defects, including abnormalities in the semicircular canals, ampullae, endolymphatic duct, and cochlea (25–28). Therefore, it is important to sort out specific functions for each member of a gene family. We have attempted to address this issue for Otx1 and -2.
Otx Genes.
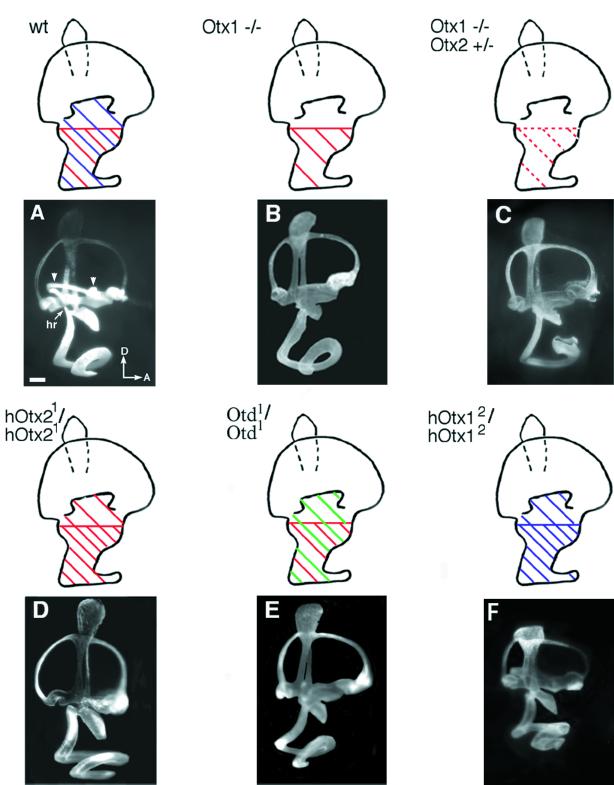
Otx1 and Otx2 are murine orthologues of the Drosophila orthodenticle gene. These genes are bicoid-like transcription factors important for the development of the head and sense organs (29–32). In the inner ear, both Otx1 and Otx2 are activated during the otocyst stage. At 10.25 dpc, Otx1 is expressed in the ventro-lateral wall of the otocyst, and Otx2 is expressed in the ventral tip of the otocyst within a portion of the Otx1-positive region (10). As development progresses, the most dorsal boundary of the Otx1 domain corresponds to the presumptive lateral canal level, and the dorsal boundary of Otx2 expression domain corresponds to the middle of the utricular anlage (Fig. 3A, schematic). In Otx1-knockout mice, the lateral canal and ampulla are missing, the cochlea is misshapen, and the utricle and saccule fail to form separate chambers (Fig. 3B; refs. 10 and 29). Otx2-knockout mice die around 10 dpc before any significant inner ear development (30, 33). To address the possible functions of Otx2 in inner ear development, inner ears of Otx1-knockout mice with one disrupted allele of Otx2 (Otx1−/−; Otx2+/−) were examined. These mice show much more severe defects, particularly in ventral structures, including the saccule and cochlea, which normally express Otx2 (Fig. 3C and Table 2).
Figure 3.
Lateral views of paint-filled inner ears from wild-type (A), Otx1−/− (B), Otx1−/−; Otx2+/− (C), hOtx21/hOtx21 (D), otd1/otd1 (E) and hOtx12/hOtx12 (F) mice. Domains of Otx1 (blue), Otx2 (red), and otd (green) expression are shown as schematics above examples of paint-filled inner ears. In Otx1−/− mutants (B), the lateral canal and ampulla are missing. The utricle and saccule are incompletely separated, and the shapes of the saccule and cochlea often are malformed. In Otx1−/−; Otx2+/− mutants (C), the phenotypes of the saccule and cochlea are more severe than those in Otx1−/− mice. In hOtx21/hOtx21 mice (D), the lateral canal and ampulla are missing. The shapes of the saccule and cochlea are normal but the cochlea is sometimes shortened. In otd1/otd1 mice (E), the phenotype is similar to that of hOtx21/hOtx21, with no lateral canal and ampulla formation. In addition, the saccule also is malformed. In hOtx12/hOtx12 mice (F), both the saccule and cochlea are malformed but the lateral canal and ampulla are normal. hr, hook region. Arrowheads in A indicate the lateral canal and ampulla, which are missing in B, C, D, and E. Orientation as per Fig. 1. (Scale bar = 300 μm.)
