Abstract
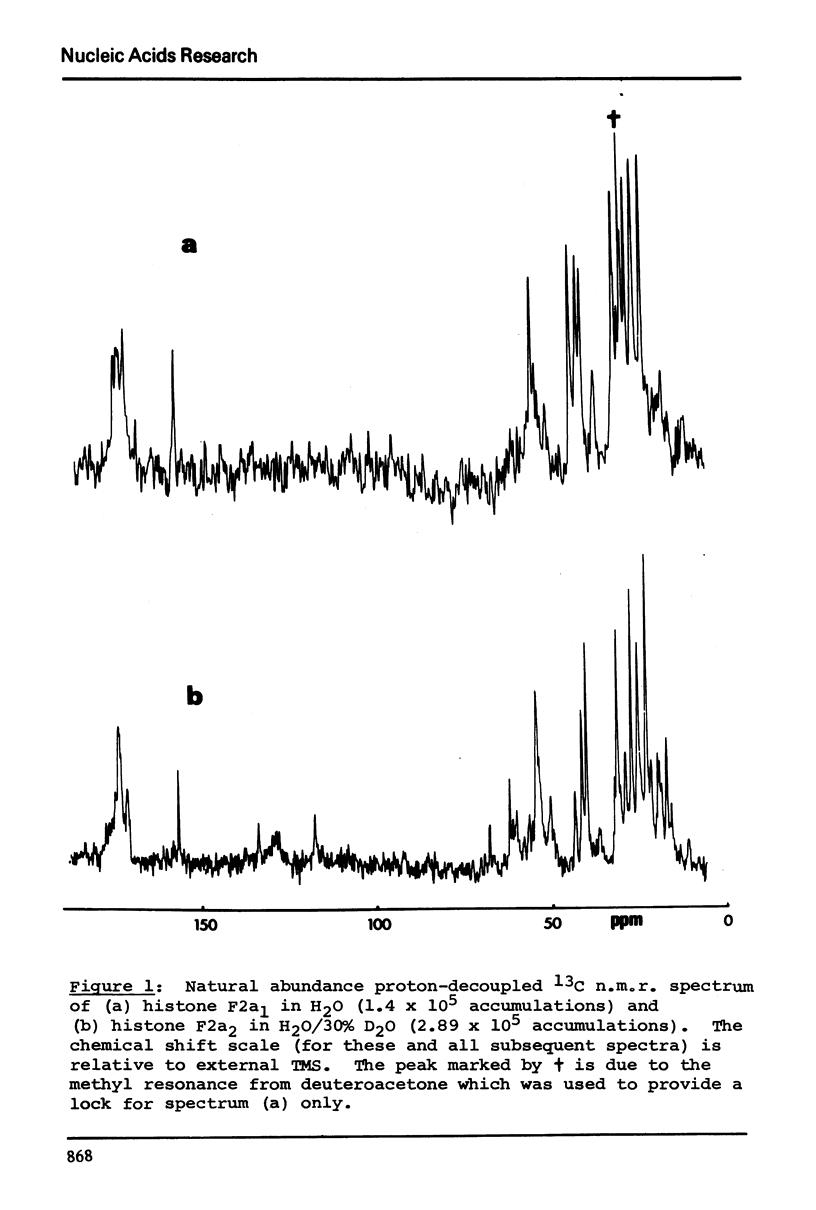
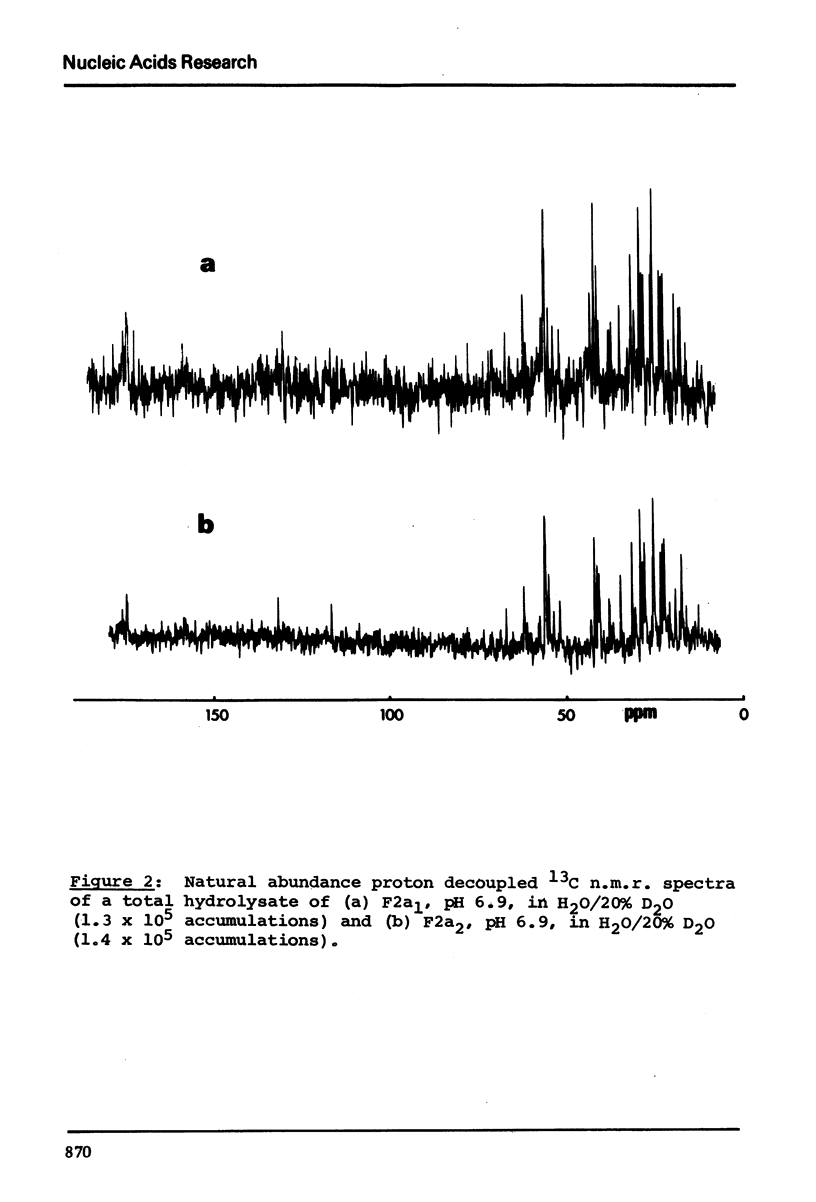
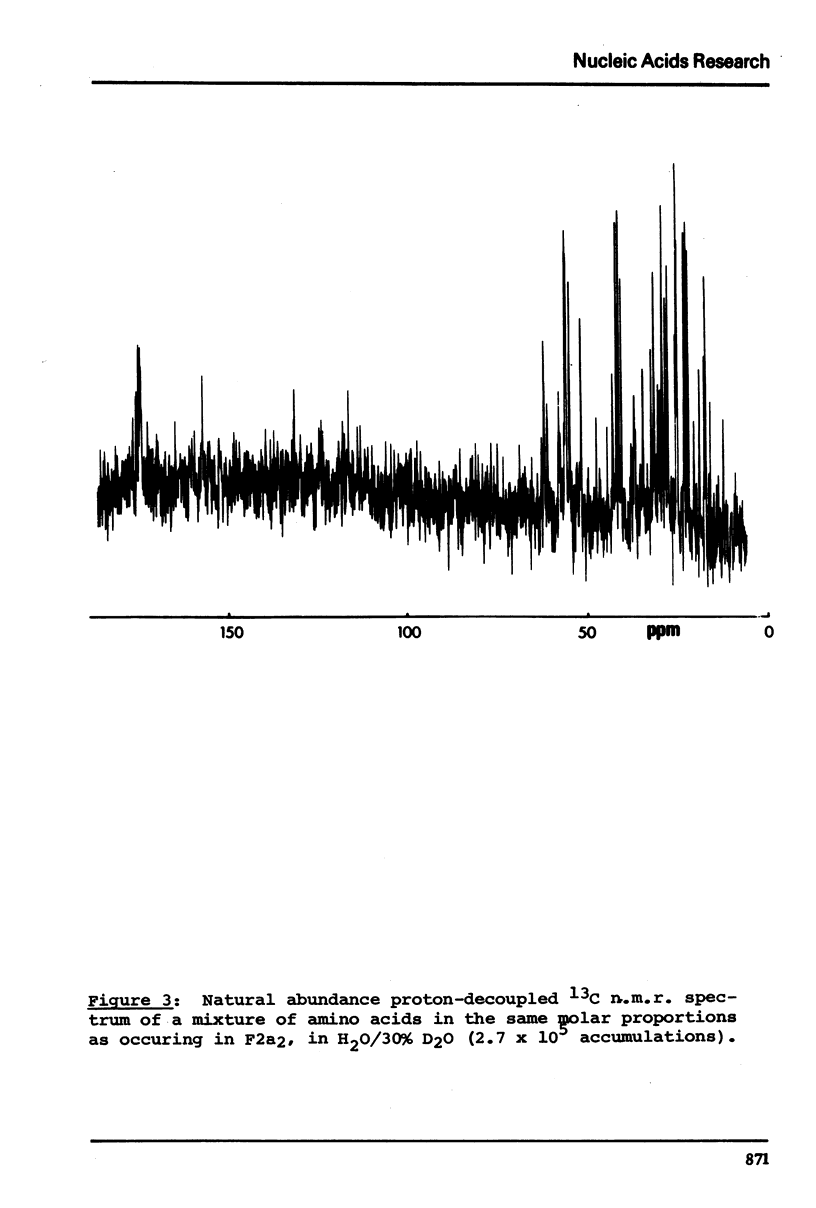
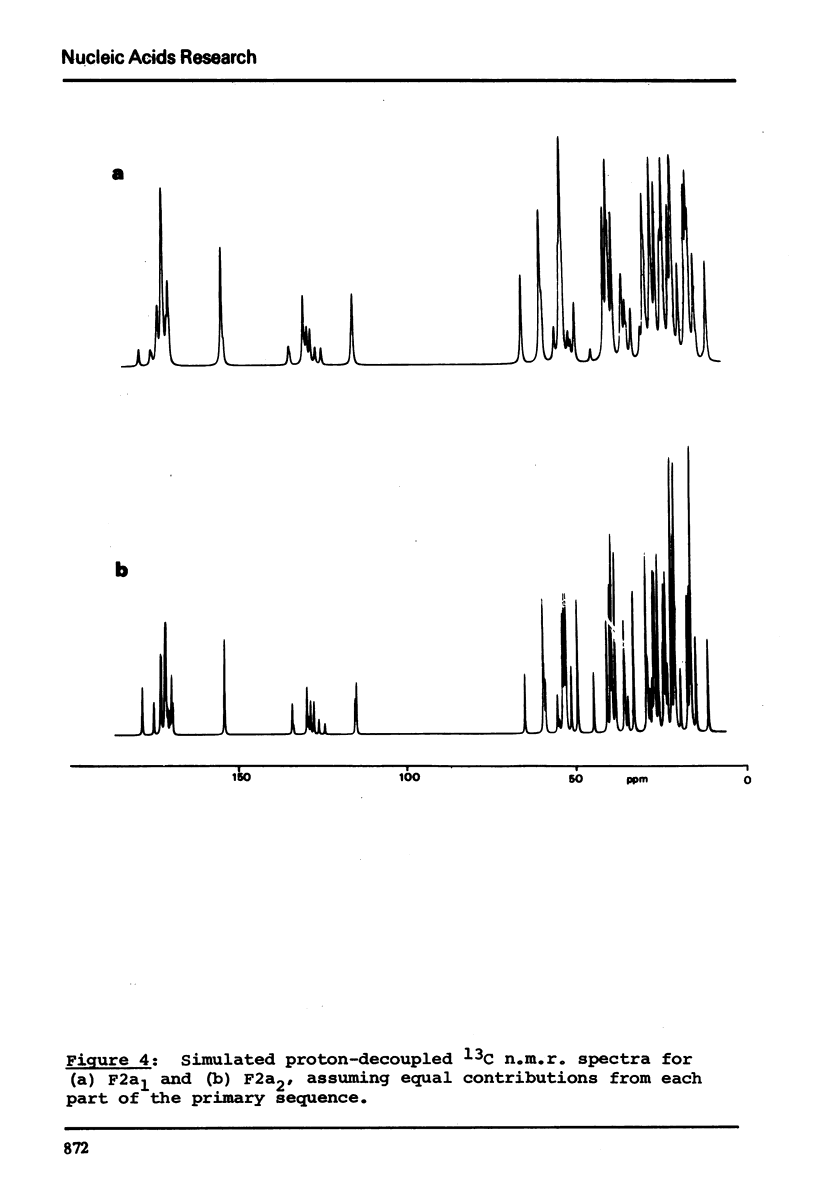
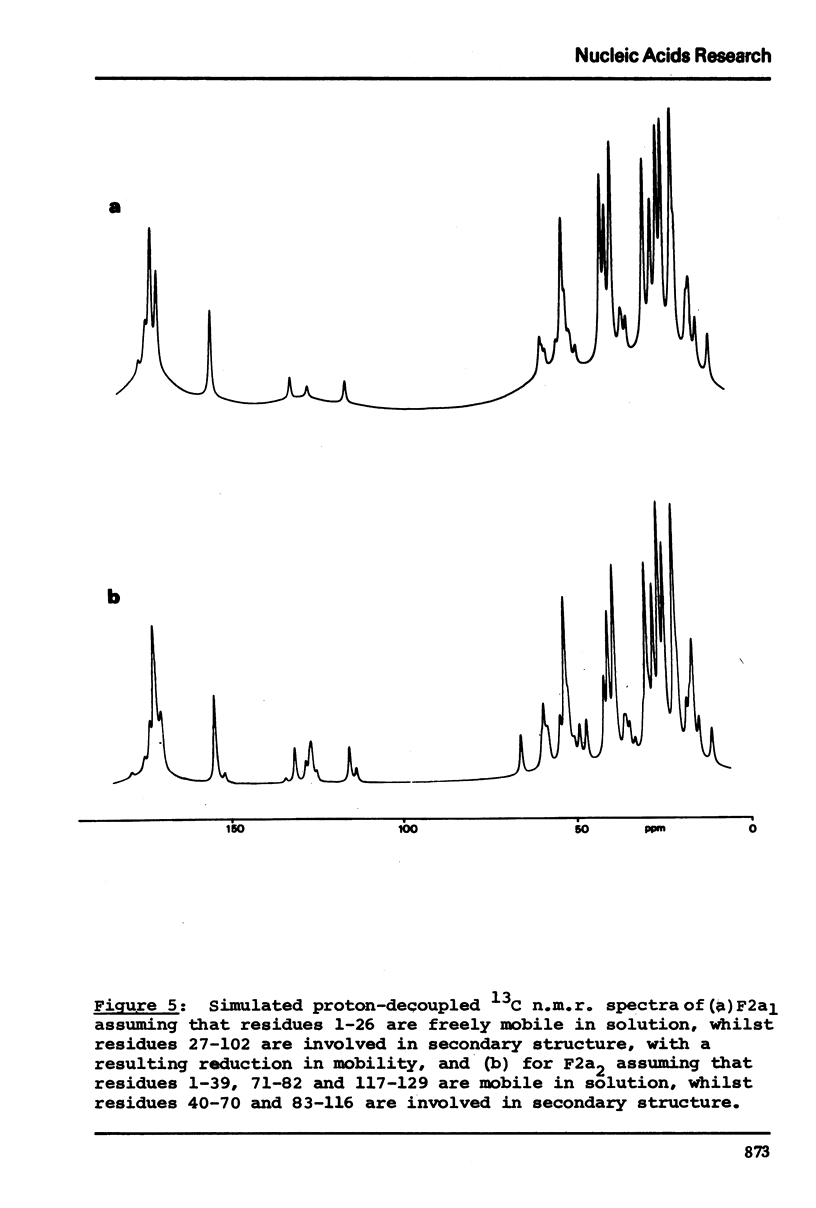
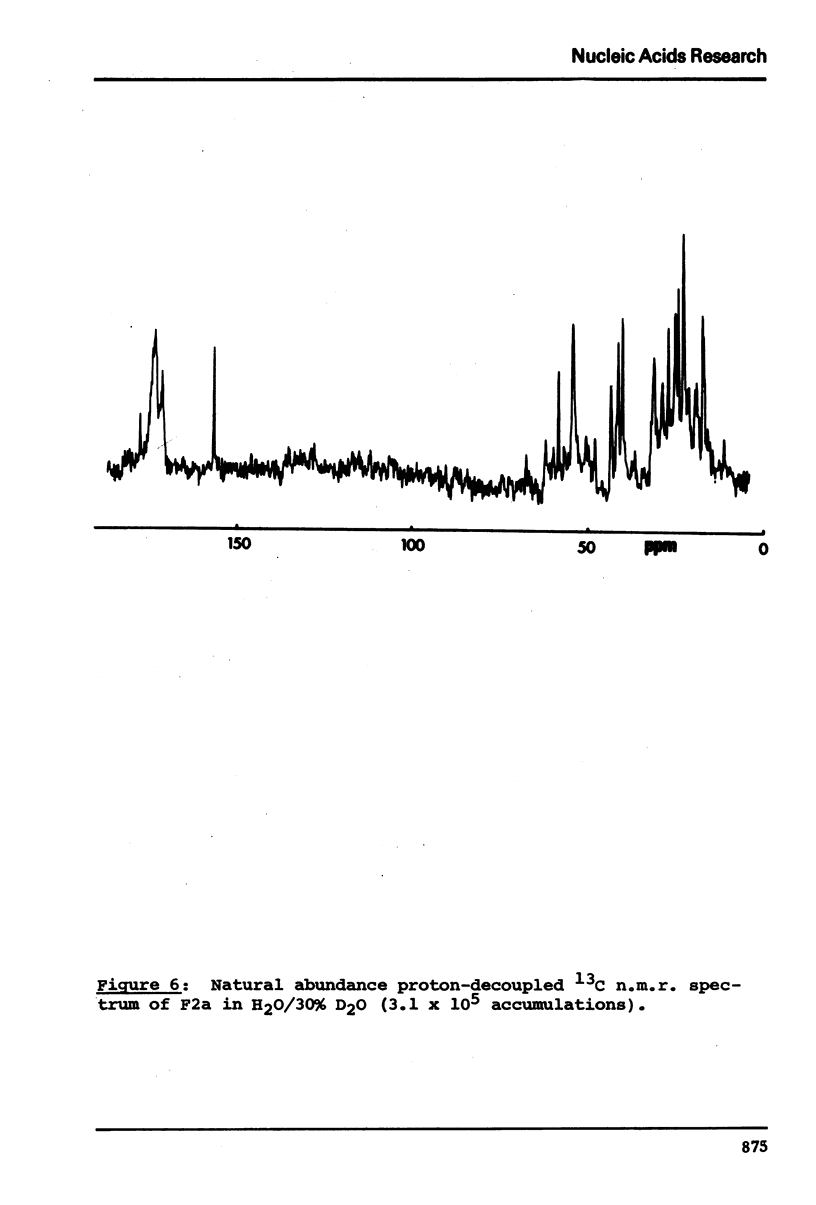
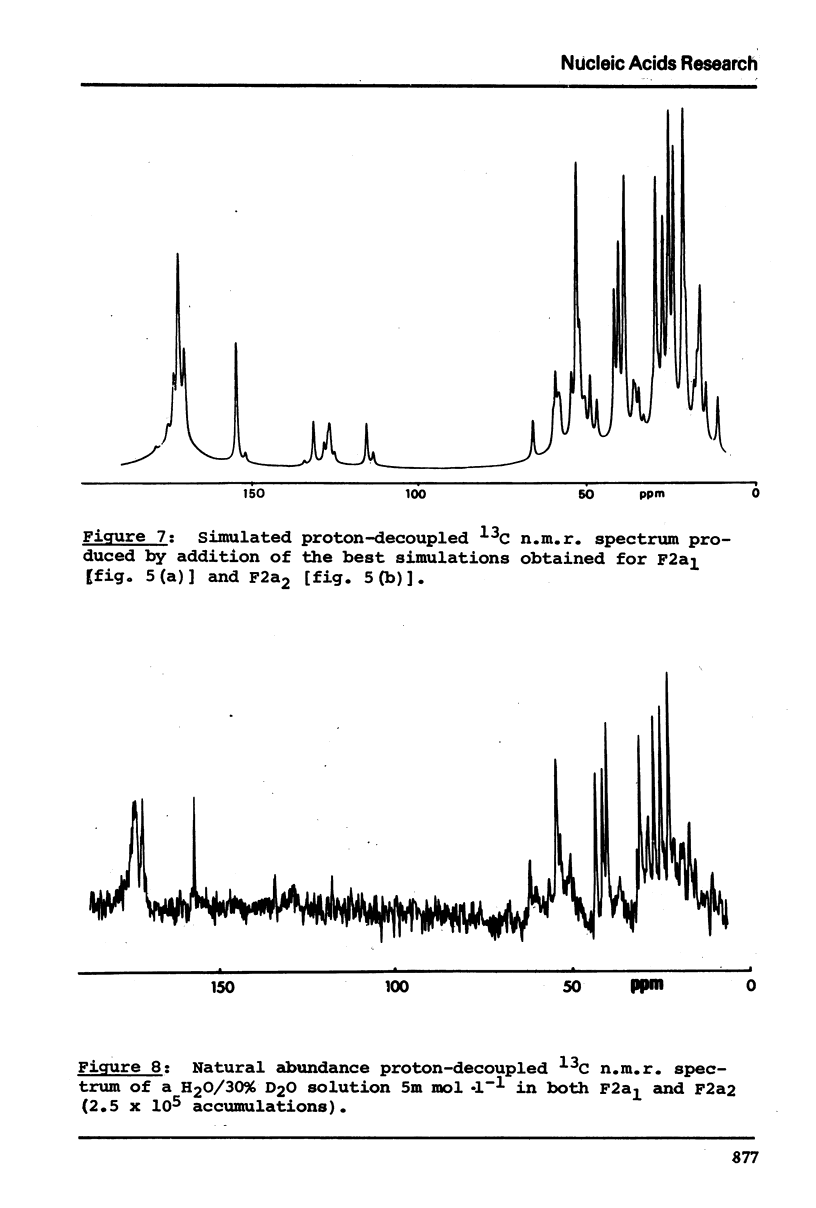
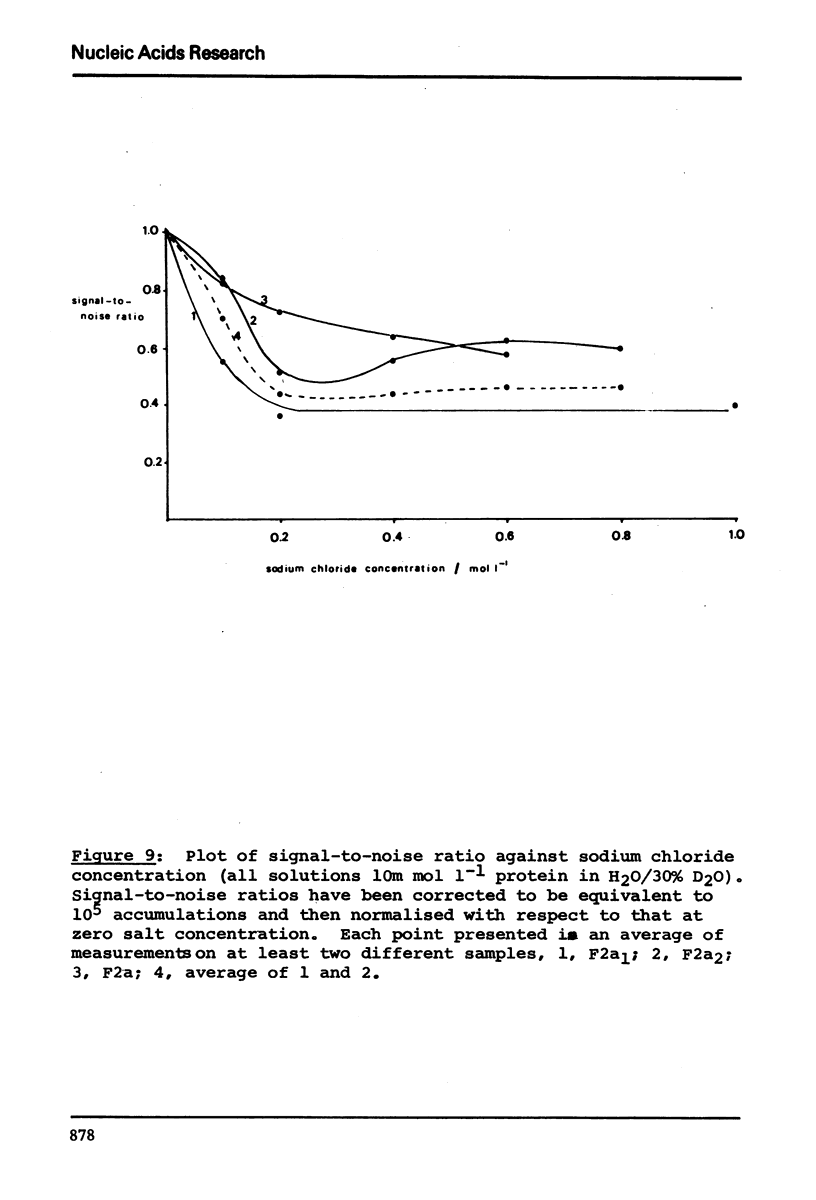
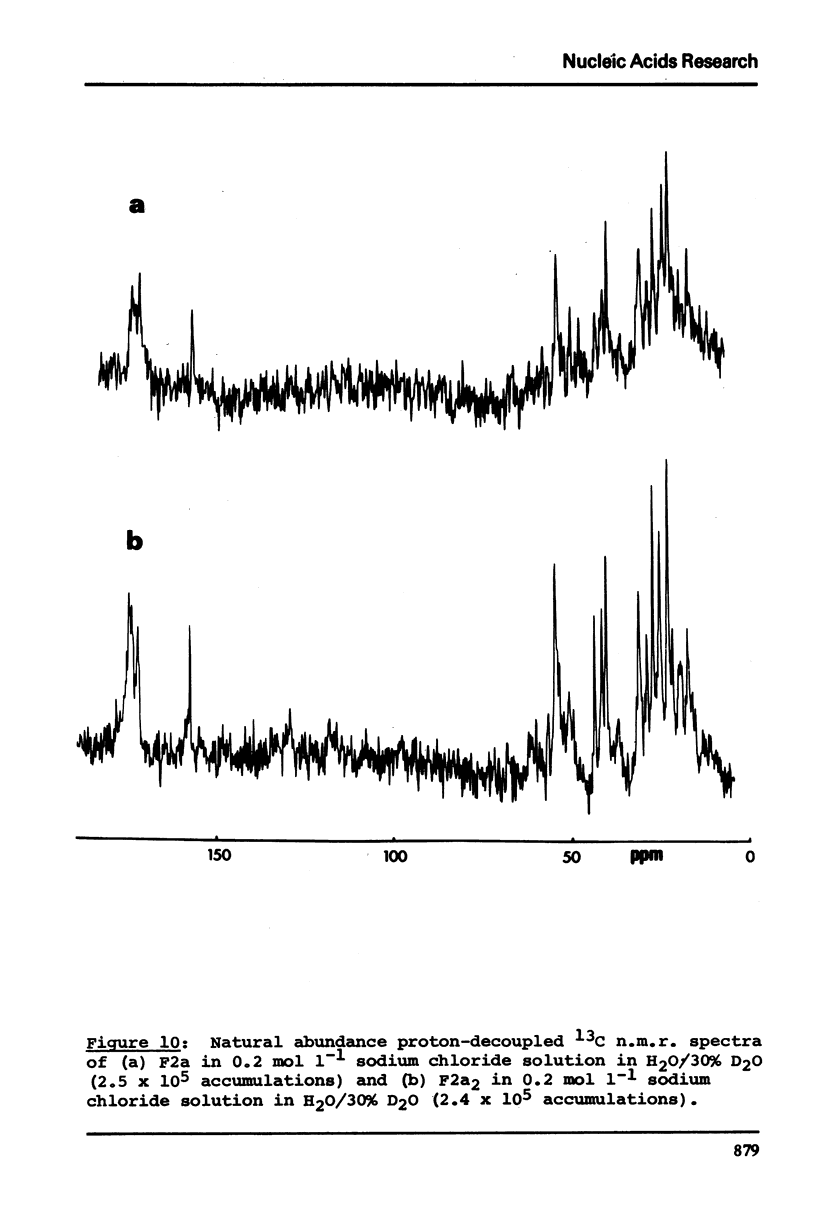
13C n.m.r. spectra have been obtained for aqueous solutions of histones F2a1 and F2a2, for the group F2a, for the appropriate amino acid