Table 2.
Frequencies of various phenotypes in inner ears of Otx1 and Otx2 mutants
| Genotype | No. of animals | Lack of lateral canal and ampulla | Lack of separation of utricle and saccule | Lack or aberrant cochleo saccular duct | Aberrant saccule | Misshapen cochlea
|
||
|---|---|---|---|---|---|---|---|---|
| Hook region | No. of coils | Aberrant shape | ||||||
| Otx1 −/− | 11 | 11 | 11 | 11 | 6 | 11 | 9 | 5 |
| Otx1 −/− Otx2 +/− | 9 | 9 | 9 | 9 | 7 | 9 | 8 | 7 |
| hOtx21/hOtx21 | 7 | 7 | 6 | 5 | 0 | 5 | 1 | 0 |
| Otd1/Otd1 | 6 | 6 | 6 | 5 | 6 | 5 | 0 | 0 |
| hOtx12/hOtx12 | 10 | 0 | 9 | 9 | 10 | 10 | 9 | 10 |
All mutant mice were scored between 15 to 16.5 dpc. At these stages, the cochlea should coil from 1.5 to a mature 1.75 turns. Any cochlea that had 1.5 turns was considered normal in order to accommodate for variability in staging and possible developmental delay of mutants.
The predicted murine OTX1 and OTX2 proteins show extensive homology between the N terminus and the end of the homeodomain. otd and its orthologue, Otx2, have been shown to largely substitute for Otx1 functions in the brain (34, 35). Therefore, we attempted to determine whether Otx2 and otd could also substitute for Otx1 in inner ear development, especially in the lateral canal region where normally only Otx1 is expressed. To achieve this substitution, the full-length human Otx2 cDNA was introduced into a disrupted Otx1 locus (hOtx21/hOtx21) and thus placed under transcriptional regulation of Otx1. The inner ears of these mice had no lateral canal or ampulla, indicating that Otx2 was not able to functionally compensate for Otx1 in forming these structures (refs. 10 and 34; Fig. 3D). In regions where the two genes are normally coexpressed, there was a partial rescue of saccular and cochlear phenotypes, as well as the separation of utricle and saccule (Table 2). Introduction of otd into the disrupted Otx1 locus also failed to restore the formation of the lateral canal and ampulla (ref. 35; Fig. 3E). In addition, although the shape of the cochlea in these mice was similar to the cochlea of wild-type and hOtx21/hOtx21 mice, the saccule was often smaller in size, indicating that otd might be less effective at compensating for Otx1 functions than Otx2 (Fig. 3E and Table 2).
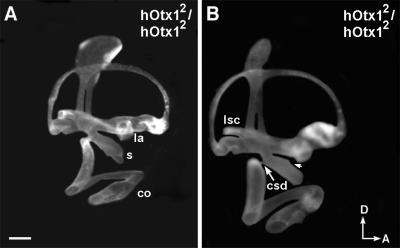
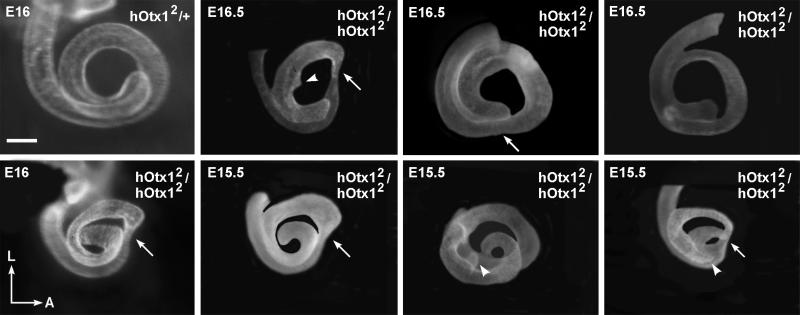
When the human Otx1 cDNA was introduced into a disrupted Otx2 locus (hOtx12/hOtx12), embryogenesis proceeded much further than in Otx2-null mice (36). The expression of Otx1 in the visceral endoderm was able to rescue gastrulation and specification of rostral neuroectoderm that were defective in Otx2−/− mice. However, despite the presence of Otx1 mRNA, no OTX1 protein was detected in the epiblast of these mice. As a result, hOtx12/hOtxl2 mice lacked forebrain and midbrain structures, displaying a headless phenotype from 9 dpc onward. Among all of the specimens examined between 15 to 16.5 dpc for inner ear defects, the coiling and the shape of the cochlea were invariably affected (Figs. 4 and 5). Most specimens show an incomplete separation of the utricle and saccule (Table 2 and Fig. 4). The shape of the saccule often appeared thinner than that of wild type (Fig. 4A), and sometimes displayed aberrant notches (Fig. 4B, arrowhead). Compared with saccules of hOtx21/hOtx21 mice, they were also more affected (compare Fig. 3 D with F and Fig. 4). The distribution of human OTX1 protein in the hOtx12/hOtx12 inner ears has not been examined, so the inability of Otx1 to substitute for Otx2 functions in the inner ear could also be caused by a posttranscriptional problem, similar to the situation in the epiblast. The lateral canal and ampulla in these inner ears were normal; an expected result because the Otx1 locus was not disrupted in these mice (Fig. 4). Taken together, these results suggest that Otx1 and Otx2 have both overlapping and specific functions in the patterning of the inner ear. Otx1 is essential for the formation of the lateral canal and ampulla, whereas Otx2 plays a critical role in the patterning of ventral structures such as the cochlea and saccule (Table 2).
Figure 4.
Lateral views of paint-filled hOtx12/hOtx12 inner ears from 16 (A) and 16.5 (B) dpc. The lateral canal and ampulla are normal. The saccule and cochleosaccular ducts are affected in both specimens, but B is more severe than A is. Arrowhead in B indicates an aberrant notch in the saccule. Orientation and abbreviations as per Fig. 1. (Scale bar = 200 μm.)
Figure 5.
Ventral views of cochleae from heterozygous and hOtx12/hOtx12 mutant mice. Each of the mutant cochleae demonstrates abnormalities in both number of coils and shape (arrows). In addition, some coils have aberrant protrusions (arrowheads). En, embryonic day n. Orientation: A, anterior; L, lateral. (Scale bar = 200 μm.)
Genes Expressed in the VIIIth Cranial Ganglion/Neurogenic Region.
Neurons of the VIIIth ganglion are derived from otic epithelial cells that delaminate from the antero-lateral region of the otic cup/otocyst (37). Thus far, there is no direct evidence suggesting that normal formation of the VIIIth ganglion affects inner ear development. However, from gene expression studies, the neurogenic region and the presumptive sensory organs of the utricle, saccule, and cochlea most likely share a common Lunatic fringe (L-fng) expression domain (4, 38, 39). Interestingly, mice with a deletion of a basic helix–loop–helix gene, neurogenin 1 (ngn 1), fail to form the VIIIth ganglion, and maculae of both the utricle and saccule are smaller in size (11). The length of the cochlear duct is also shorter compared with that of wild type. An attractive interpretation of these results is that the absence of ngn 1 causes the loss of progenitor cells that normally give rise to sensory neurons as well as sensory hair cells and supporting cells (11). As a result, defects are observed in both the ganglion and sensory epithelia. However, even though ngn 1 is expressed in the expected neurogenic region in the otocyst stage (40), the expression of ngn 1 in later stages of inner ear development has not been reported. Therefore, it is equally likely that ngn 1 is expressed in both the neurogenic and sensory regions, and that this gene is independently required for the normal development of these regions. In this scenario, the development of the neurogenic and sensory fates are not related.