mixturesand for the corresponding hydrolysates. These, when compared with computer simulated spectra give good agreement for secondary structure with that calculated from the known primary structure of the proteins. Evidence based on the spectra obtained at various salt concentrations leads to the conclusion that F2a is not a simple mixture but an interacting heterologous group of histones F2a1 and F2a2.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allerhand A., Oldfield E. Determination of rotational mobilities of backbone and side-chain carbons of poly(gamma-benzyl L-glutamate) in the helical and random-coil states from measurements of carbon-13 relaxation times and nuclear Overhauser enhancements. Biochemistry. 1973 Aug 28;12(18):3428–3433. doi: 10.1021/bi00742a011. [DOI] [PubMed] [Google Scholar]
- Boublík M., Bradbury E. M., Crane-Robinson C. An investigation of the conformational changes in histones F1 and F2a1 by proton magnetic resonance spectroscopy. Eur J Biochem. 1970 Jul;14(3):486–497. doi: 10.1111/j.1432-1033.1970.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Bradbury J. H., Norton R. S. Carbon-13 NMR spectra of tryptophan, tryptophan peptides and of native and denatured proteins. Biochim Biophys Acta. 1973 Nov 11;328(1):10–19. doi: 10.1016/0005-2795(73)90324-3. [DOI] [PubMed] [Google Scholar]
- Browne D. T., Kenyon G. L., Packer E. L., Sternlicht H., Wilson D. M. Studies of macromolecular structure by 13 C nuclear magnetic resonance. II. A specific labeling approach to the study of histidine residues in proteins. J Am Chem Soc. 1973 Feb 21;95(4):1316–1323. doi: 10.1021/ja00785a050. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Jr, Isenberg I. A complex of histones IIb2 and IV. Biochemistry. 1973 Mar 13;12(6):1035–1043. doi: 10.1021/bi00730a003. [DOI] [PubMed] [Google Scholar]
- Freedman M. H., Cohen J. S., Chaiken I. M. Carbon-13 Fourier transform nuclear magnetic resonance studies of peptides. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1148–1155. doi: 10.1016/0006-291x(71)90025-8. [DOI] [PubMed] [Google Scholar]
- Freedman M. H., Lyerla J. R., Jr, Chaiken I. M., Cohen J. S. Carbon-13 nuclear-magnetic-resonance studies on selected amino acids, peptides, and proteins. Eur J Biochem. 1973 Jan 15;32(2):215–226. doi: 10.1111/j.1432-1033.1973.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Glushko V., Lawson P. J., Gurd F. R. Conformational states of bovine pancreatic ribonuclease A observed by normal and partially relaxed carbon 13 nuclear magnetic resonance. J Biol Chem. 1972 May 25;247(10):3176–3185. [PubMed] [Google Scholar]
- Johns E. W. A method for the selective extraction of histone fractions f2(a)1 and f2(a)2 from calf thymus deoxyribonucleoprotein at pH7. Biochem J. 1967 Nov;105(2):611–614. doi: 10.1042/bj1050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns E. W. The electrophoresis of histones in polyacrylamide gel and their quantitative determination. Biochem J. 1967 Jul;104(1):78–82. doi: 10.1042/bj1040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon J. F., Wilkins M. H. A super-coil model for nucleohistone. J Mol Biol. 1972 Jul 14;68(1):115–124. doi: 10.1016/0022-2836(72)90267-7. [DOI] [PubMed] [Google Scholar]
- Prothero J. W. Correlation between the distribution of amino acids and alpha helices. Biophys J. 1966 May;6(3):367–370. doi: 10.1016/S0006-3495(66)86662-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards B. M., Pardon J. F. The molecular structure of nucleohistone (DNH). Exp Cell Res. 1970 Sep;62(1):184–196. doi: 10.1016/0014-4827(79)90519-6. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]