In addition, the absence of the spiral ganglion may affect the proper formation of the modiolus (the bony tube that forms the central axis of the cochlea), which in turn may have a secondary effect on the final shape of the cochlea. In ngn 1-knockout mice, the modiolus is also missing and the coiling of the cochlea is tighter than is observed in wild-type mice (11). Despite the defects in the ganglion and sensory organs of ngn 1-mutant mice, the sensory hair cells appeared normal. This observation is consistent with the idea that normal innervation is not required for hair cell differentiation, at least until birth (41, 42).
Relationship Between Sensory Organ Specification and Gross Patterning of the Inner Ear.
Two lines of evidence suggest that in the developing inner ear, sensory tissues are specified before nonsensory structures. First, there are no examples of either zebrafish or mouse mutants in which nonsensory structures develop normally in the absence of any sensory tissues. However, there are examples of mutants that lack nonsensory structures but develop normal sensory structures (12, 43–45). These observations suggest that nonsensory structures do not develop without the prior specification of some sensory tissues.
The second line of evidence stems from transplantation experiments. When the antero-posterior (A/P) axis of the chicken inner ear is surgically reversed at a stage when the otocyst is almost closed, the A/P axis of the sensory organs in this transplanted inner ear is already specified by the donor. In contrast, the A/P axis of the nonsensory structures such as the semicircular canals are respecified according to the new axial information from the host (46). As a result, in such a transplanted inner ear, the posterior crista, for example, is now located in the anterior region of the inner ear, and the posterior semicircular canal that is normally connected to the posterior ampulla is positioned anteriorly and adopts the pattern of an anterior canal. This evidence strongly suggests that there is a temporal delay in the specification of sensory versus nonsensory tissues.
Thus far, there are no reported mutants in zebrafish or mice that have normal semicircular canals (nonsensory) but lack their corresponding sensory tissues, the cristae (12, 43–45). This observation raises an interesting possibility that the development of nonsensory tissues within the inner ear is under the influence of sensory structures. The identification of such signaling molecules produced by sensory tissues will be essential in unraveling the formation of this complex organ. Recently, it has been suggested that fibroblast growth factor (FGF) 10, produced in the sensory regions, is one of the ligands directing development of adjacent nonsensory structures that express its receptor, Fgfr2 (47). These results are supported by the fact that knockout of Fgfr2 IIIb, one of the two functional isoforms of Fgfr2, yielded an inner ear with poor vestibular as well as cochlear development. However, sensory patches in these mutant mice are also rudimentary, suggesting that nonsensory tissues may also feedback on sensory tissues for their further development. As more and more of these signaling molecules are identified, it should be feasible to establish a hierarchy of molecular events starting from otic induction to a mature inner ear.
Most existing inner ear mutants display defects in both sensory and nonsensory structures (12, 43–45). The genes involved may play a role in specifying or coordinating sensory and nonsensory development. Depending on the domain of expression and the type of gene product, these genes could also be independently required for the formation of the structures involved. In Otx1-knockout mice, both the lateral canal and crista do not form (10). Gene expression and paint-fill data suggest that the presumptive lateral crista and the lateral canal are present initially but fail to develop in the mutant. Therefore, Otx1 is most likely playing a role in the continued development of the prespecified lateral crista and canal. It remains to be determined whether Otx1 plays a role in coordinating the development of these two structures (10).
Conclusion
Knockout and mutant mice will continue to be an indispensable tool in understanding normal development of the inner ear. However, to decipher the molecular mechanisms that underlie the normal developmental process, efforts must be invested beyond mere documentation of mutant phenotypes. For any given gene, it is important to determine where along the developmental cascade a given gene acts, with whom it interacts, and how its functions. Correlating pattern of expression with phenotype is a first step toward achieving that goal. More sophisticated gene targeting approaches designed to remove gene functions in a spatially or temporally restricted manner will also facilitate the deciphering of the development of this complex organ.
Acknowledgments
We thank Drs. Susan Sullivan and Bernd Fritzsch for critically reviewing this manuscript.
Abbreviation
- dpc
days postcoitium
Footnotes
This paper was presented at the National Academy of Sciences colloquium “Auditory Neuroscience: Development, Transduction, and Integration,” held May 19–21, 2000, at the Arnold and Mabel Beckman Center in Irvine, CA.
References
- 1.Hudspeth A J. Nature (London) 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- 2.Echteler S M, Fay R R, Popper A N. In: Comparative Hearing: Mammals. Fay R R, Popper A N, editors. New York: Springer; 1994. pp. 134–171. [Google Scholar]
- 3.Lewis E R, Leverenz E L, Bialek W. The Vertebrate Inner Ear. Boca Raton, FL: CRC; 1985. [Google Scholar]
- 4.Morsli H, Choo D, Ryan A, Johnson R, Wu D K. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruben R J. Acta Otolaryngol Suppl. 1967;220:1–44. [PubMed] [Google Scholar]
- 6.Wangeman P, Schacht J. In: The Cochlea. Dallos P, Popper A N, Fay R R, editors. Vol. 8. New York: Springer; 1996. p. 551. [Google Scholar]
- 7.Cowan C A, Yokoyama N, Bianchi L M, Henkemeyer M, Fritzsch B. Neuron. 2000;26:417–430. doi: 10.1016/s0896-6273(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 8.Hadrys T, Braun T, Rinkwitz-Brandt S, Arnold H H, Bober E. Development (Cambridge, UK) 1998;125:33–39. doi: 10.1242/dev.125.1.33. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Van De Water T, Lufkin T. Development (Cambridge, UK) 1998;125:621–634. doi: 10.1242/dev.125.4.621. [DOI] [PubMed] [Google Scholar]
- 10.Morsli H, Tuorto F, Choo D, Postiglione M P, Simeone A, Wu D K. Development (Cambridge, UK) 1999;126:2335–2343. doi: 10.1242/dev.126.11.2335. [DOI] [PubMed] [Google Scholar]
- 11.Ma, Q., Anderson, D. J. & Fritzsch, B. (2000) J. Assoc. Res. Otolaryngol., in press. [DOI] [PMC free article] [PubMed]
- 12.Fekete D M. Trends Neurosci. 1999;22:263–269. doi: 10.1016/s0166-2236(98)01366-6. [DOI] [PubMed] [Google Scholar]
- 13.Torres M, Giraldez F. Mech Dev. 1998;71:5–21. doi: 10.1016/s0925-4773(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 14.Fritzsch B, Barald K F, Lomax M I. In: Springer Handbook of Auditory Research. Rubel E W, Popper A N, Fay RR, editors. Vol. 12. New York: Springer; 1998. pp. 81–145. [Google Scholar]
- 15.Fekete D M. Curr Opin Neurobiol. 1996;6:533–541. doi: 10.1016/s0959-4388(96)80061-4. [DOI] [PubMed] [Google Scholar]
- 16.Noden D M, Van de Water T R. Trends Neurosci. 1992;15:235–237. doi: 10.1016/0166-2236(92)90056-e. [DOI] [PubMed] [Google Scholar]
- 17.Torres M, Gomez-Pardo E, Gruss P. Development (Cambridge, UK) 1996;122:3381–3391. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- 18.Xu P X, Adams J, Peters H, Brown M C, Heaney S, Maas R. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- 19.Rinkwitz-Brandt S, Arnold H H, Bober E. Hear Res. 1996;99:129–138. doi: 10.1016/s0378-5955(96)00093-7. [DOI] [PubMed] [Google Scholar]
- 20.Nornes H O, Dressler G R, Knapik E W, Deutsch U, Gruss P. Development (Cambridge, UK) 1990;109:797–809. doi: 10.1242/dev.109.4.797. [DOI] [PubMed] [Google Scholar]
- 21.Mansouri A, Chowdhury K, Gruss P. Nat Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- 22.Xu P X, Woo I, Her H, Beier D R, Maas R L. Development (Cambridge, UK) 1997;124:219–231. doi: 10.1242/dev.124.1.219. [DOI] [PubMed] [Google Scholar]
- 23.Simeone A, Acampora D, Pannese M, D'Esposito M, Stornaiuolo A, Gulisano M, Mallamaci A, Kastury K, Druck T, Huebner K, et al. Proc Natl Acad Sci USA. 1994;91:2250–2254. doi: 10.1073/pnas.91.6.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson G W, Mahon K A. Mech Dev. 1994;48:199–215. doi: 10.1016/0925-4773(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 25.Acampora D, Merlo G R, Paleari L, Zerega B, Postiglione M P, Mantero S, Bober E, Barbieri O, Simeone A, Levi G. Development (Cambridge, UK) 1999;126:3795–3809. doi: 10.1242/dev.126.17.3795. [DOI] [PubMed] [Google Scholar]
- 26.Depew M J, Liu J K, Long J E, Presley R, Meneses J J, Pedersen R A, Rubenstein J L. Development (Cambridge, UK) 1999;126:3831–3846. doi: 10.1242/dev.126.17.3831. [DOI] [PubMed] [Google Scholar]
- 27.Qiu M, Bulfone A, Martinez S, Meneses J J, Shimamura K, Pedersen R A, Rubenstein J L. Genes Dev. 1995;9:2523–2538. doi: 10.1101/gad.9.20.2523. [DOI] [PubMed] [Google Scholar]
- 28.Morasso M I, Grinberg A, Robinson G, Sargent T D, Mahon K A. Proc Natl Acad Sci USA. 1999;96:162–167. doi: 10.1073/pnas.96.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acampora D, Mazan S, Avantaggiato V, Barone P, Tuorto F, Lallemand Y, Brulet P, Simeone A. Nat Genet. 1996;14:218–222. doi: 10.1038/ng1096-218. [DOI] [PubMed] [Google Scholar]
- 30.Acampora D, Mazan S, Lallemand Y, Avantaggiato V, Maury M, Simeone A, Brulet P. Development (Cambridge, UK) 1995;121:3279–3290. doi: 10.1242/dev.121.10.3279. [DOI] [PubMed] [Google Scholar]
- 31.Royet J, Finkelstein R. Development (Cambridge, UK) 1995;121:3561–3572. doi: 10.1242/dev.121.11.3561. [DOI] [PubMed] [Google Scholar]
- 32.Hirth F, Therianos S, Loop T, Gehring W J, Reichert H, Furukubo-Tokunaga K. Neuron. 1995;15:769–778. doi: 10.1016/0896-6273(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 33.Ang S L, Jin O, Rhinn M, Daigle N, Stevenson L, Rossant J. Development (Cambridge, UK) 1996;122:243–252. doi: 10.1242/dev.122.1.243. [DOI] [PubMed] [Google Scholar]
- 34.Acampora D, Avantaggiato V, Tuorto F, Barone P, Perera M, Choo D, Wu D, Corte G, Simeone A. Development (Cambridge, UK) 1999;126:1417–1426. doi: 10.1242/dev.126.7.1417. [DOI] [PubMed] [Google Scholar]
- 35.Acampora D, Avantaggiato V, Tuorto F, Barone P, Reichert H, Finkelstein R, Simeone A. Development (Cambridge, UK) 1998;125:1691–1702. doi: 10.1242/dev.125.9.1691. [DOI] [PubMed] [Google Scholar]
- 36.Acampora D, Avantaggiato V, Tuorto F, Briata P, Corte G, Simeone A. Development (Cambridge, UK) 1998;125:5091–5104. doi: 10.1242/dev.125.24.5091. [DOI] [PubMed] [Google Scholar]
- 37.Carney P R, Couve E. Anat Rec. 1989;225:156–164. doi: 10.1002/ar.1092250211. [DOI] [PubMed] [Google Scholar]
- 38.Cole L K, Le Roux I, Nunes F, Laufer E, Lewis J, Wu D K. J Comp Neurol. 2000;42:509–520. doi: 10.1002/1096-9861(20000828)424:3<509::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 39.Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Development (Cambridge, UK) 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- 40.Ma Q, Chen Z, Barrantes I, de la Pompa J L, Anderson D J. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- 41.Fritzsch B, Silos-Santiago I, Bianchi L M, Farinas I. Semin Cell Dev Biol. 1997;8:277–284. [PubMed] [Google Scholar]
- 42.Silos-Santiago I, Fagan A M, Garber M, Fritzsch B, Barbacid M. Eur J Neurosci. 1997;9:2045–2056. doi: 10.1111/j.1460-9568.1997.tb01372.x. [DOI] [PubMed] [Google Scholar]
- 43.Whitfield T T, Granato M, van Eeden F J, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg C P, Jiang Y J, et al. Development (Cambridge, UK) 1996;123:241–254. doi: 10.1242/dev.123.1.241. [DOI] [PubMed] [Google Scholar]
- 44.Malicki J, Schier A F, Solnica-Krezel L, Stemple D L, Neuhauss S C, Stainier D Y, Abdelilah S, Rangini Z, Zwartkruis F, Driever W. Development (Cambridge, UK) 1996;123:275–283. doi: 10.1242/dev.123.1.275. [DOI] [PubMed] [Google Scholar]
- 45.Deol M S. In: Development of Auditory and Vestibular Systems. Romand R, editor. London: Academic; 1983. pp. 309–333. [Google Scholar]
- 46.Wu D K, Nunes F D, Choo D. Development (Cambridge, UK) 1998;125:11–20. doi: 10.1242/dev.125.1.11. [DOI] [PubMed] [Google Scholar]
- 47.Pirvola U, Spencer-Dene B, Xing-Qun L, Kettunen P, Thesleff I, Fritzsch B, Dickson C, Ylikoski J. J Neurosci. 2000;20:6125–6134. doi: 10.1523/JNEUROSCI.20-16-06125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Kok Y J, van der Maarel S M, Bitner-Glindzicz M, Huber I, Monaco A P, Malcolm S, Pembrey M E, Ropers H H, Cremers F P. Science. 1995;267:685–688. doi: 10.1126/science.7839145. [DOI] [PubMed] [Google Scholar]
- 49.Phippard D, Heydemann A, Lechner M, Lu L, Lee D, Kyin T, Crenshaw E B., 3rd Hear Res. 1998;120:77–85. doi: 10.1016/s0378-5955(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 50.Phippard D, Lu L, Lee D, Saunders J C, Crenshaw E B., 3rd J Neurosci. 1999;19:5980–5989. doi: 10.1523/JNEUROSCI.19-14-05980.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minowa O, Ikeda K, Sugitani Y, Oshima T, Nakai S, Katori Y, Suzuki M, Furukawa M, Kawase T, Zheng Y, et al. Science. 1999;285:1408–1411. doi: 10.1126/science.285.5432.1408. [DOI] [PubMed] [Google Scholar]
- 52.Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, et al. Nat Genet. 1997;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- 53.Kalatzis V, Sahly I, El-Amraoui A, Petit C. Dev Dyn. 1998;213:486–499. doi: 10.1002/(SICI)1097-0177(199812)213:4<486::AID-AJA13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 54.Mansour S L. Mol Reprod Dev. 1994;39:62–68. doi: 10.1002/mrd.1080390111. [DOI] [PubMed] [Google Scholar]
- 55.Mansour S L, Goddard J M, Capecchi M R. Development (Cambridge, UK) 1993;117:13–28. doi: 10.1242/dev.117.1.13. [DOI] [PubMed] [Google Scholar]
- 56.McKay I J, Lewis J, Lumsden A. Dev Biol. 1996;174:370–378. doi: 10.1006/dbio.1996.0081. [DOI] [PubMed] [Google Scholar]
- 57.Wilkinson D G, Bhatt S, McMahon A P. Development (Cambridge, UK) 1989;105:131–136. doi: 10.1242/dev.105.1.131. [DOI] [PubMed] [Google Scholar]
- 58.Mark M, Lufkin T, Vonesch J L, Ruberte E, Olivo J C, Dolle P, Gorry P, Lumsden A, Chambon P. Development (Cambridge, UK) 1993;119:319–338. doi: 10.1242/dev.119.2.319. [DOI] [PubMed] [Google Scholar]
- 59.Murphy P, Hill R E. Development (Cambridge, UK) 1991;111:61–74. doi: 10.1242/dev.111.1.61. [DOI] [PubMed] [Google Scholar]
- 60.Chisaka O, Musci T S, Capecchi M R. Nature (London) 1992;355:516–520. doi: 10.1038/355516a0. [DOI] [PubMed] [Google Scholar]
- 61.Lufkin T, Dierich A, LeMeur M, Mark M, Chambon P. Cell. 1991;66:1105–1119. doi: 10.1016/0092-8674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- 62.Gavalas A, Studer M, Lumsden A, Rijli F M, Krumlauf R, Chambon P. Development (Cambridge, UK) 1998;125:1123–1136. doi: 10.1242/dev.125.6.1123. [DOI] [PubMed] [Google Scholar]
- 63.Maconochie M, Nonchev S, Morrison A, Krumlauf R. Annu Rev Genet. 1996;30:529–556. doi: 10.1146/annurev.genet.30.1.529. [DOI] [PubMed] [Google Scholar]
- 64.Rijli F M, Mark M, Lakkaraju S, Dierich A, Dolle P, Chambon P. Cell. 1993;75:1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- 65.Cordes S P, Barsh G S. Cell. 1994;79:1025–1034. doi: 10.1016/0092-8674(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 66.Deol M S. J Embryol Exp Morph. 1964;12:475–490. [PubMed] [Google Scholar]
- 67.Ruben R. Laryngoscope. 1973;83:1440–1468. doi: 10.1288/00005537-197309000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, Senft E, Wurst W, Blanquet V, Grimes P, Sporle R, Schughart K. Proc Natl Acad Sci USA. 1996;93:13870–13875. doi: 10.1073/pnas.93.24.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goulding M D, Chalepakis G, Deutsch U, Erselius J R, Gruss P. EMBO J. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goulding M D, Lumsden A, Gruss P. Development (Cambridge, UK) 1993;117:1001–1016. doi: 10.1242/dev.117.3.1001. [DOI] [PubMed] [Google Scholar]
- 71.Goulding M, Sterrer S, Fleming J, Balling R, Nadeau J, Moore K J, Brown S D, Steel K P, Gruss P. Genomics. 1993;17:355–363. doi: 10.1006/geno.1993.1332. [DOI] [PubMed] [Google Scholar]
- 72.Tassabehji M, Read A P, Newton V E, Harris R, Balling R, Gruss P, Strachan T. Nature (London) 1992;355:635–636. doi: 10.1038/355635a0. [DOI] [PubMed] [Google Scholar]
- 73.Deol M. Nature (London) 1966;209:219–220. doi: 10.1038/209219a0. [DOI] [PubMed] [Google Scholar]